Amomum subulatum Fruit Extract Mediated Green Synthesis of Silver and Copper Oxide Nanoparticles: Synthesis, Characterization, Antibacterial and Anticancer Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Amomum Subulatum Fruit Extract
2.3. Qualitatively Preliminary Phytochemical Analysis of the Extract
2.3.1. Test for Primary Metabolites
2.3.2. Test for Secondary Metabolites
2.4. Preparation of Metal Solutions
2.5. Synthesis of Silver NPs
2.6. Synthesis of Copper Oxide NPs
2.7. Optimisation of NPs
2.8. Stability of Biosynthesized AgNPs and CuONPs
2.9. Characterization
2.10. Antibacterial Activity of Silver and Copper Oxide NPs by Well Diffusion Method
2.11. MTT Assay for Cytotoxicity Evaluation
2.12. Statistical Evaluation
3. Results
3.1. Phytochemical Analysis
3.2. Visible Observation
3.3. Ag and CuONPs Characterization
3.3.1. UV-Visible Analysis
3.3.2. Stability Study of AgNPs and CuONPs
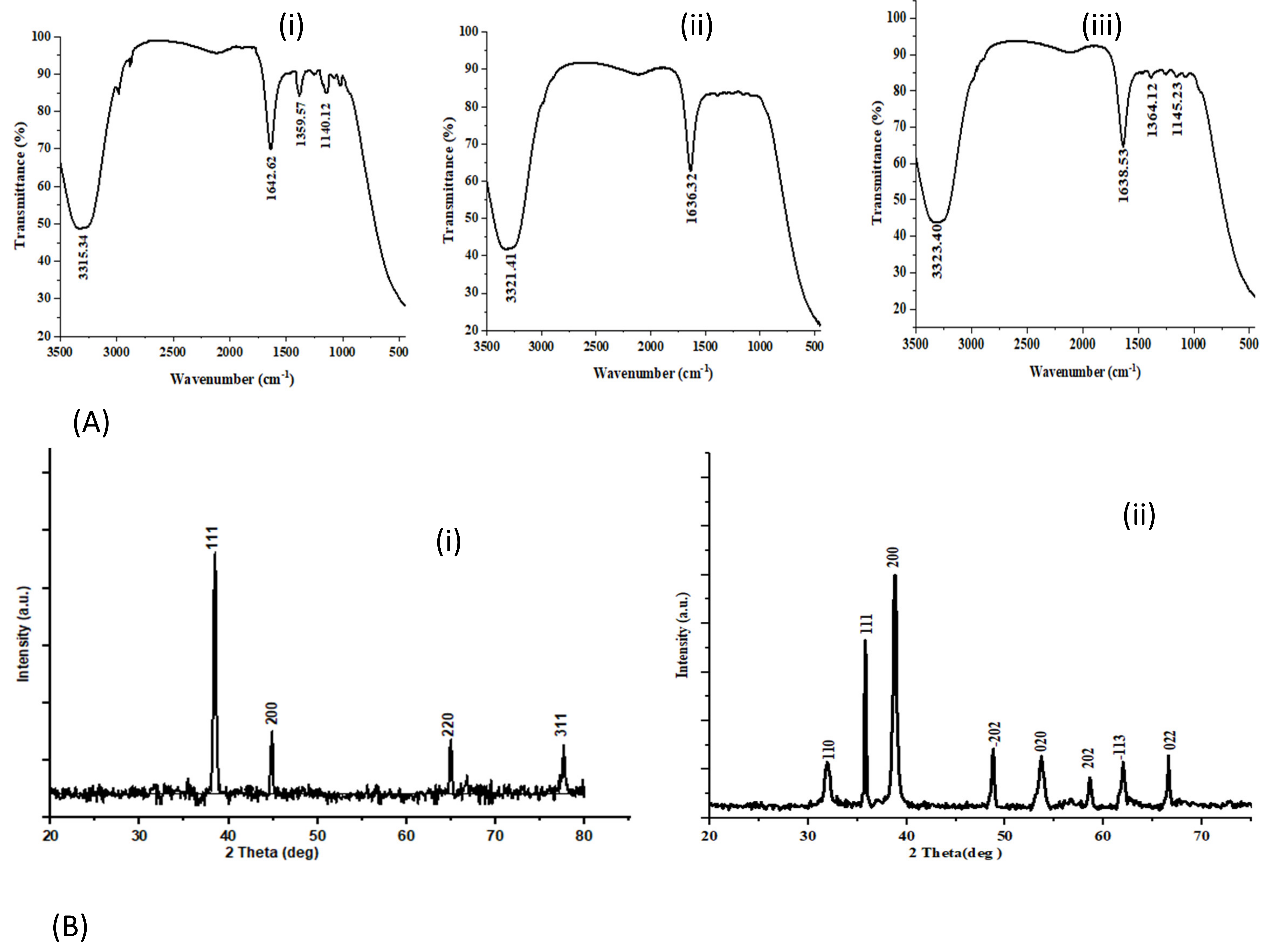
3.3.3. Infrared Spectral Study and Powder XRD Diffraction Study
3.3.4. Dynamic Light Scattering and Zeta Potential Analysis
3.3.5. Transmission Electron Microscopy (TEM) Analysis
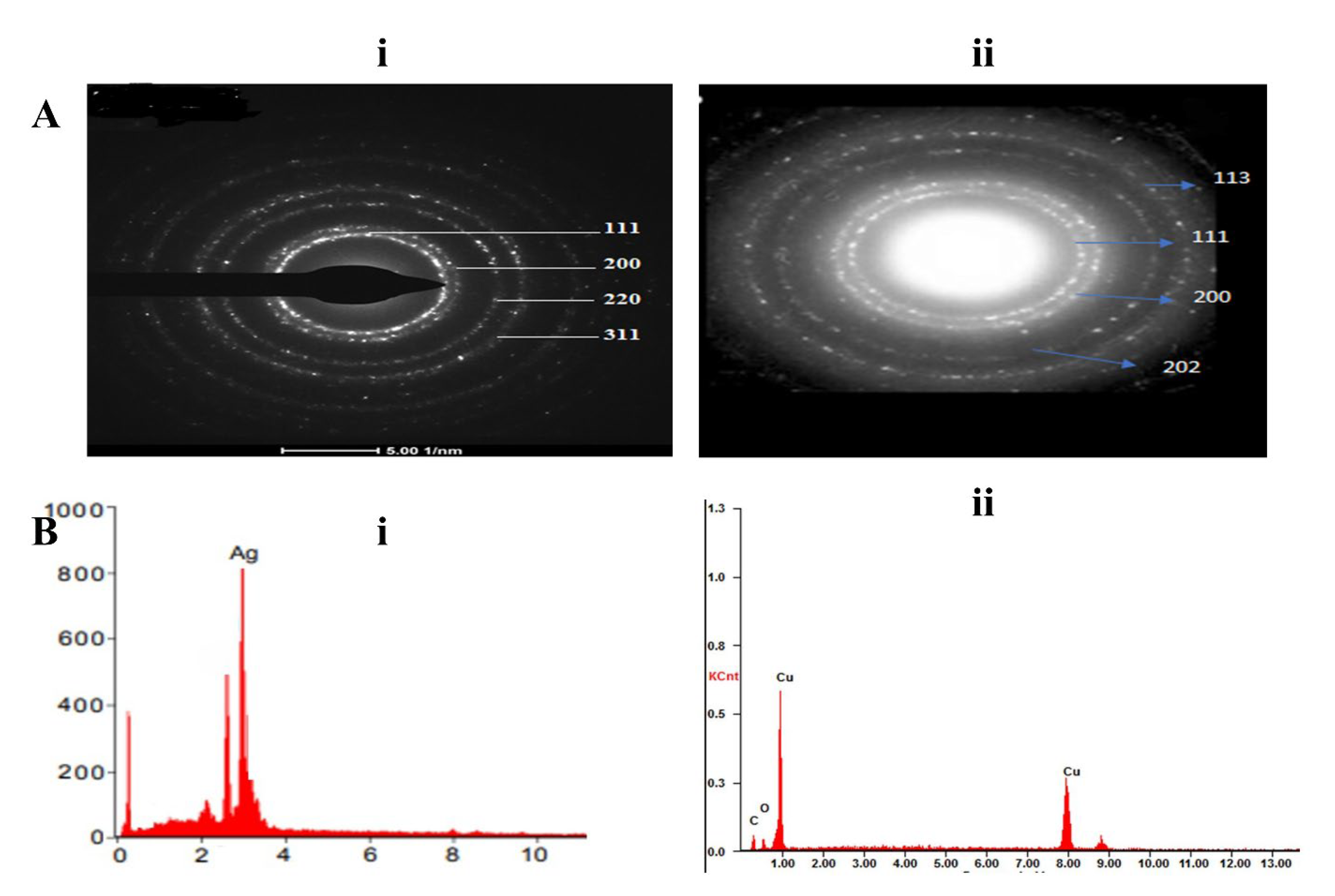
3.3.6. Selected Area Electron Diffraction (SAED) Analysis
3.3.7. Energy Dispersive X-ray Spectroscopy Study
3.4. Applications of Silver and Copper Oxide NPs
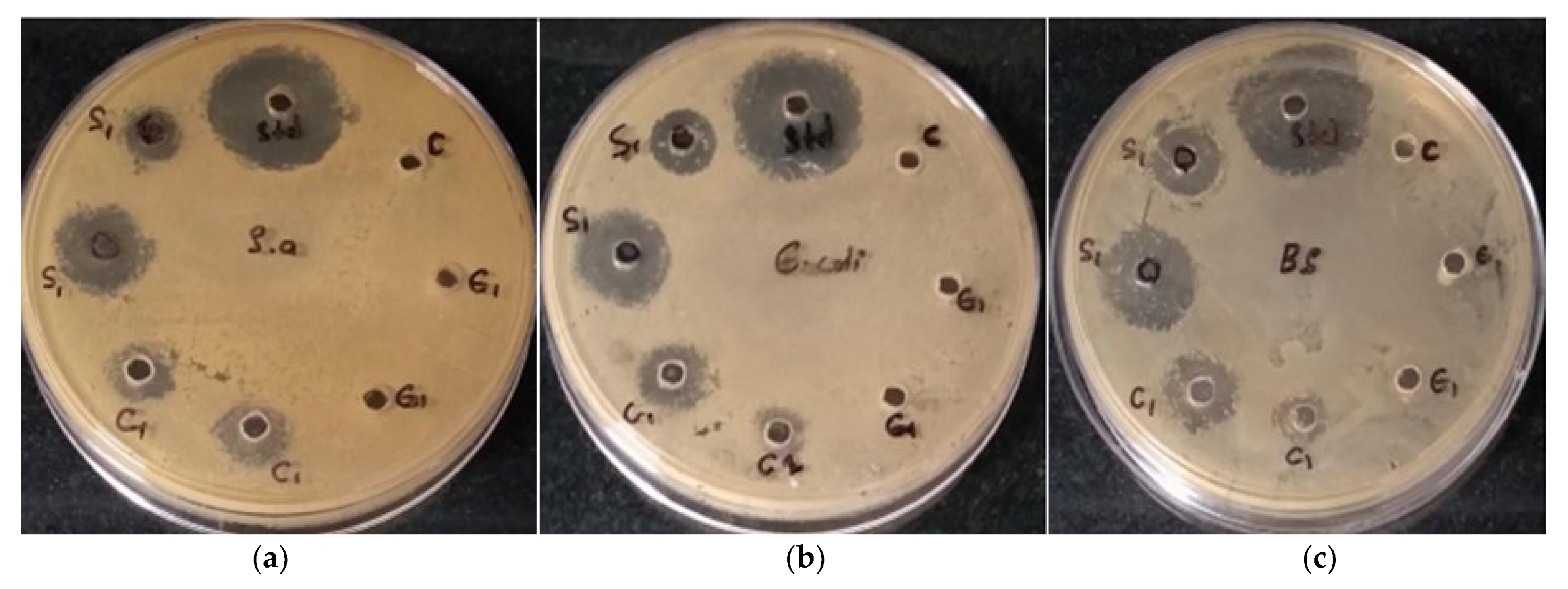
3.4.1. Antimicrobial Potential
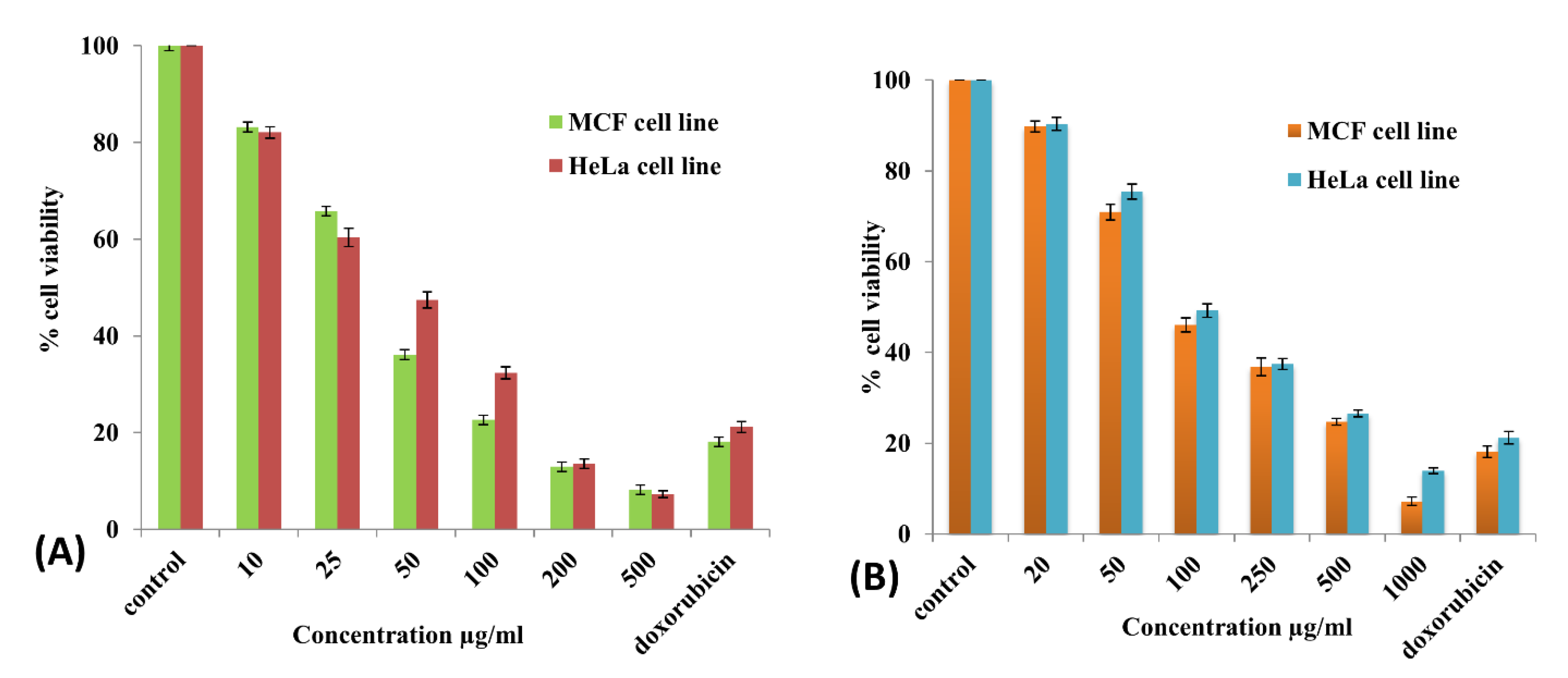
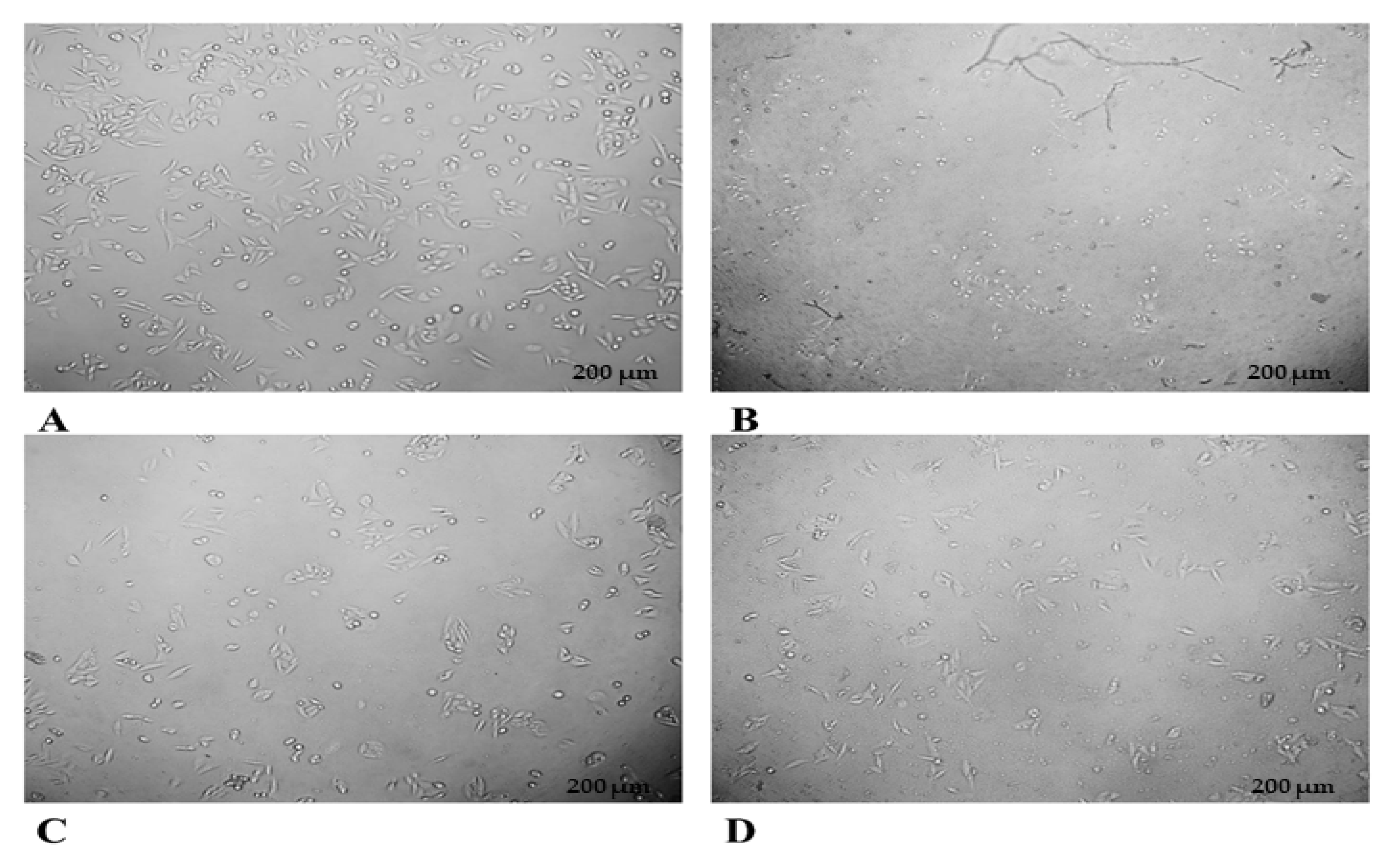
3.4.2. In Vitro Cytotoxicity (MTT Assay)
4. Discussion
5. Conclusions
6. Future Perspective and Limitations
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Maldonado, A.; Bustos-Guadarrama, S.; Espinoza-Gomez, H.; Flores-López, L.Z.; Ramirez-Acosta, K.; Alonso-Nuñez, G.; Cadena-Nava, R.D. Green Synthesis of Copper Nanoparticles Using Different Plant Extracts and Their Antibacterial Activity. J. Environ. Chem. Eng. 2022, 10, 107130. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Sharma, A.; Pathak, K. Codelivery of Phytochemicals with Conventional Anticancer Drugs in Form of Nanocarriers. Pharmaceutics 2023, 15, 889. [Google Scholar] [CrossRef] [PubMed]
- Alhalmi, A.; Beg, S.; Almalki, W.H.; Alghamdi, S.; Kohli, K. Recent Advances in Nanotechnology-Based Targeted Therapeutics for Breast Cancer Management. Curr. Drug Metab. 2022, 23, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Alhalmi, A.; Beg, S.; Kohli, K.; Waris, M.; Singh, T. Nanotechnology Based Approach for Hepatocellular Carcinoma Targeting. Curr. Drug Targets 2021, 22, 779–792. [Google Scholar] [CrossRef]
- Virmani, T.; Kumar, G.; Sharma, A.; Pathak, K.; Akhtar, M.S.; Afzal, O.; Altamimi, A.S.A. Amelioration of Cancer Employing Chitosan, Its Derivatives, and Chitosan-Based Nanoparticles: Recent Updates. Polymers 2023, 15, 2928. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Pathak, K.; Alhalmi, A. A Revolutionary Blueprint for Mitigation of Hypertension via Nanoemulsion. BioMed Res. Int. 2022, 2022, e4109874. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical Applications of Metallic Nanoparticles in Cancer: Current Status and Future Perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Virmani, T.; Kumar, G.; Virmani, R.; Sharma, A.; Pathak, K. Nanocarrier-Based Approaches to Combat Chronic Obstructive Pulmonary Disease. Nanomedicine 2022, 17, 1833–1854. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; El-Sayyad, G.S. Antimicrobial and Anticancer Activities of Biosynthesized Bimetallic Silver-Zinc Oxide Nanoparticles (Ag-ZnO NPs) Using Pomegranate Peel Extract. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. NSA 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, A.; Waseem, M.; Javed, A.; Baig, S.; Ismail, B.; Baig, A.; Shahzadi, I.; Nawazish, S.; Zaman, I. Augmented Anticancer Effect and Antibacterial Activity of Silver Nanoparticles Synthesized by Using Taxus Wallichiana Leaf Extract. PeerJ 2022, 10, e14391. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef] [PubMed]
- Alruhaili, M.H.; Almuhayawi, M.S.; Gattan, H.S.; Alharbi, M.T.; Nagshabandi, M.K.; Jaouni, S.K.A.; Selim, S.; AbdElgawad, H. Insight into the Phytochemical Profile and Antimicrobial Activities of Amomum Subulatum and Amomum Xanthioides: An in Vitro and in Silico Study. Front. Plant Sci. 2023, 14, 1136961. [Google Scholar] [CrossRef] [PubMed]
- Ihsan, M.; Niaz, A.; Rahim, A.; Zaman, M.I.; Arain, M.B.; Sirajuddin; Sharif, T.; Najeeb, M. Biologically Synthesized Silver Nanoparticle-Based Colorimetric Sensor for the Selective Detection of Zn2+. RSC Adv. 2015, 5, 91158–91165. [Google Scholar] [CrossRef]
- Ismail, M.; Xiangke, W.; Khan, A.A.; Khan, Q. Amomum Subalatum Leaf Extract Derived Silver Nanoparticles for Eco-Friendly Spectrophotometric Detection of Hg (II) Ions in Water. Chem. Phys. Impact 2023, 6, 100148. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Purniawan, A.; Lusida, M.I.; Pujiyanto, R.W.; Nastri, A.M.; Permanasari, A.A.; Harsono, A.A.H.; Oktavia, N.H.; Wicaksono, S.T.; Dewantari, J.R.; Prasetya, R.R.; et al. Synthesis and Assessment of Copper-Based Nanoparticles as a Surface Coating Agent for Antiviral Properties against SARS-CoV-2. Sci. Rep. 2022, 12, 4835. [Google Scholar] [CrossRef]
- Ghosh, S.; More, P.; Nitnavare, R.; Jagtap, S.; Chippalkatti, R.; Derle, A.; Kitture, R.; Asok, A.; Kale, S.; Ramanamurthy, B.; et al. Antidiabetic and Antioxidant Properties of Copper Nanoparticles Synthesized by Medicinal Plant Dioscorea Bulbifera. J. Nanomed. Nanotechnol. 2015, S6, 7. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Bo, Y.; Folorunso, A.S. A Review on Synthesis, Optimization, Mechanism, Characterization, and Antibacterial Application of Silver Nanoparticles Synthesized from Plants. J. Chem. 2020, 2020, e3189043. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Jalal, M.; Ahmad, H.; Sharma, D.; Ahmad, A.; Umar, K.; Khan, H. Green Synthesis of Silver Nanoparticles from Camellia Sinensis and Its Antimicrobial and Antibiofilm Effect against Clinical Isolates. Materials 2022, 15, 6978. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.U.; Shah, A.; Haleem, A.; Shah, S.M.; Shah, I. Eucalyptus Globulus Mediated Green Synthesis of Environmentally Benign Metal Based Nanostructures: A Review. Nanomaterials 2023, 13, 2019. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.A.; Patlolla, A.K.; Madhusudhanachary, P. Biosynthesis of Silver Nanoparticles Using Azadirachta Indica and Their Antioxidant and Anticancer Effects in Cell Lines. Micromachines 2022, 13, 1416. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Batool, S.; Kalsoom, T.; Raina, S.; Sharif, H.M.A.; Yasmeen, S. Biosynthesis of Silver Nanoparticles Using Sida Acuta Extract for Antimicrobial Actions and Corrosion Inhibition Potential. Env. Technol. 2019, 40, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.R.; Pant, N.D.; Saru, D.B.; Yadav, U.N.; Khanal, D.P. Phytochemical Screening and Study of Antioxidant, Antimicrobial, Antidiabetic, Anti-Inflammatory and Analgesic Activities of Extracts from Stem Wood of Pterocarpus Marsupium Roxburgh. J. Intercult. Ethnopharmacol. 2017, 6, 170–176. [Google Scholar] [CrossRef]
- Cyril, N.; George, J.B.; Joseph, L.; Raghavamenon, A.C.; Sylas, V.P. Assessment of Antioxidant, Antibacterial and Anti-Proliferative (Lung Cancer Cell Line A549) Activities of Green Synthesized Silver Nanoparticles from Derris trifoliata. Toxicol. Res. 2019, 8, 297–308. [Google Scholar] [CrossRef]
- Jurado Gonzalez, P.; Sörensen, P.M. Characterization of Saponin Foam from Saponaria Officinalis for Food Applications. Food Hydrocoll. 2020, 101, 105541. [Google Scholar] [CrossRef]
- Auwal, M.S.; Saka, S.; Mairiga, I.A.; Sanda, K.A.; Shuaibu, A.; Ibrahim, A. Preliminary Phytochemical and Elemental Analysis of Aqueous and Fractionated Pod Extracts of Acacia Nilotica (Thorn Mimosa). Vet. Res. Forum. 2014, 5, 95–100. [Google Scholar] [PubMed]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Gogoi, S.K.; Kakati, N.; Baishya, D.; Kari, Z.A.; Edinur, H.A. Green Synthesis of Silver Nanoparticles Using Diospyros Malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-Nitrophenol (4-Np). Nanomaterials 2021, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
- Ahalwat, S.; Bhatt, D.C.; Rohilla, S.; Jogpal, V.; Sharma, K.; Virmani, T.; Kumar, G.; Alhalmi, A.; Alqahtani, A.S.; Noman, O.M.; et al. Mannose-Functionalized Isoniazid-Loaded Nanostructured Lipid Carriers for Pulmonary Delivery: In Vitro Prospects and In Vivo Therapeutic Efficacy Assessment. Pharmaceuticals 2023, 16, 1108. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nakkala, J.R.; Mata, R.; Gupta, A.K.; Sadras, S.R. Biological Activities of Green Silver Nanoparticles Synthesized with Acorous Calamus Rhizome Extract. Eur. J. Med. Chem. 2014, 85, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Pathak, D.; Patel, A.; Dalwadi, P.; Prasad, R.; Patel, P.; Selvaraj, K. Biogenic Synthesis of Silver Nanoparticles Using Nicotiana Tobaccum Leaf Extract and Study of Their Antibacterial Effect. Afr. J. Biotechnol. 2011, 10, 8122–8130. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Muthuraman, P.; Sreekanth, T.V.M.; Kim, D.H.; Shim, J. Green Synthesis: In-Vitro Anticancer Activity of Copper Oxide Nanoparticles against Human Cervical Carcinoma Cells. Arab. J. Chem. 2017, 10, 215–225. [Google Scholar] [CrossRef]
- Letchumanan, D.; Sok, S.P.M.; Ibrahim, S.; Nagoor, N.H.; Arshad, N.M. Plant-Based Biosynthesis of Copper/Copper Oxide Nanoparticles: An Update on Their Applications in Biomedicine, Mechanisms, and Toxicity. Biomolecules 2021, 11, 564. [Google Scholar] [CrossRef]
- Vincent, J.; Lau, K.S.; Evyan, Y.C.Y.; Chin, S.X.; Sillanpää, M.; Chia, C.H. Biogenic Synthesis of Copper-Based Nanomaterials Using Plant Extracts and Their Applications: Current and Future Directions. Nanomaterials 2022, 12, 3312. [Google Scholar] [CrossRef]
- Vidovix, T.B.; Quesada, H.B.; Januário, E.F.D.; Bergamasco, R.; Vieira, A.M.S. Green Synthesis of Copper Oxide Nanoparticles Using Punica Granatum Leaf Extract Applied to the Removal of Methylene Blue. Mater. Lett. 2019, 257, 126685. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Menon, S.; Venkat Kumar, S.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Satija, S.; Gupta, G.; Chellappan, D.K.; Thangavelu, L.; et al. Antibacterial and Antioxidant Potential of Biosynthesized Copper Nanoparticles Mediated through Cissus Arnotiana Plant Extract. J. Photochem. Photobiol. B Biol. 2019, 197, 111531. [Google Scholar] [CrossRef] [PubMed]
- Chand Mali, S.; Raj, S.; Trivedi, R. Biosynthesis of Copper Oxide Nanoparticles Using Enicostemma Axillare (Lam.) Leaf Extract. Biochem. Biophys. Rep. 2019, 20, 100699. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pallela, P.N.V.K.; Pammi, S.V.N.; Padavala, V.S.; Kolapalli, V.R.M. Synergetic Antibacterial and Anticarcinogenic Effects of Annona Squamosa Leaf Extract Mediated Silver Nano Particles. Mater. Sci. Semicond. Process. 2019, 100, 301–309. [Google Scholar] [CrossRef]
- Souri, M.; Hoseinpour, V.; Ghaemi, N.; Shakeri, A. Procedure Optimization for Green Synthesis of Manganese Dioxide Nanoparticles by Yucca Gloriosa Leaf Extract. Int. Nano Lett. 2019, 9, 73–81. [Google Scholar] [CrossRef]
- Kumar, S.V.; Bafana, A.P.; Pawar, P.; Faltane, M.; Rahman, A.; Dahoumane, S.A.; Kucknoor, A.; Jeffryes, C.S. Optimized Production of Antibacterial Copper Oxide Nanoparticles in a Microwave-Assisted Synthesis Reaction Using Response Surface Methodology. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 170–178. [Google Scholar] [CrossRef]
- Talank, N.; Morad, H.; Barabadi, H.; Mojab, F.; Amidi, S.; Kobarfard, F.; Mahjoub, M.A.; Jounaki, K.; Mohammadi, N.; Salehi, G.; et al. Bioengineering of Green-Synthesized Silver Nanoparticles: In Vitro Physicochemical, Antibacterial, Biofilm Inhibitory, Anticoagulant, and Antioxidant Performance. Talanta 2022, 243, 123374. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and Antibacterial Activity of Silver Nanoparticles Synthesized by Cestrum Nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ahn, E.Y.; Park, Y. Shape-Dependent Cytotoxicity and Cellular Uptake of Gold Nanoparticles Synthesized Using Green Tea Extract. Nanoscale Res. Lett. 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Barabadi, H.; Webster, T.J.; Vahidi, H.; Sabori, H.; Damavandi Kamali, K.; Jazayeri Shoushtari, F.; Mahjoub, M.A.; Rashedi, M.; Mostafavi, E.; Cruz, D.M.; et al. Green Nanotechnology-Based Gold Nanomaterials for Hepatic Cancer Therapeutics: A Systematic Review. Iran. J. Pharm. Res. IJPR 2020, 19, 3–17. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, M.S.; Nayak, A.K. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Ashokkumar, T.; Prabhu, D.; Geetha, R.; Govindaraju, K.; Manikandan, R.; Arulvasu, C.; Singaravelu, G. Apoptosis in Liver Cancer (HepG2) Cells Induced by Functionalized Gold Nanoparticles. Colloids Surf. B Biointerfaces 2014, 123, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Cuong, H.N.; Pansambal, S.; Ghotekar, S.; Oza, R.; Thanh Hai, N.T.; Viet, N.M.; Nguyen, V.-H. New Frontiers in the Plant Extract Mediated Biosynthesis of Copper Oxide (CuO) Nanoparticles and Their Potential Applications: A Review. Environ. Res. 2022, 203, 111858. [Google Scholar] [CrossRef] [PubMed]
- Osibe, D.A.; Aoyagi, H. A Novel Strategy for the Synthesis of Gold Nanoparticles with Catharanthus Roseus Cell Suspension Culture. Mater. Lett. 2019, 238, 317–320. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Abdulhaq, A.; Ragavendran, C.; Mohan, S. Phytoconstituents Assisted Biofabrication of Copper Oxide Nanoparticles and Their Antiplasmodial, and Antilarval Efficacy: A Novel Approach for the Control of Parasites. Molecules 2022, 27, 8269. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Iqubal, S.M.S.; Khan, A.A.; Mohammed, T.; Alshabi, A.M.; Aazam, E.S.; Rafiquee, M.Z.A. Effect of CTABr (Surfactant) on the Kinetics of Formation of Silver Nanoparticles by Amla Extract. J. Mol. Liq. 2021, 329, 115537. [Google Scholar] [CrossRef]
- Tuama, A.A.; Mohammed, A.A. Phytochemical Screening and in Vitro Antibacterial and Anticancer Activities of the Aqueous Extract of Cucumis Sativus. Saudi J. Biol. Sci. 2019, 26, 600–604. [Google Scholar] [CrossRef]
- Waghmode, S.; Chavan, P.; Kalyankar, V.; Dagade, S. Synthesis of Silver Nanoparticles Using Triticum Aestivum and Its Effect on Peroxide Catalytic Activity and Toxicology. J. Chem. 2013, 2013, 265864. [Google Scholar] [CrossRef]
- Sundaramurthy, N.; Parthiban, C. Biosynthesis of Copper Oxide Nanoparticles Using Pyrus Pyrifolia Leaf Extract and Evolve the Catalytic Activity. Int. Res. J. Eng. Technol. 2015, 2, 332–338. [Google Scholar]
- Kumar, P.P.N.V.; Shameem, U.; Kollu, P.; Kalyani, R.L.; Pammi, S.V.N. Green Synthesis of Copper Oxide Nanoparticles Using Aloe Vera Leaf Extract and Its Antibacterial Activity Against Fish Bacterial Pathogens. BioNanoScience 2015, 5, 135–139. [Google Scholar] [CrossRef]
- Khatua, A.; Prasad, A.; Priyadarshini, E.; Patel, A.K.; Naik, A.; Saravanan, M.; Barabadi, H.; Ghosh, L.; Paul, B.; Paulraj, R.; et al. Emerging Antineoplastic Plant-Based Gold Nanoparticle Synthesis: A Mechanistic Exploration of Their Anticancer Activity Toward Cervical Cancer Cells. J. Clust. Sci. 2019, 31, 1329–1340. [Google Scholar] [CrossRef]
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green Synthesis of Copper Nanoparticles Using Celastrus Paniculatus Willd. Leaf Extract and Their Photocatalytic and Antifungal Properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef]
- Thirumagal, N.; Jeyakumari, A.P. Structural, Optical and Antibacterial Properties of Green Synthesized Silver Nanoparticles (AgNPs) Using Justicia Adhatoda L. Leaf Extract. J. Clust. Sci. 2020, 31, 487–497. [Google Scholar] [CrossRef]
- Alhalmi, A.; Amin, S.; Khan, Z.; Beg, S.; Al, O.; Saleh, A.; Kohli, K. Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies. Pharmaceutics 2022, 14, 1771. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Virmani, T.; Pathak, K.; Kamaly, O.A.; Saleh, A. Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation. Pharmaceuticals 2022, 15, 1343. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Chakraborty, B.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Kotresha, D.; Pallavi, S.S.; Dhanyakumara, S.B.; Shashiraj, K.N.; Nayaka, S. Biogenic Synthesis, Characterization and Antimicrobial Activity of Ixora Brachypoda (DC) Leaf Extract Mediated Silver Nanoparticles. J. King Saud Univ.–Sci. 2021, 33, 101296. [Google Scholar] [CrossRef]
- Verma, A.; Bharadvaja, N. Plant-Mediated Synthesis and Characterization of Silver and Copper Oxide Nanoparticles: Antibacterial and Heavy Metal Removal Activity. J. Clust. Sci. 2022, 33, 1697–1712. [Google Scholar] [CrossRef]
- Devanesan, S.; AlSalhi, M.S. Green Synthesis of Silver Nanoparticles Using the Flower Extract of Abelmoschus Esculentus for Cytotoxicity and Antimicrobial Studies. Int. J. Nanomed. 2021, 16, 3343–3356. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green Synthesis of Silver Nanoparticles from Tectona Grandis Seeds Extract: Characterization and Mechanism of Antimicrobial Action on Different Microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Kasi, S.D.; Ramasamy, J.M.; Nagaraj, D.; Santiyagu, V.; Ponraj, J.S. Biogenic Synthesis of Copper Oxide Nanoparticles Using Leaf Extracts of Cissus Quadrangularis and Piper Betle and Its Antibacterial Effects. Micro Nano Lett. 2021, 16, 419–424. [Google Scholar] [CrossRef]
- Naveed, M.; Bukhari, B.; Aziz, T.; Zaib, S.; Mansoor, M.A.; Khan, A.A.; Shahzad, M.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A.; et al. Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer Oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches. Molecules 2022, 27, 4226. [Google Scholar] [CrossRef] [PubMed]
- Bhavyasree, P.G.; Xavier, T.S. Green Synthesised Copper and Copper Oxide Based Nanomaterials Using Plant Extracts and Their Application in Antimicrobial Activity: Review. Curr. Res. Green Sustain. Chem. 2022, 5, 100249. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ouahrani, M.R. A Review on Green Synthesis of CuO Nanoparticles Using Plant Extract and Evaluation of Antimicrobial Activity A Review on Green Synthesis of CuO Nanoparticles Using Plant Extract and Evaluation of Antimicrobial Activity. Asian J. Res. Chem. 2020, 13, 65–70. [Google Scholar] [CrossRef]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The Effect of Particle Size on the Cytotoxicity, Inflammation, Developmental Toxicity and Genotoxicity of Silver Nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of Silver Nanoparticles Using Flavonoids: Hesperidin, Naringin and Diosmin, and Their Antibacterial Effects and Cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Gomathi, A.C.; Xavier Rajarathinam, S.R.; Mohammed Sadiq, A.; Rajeshkumar, S. Anticancer Activity of Silver Nanoparticles Synthesized Using Aqueous Fruit Shell Extract of Tamarindus Indica on MCF-7 Human Breast Cancer Cell Line. J. Drug Deliv. Sci. Technol. 2020, 55, 101376. [Google Scholar] [CrossRef]
- Thangam, R.; Senthilkumar, D.; Suresh, V.; Sathuvan, M.; Sivasubramanian, S.; Pazhanichamy, K.; Gorlagunta, P.K.; Kannan, S.; Gunasekaran, P.; Rengasamy, R.; et al. Induction of ROS-Dependent Mitochondria-Mediated Intrinsic Apoptosis in MDA-MB-231 Cells by Glycoprotein from Codium Decorticatum. J. Agric. Food Chem. 2014, 62, 3410–3421. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Masuda, A.; Sun, M.; Centonze, V.E.; Herman, B. Oxidative Stress-Induced Apoptosis Is Associated with Alterations in Mitochondrial Caspase Activity and Bcl-2-Dependent Alterations in Mitochondrial PH (PHm). Brain Res. Bull. 2004, 62, 497–504. [Google Scholar] [CrossRef]
- Andualem, W.W.; Sabir, F.K.; Mohammed, E.T.; Belay, H.H.; Gonfa, B.A. Synthesis of Copper Oxide Nanoparticles Using Plant Leaf Extract of Catha Edulis and Its Antibacterial Activity. J. Nanotechnol. 2020, 2020, e2932434. [Google Scholar] [CrossRef]
- Velsankar, K.; Aswin Kumar, R.M.; Preethi, R.; Muthulakshmi, V.; Sudhahar, S. Green Synthesis of CuO Nanoparticles via Allium Sativum Extract and Its Characterizations on Antimicrobial, Antioxidant, Antilarvicidal Activities. J. Environ. Chem. Eng. 2020, 8, 104123. [Google Scholar] [CrossRef]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Tripathy, M.; Paliwal, N. Green Synthesis, Characterization, Antibacterial, Antioxidant and Photocatalytic Activity of Parkia Speciosa Leaves Extract Mediated Silver Nanoparticles. Results Phys. 2019, 15, 102565. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Mutairi, A.; Al-Shamri, B.; Aabed, K.F. Antibacterial and Cytotoxic Potential of Biosynthesized Silver Nanoparticles by Some Plant Extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Sankar, R.; Maheswari, R.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Anticancer Activity of Ficus Religiosa Engineered Copper Oxide Nanoparticles. Mater. Sci. Eng. C 2014, 44, 234–239. [Google Scholar] [CrossRef] [PubMed]






| Category | Test | Aqueous Extract |
|---|---|---|
| Carbohydrates | Molisch | + |
| Proteins | Millon | + |
| Flavonoids | Shinoda | + |
| Alkaloids | Dragendorff’s | − |
| Saponins | Foam | + |
| Glycosides | Killer killani | + |
| Phenolic and tannins | Ferric chloride | + |
| Terpenoids | Salkowski | − |
| Amino acids | Ninhydrin | + |
| Test Compounds | Volume per Well | S. aureus | E. coli | B. subtilis |
|---|---|---|---|---|
| Zone of inhibition (mm) | ||||
| Distilled water (C) | 50 µL | 0 | 0 | 0 |
| Plant extract (E) | 100 µL | 0 | 0 | 0 |
| 50 µL | 0 | 0 | 0 | |
| Gentamycin (Std) | 50 µL | 22 ± 0.1 | 22 ± 0.05 | 24 ± 0.12 |
| AgNPs (S1) | 100 µL | 18 ± 0.5 | 19 ± 0.12 | 18 ± 0.02 |
| 50 µL | 11 ± 0.2 | 12 ± 0.05 | 13 ± 0.01 | |
| CuONPs (C1) | 100 µL | 14 ± 0.15 | 14.5 ± 0.05 | 14 ± 0.12 |
| 50 µL | 10 ± 0.2 | 10 ± 0.12 | 10 ± 0.11 | |
| Type of Metal NPs | Plant Extract | Antimicrobial Activity (Zone of Inhibition) | Anticancer Activity (IC50 Value) | Reference |
|---|---|---|---|---|
| AgNPs | Amomum subulatum | S. aureus (18 mm) E. coli (19 mm) B. subtilis (18 mm) | MCF Cells (39.79 µg/mL) HeLa cells (45.5 µg/mL) | Present study |
| CuONPs | Amomum subulatum | S. aureus (14 mm) E. coli (14.5 mm) B. subtilis (14 mm) | MCF Cells (83.89 µg/mL) HeLa cells (97.07 µg/mL) | Present study |
| CuONPs | Catha edulis | S. aureus (22 ± 0.01 mm) genes (24 ± 0.02 mm) E. Coli (32 ± 0.02 mm) | [79] | |
| CuONPs | Allium sativum | Escherichia coli (7 mm) S. aureus (8 mm) B. Subtilis (7 mm) S. phogenes (7 mm) | [80] | |
| AgNPs | Parkia speciosa leaves | Escherichia coli (9 mm) S. aureus (10 mm) P. aeruginosa (7 mm) B. subtilis (7 mm) | [81] | |
| AgNPs | Phoenix dactylifera Ferula asafetida Acacia nilotica plant | 46.15 ± 2.0 µg/mL 58.02 ± 2.1 µg/mL 69.73 ± 2.02 µg/mL | [82] | |
| CuONPs | Ficus religiosa | 200 µg/mL | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhir, S.; Dutt, R.; Singh, R.P.; Chauhan, M.; Virmani, T.; Kumar, G.; Alhalmi, A.; Aleissa, M.S.; Rudayni, H.A.; Al-Zahrani, M. Amomum subulatum Fruit Extract Mediated Green Synthesis of Silver and Copper Oxide Nanoparticles: Synthesis, Characterization, Antibacterial and Anticancer Activities. Processes 2023, 11, 2698. https://doi.org/10.3390/pr11092698
Dhir S, Dutt R, Singh RP, Chauhan M, Virmani T, Kumar G, Alhalmi A, Aleissa MS, Rudayni HA, Al-Zahrani M. Amomum subulatum Fruit Extract Mediated Green Synthesis of Silver and Copper Oxide Nanoparticles: Synthesis, Characterization, Antibacterial and Anticancer Activities. Processes. 2023; 11(9):2698. https://doi.org/10.3390/pr11092698
Chicago/Turabian StyleDhir, Sarika, Rohit Dutt, Rahul Pratap Singh, Mahima Chauhan, Tarun Virmani, Girish Kumar, Abdulsalam Alhalmi, Mohammed S. Aleissa, Hassan A. Rudayni, and Mohammed Al-Zahrani. 2023. "Amomum subulatum Fruit Extract Mediated Green Synthesis of Silver and Copper Oxide Nanoparticles: Synthesis, Characterization, Antibacterial and Anticancer Activities" Processes 11, no. 9: 2698. https://doi.org/10.3390/pr11092698








