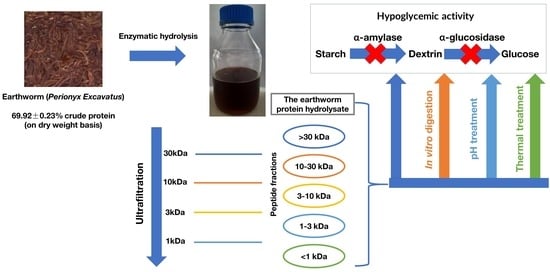
Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions
Abstract
Share and Cite
Bui, P.T.; Pham, K.T.; Vo, T.D.L. Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions. Processes 2023, 11, 2490. https://doi.org/10.3390/pr11082490
Bui PT, Pham KT, Vo TDL. Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions. Processes. 2023; 11(8):2490. https://doi.org/10.3390/pr11082490
Chicago/Turabian StyleBui, Phong T., Khoa T. Pham, and Tam D. L. Vo. 2023. "Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions" Processes 11, no. 8: 2490. https://doi.org/10.3390/pr11082490
APA StyleBui, P. T., Pham, K. T., & Vo, T. D. L. (2023). Earthworm (Perionyx excavatus) Protein Hydrolysate: Hypoglycemic Activity and Its Stability for the Hydrolysate and Its Peptide Fractions. Processes, 11(8), 2490. https://doi.org/10.3390/pr11082490