Study on Process Parameters in Hydrothermal Liquefaction of Rice Straw and Cow Dung: Product Distribution and Application of Biochar in Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Biomass Collection and Characterization
2.3. Hydrothermal Optimization Experiments
2.4. Hydrothermal Products Separation and Recovery
2.5. HTL Products Characterization
2.5.1. Bio-Oil Characterization (GC–MS)
2.5.2. Biochar Characterization
2.6. Biochar Recovery and Activation for Adsorption Experiments
3. Results and Discussion
3.1. Biomass Characterization
3.2. Hydrothermal Experiments
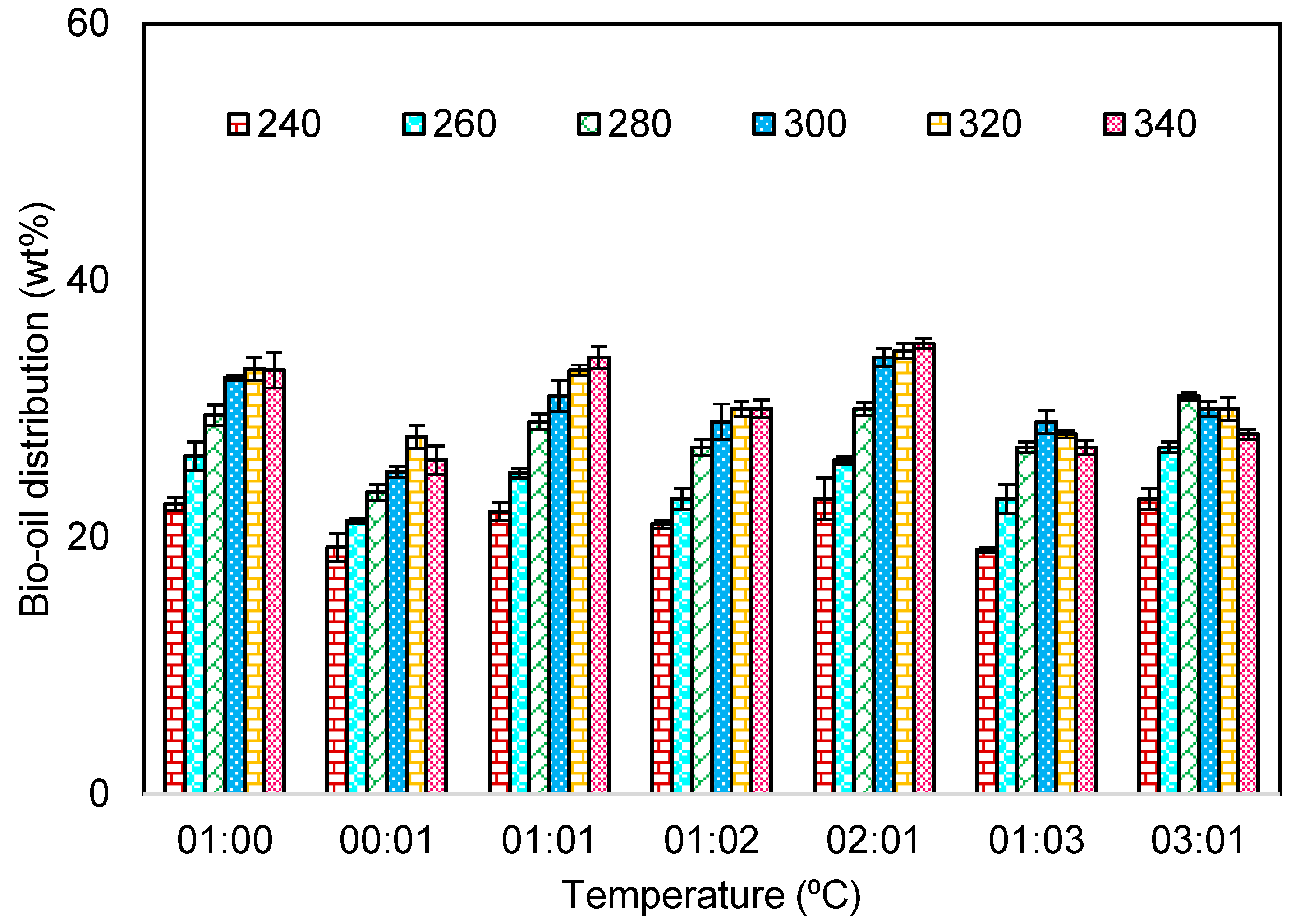
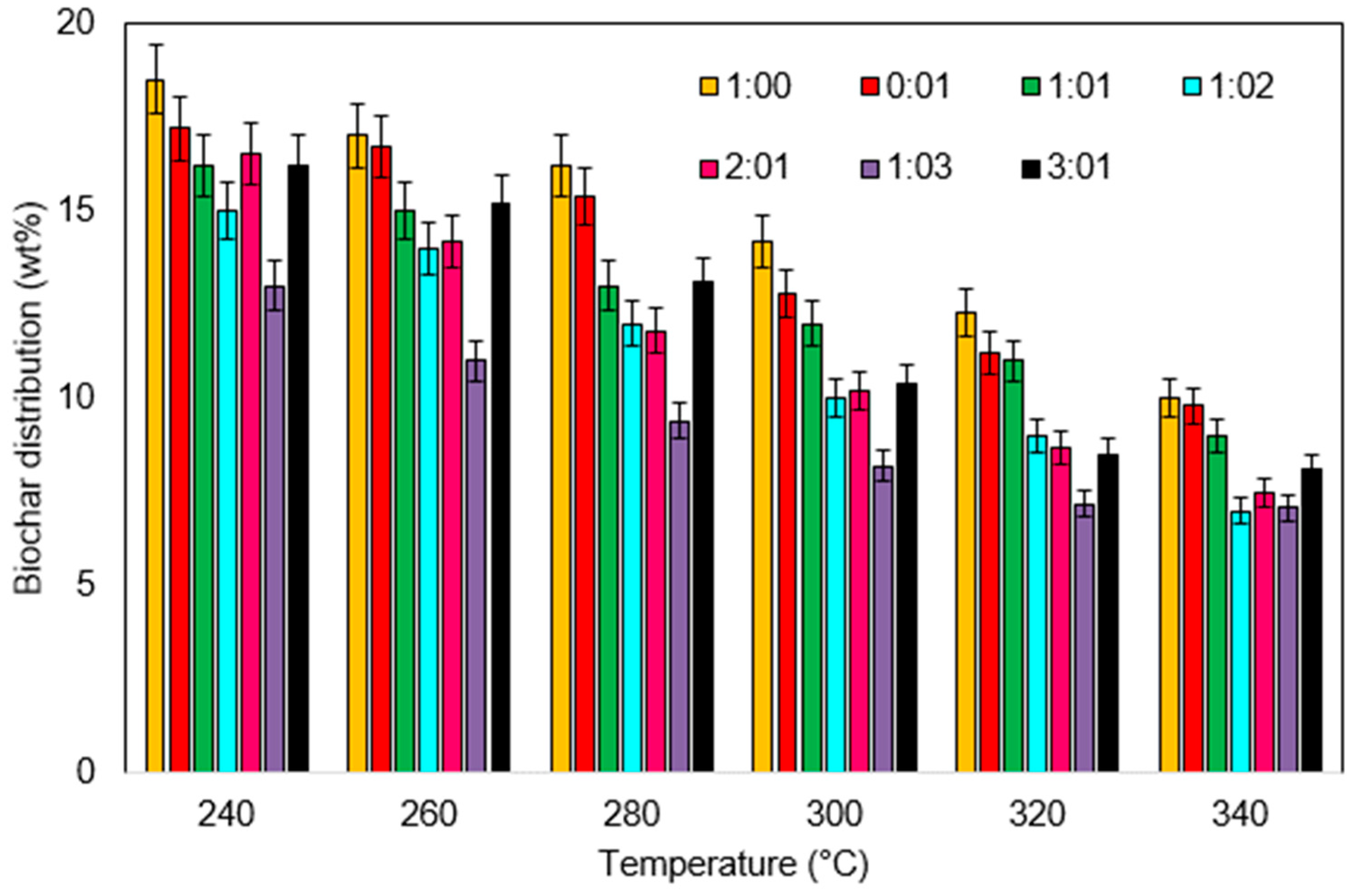
3.2.1. Effect of Temperature
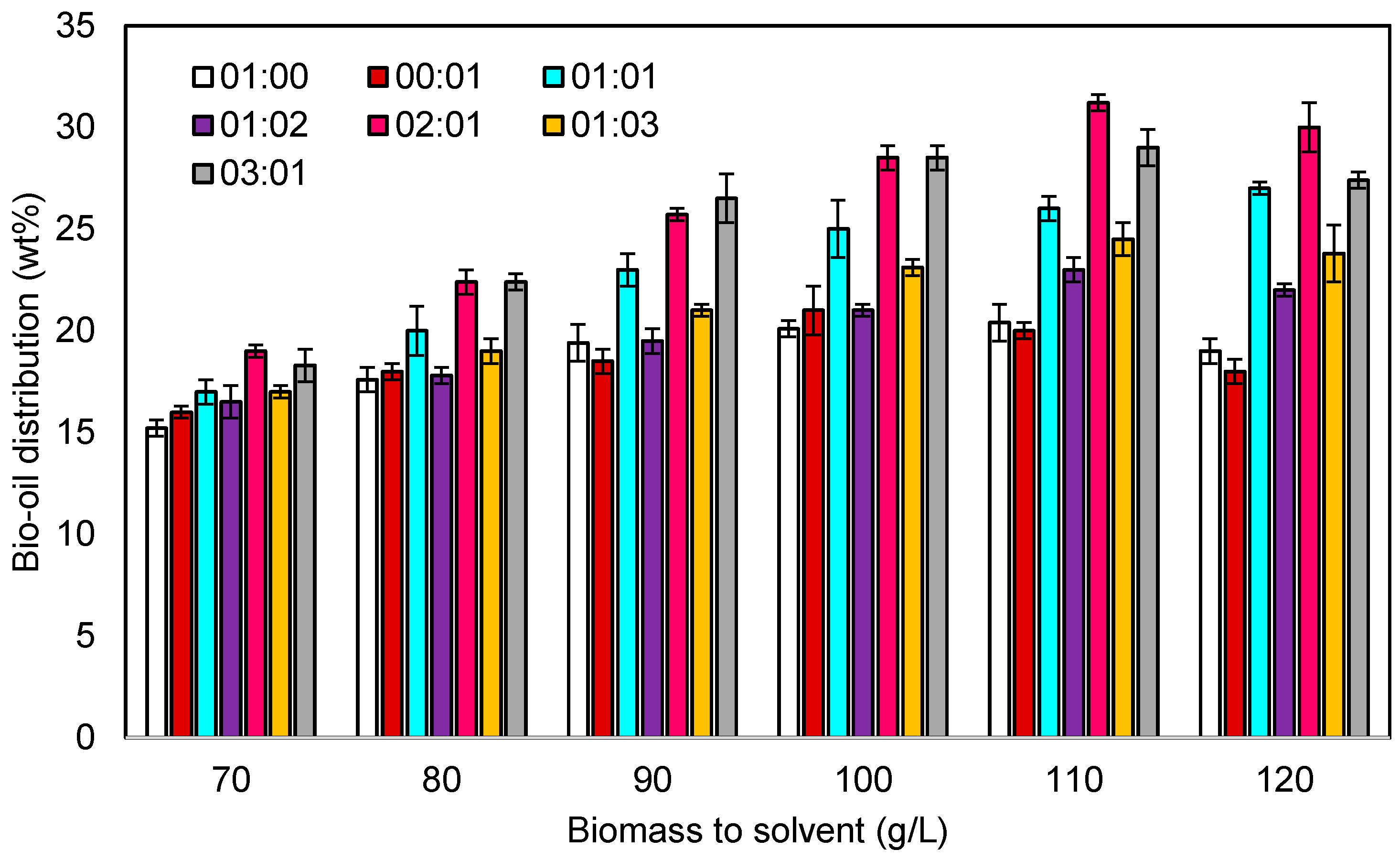
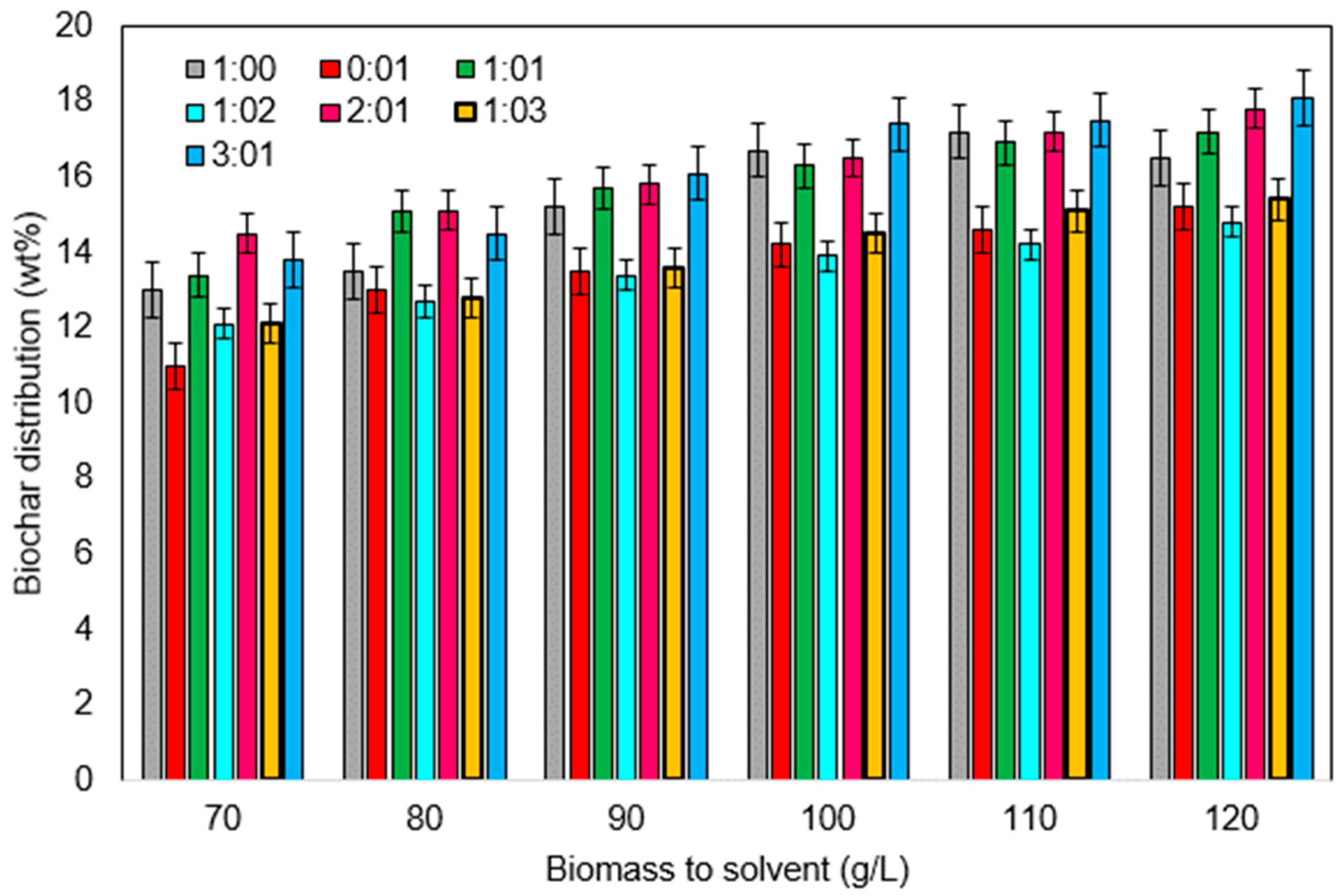
3.2.2. Effect of Solvent to Biomass Ratio
3.3. HTL Product Analysis
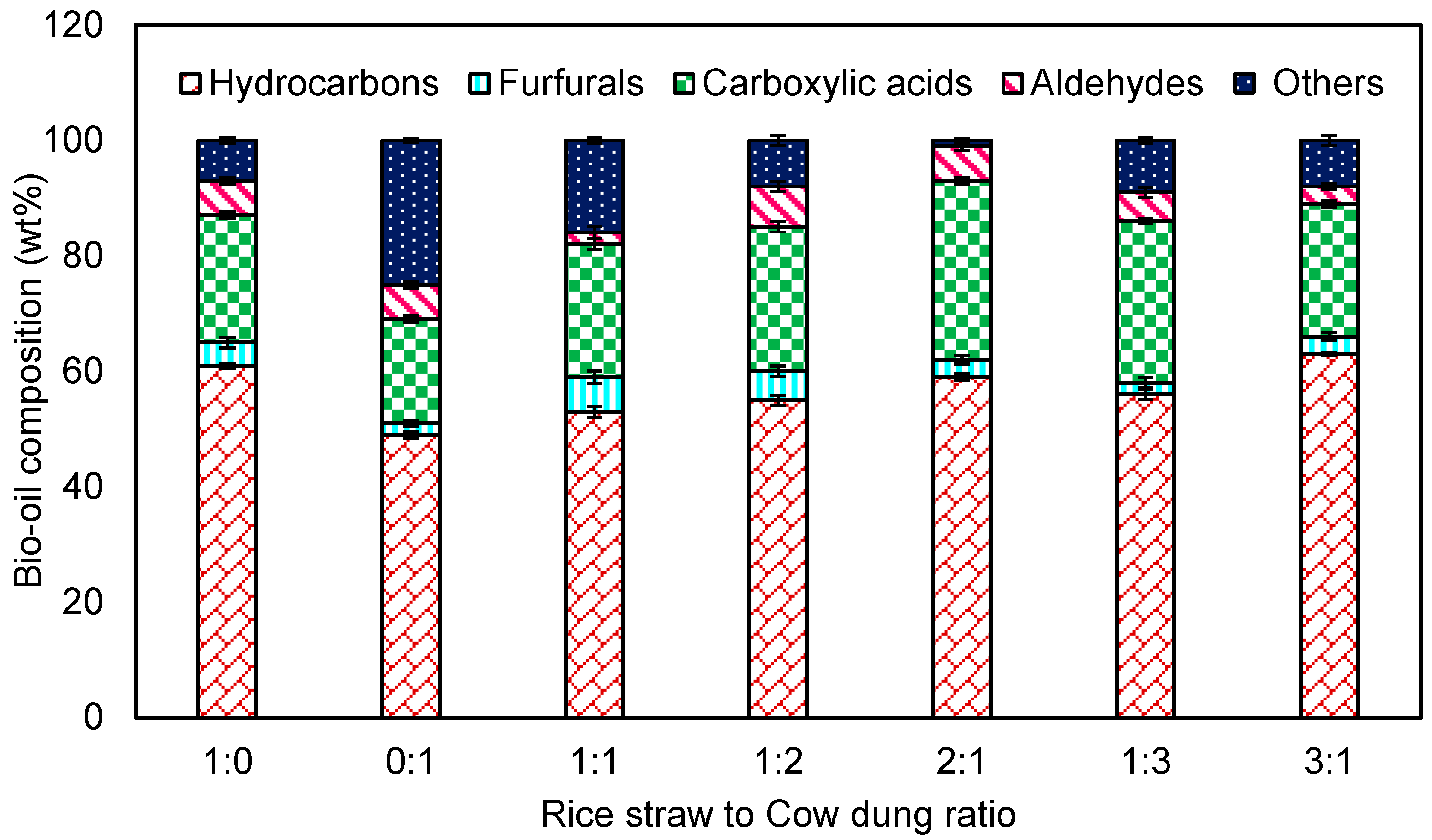
3.3.1. GC–FID for Bio-Oil
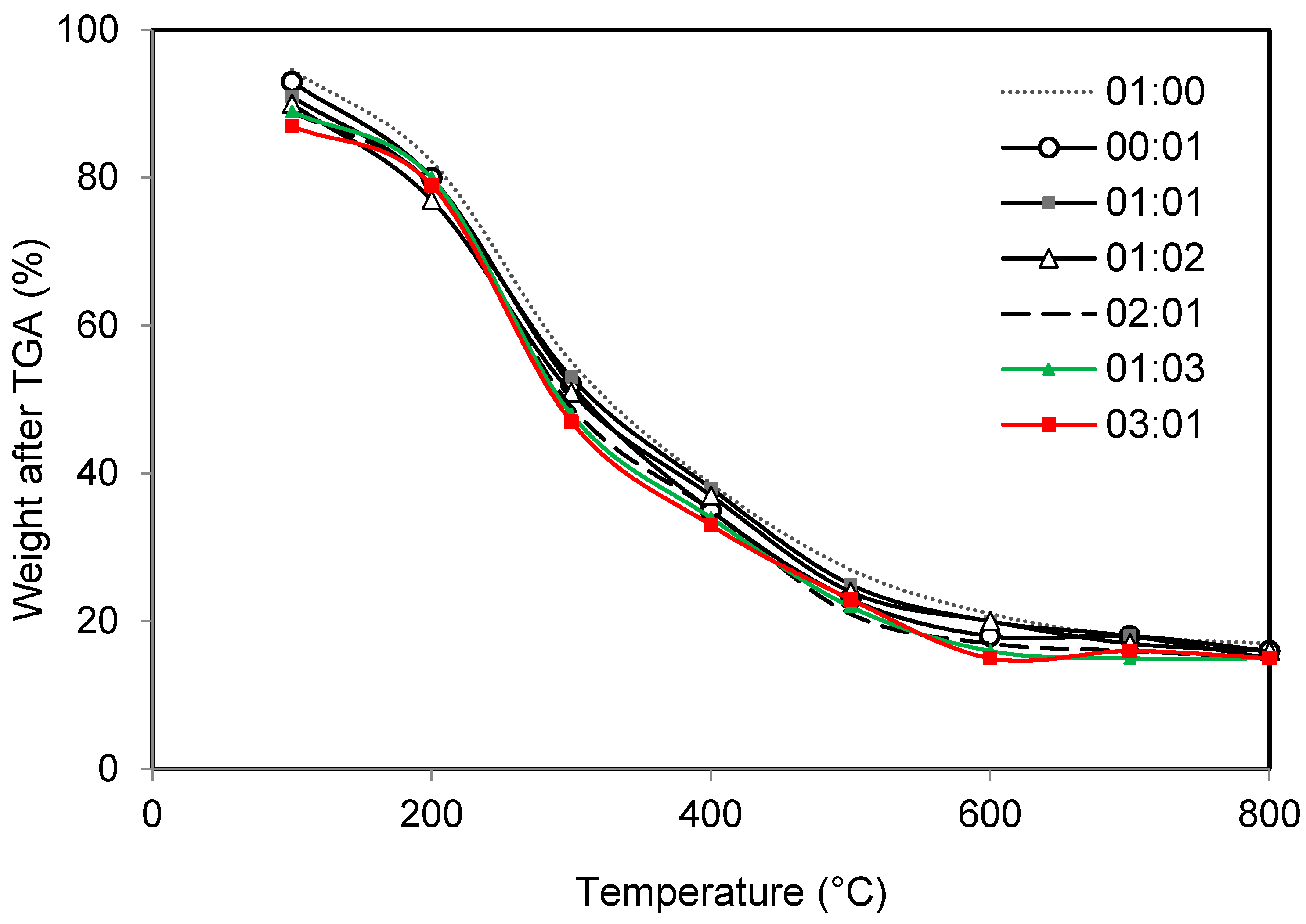
3.3.2. Proximate and Ultimate Analyses of Bio-Oil and Biomass
3.4. Biochar Analysis
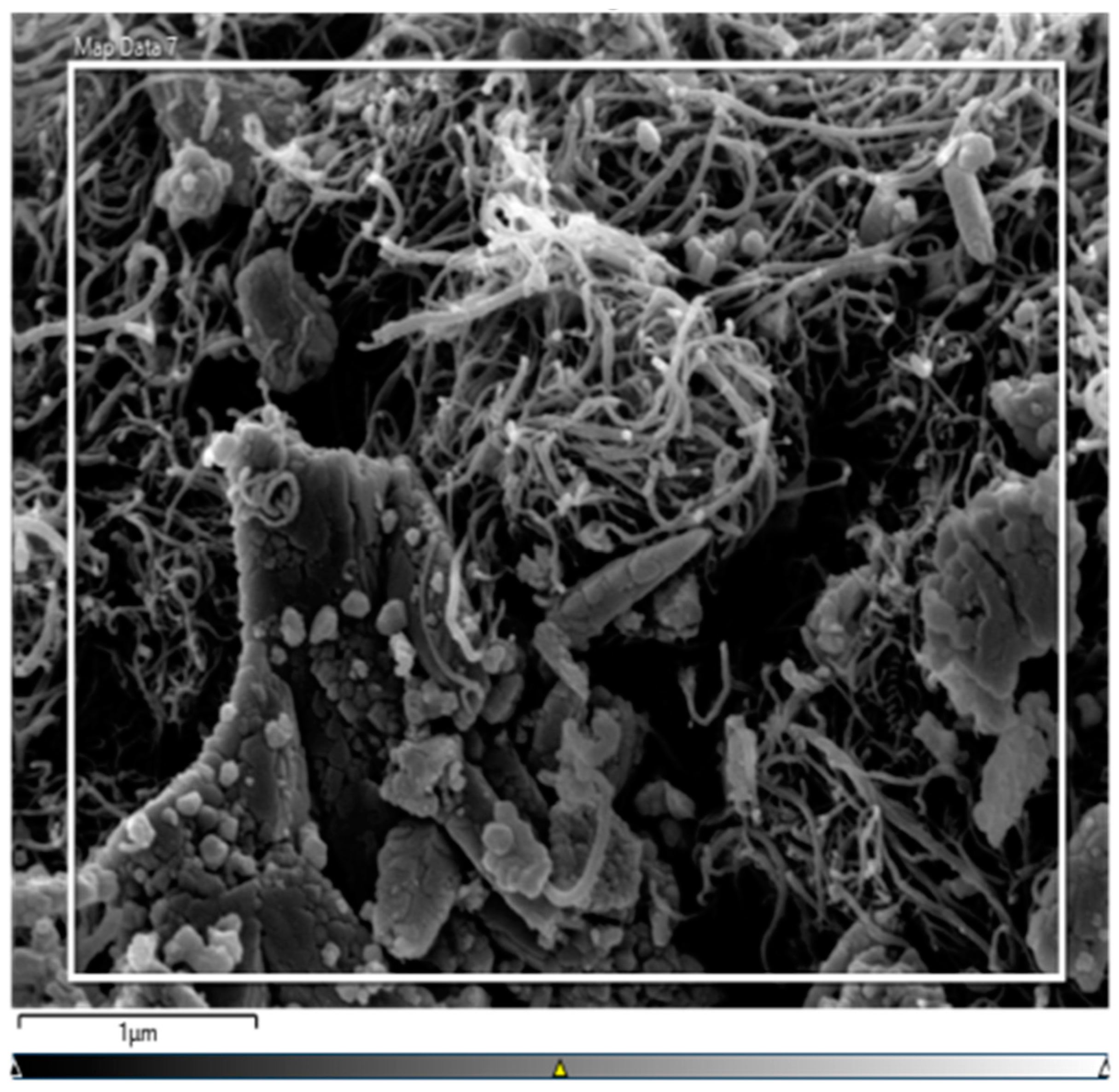
3.4.1. SEM and BET Analysis of Biochar
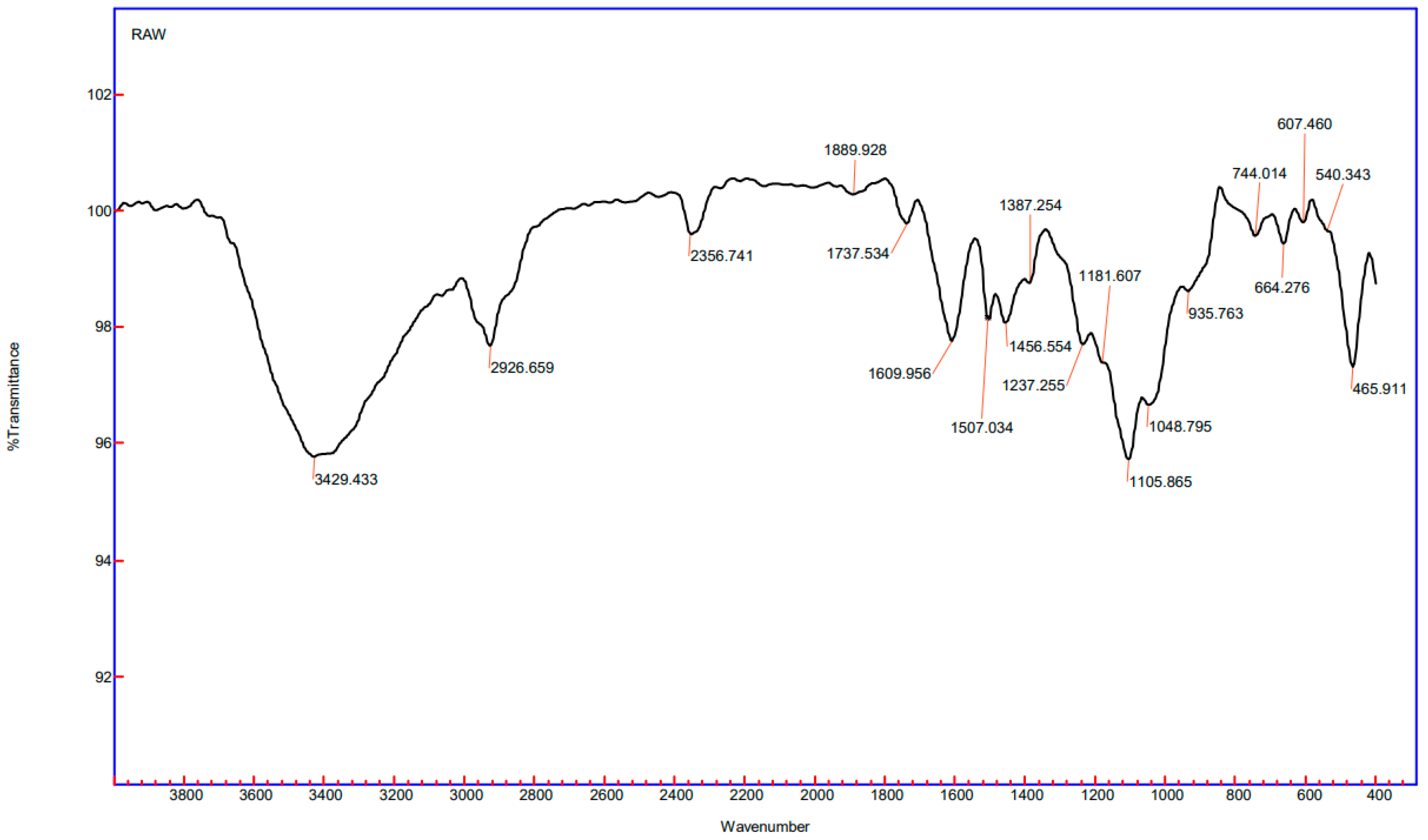
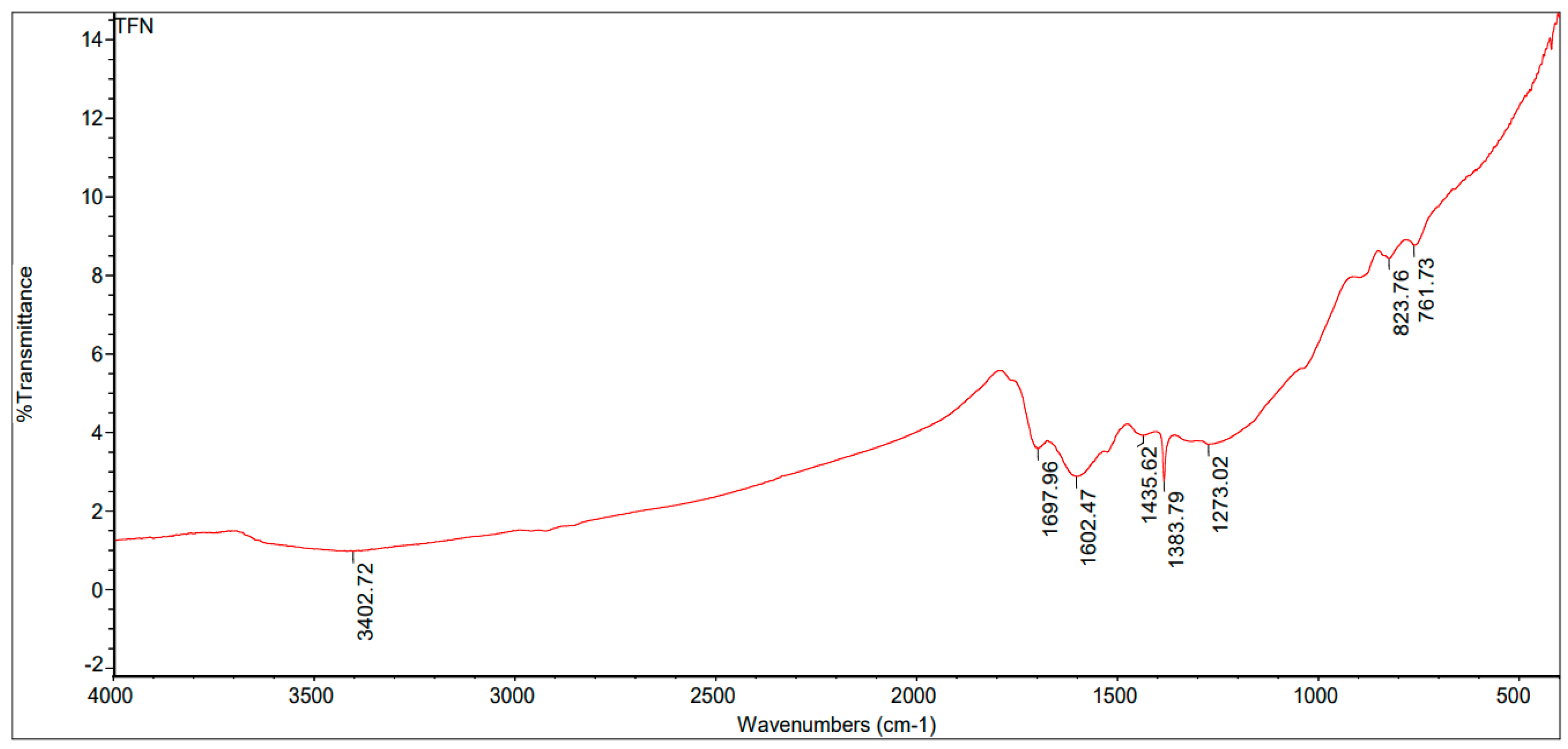
3.4.2. FTIR for Biochar
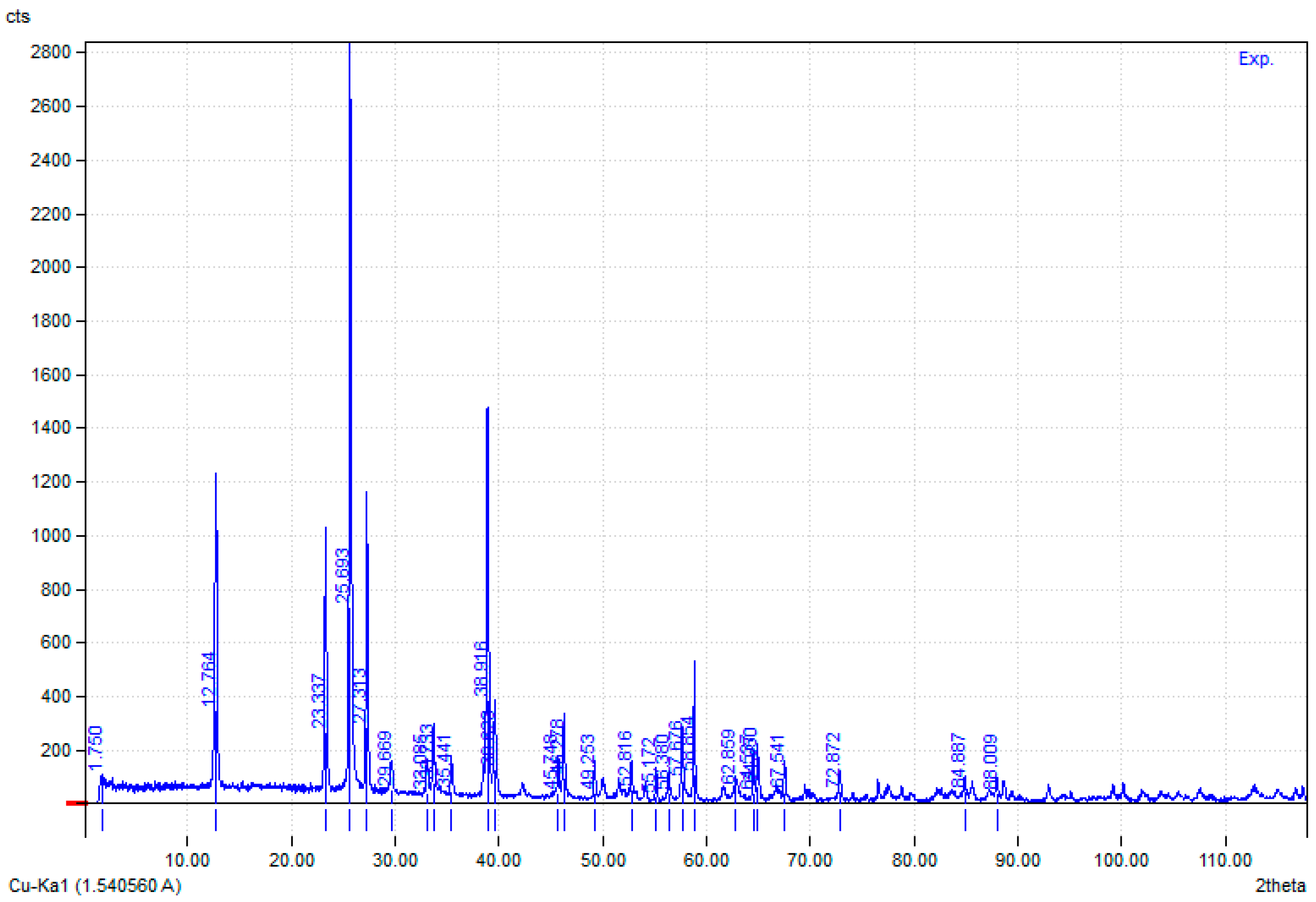
3.4.3. XRD Analysis
3.4.4. FT-Raman Analysis
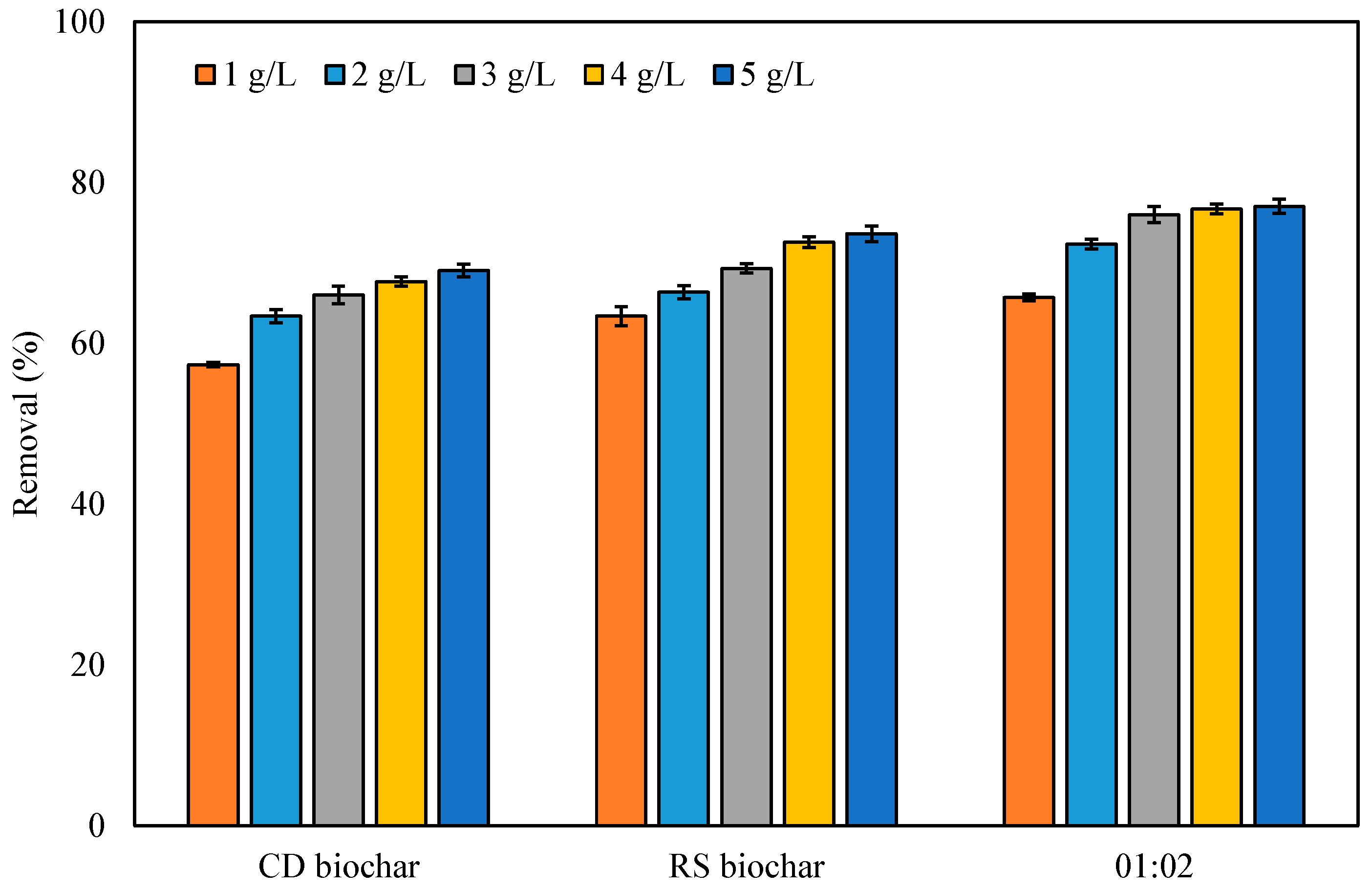
3.5. Adsorption Experiments
Effect of Biochar Dosage on Dye Removal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, N.; Chowdhary, P.; Ahmad, A.; Shekher Giri, B.; Chaturvedi, P. Hydrothermal liquefaction of rice husk and cow dung in Mixed-Bed-Rotating Pyrolyzer and application of biochar for dye removal. Bioresour. Technol. 2020, 309, 123294. [Google Scholar] [CrossRef]
- EswaryDevi, T.; Parthiban, R.; Arun, J.; Gopinath, K.P. Processing of marine microalgae biomass via hydrothermal liquefaction for bio-oil production: Study on algae cultivation, harvesting, and process parameters. Biomass Convers. Biorefin. 2022, 1–14. [Google Scholar] [CrossRef]
- López-García, M.; Lodeiro, P.; Herrero, R.; Barriada, J.L.; Rey-Castro, C.; David, C.; Sastre de Vicente, M.E. Experimental evidences for a new model in the description of the adsorption-coupled reduction of Cr(VI) by protonated banana skin. Bioresour. Technol. 2013, 139, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K. Value added liquid products from waste biomass pyrolysis using pretreatments. Sci. Total Environ. 2015, 538, 145–151. [Google Scholar] [CrossRef]
- Durán-Sarmiento, M.A.; Del Portillo-Valdés, L.A.; Rueda-Ordonez, Y.J.; Florez-Rivera, J.S.; Rincón-Quintero, A.D. Study on the gasification process of the biomass obtained from agricultural waste with the purpose of estimating the energy potential in the Santander region and its surroundings. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1253, 012001. [Google Scholar] [CrossRef]
- Doukeh, R.; Bombos, D.; Bombos, M.; Oprescu, E.E.; Dumitrascu, G.; Vasilievici, G.; Calin, C. Catalytic hydrotreating of bio-oil and evaluation of main noxious emissions of gaseous phase. Sci. Rep. 2021, 11, 6176. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.-T.; Show, P.-L. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Srifa, A.; Chaiwat, W.; Pitakjakpipop, P.; Anutrasakda, W.; Faungnawakij, K. Advances in bio-oil production and upgrading technologies. In Sustainable Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–198. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the applications of bio-oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Lazzari, E.; dos Santos Polidoro, A.; Onorevoli, B.; Schena, T.; Silva, A.N.; Scapin, E.; Jacques, R.A.; Caramão, E.B. Production of rice husk bio-oil and comprehensive characterization (qualitative and quantitative) by HPLC/PDA and GC × GC/qMS. Renew. Energy 2019, 135, 554–565. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Mohd Zulkifli, N.W.; Ibrahim, S.; Chen, W.H.; Chong, C.T.; Ok, Y.S. Valorization of animal manure via pyrolysis for bioenergy: A review. J. Clean. Prod. 2022, 343, 130965. [Google Scholar] [CrossRef]
- Saxena, D.; Sandhwar, V.K. Cow farm wastes: A bioresource for sustainable development. In Bio-Based Materials and Waste for Energy Generation and Resource Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 411–429. [Google Scholar] [CrossRef]
- ASTM E871-82; Standard Test Method for Moisture Analysis of Particulate Wood Fuels. Available online: https://www.astm.org/e0871-82r19.html (accessed on 10 August 2023).
- ASTM E1755-01; Standard Test Method for Ash in Biomass. Available online: https://www.astm.org/e1755-01.html (accessed on 10 August 2023).
- Vaithyanathan, V.K.; Goyette, B.; Rajagopal, R. A critical review of the transformation of biomass into commodity chemicals: Prominence of pretreatments. Environ. Chall. 2023, 11, 100700. [Google Scholar] [CrossRef]
- Biswas, B.; Arun Kumar, A.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Effects of temperature and solvent on hydrothermal liquefaction of Sargassum tenerrimum algae. Bioresour. Technol. 2017, 242, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Mathanker, A.; Pudasainee, D.; Kumar, A.; Gupta, R. Hydrothermal liquefaction of lignocellulosic biomass feedstock to produce biofuels: Parametric study and products characterization. Fuel 2020, 271, 117534. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; SundarRajan, P.S.; Malolan, R.; AjaySrinivaasan, P. Hydrothermal liquefaction and pyrolysis of Amphiroa fragilissima biomass: Comparative study on oxygen content and storage stability parameters of bio-oil. Bioresour. Technol. Rep. 2020, 11, 100465. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; SundarRajan, P.S.; Malolan, R.; Adithya, S.; Sai Jayaraman, R.; Srinivaasan Ajay, P. Hydrothermal liquefaction of Scenedesmus obliquus using a novel catalyst derived from clam shells: Solid residue as catalyst for hydrogen production. Bioresour. Technol. 2020, 310, 123443. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; Vigneshwar, S.S.; Swetha, A. Sustainable and eco-friendly approach for phosphorus recovery from wastewater by hydrothermally carbonized microalgae: Study on spent bio-char as fertilizer. J. Water Process. Eng. 2020, 38, 101567. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; Sivaramakrishnan, R.; Shyam, S.; Mayuri, N.; Manasa, S.; Pugazhendhi, A. Hydrothermal liquefaction of Prosopis juliflora biomass for the production of ferulic acid and bio-oil. Bioresour. Technol. 2021, 319, 124116. [Google Scholar] [CrossRef]
- Ibrahim, I.; Tsubota, T.; Hassan, M.A.; Andou, Y. Surface Functionalization of Biochar from Oil Palm Empty Fruit Bunch through Hydrothermal Process. Processes 2021, 9, 149. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Shirley, V.; Cheng, D. Hydrothermal pyrolysis of swine manure to bio-oil: Effects of operating parameters on products yield and characterization of bio-oil. J. Anal. Appl. Pyrolysis 2010, 88, 73–79. [Google Scholar] [CrossRef]
- Tahir, M.H.; Cheng, X.; Irfan, R.M.; Ashraf, R.; Zhang, Y. Comparative chemical analysis of pyrolyzed bio oil using online TGA-FTIR and GC-MS. J. Anal. Appl. Pyrolysis 2020, 150, 104890. [Google Scholar] [CrossRef]
- Arellano, O.; Flores, M.; Guerra, J.; Hidalgo, A.; Rojas, D.; Strubinger, A. Hydrothermal Carbonization of Corncob and Characterization of the Obtained Hydrochar. Chem. Eng. Trans. 2016, 50, 235–240. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. Hydrothermal liquefaction of beech wood using a natural calcium borate mineral. J. Supercrit. Fluids 2012, 72, 134–139. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor. Chem. Eng. J. 2019, 360, 212–220. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Feng, S.; Xu, C.C.; Bassi, A. A review of recent developments of pre-treatment technologies and hydrothermal liquefaction of microalgae for bio-crude oil production. Renew. Sustain. Energy Rev. 2019, 101, 476–492. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Mohamad, N.; Jouhara, H. Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]






| Biomass Mixture (RS:CD) | C | H | N | S | O | HHV (MJ/Kg) | H/C | O/C | |
|---|---|---|---|---|---|---|---|---|---|
| Biomass | 01:00 | 47.51 | 9.34 | 5.23 | 2.45 | 35.47 | 23.06451 | 2.359082 | 0.559935 |
| 00:01 | 37.65 | 12.2 | 9.12 | 1.05 | 39.95 | 23.05907 | 3.898008 | 0.795817 | |
| 01:01 | 45.23 | 8.6 | 4.23 | 1.45 | 40.49 | 20.34108 | 2.281671 | 0.671402 | |
| 01:02 | 41.56 | 9.56 | 4.89 | 2.1 | 41.89 | 20.2216 | 2.760346 | 0.755955 | |
| 02:01 | 48.71 | 9.74 | 3.71 | 0.78 | 37.06 | 23.75749 | 2.399507 | 0.570622 | |
| 01:03 | 40.81 | 8.12 | 3.59 | 1.07 | 46.41 | 17.10496 | 2.38765 | 0.852916 | |
| 03:01 | 45.23 | 9.03 | 3.18 | 1.23 | 41.33 | 20.80518 | 2.395755 | 0.685331 | |
| Bio-oil | 01:00 | 71.23 | 7.12 | 3.12 | 0.82 | 17.71 | 31.08187 | 1.199495 | 0.186473 |
| 00:01 | 64.23 | 9.45 | 4.18 | 1.12 | 21.02 | 31.45227 | 1.76553 | 0.245446 | |
| 01:01 | 66.89 | 8.23 | 3.58 | 0.91 | 20.39 | 30.72165 | 1.476454 | 0.228622 | |
| 01:02 | 61.58 | 9.56 | 2.45 | 1.49 | 24.92 | 30.0175 | 1.862943 | 0.303508 | |
| 02:01 | 70.23 | 8.56 | 1.15 | 0.45 | 19.61 | 32.46104 | 1.462623 | 0.209419 | |
| 01:03 | 67.23 | 7.89 | 2.23 | 0.76 | 21.89 | 30.0833 | 1.4083 | 0.244199 | |
| 03:01 | 68.91 | 6.78 | 1.97 | 0.49 | 21.85 | 29.0732 | 1.18067 | 0.23781 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seikh, A.H.; Alharbi, H.F.; Alnaser, I.A.; Karim, M.R.; Mohammed, J.A.; Aijaz, M.O.; Hassan, A.; Abdo, H.S. Study on Process Parameters in Hydrothermal Liquefaction of Rice Straw and Cow Dung: Product Distribution and Application of Biochar in Wastewater Treatment. Processes 2023, 11, 2779. https://doi.org/10.3390/pr11092779
Seikh AH, Alharbi HF, Alnaser IA, Karim MR, Mohammed JA, Aijaz MO, Hassan A, Abdo HS. Study on Process Parameters in Hydrothermal Liquefaction of Rice Straw and Cow Dung: Product Distribution and Application of Biochar in Wastewater Treatment. Processes. 2023; 11(9):2779. https://doi.org/10.3390/pr11092779
Chicago/Turabian StyleSeikh, Asiful H., Hamad F. Alharbi, Ibrahim A. Alnaser, Mohammad R. Karim, Jabair A. Mohammed, Muhammad Omer Aijaz, Ahmed Hassan, and Hany S. Abdo. 2023. "Study on Process Parameters in Hydrothermal Liquefaction of Rice Straw and Cow Dung: Product Distribution and Application of Biochar in Wastewater Treatment" Processes 11, no. 9: 2779. https://doi.org/10.3390/pr11092779








