Photo-Programmable Processes in Bithiophene–Azobenzene Monolayers on Gold Probed via Simulations
Abstract
:1. Introduction
- Influenced the extent of conjugation;
- Affected dipole moment;
- Changed backbone rigidity,
- Brought new functionalities such as strong stacking between the aromatic units,
- Altered the system’s response to light stimulus (i.e., accelerated collective switching behavior or caused significant broadening of the optical absorption spectra);
- Emphasized the role of spacer length [14]: a shorter spacer should facilitate the charge transfer rate through the junction and increase conductance, while a longer spacer is prone to decoupling of the Azo from the electrode;
- Allowed larger dynamics of the switching event;
- Allowed a larger ON/OFF conductance ratio.
2. Models and Methods
3. Results
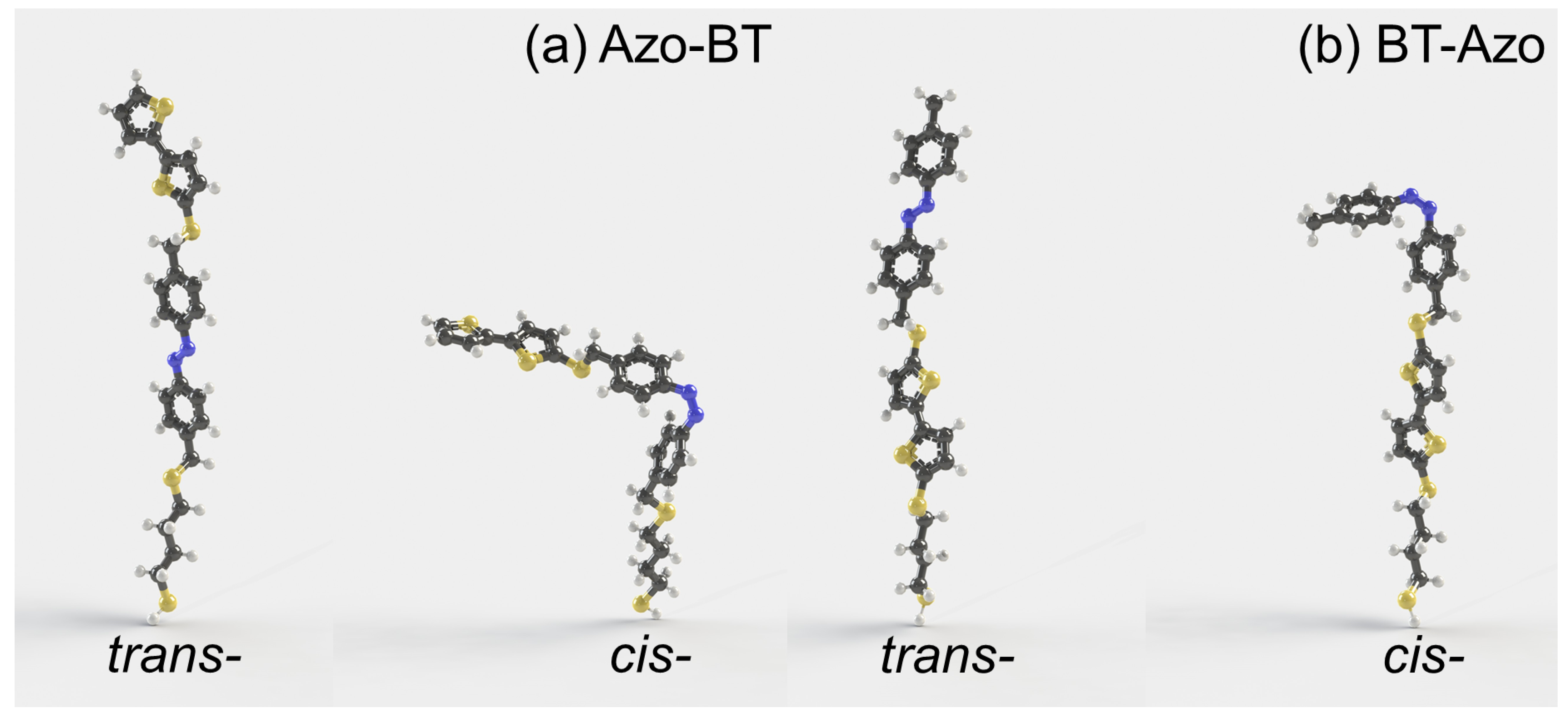
3.1. Properties of Isolated Molecules
- The values of the dipole moments (1.34 and 3.22 D for trans- and cis-BT-Azo; 1.67 and 3.52 D for trans- and cis-Azo-BT);
- The differences in the length of trans- and cis-isomers h are 6.05 Å and 14.69 Å for BT-Azo and Azo-BT, respectively; the experimental value of h = 5.1 Å for the layer of BT-Azo [25];
- Even though the molecular volume reduces upon the trans-cis isomerization for both MS, intermolecular sterical clashes may arise for cis-populated layers, especially for Azo-BT MS.
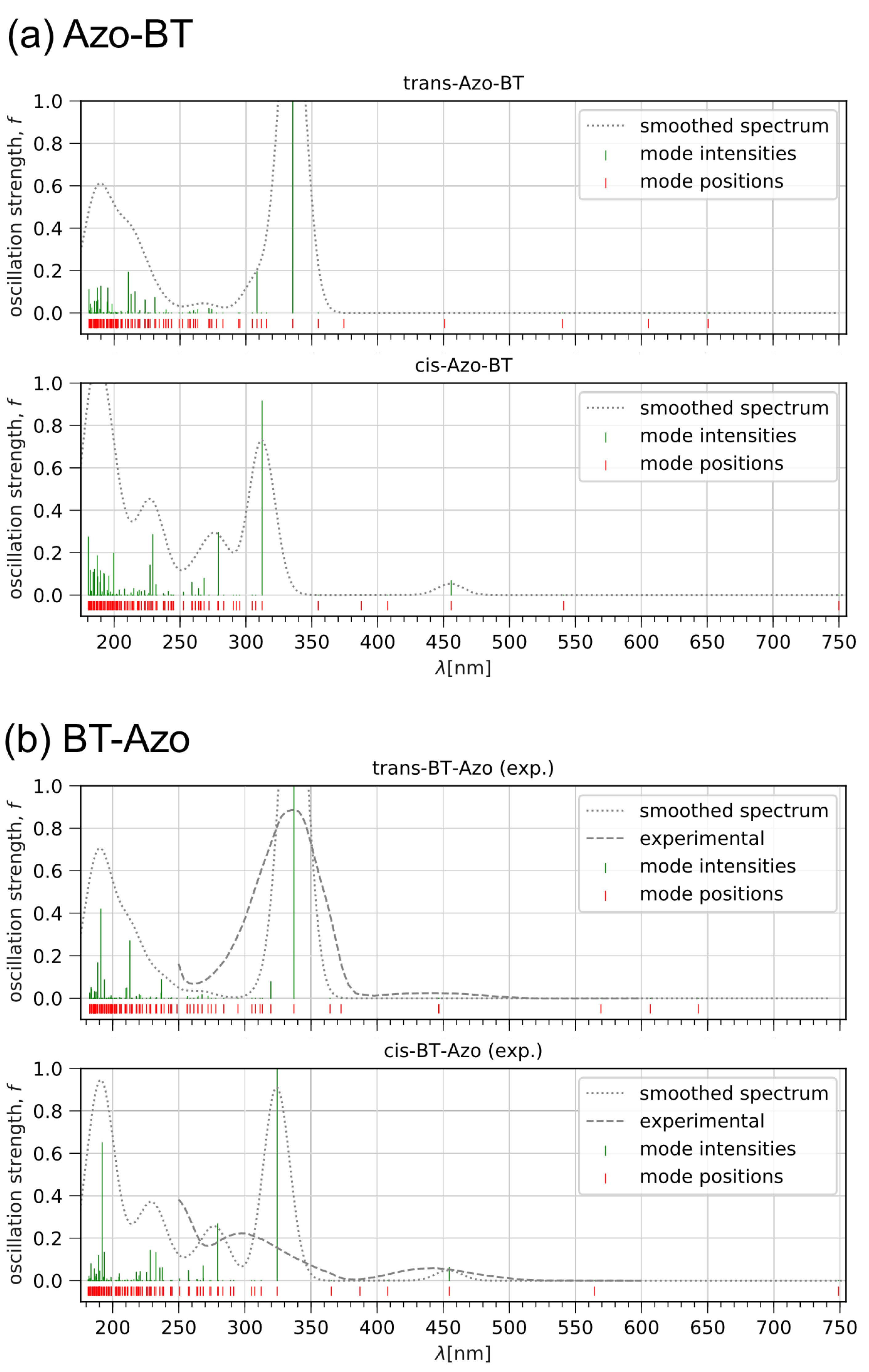
3.1.1. Optical Properties
3.1.2. Reorganization Energies
3.1.3. Gibbs Free Energy of Solvation and Its Changes upon Light Stimulus
3.2. Properties of Chemisorbed Monolayers
3.2.1. Structural Properties
3.2.2. Photo-Programmable Charge Transfer
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Azo | Azobenzene |
| BT | Bithiophene |
| MS | Molecular switch |
| MJ | Molecular junction |
| DFT | Density functional theory |
| MD | Molecular dynamics simulation |
References
- Fuentes, N.; Martin-Lasanta, A.; de Cienfuegos, L.Á.; Ribagorda, M.; Parra, A.; Cuerva, J.M. Organic-based molecular switches for molecular electronics. Nanoscale 2011, 3, 4003–4014. [Google Scholar] [CrossRef]
- Klajn, R. Immobilized azobenzenes for the construction of photoresponsive materials. Pure Appl. Chem. 2010, 82, 2247–2279. [Google Scholar] [CrossRef]
- Delorme, N.; Bardeau, J.-F.; Bulou, A.; Poncin-Epaillard, F. Azobenzene-containing monolayer with photoswitchable wettability. Langmuir 2005, 21, 12278–12282. [Google Scholar] [CrossRef]
- Takahashi, I.; Honda, Y.; Hirota, S. Regulating copper-binding affinity with photoisomerizable azobenzene ligand by construction of a self-assembled monolayer. Angew. Chem. Int. Ed. 2009, 48, 6065–6068. [Google Scholar] [CrossRef] [PubMed]
- Callari, F.L.; Petralia, S.; Conoci, S.; Sortino, S. Light-triggered DNA release by dynamic monolayer films. New J. Chem. 2008, 32, 1899–1903. [Google Scholar] [CrossRef]
- Kano, K.; Tanaka, Y.; Ogawa, T.; Shimomura, M.; Okahata, Y.; Kunitake, T. Photoresponsive memnranes. Regulation of membrane properties by photoreversible cis-trans isomerization of azobenzenes. Chem. Lett. 1980, 9, 421–424. [Google Scholar] [CrossRef]
- Bisby, R.H.; Mead, C.; Morgan, C.G. Wavelength-programmed solute release from photoresponsive liposomes. Biochem. Biophys. Res. Commun. 2000, 276, 169–173. [Google Scholar] [CrossRef]
- Morgan, C.G.; Thomas, E.W.; Sandhu, S.S.; Yianni, Y.P.; Mitchell, A.C. Light-induced fusion of liposomes with release of trapped marker dyu is sensitised by photochromic phospholipid. Biochim. Biophys. Acta (BBA) Biomembr. 1987, 903, 504–509. [Google Scholar] [CrossRef]
- Huang, X.; Li, T. Recent progress in the development of molecular-scale electronics based on photoswitchable molecules. J. Mater. Chem. C 2020, 8, 821–848. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, X.; Soni, S.; Chiechi, R.C. Charge transport through molecular ensembles: Recent progress in molecular electronics. Chem. Phys. Rev. 2021, 2, 021303. [Google Scholar] [CrossRef]
- Tang, C.; Shiri, M.; Zhang, H.; Ayinla, R.T.; Wang, K. Light-driven charge transport and optical sensing in molecular junctions. Nanomaterials 2022, 12, 698. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Hassan, S.Z.; So, C.; Kang, M.; Chung, D.S. Molecular-switch-embedded Solution-processed Semiconductors. Adv. Mater. 2023, 35, 2203401. [Google Scholar] [CrossRef] [PubMed]
- Karpe, S.; Oçafrain, M.; Smaali, K.; Lenfant, S.; Vuillaume, D.; Blanchard, P.; Roncali, J. Oligothiophene-derivatized azobenzene as immobilized photoswitchable conjugated systems. Chem. Commun. 2010, 46, 3657–3659. [Google Scholar] [CrossRef] [PubMed]
- Smaali, K.; Lenfant, S.; Karpe, S.; Oçafrain, M.; Blanchard, P.; Deresmes, D.; Godey, S.; Rochefort, A.; Roncali, J.; Vuillaume, D. High ON-OFF conductance switching ratio in optically-driven self-assembled conjugated molecular systems. ACS Nano 2010, 4, 2411–2421. [Google Scholar] [CrossRef]
- Ferri, V.; Elbing, M.; Pace, G.; Dickey, M.D.; Zharnikov, M.; Samorì, P.; Mayor, M.; Rampi, M.A. Light-powered electrical switch based on cargo-lifting azobenzene monolayers. Angew. Chem. Int. Ed. 2008, 18, 3455–3457. [Google Scholar] [CrossRef]
- Schuster, S.; Füser, M.; Asyuda, A.; Cyganik, P.; Terfort, A.; Zharnikov, M. Photoisomerization of azobenzene-substituted alkanethiolates on Au (111) substrates in the context of work function variation: The effect of structure and packing density. Phys. Chem. Chem. Phys. 2019, 21, 9098–9105. [Google Scholar] [CrossRef]
- Mativetsky, J.M.; Pace, G.; Elbing, M.; Rampi, M.A.; Mayor, M.; Samori, P. Azobenzenes as light-controlled molecular electronic switches in nanoscale metal- molecule- metal junctions. J. Am. Chem. Soc. 2008, 130, 9192–9193. [Google Scholar] [CrossRef]
- Thomas, L.; Arbouch, I.; Guérin, D.; Wallart, X.; Van Dyck, C.; Mélin, T.; Cornil, J.; Vuillaume, D.; Lenfant, S. Conductance switching of azobenzene-based self-assembled monolayers on cobalt probed by UHV conductive-AFM. Nanoscale 2021, 13, 6977–6990. [Google Scholar] [CrossRef]
- Crivillers, N.; Liscio, A.; Di Stasio, F.; Van Dyck, C.; Osella, S.; Cornil, D.; Mian, S.; Lazzerini, G.M.; Fenwick, O.; Orgiu, E.; et al. Photoinduced work function changes by isomerization of a densely packed azobenzene-based SAM on Au: A joint experimental and theoretical study. Phys. Chem. Chem. Phys. 2011, 13, 14302–14310. [Google Scholar] [CrossRef]
- Masillamani, A.M.; Osella, S.; Liscio, A.; Fenwick, O.; Reinders, F.; Mayor, M.; Palermo, V.; Cornil, J.; Samorì, P. Light-induced reversible modification of the work function of a new perfluorinated biphenyl azobenzene chemisorbed on Au (111). Nanoscale 2014, 6, 8969–8977. [Google Scholar] [CrossRef]
- Ah, Q.; Lloyd, F.N.; Akiyama, H.; Nagahiro, T.; Tamada, K.; Wee, A.T.S. Reversible work function changes induced by photoisomerization of asymmetric azobenzene dithiol self-assembled monolayers on gold. Appl. Phys. Lett. 2008, 93, 083109. [Google Scholar]
- Yamamoto, T.; Natsui, K.; Einaga, Y. Photo-Modulation of Superconducting and Magnetic Properties. In Photon-Working Switches; Springer: Berlin/Heidelberg, Germany, 2017; pp. 285–299. [Google Scholar]
- Suda, M.; Kameyama, N.; Ikegami, A.; Suzuki, M.; Kawamura, N.; Einaga, Y. Size-reduction induced ferromagnetism and photo-magnetic effects in azobenzene-thiol-passivated gold nanoparticles. Polyhedron 2009, 28, 1868–1874. [Google Scholar] [CrossRef]
- Karthäuser, S.; Peter, S.; Simon, U. Integration of individual functionalized gold nanoparticles into nanoelectrode configurations: Recent advances. Eur. J. Inorg. Chem. 2020, 40, 3798–3810. [Google Scholar] [CrossRef]
- Viero, Y.; Copie, G.; Guerin, D.; Krzeminski, C.; Vuillaume, D.; Lenfant, S.; Cleri, F. High conductance ratio in molecular optical switching of functionalized nanoparticle self-assembled nanodevices. J. Phys. Chem. C 2015, 119, 21173–21183. [Google Scholar] [CrossRef]
- Ahonen, P.; Laaksonen, T.; Schiffrin, D.J.; Kontturi, K. Photoswitching electron transport properties of an azobenzene containing thiol-SAM. Phys. Chem. Chem. Phys. 2007, 9, 4898–4901. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Yang, M.; Shin, N.; Hong, S. Mapping reversible photoswitching of molecular resistance fluctuations during the conformational transformation of azobenzene-terminated molecular switches. Nanotechnology 2018, 29, 365704. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Ye, T.; Takami, T.; Yu, B.-C.; Flatt, A.K.; Tour, J.M.; Weiss, P.S. Reversible photo-switching of single azobenzene molecules in controlled nanoscale environments. Nano Lett. 2008, 8, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, M.; Gutiérrez, R.; Tejedor, C.; Cuniberti, G. Tuning the conductance of a molecular switch. Nat. Nanotechnol. 2007, 2, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Saphiannikova, M.; Santer, S.; Guskova, O. Photoisomers of azobenzene star with a flat core: Theoretical insights into multiple states from DFT and MD perspective. J. Phys. Chem. B 2017, 121, 8854–8867. [Google Scholar] [CrossRef]
- Montagna, M.; Guskova, O. Photosensitive cationic azobenzene surfactants: Thermodynamics of hydration and the complex formation with poly (methacrylic acid). Langmuir 2018, 34, 311–321. [Google Scholar] [CrossRef]
- Savchenko, V.; Guskova, O. Molecular switch based on bithiophene-azobenzene: How to control conductance through the monolayer using light. Her. Tver State Univ. Ser. Chem. 2021, 3, 7–20. [Google Scholar]
- Kaneta, M.; Honda, T.; Onda, K.; Han, M. Repeated photoswitching performance of azobenzenes adsorbed on gold surfaces: A balance between space, intermolecular interactions, and phase separation. New J. Chem. 2017, 41, 1827–1833. [Google Scholar] [CrossRef]
- Tian, Z.; Wen, J.; Ma, J. Dynamic simulations of stimuli-responsive switching of azobenzene derivatives in self-assembled monolayers: Reactive rotation potential and switching functions. Mol. Simul. 2015, 41, 28–42. [Google Scholar] [CrossRef]
- Cantatore, V.; Granucci, G.; Rousseau, G.; Padula, G.; Persico, M. Photoisomerization of self-assembled monolayers of azobiphenyls: Simulations highlight the role of packing and defects. J. Phys. Chem. Lett. 2016, 7, 4027–4031. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Tian, Z.; Ma, J. Light-and electric-field-induced switching of thiolated azobenzene self-assembled monolayer. J. Phys. Chem. C 2013, 117, 19934–19944. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, D.; Hu, W.; Zhu, Q.; Tian, Z.; Zhao, J.; Zhu, Y.; Ma, J. Tuning the collective switching behavior of azobenzene/Au hybrid materials: Flexible versus rigid azobenzene backbones and Au (111) surfaces versus curved Au nanoparticles. Nanoscale 2017, 9, 16700–16710. [Google Scholar] [CrossRef]
- Fast, E.; Schlimm, A.; Lautenschläger, I.; Clausen, K.U.; Strunskus, T.; Spormann, C.; Lindhorst, T.K.; Tuczek, F. Improving the switching capacity of glyco-self-assembled monolayers on Au (111). Chem. Eur. J. 2020, 26, 485–501. [Google Scholar] [CrossRef]
- Rashid, M.A.M.; Hayati, D.; Kwak, K.; Hong, J. Theoretical investigation of azobenzene-based photochromic dyes for dye-sensitized solar cells. Nanomaterials 2020, 10, 914. [Google Scholar] [CrossRef]
- Crivillers, N.; Orgiu, E.; Reinders, F.; Mayor, M.; Samorì, P. Optical modulation of the charge injection in an organic field-effect transistor based on photochromic self-assembled-monolayer-functionalized electrodes. Adv. Mat. 2011, 23, 1447–1452. [Google Scholar] [CrossRef]
- Tirosh, E.; Benassi, E.; Pipolo, S.; Mayor, M.; Valášek, M.; Frydman, V.; Corni, S.; Cohen, S.R. Direct monitoring of opto-mechanical switching of self-assembled monolayer films containing the azobenzene group. Beilstein J. Nanotechnol. 2011, 2, 834–844. [Google Scholar] [CrossRef]
- Yu, S.H.; Hassan, S.Z.; Nam, G.-H.; An, S.; Kang, B.; Chung, D.S. Consideration of azobenzene-based self-assembled monolayer deposition conditions for maximizing optoelectronic switching performances. Chem. Mater. 2021, 33, 5991–6002. [Google Scholar] [CrossRef]
- Crivillers, N.; Osella, S.; Van Dyck, C.; Lazzerini, G.M.; Cornil, D.; Liscio, A.; Di Stasio, F.; Mian, S.; Fenwick, O.; Reinders, F.; et al. Large work function shift of gold induced by a novel perfluorinated azobenzene-based self-assembled monolayer. Adv. Mater. 2013, 25, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Osella, S.; Samori, P.; Cornil, J. Photoswitching azobenzene derivatives in single molecule junctions: A theoretical insight into the I/V characteristics. J. Phys. Chem. C 2014, 118, 18721–18729. [Google Scholar] [CrossRef]
- Van Dyck, C.; Bergren, A.J.; Mukundan, V.; Fereiro, J.A.; DiLabio, G.A. Extent of conjugation in diazonium-derived layers in molecular junction devices determined by experiment and modelling. Phys. Chem. Chem. Phys. 2019, 21, 16762–16770. [Google Scholar] [CrossRef]
- Rego, L.G.C.; Bortolini, G. Modulating the photoisomerization mechanism of semiconductor-bound azobenzene-functionalized compounds. J. Phys. Chem. C 2019, 123, 5692–5698. [Google Scholar] [CrossRef]
- Bang, G.S.; Lee, J.; Baek, H.Y.; Lee, H.; Jun, K.; Shin, S.R. Adsorption of azothiophene dye having an n-bridging bidentate tail group on gold. Langmuir 2009, 25, 10788–10793. [Google Scholar] [CrossRef] [PubMed]
- Viero, Y.; Guérin, D.; Vladyka, A.; Alibart, F.; Lenfant, S.; Calame, M.; Vuillaume, D. Light-stimulatable molecules/nanoparticles networks for switchable logical functions and reservoir computing. Adv. Func. Mater. 2018, 28, 1801506. [Google Scholar] [CrossRef]
- Stiévenard, D.; Guérin, D.; Lenfant, S.; Lévêque, G.; Nijhuis, C.A.; Vuillaume, D. Electrical detection of plasmon-induced isomerization in molecule–nanoparticle network devices. Nanoscale 2018, 10, 23122–23130. [Google Scholar] [CrossRef]
- Lenfant, S. Charge transport in dynamic molecular junctions. In Molecular Electronics: An Experimental and Theoretical Approach; CRC Press: Boca Raton, FL, USA, 2016; pp. 65–99. [Google Scholar]
- Guskova, O.A. On the inter-ring torsion potential of 2, 2’-bithiophene: A review of open problems and current proposals. In Quantum Systems in Physics, Chemistry, and Biology: Advances in Concepts and Applications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 209–230. [Google Scholar]
- Mennucci, B.; Cances, E.; Tomasi, J. Evaluation of solvent effects in isotropic and anisotropic dielectrics and in ionic solutions with a unified integral equation method: Theoretical bases, computational implementation, and numerical applications. J. Phys. Chem. B 1997, 101, 10506–10517. [Google Scholar] [CrossRef]
- Malyar, I.V.; Titov, E.; Lomadze, N.; Saalfrank, P.; Santer, S. Photoswitching of azobenzene-containing self-assembled monolayers as a tool for control over silicon surface electronic properties. J. Chem. Phys. 2017, 146, 104703. [Google Scholar] [CrossRef]
- Pipolo, S.; Benassi, E.; Corni, S. Structural properties of azobenzene self-assembled monolayers by atomistic simulations. Langmuir 2017, 29, 10505–10512. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, M.; Brédas, J.-L. Density functional theory study of the geometric structure and energetics of triphenylamine-based hole-transporting molecules. Chem. Phys. Lett. 2000, 327, 13–17. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Blackstock, S.C.; Kim, Y. Estimation of inner shell Marcus terms for amino nitrogen compounds by molecular orbital calculations. J. Am. Chem. Soc. 1987, 109, 677–682. [Google Scholar] [CrossRef]
- Koh, S.E.; Risko, C.; da Silva Filho, D.A.; Kwon, O.; Facchetti, A.; Brédas, J.-L.; Marks, T.J.; Ratner, M.A. Modeling electron and hole transport in fluoroarene-oligothiopene semiconductors: Investigation of geometric and electronic structure properties. Adv. Funct. Mater. 2008, 18, 332–340. [Google Scholar] [CrossRef]
- Savchenko, V.A.; Guskova, O.A. The effect of alkyl substitutes on the characteristics of charge transfer in stacks of D-π-A-π-D molecules. Rev. Adv. Chem. 2022, 12, 214–221. [Google Scholar] [CrossRef]
- Raychev, D.; Méndez López, R.D.; Kiriy, A.; Seifert, G.; Sommer, J.-U.; Guskova, O. Copolymers of diketopyrrolopyrrole and benzothiadiazole: Design and function from simulations with experimental support. Macromolecules 2019, 52, 904–914. [Google Scholar] [CrossRef]
- Makarova, M.V.; Semenov, S.G.; Guskova, O.A. Computational study of structure, electronic, and microscopic charge transport properties of small conjugated diketopyrrolopyrrole-thiophene molecules. Int. J. Quant. Chem. 2016, 116, 1459–1466. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- BIOVIA, Dassault Systèmes, Materials Studio 09; Dassault Systèmes: San Diego, CA, USA, 2019.
- Tian, Z.; Wen, J.; Ma, J. Reactive molecular dynamics simulations of switching processes of azobenzene-based monolayer on surface. J. Chem. Phys. 2013, 139, 014706. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B. Organic Solvents: Physical Properties and Methods of Purification, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1986; pp. 66–69. [Google Scholar]
- Savchenko, V.; Lomadze, N.; Santer, S.; Guskova, O. Spiropyran/Merocyanine Amphiphile in Various Solvents: A Joint Experimental–Theoretical Approach to Photophysical Properties and Self-Assembly. Int. J. Mol. Sci. 2022, 23, 11535. [Google Scholar] [CrossRef]



| Reorganization Energy | Trans-BT-Azo | cis-BT-Azo | Trans-Azo-BT | cis-Azo-BT |
|---|---|---|---|---|
| [eV] | 1.030 | 0.919 | 0.584 | 0.499 |
| [eV] | 0.309 | 0.214 | 0.366 | 0.826 |
| Contributions [kcal mol] | Trans-BT-Azo | cis-BT-Azo | Trans-Azo-BT | cis-Azo-BT |
|---|---|---|---|---|
| (a) CHCl | ||||
| ideal term | −3.19 | −7.54 | −7.13 | −7.46 |
| van der Waals term | −15.96 | −15.49 | −17.73 | −16.53 |
| electrostatic term | 1.72 | 5.74 | 5.67 | 5.72 |
| −17.34 ± 0.69 | −17.30 ± 0.51 | −19.19 ± 0.72 | −18.26 ± 0.94 | |
| (b) HO | ||||
| ideal term | −3.62 | −3.94 | −7.48 | −7.80 |
| van der Waals term | 6.32 | 6.18 | 5.87 | 4.71 |
| electrostatic term | −1.53 | −1.54 | 1.87 | 1.70 |
| 1.16 ± 0.05 | 0.71 ± 0.04 | 0.26 ± 0.05 | −1.39 ± 0.09 |
| Property | Trans-BT-Azo | cis-BT-Azo | Trans-Azo-BT | cis-Azo-BT |
|---|---|---|---|---|
| [eV] | 0.139 ± 0.115 | 0.205 ± 0.128 | 0.179 ± 0.112 | 0.162 ± 0.167 |
| [eV] | 0.189 ± 0.119 | 0.189 ± 0.153 | 0.168 ± 0.122 | 0.273 ± 0.192 |
| [nm] | 0.75 ± 0.22 | 0.69 ± 0.16 | 0.67 ± 0.13 | 0.66 ± 0.12 |
| k10 [s] | 0.014 | 0.096 | 2.392 | 4.849 |
| k10 [s] | 53.328 | 161.569 | 22.228 | 0.443 |
| [cm V s] | 1.54910 | 8.89310 | 0.209 | 0.411 |
| [cm V s] | 5.840 | 1.497 | 1.942 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savchenko, V.; Hadjab, M.; Pavlov, A.S.; Guskova, O. Photo-Programmable Processes in Bithiophene–Azobenzene Monolayers on Gold Probed via Simulations. Processes 2023, 11, 2657. https://doi.org/10.3390/pr11092657
Savchenko V, Hadjab M, Pavlov AS, Guskova O. Photo-Programmable Processes in Bithiophene–Azobenzene Monolayers on Gold Probed via Simulations. Processes. 2023; 11(9):2657. https://doi.org/10.3390/pr11092657
Chicago/Turabian StyleSavchenko, Vladyslav, Moufdi Hadjab, Alexander S. Pavlov, and Olga Guskova. 2023. "Photo-Programmable Processes in Bithiophene–Azobenzene Monolayers on Gold Probed via Simulations" Processes 11, no. 9: 2657. https://doi.org/10.3390/pr11092657
APA StyleSavchenko, V., Hadjab, M., Pavlov, A. S., & Guskova, O. (2023). Photo-Programmable Processes in Bithiophene–Azobenzene Monolayers on Gold Probed via Simulations. Processes, 11(9), 2657. https://doi.org/10.3390/pr11092657







