Consistency Checks for Pressure-Volume-Temperature Experiment of Formation Oil and Gas at High Temperature
Abstract
:1. Introduction
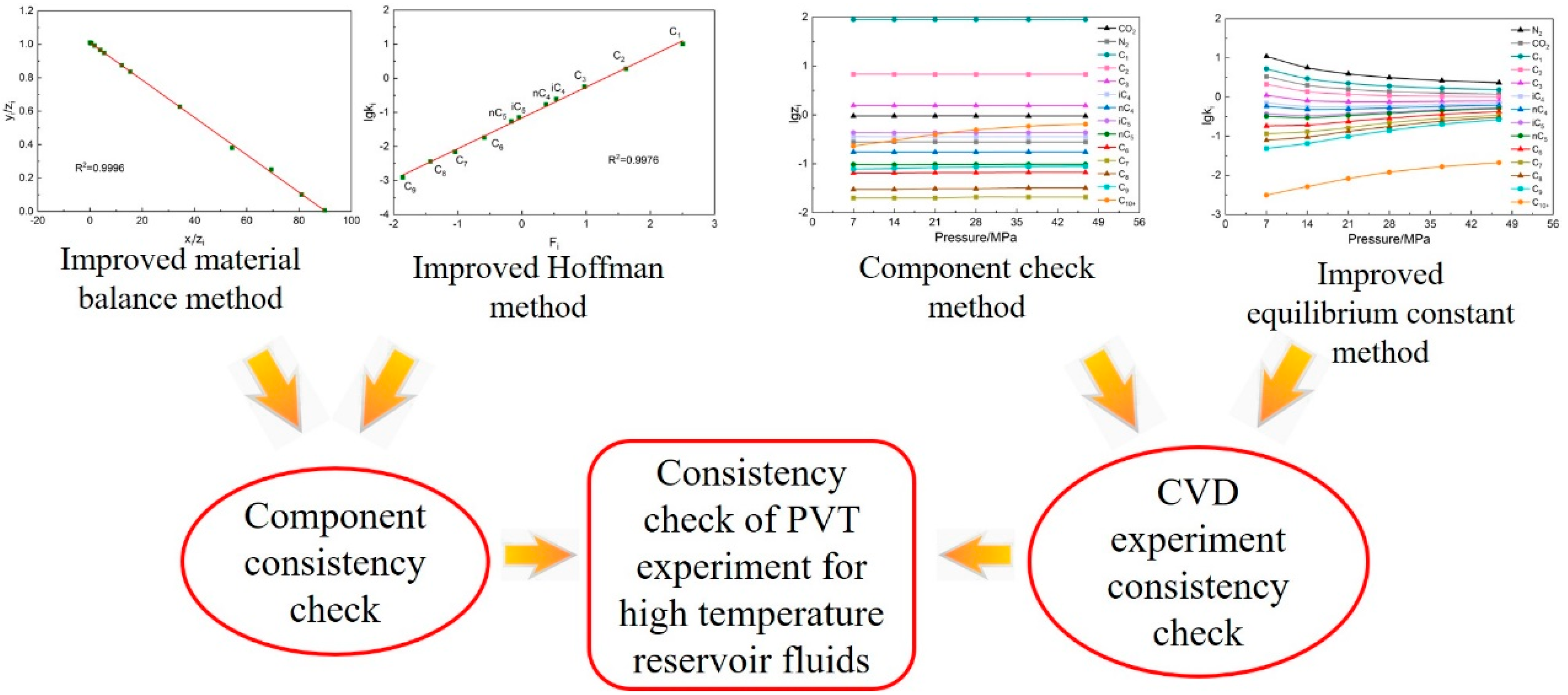
2. Check Method
2.1. Component Consistency Check
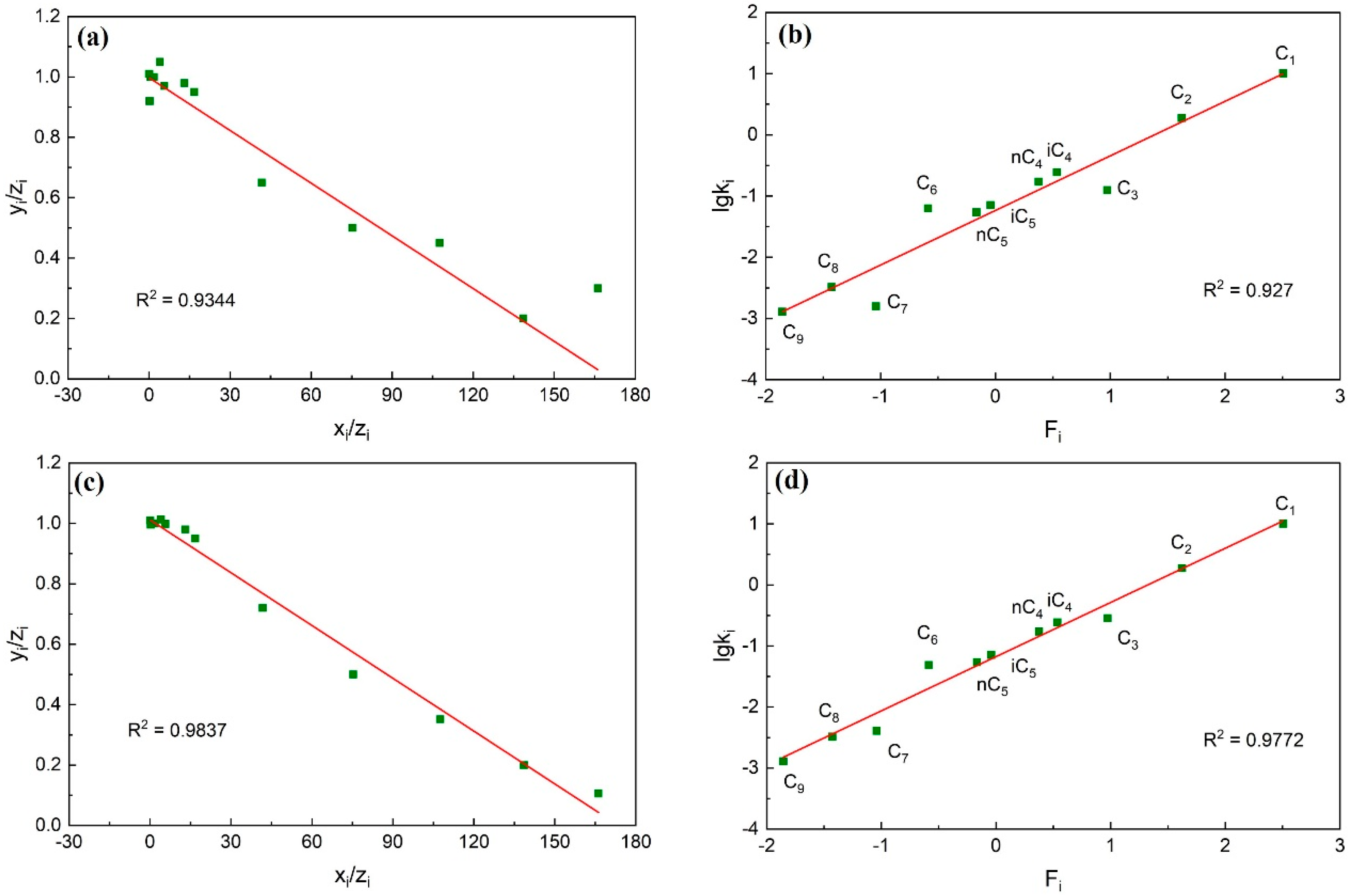
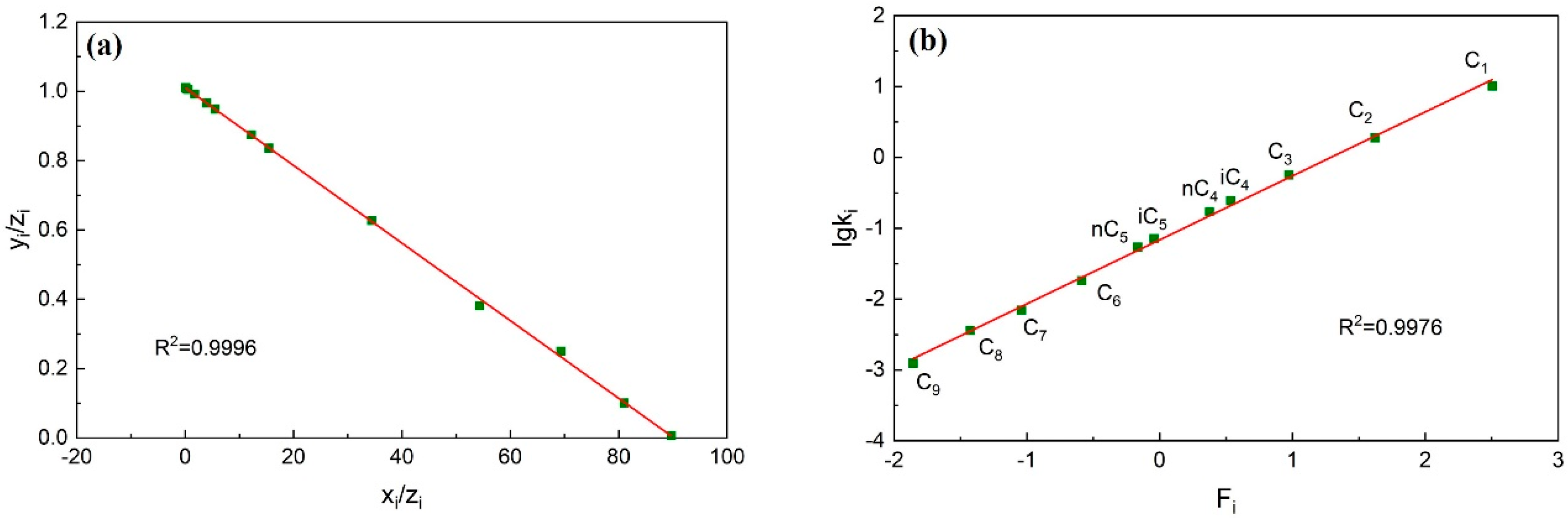
2.1.1. Material Balance Method
2.1.2. Hoffman Method
2.2. Constant Volume Depletion Experiment Consistency Check
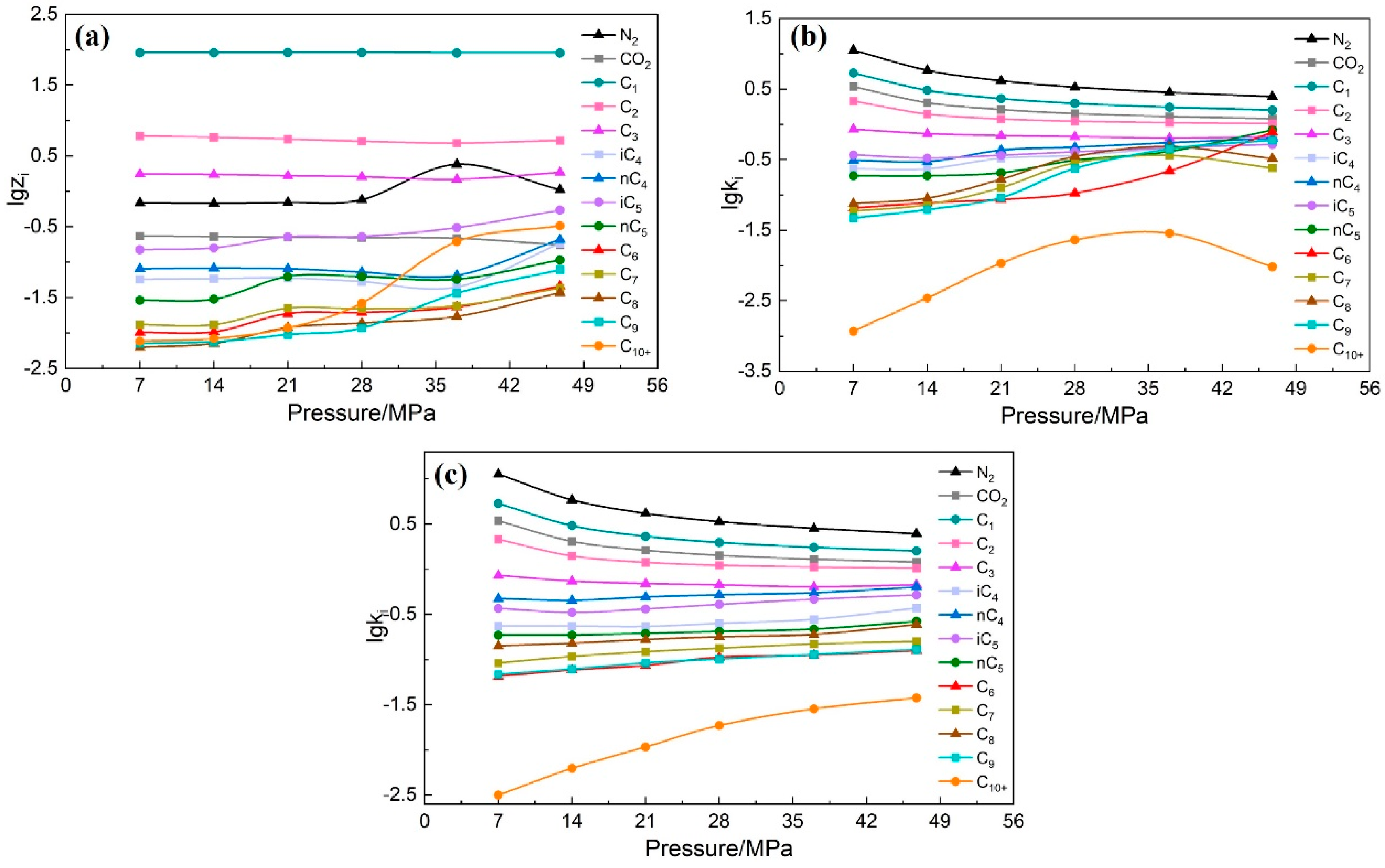
2.2.1. Component Check Method
2.2.2. Equilibrium Constant Method
2.3. Check Steps
- (1)
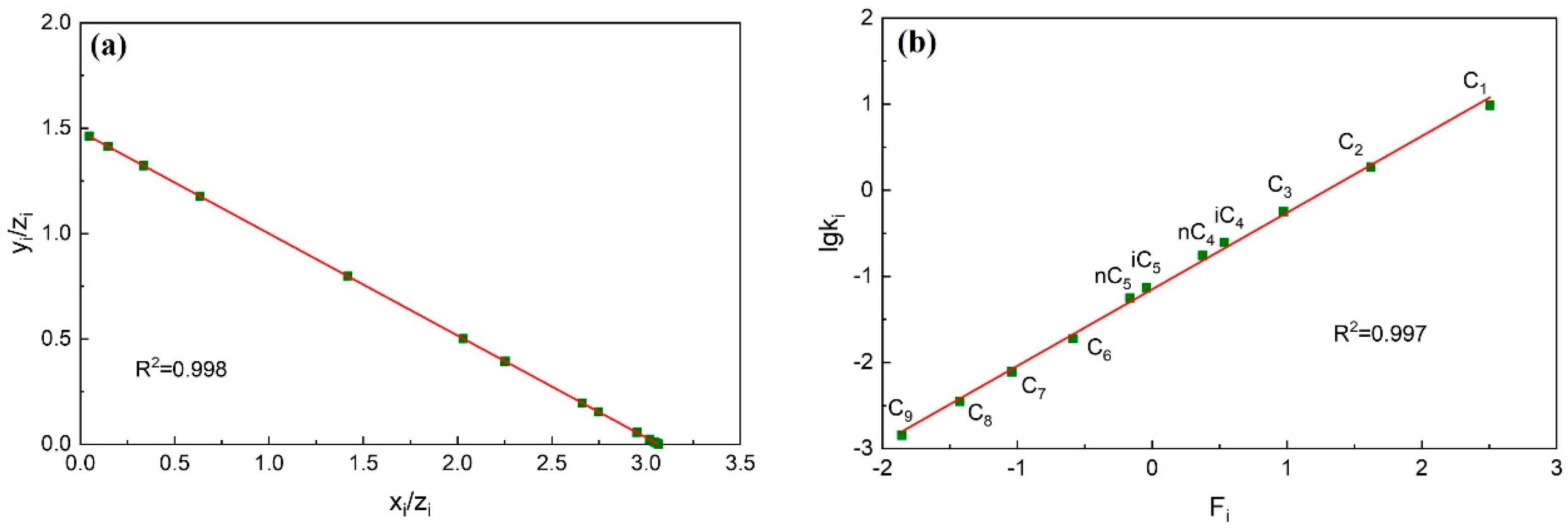
- Calculate the data of each component using the improved material balance method. The data points of each component are calculated and form a trend line. Check whether each component data point deviates from the trend line, whether the linear correlation coefficient is greater than 0.95, and whether the overall consistency of the component is strong.
- (2)
- Calculate the data of each component using the improved Hoffman method. Similar to step (1), the data points of each component are calculated and form a trend line. Check whether the data points of non-heavy hydrocarbon components deviate from the trend line and whether the linear correlation coefficient is greater than 0.95.
- (3)
- If the plots formed in steps (1) and (2) all show that the data points do not deviate from the trend line and the linear correlation coefficient is greater than 0.95, it indicates that the consistency of this fluid system component data is strong. The accuracy of the component data is proved to be high.
- (4)
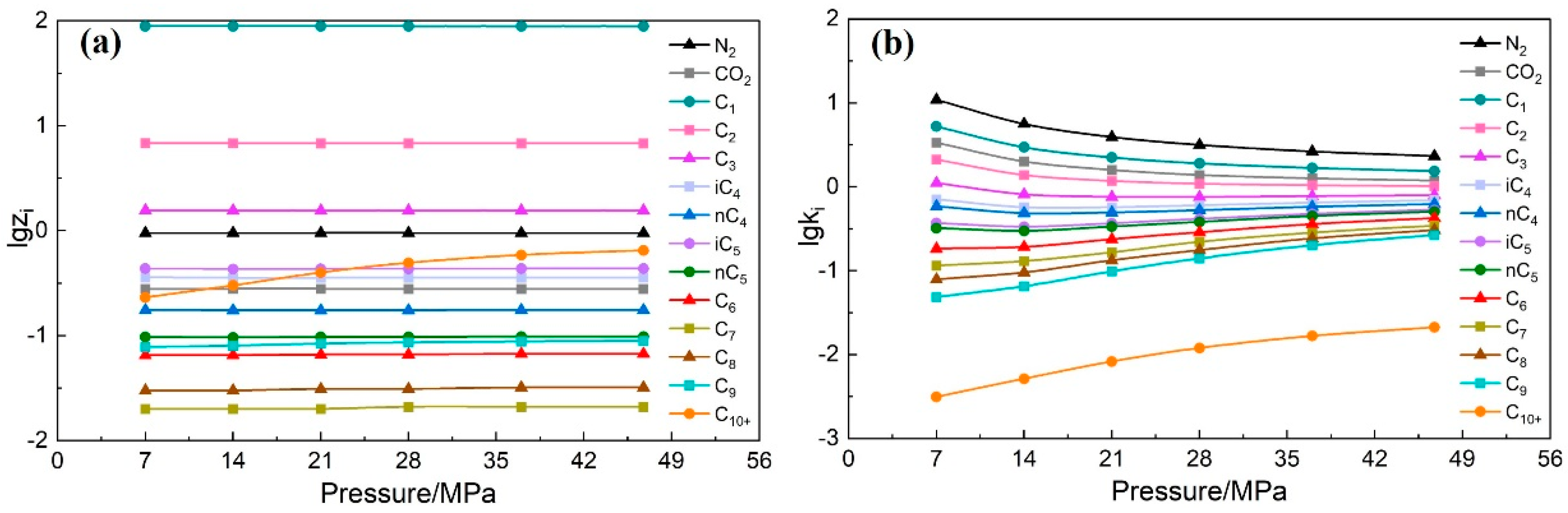
- According to the component data under different depletion pressures and depletion pressures of the CVD experiment data, draw the semi-log curve plot. Check whether the curves of each component cross each other and whether the monotonicity is strong to conduct preliminary verification of the CVD experiment data.
- (5)
- Calculate the CVD experiment data using the improved equilibrium constant method. Similar to step (4), the semi-log curves plot of all components at different depletion pressures is drawn. Check whether each component curve jumps and crosses each other.
- (6)
- If the plots drawn in steps (4) and (5) all show that the curves do not jump or cross each other and the monotonicity of the curves is strong, it indicates that the consistency of the CVD experiment data is strong. The accuracy of the CVD experiment data is proved to be high.
3. Method Application
3.1. Gas Condensate
3.1.1. Fluid and Reservoir Properties
3.1.2. Check Result
3.2. Volatile Oil
3.2.1. Fluid and Reservoir Properties
3.2.2. Check Result
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| xi | liquid phase mole fraction of component i |
| yi | gas phase mole fraction of component i |
| zi | global mole fraction of component i |
| ki | equilibrium constant of component i |
| ΔHvapi | enthalpy change of component i in the gas phase, kJ/mol |
| vsi | partial molar volume of solvent at infinite dilution of component i, m3/mol |
| φsi | fugacity coefficient of component i |
| Ai | constant, determined by the chemical properties of component i |
| P | pressure during the experiment, kPa |
| P0i | reference pressure of component i, kPa |
| T | absolute temperature, K |
| R | universal gas constant, kJ/(K·mol) |
| Tbi | boiling temperature of component i, K |
| Tci | critical temperature of component i, K |
| Ts | separator temperature, K |
| pci | critical pressure of component i, kPa |
| psc | pressure of standard conditions, kPa |
References
- Liu, B.; Gao, S.; Mohammadian, E.; Hadavimoghaddam, F.; Tian, S.; Xu, Y.; Pan, Z. Comprehensive Outlook into Critical Roles of Pressure, Volume, and Temperature (PVT) and Phase Behavior on the Exploration and Development of Shale Oil. Energy Fuels 2022, 36, 14534–14553. [Google Scholar] [CrossRef]
- Ghasemi, M.; Whitson, C.H. PVT modeling of complex heavy oil mixtures. J. Pet. Sci. Eng. 2021, 205, 108510. [Google Scholar] [CrossRef]
- Bi, R.; Nasrabadi, H. Molecular simulation of the constant composition expansion experiment in shale multi-scale systems. Fluid Phase Equilib. 2019, 495, 59–68. [Google Scholar] [CrossRef]
- Panhuis, P.; Van Jaarsveld, J.; Cornelisse, P.; Ramaswami, S.; Janson, D.; Rien, D. PVT Data Making the Difference: Delineating the Size of Gas Caps in Stacked Reservoirs. In Proceedings of the SPE Reservoir Characterisation and Simulation Conference and Exhibition, Abu Dhabi, United Arab Emirates, 24–26 January 2023. [Google Scholar]
- Qian, K.; Yang, S.; Dou, H.; Wang, Q.; Huang, Y.; Wan, T.; Zhang, Y. Interaction of the CO2-oil system and displacement mechanisms during CO2 flooding. Pet. Sci. Bull. 2019, 4, 69–82. [Google Scholar]
- Li, J.; Yuan, B.; Clarkson, C.R.; Tian, J. A semi-analytical rate-transient analysis model for light oil reservoirs exhibiting reservoir heterogeneity and multiphase flow. Pet. Sci. 2023, 20, 309–321. [Google Scholar] [CrossRef]
- Liu, L.; Yao, J.; Sun, H.; Huang, Z.; Yan, X.; Li, L. Compositional modeling of shale condensate gas flow with multiple transport mechanisms. J. Pet. Sci. Eng. 2019, 172, 1186–1201. [Google Scholar]
- Apasov, R.T.; Apasov, G.T.; Varavva, A.I.; Vinogradov, D.V.; Polkovnikov, F.I.; Platonov, D.V.; Samolovov, D.A. Creating and Maintenance of Well’s Lift Models at Oil Rim Field’s Under-Gas-Cap Zones—Experience Novoport Oil and Gas-Condensate Field and Tazovskoe Oil and Gas-Condensate Field. In Proceedings of the SPE Russian Petroleum Technology Conference, Virtual, 26–29 October 2020. [Google Scholar]
- Luo, L.; Lee, J. Rate Transient Analysis in Unconventional Volatile Oil Reservoirs. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Virtual, 20–22 July 2020. [Google Scholar]
- Suhendro, S.; Energi, P.H. Review of 20 Years Hydrocarbon Gas Cycling in the Arun Gas Field. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017. [Google Scholar]
- Franco, C.; Botero, O.; Zapata, J.; Mora, E.; Candela, C.; Castillo, A. Inhibited Gas Stimulation to Mitigate Condensate Banking and Maximize Recovery in Cupiagua Field. SPE Prod. Oper. 2013, 28, 154–167. [Google Scholar] [CrossRef]
- Phi, T.; Schechter, D. CO2 EOR Simulation in Unconventional Liquid Reservoirs: An Eagle Ford Case Study. In Proceedings of the SPE Unconventional Resources Conference, Calgary, AB, Canada, 15–16 February 2017. [Google Scholar]
- Qiao, R.; Chen, Z. Petroleum phase evolution at high temperature: A combined study of oil cracking experiment and deep oil in Dongying Depression, eastern China. Fuel 2022, 326, 124978. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; He, M.; Li, X.; Yang, W.; Lu, J. Asphaltene precipitation and reservoir damage characteristics of CO2 flooding in different microscopic structure types in tight light oil reservoirs. Fuel 2022, 312, 122943. [Google Scholar] [CrossRef]
- Long, K.; Tang, Y.; He, Y.; Wang, Y.; Qin, J.; Tang, L. Fluid phase behavior during multi-cycle injection and production of underground gas storage based on gas-condensate reservoirs with oil rim. Geoenergy Sci. Eng. 2023, 226, 211769. [Google Scholar] [CrossRef]
- Li, G.; Xian, C.; Liu, H. A “One Engine with Six Gears” System Engineering Methodology for the Economic Development of Unconventional Oil and Gas in China. Engineering 2022, 18, 105–115. [Google Scholar] [CrossRef]
- Jia, H.; Yu, H.; Xie, F.; Yuan, Z.; Xu, K.; Wang, Y. Research on CO2 microbubble dissolution kinetics and enhanced oil recovery mechanisms. Chin. J. Theor. Appl. Mech. 2023, 55, 755–764. [Google Scholar]
- Qi, S.; Yu, H.; Yang, H.; Wang, Y.; Yang, Z. Experimental research on quantification of countercurrent imbibition distance for tight sandstone. Chin. J. Theor. Appl. Mech. 2021, 53, 2603–2611. [Google Scholar]
- Bai, L.-H.; Liu, B.; Du, Y.-J.; Wang, B.-Y.; Tian, S.-S.; Wang, L.; Xue, Z.-Q. Distribution characteristics and oil mobility thresholds in lacustrine shale reservoir: Insights from N2 adsorption experiments on samples prior to and following hydrocarbon extraction. Pet. Sci. 2022, 19, 486–497. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Jia, N.; Zhang, K.; Yang, R.; Yang, S. Prospects and key scientific issues of CO2 near-miscible flooding. Pet. Sci. Bull. 2020, 5, 392–401. [Google Scholar]
- Tang, M.; Wang, C.; Deng Xa Yang, H.; Lu, J.; Yu, H. Experimental investigation on plugging performance of nanospheres in low permeability reservoir with bottom water. Adv. Geo-Energy Res. 2022, 6, 95–103. [Google Scholar] [CrossRef]
- Yu, H.; Wang, S.; Yang, H.; Yang, Y.; Zhang, J.; Wang, Y.; Yang, W.; Lu, J. A Systematic Method to Investigate the EOR Mechanism of Nanospheres: Laboratory Experiments from Core to Micro Perspective. Energy Fuels 2023, 37, 2053–2065. [Google Scholar] [CrossRef]
- White, A.; Prefontaine, N.; Thomas, F.B. Pseudo Formation Volume Factor: A Consistent and Continuous Volumetric Assessment Across Multiphase Reservoirs, a Wet Gas to Black Oil Duvernay Formation Example. In Proceedings of the SPE Annual Technical Conference and Exhibition, Calgary, AB, Canada, 30 September–2 October 2019. [Google Scholar]
- Yao, J.; Gu, Y. A new experimental method for measuring the bubble-point pressures of the light crude oil–CO2 system at different CO2 concentrations. Fuel 2022, 311, 122526. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Z. Hydrocarbon mixture and CO2 adsorptions in A nanopore-bulk multiscale system in relation to CO2 enhanced shale gas recovery. Chem. Eng. J. 2021, 415, 128398. [Google Scholar] [CrossRef]
- Hamdi, H.; Clarkson, C.R.; Esmail, A.; Sousa, M.C. Importance of Multiple-Contact and Swelling Tests for Huff-n-Puff Simulations: A Montney Shale Example. In Proceedings of the SPE Annual Technical Conference and Exhibition, Virtual, 27–29 October 2020. [Google Scholar]
- Li, D.; Saraji, S.; Jiao, Z.; Zhang, Y. CO2 injection strategies for enhanced oil recovery and geological sequestration in a tight reservoir: An experimental study. Fuel 2021, 284, 119013. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, H.; Guo, P.; Zeng, F.; Sang, T.; Wang, Z. Fluid behavior of gas condensate system with water vapor. Fluid Phase Equilib. 2017, 438, 67–75. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, Z. Hydrocarbon mixture phase behavior in multi-scale systems in relation to shale oil recovery: The effect of pore size distributions. Fuel 2021, 291, 120141. [Google Scholar] [CrossRef]
- Spivey, J.P. Comprehensive Quality Control for Constant Volume Depletion Laboratory Tests for Volatile Oils and Retrograde Gases. In Proceedings of the SPE Annual Technical Conference and Exhibition, Virtual, 27–29 October 2020. [Google Scholar]
- Luo, S.; Lutkenhaus, J.; Nasrabadi, H. A Framework for Incorporating Nanopores in Compositional Simulation to Model the Unusually High GOR Observed in Shale Reservoirs. In Proceedings of the SPE Reservoir Simulation Conference, Galveston, TX, USA, 10–11 April 2019. [Google Scholar]
- Tran, S.; Eghbali, S.; Dehghanpour, H. Studying Phase Behavior of Oil/Natural-Gas Systems for Designing Gas-Injection Operations: A Montney Case Study. SPE Res. Eval. Eng. 2020, 23, 943–961. [Google Scholar] [CrossRef]
- Hoffman, A.E.; Crump, J.S.; Hocott, C.R. Equilibrium constants for a gas-condensate system. J. Pet. Technol. 1953, 198, 1–10. [Google Scholar] [CrossRef]
- Bashbush, J.L. A method to determine k-values from laboratory data and its applications. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 4–7 October 1981. [Google Scholar]
- Whitson, C.H.; Torp, S.B. Evaluating Constant-Volume Depletion Data. J. Pet. Technol. 1983, 35, 610–620. [Google Scholar] [CrossRef]
- Akpabio, J.U.; Udofia, E.E.; Ogbu, M. PVT fluid characterization and consistency check for retrograde condensate reservoir modeling. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 5–7 August 2014. [Google Scholar]
- Chen, C.; Wang, Y.; Beagle, J.R.; Liao, L.; Shi, S.; Deng, R. Reconstruction of the evolution of deep fluids in light oil reservoirs in the Central Tarim Basin by using PVT simulation and basin modeling. Mar. Pet. Geol. 2019, 107, 116–126. [Google Scholar] [CrossRef]
- Varzandeh, F.; Stenby, E.H.; Yan, W. General approach to characterizing reservoir fluids for EoS models using a large PVT database. Fluid Phase Equilib. 2017, 433, 97–111. [Google Scholar] [CrossRef]
- Ghorayeb, K.; Mawlod, A.A.; Maarouf, A.; Sami, Q.; El Droubi, N.; Merrill, R.; El Jundi, O.; Mustapha, H. Chain-based machine learning for full PVT data prediction. J. Pet. Sci. Eng. 2022, 208, 109658. [Google Scholar] [CrossRef]
- Zirrahi, M.; Hassanzadeh, H.; Abedi, J. Experimental Measurements and Correlation of K-value, Viscosity, and Density Data for Mixtures of Light to Heavy Solvents and Athabasca Bitumen with Applications of ES-SAGD Process. In Proceedings of the SPE Canada Heavy Oil Technical Conference, Calgary, AB, Canada, 7–9 June 2016. [Google Scholar]
- Najafi-Marghmaleki, A.; Tatar, A.; Barati-Harooni, A.; Arabloo, M.; Rafiee-Taghanaki, S.; Mohammadi, A.H. Reliable modeling of constant volume depletion (CVD) behaviors in gas condensate reservoirs. Fuel 2018, 231, 146–156. [Google Scholar] [CrossRef]
- Ibeh, S.U.; Chubueze, S.E. PVT analysis: Validity checks and eos tuning techniques for retrograde gas condensate reservoirs. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 2–4 August 2016. [Google Scholar]

| Reservoir Parameters | Value |
|---|---|
| Reservoir pressure, MPa | 121.68 |
| Reservoir temperature, °C | 138.2 |
| Saturation pressure, MPa | 51.48 |
| Liquid molecular weight | 173 |
| Liquid density (20 °C), kg/m3 | 827.34 |
| Component | CO2 | N2 | C1 | C2 | C3 | iC4 | nC4 |
| Fluid X, mol% | 0.175 | 1.058 | 90.134 | 5.216 | 1.847 | 0.183 | 0.208 |
| Component | iC5 | nC5 | C6 | C7 | C8 | C9 | C10+ |
| Fluid X, mol% | 0.542 | 0.107 | 0.046 | 0.043 | 0.037 | 0.078 | 0.326 |
| Reservoir Parameters | Value |
|---|---|
| Reservoir pressure, MPa | 147.8 |
| Reservoir temperature, °C | 124.3 |
| Saturation pressure, MPa | 32.76 |
| Liquid molecular weight | 157 |
| Liquid density (20 °C), kg/m3 | 762.1 |
| Component | CO2 | N2 | C1 | C2 | C3 | iC4 | nC4 |
| Fluid Y, mol% | 1.78 | 0.45 | 55.48 | 10.43 | 4.48 | 1.78 | 2.63 |
| Component | iC5 | nC5 | C6 | C7 | C8 | C9 | C10+ |
| Fluid Y, mol% | 1.17 | 2.06 | 2.79 | 2.62 | 1.76 | 3.43 | 9.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhang, Y.; He, Y.; Shi, J.; Wang, X.; Song, J.; Yu, H. Consistency Checks for Pressure-Volume-Temperature Experiment of Formation Oil and Gas at High Temperature. Processes 2023, 11, 2727. https://doi.org/10.3390/pr11092727
Zhao L, Zhang Y, He Y, Shi J, Wang X, Song J, Yu H. Consistency Checks for Pressure-Volume-Temperature Experiment of Formation Oil and Gas at High Temperature. Processes. 2023; 11(9):2727. https://doi.org/10.3390/pr11092727
Chicago/Turabian StyleZhao, Libin, Yongling Zhang, Yuanyuan He, Jianchao Shi, Xiaopei Wang, Jiabang Song, and Haiyang Yu. 2023. "Consistency Checks for Pressure-Volume-Temperature Experiment of Formation Oil and Gas at High Temperature" Processes 11, no. 9: 2727. https://doi.org/10.3390/pr11092727
APA StyleZhao, L., Zhang, Y., He, Y., Shi, J., Wang, X., Song, J., & Yu, H. (2023). Consistency Checks for Pressure-Volume-Temperature Experiment of Formation Oil and Gas at High Temperature. Processes, 11(9), 2727. https://doi.org/10.3390/pr11092727






