Preparation of Titanium Carbide by Carburisation of Titanium Dioxide
Abstract
:1. Introduction
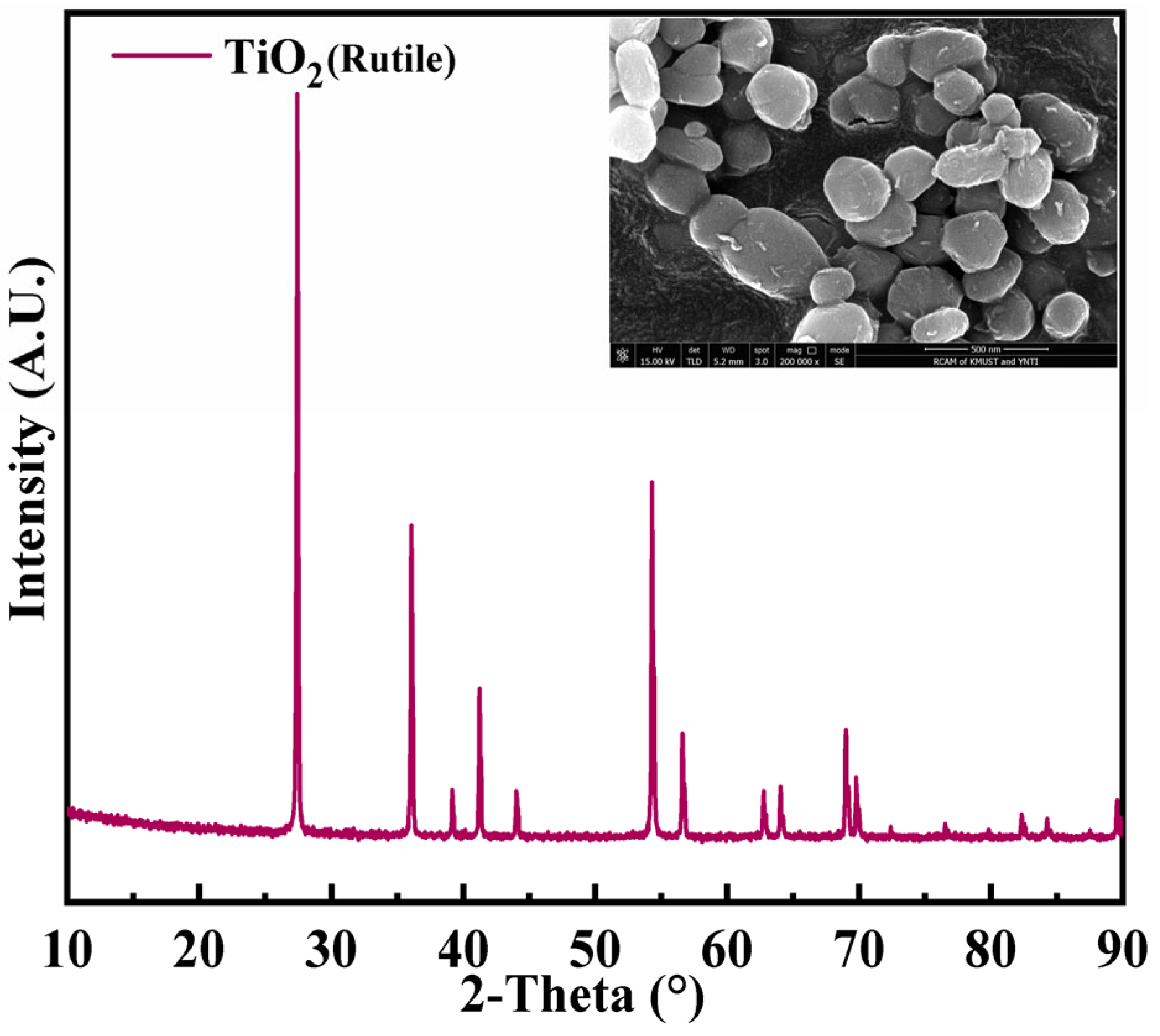
2. Materials and Methods
3. Results
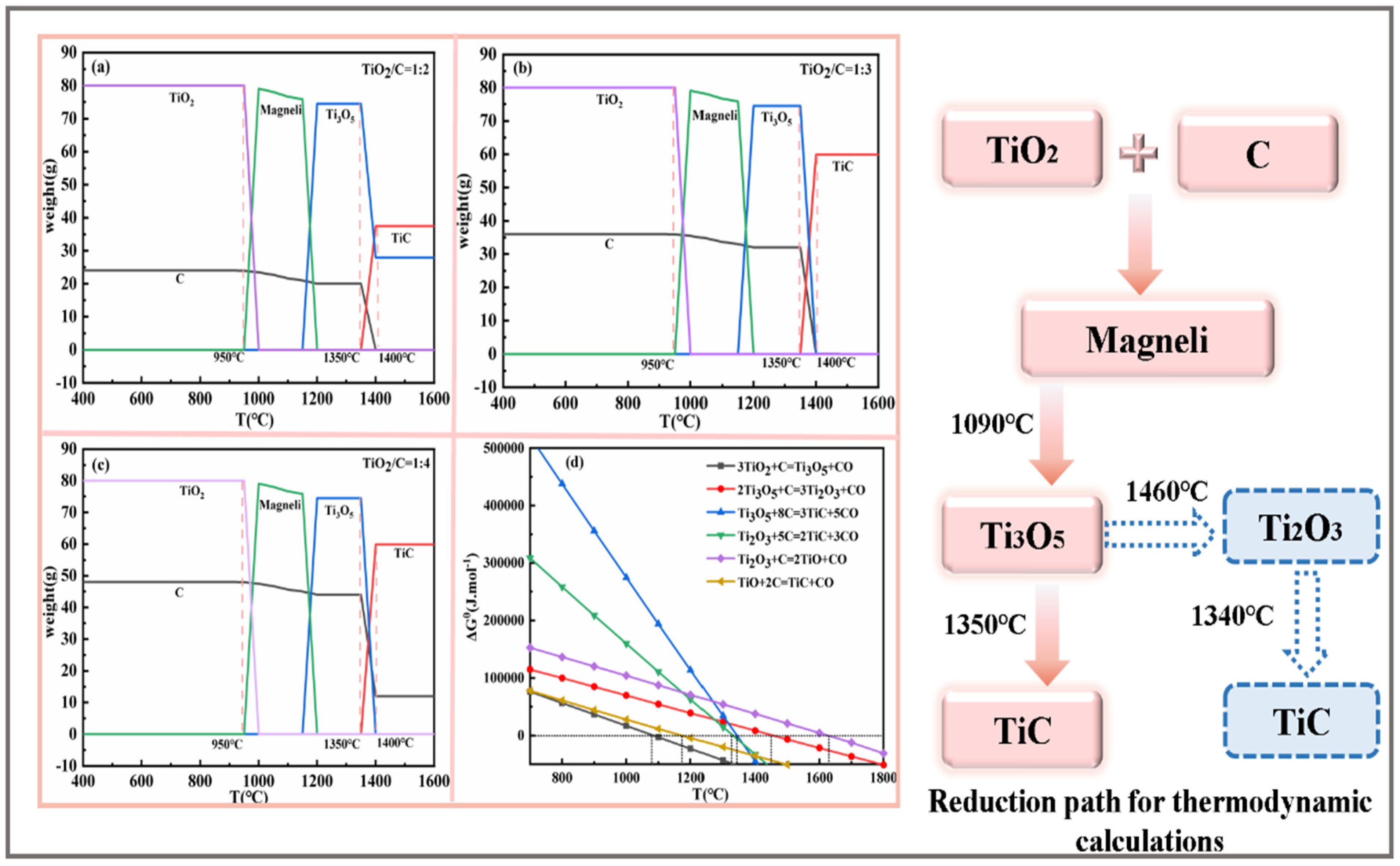
3.1. Thermodynamic Calculation
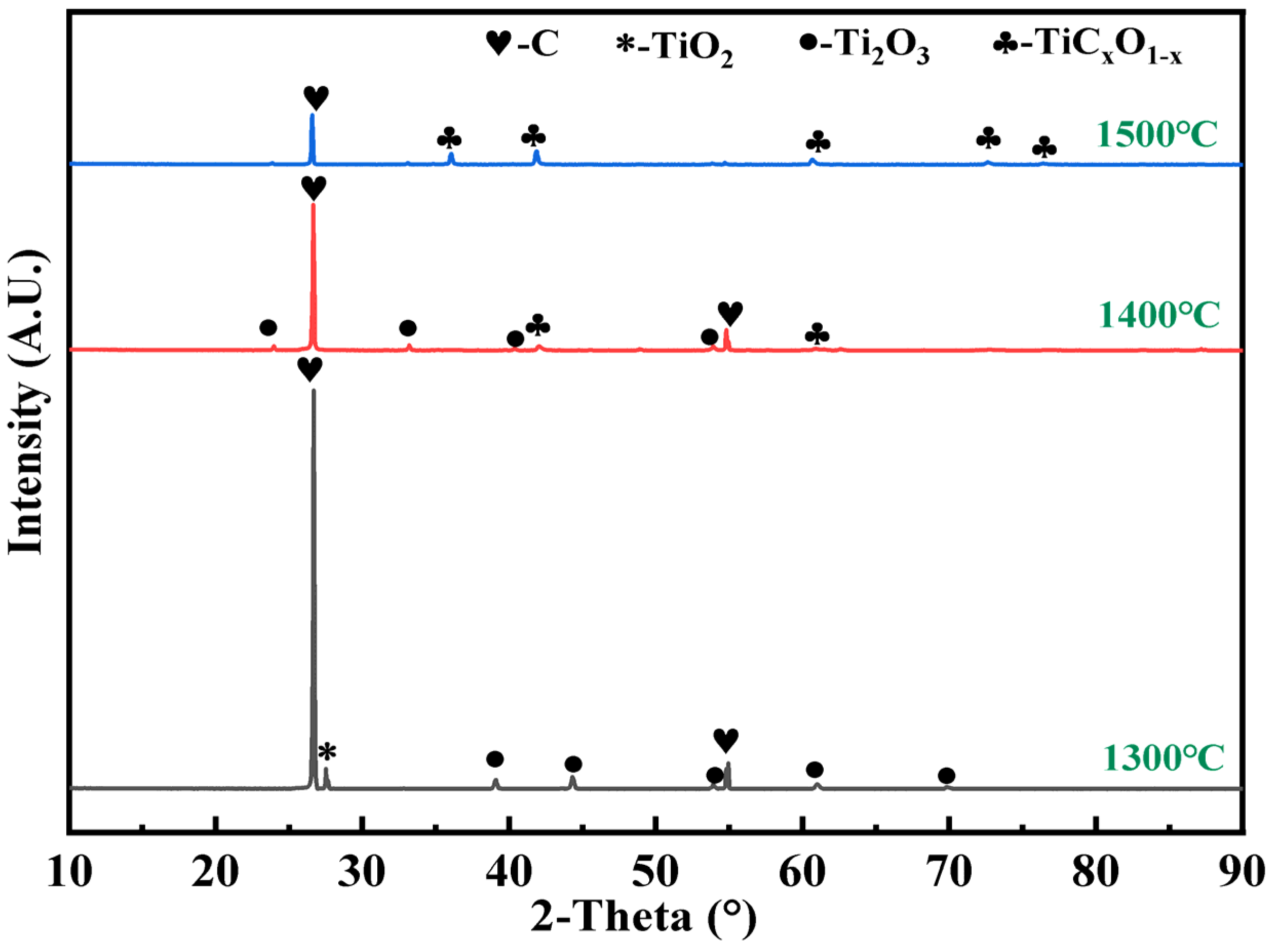
3.2. Effect of Temperature
3.3. Carbon Content
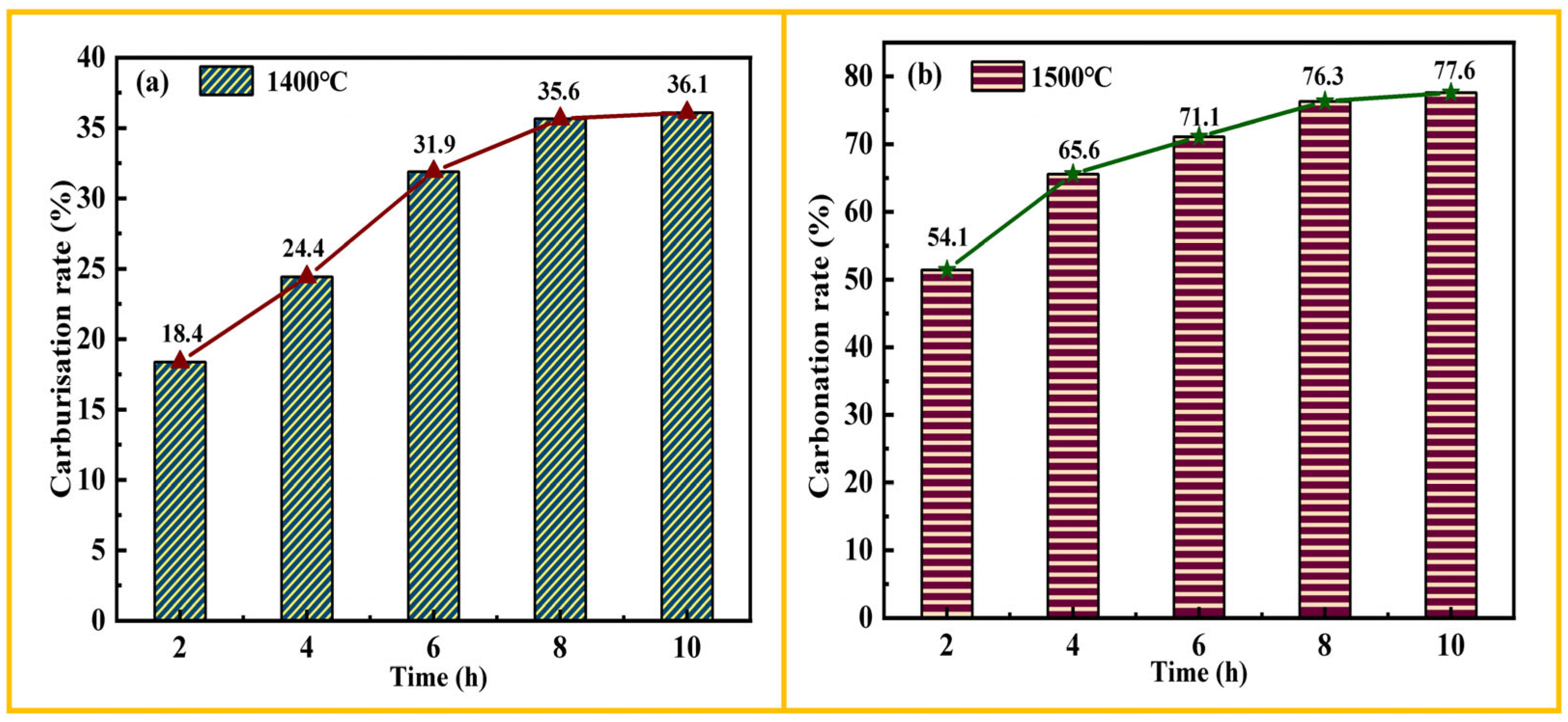
3.4. Holding Time
3.5. Carburisation Rate
4. Discussion
4.1. Reduction Route
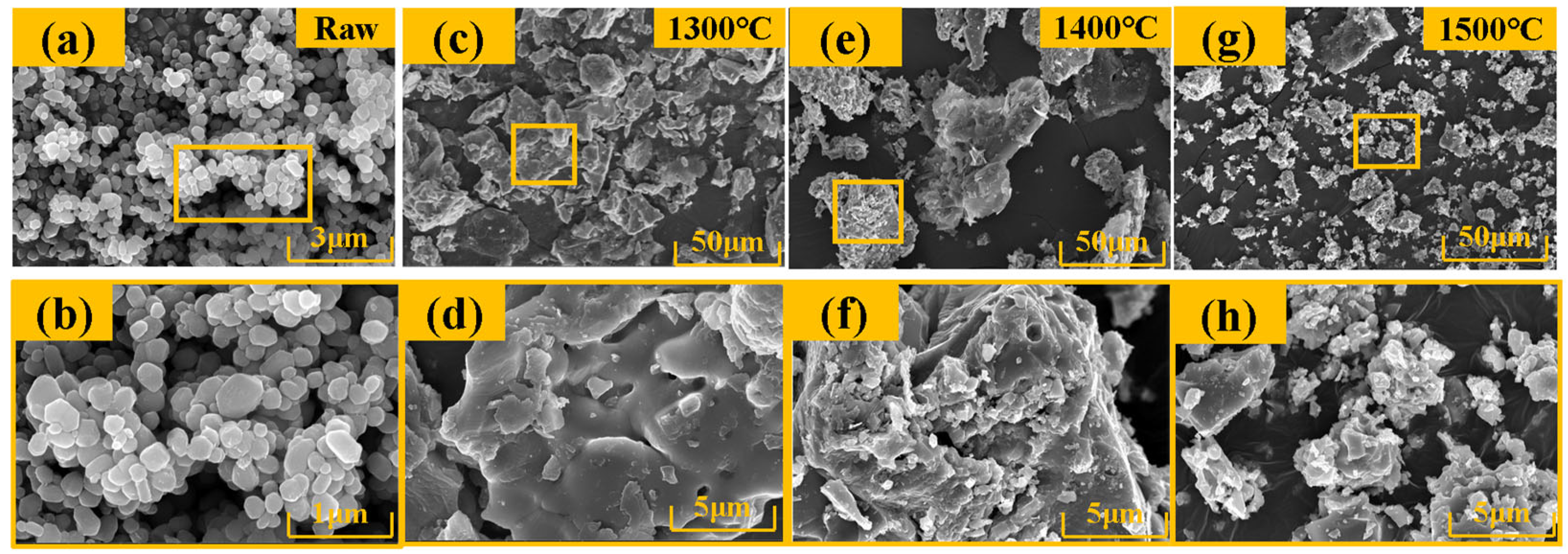
4.2. Micro-Morphology
5. Conclusions
- The temperature, carbon content, and time are three important factors in the preparation of TiCxO1−x. Increasing the temperature provides heat for the reaction and promotes the diffusion of carbon into the surrounding titanium oxide, triggering the reaction. The appropriate carbon content is crucial for the preparation of carbides. A lower amount of carbon content yields only a small quantity of carbides, whereas an excessive carbon content results in a surplus of carbon and subsequently increased costs for separation. In addition, TiCxO1−x was prepared with a molar ratio of TiO2/C = 1:3 at a temperature of 1500 °C for a duration of 10 h.
- The intermediate phases Magnéli and Ti3O5 were not detected; they were attributed to the fast reduction rate of TiO2 to Ti2O3, which does not mean that Magnéli and Ti3O5 were not produced during the reaction, and the titanium oxides were reduced stepwise. All of TiO2 was reduced to Ti2O3, suggesting that the reduction ended and was followed by the carburisation of Ti2O3. In addition, the synthesis of titanium carbide involves the main reduction path: TiO2–Magnéli–Ti3O5–Ti2O3–TiCxO1−x.
- As the temperature rises from 1300 °C to 1500 °C, the amount of carbide increases. The microscopic morphology of the material gradually deviates from the original smooth and dense cake-like structure, which becomes rough and porous. After the abundant formation of carbides, the material displays a porous layered structure of carbides, which is completely separate from the original structure.
- Temperature has a greater effect on the carburisation ratio compared to time. At a temperature of 1400 °C, with a holding time ranging from 2 h to 10 h, the carburisation ratio increases from 18.37% to 36.09%. At 1500 °C, the carburisation ratio increases from 51.43% to 77.57%. Overall, increasing the temperature and time favours the generation of titanium carbide.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mao, J.J.; Li, S.S.; Zhang, Y.X.; Zhu, X.L.; Yang, Z.X. The stability of TiC surfaces in the environment with various carbon chemical potential and surface defects. Appl. Surf. Sci. 2016, 386, 202–209. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, T.; He, X.B.; Shao, H.B.; Tang, B.; Qu, X.H. Fabrication and properties of the TiC reinforced high-strength steel matrix composite. Int. J. Refract. Met. Hard Mater. 2016, 58, 14–21. [Google Scholar] [CrossRef]
- Miao, S.; Xie, Z.M.; Zhang, T.; Wang, X.P.; Fang, Q.F.; Liu, C.S.; Luo, G.N.; Liu, X. Mechanical properties and thermal stability of rolled W-0.5 wt% TiC alloys. Mater. Sci. Eng. A 2016, 671, 87–95. [Google Scholar] [CrossRef]
- Yoshioka, S.; Boatemaa, L.; Zwaag, S.V.D.; Nokao, W.; Sloof, W.G. On the use of TiC as high-temperature healing particles in alumina based composites. J. Eur. Ceram. Soc. 2016, 36, 4155–4162. [Google Scholar] [CrossRef]
- Zhao, X.B.; Zhou, Y.G.; Liu, S.; Zhou, Y.F.; Zhao, C.C.; Wang, C.X.; Yang, Q.X. Investigation on WC/TiC interface relationship in wear-resistant coating by first-principles. Surf. Coat. Technol. 2016, 305, 200–207. [Google Scholar] [CrossRef]
- CSahoo, K.; Soni, L.; Masanta, M. Evaluation of microstructure and mechanical properties of TiC/TiC-steel composite coating produced by gas tungsten arc (GTA) coating process. Surf. Coat. Technol. 2016, 307, 17–27. [Google Scholar] [CrossRef]
- Ding, L.; Xiang, D.P.; Pan, Y.L.; Zhang, T.M.; Wu, Z.Y. In situ synthesis of TiC cermet by spark plasma reaction sintering. J. Alloys Compd. 2016, 661, 136–140. [Google Scholar] [CrossRef]
- Acharya, S.; Debata, M.; Acharya, T.S.; Acharya, P.P.; Singh, S.K. Influence of nickel boride addition on sintering behaviour and mechanical properties of TiC-Ni based cermets. J. Alloys Compd. 2016, 685, 905–912. [Google Scholar] [CrossRef]
- Oláh, N.; Fogarassy, Z.; Sulyok, A.; Szívós, J.; Csanádi, T.; Balázsi, K. Ceramic TiC/C protective nanocomposite coatings: Structure and composition versus mechanical properties and tribology. Ceram. Int. 2016, 42, 12215–12220. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yang, F.; Zhang, T.G.; Zhang, H.W.; Zhang, D.L. Research Progress of Laser Cladding Titanium Carbide Reinforced Titanium-based Composite Coating. Surf. Technol. 2020, 49, 138. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.M.; Zhao, H.; Yao, F.B.; Cao, J.; Chen, Z.; Huang, X.D.; Wang, D.B.; Yang, Q. Recent advances in transition metal carbides and nitrides (MXenes): Characteristics, environmental remediation and challenges. Chem. Eng. J. 2021, 418, 129296. [Google Scholar] [CrossRef]
- Dunmead, S.; Munir, Z.A.; Holt, J.B. Temperature Profile Analysis in Combustion Synthesis: II, Experimental Observations. J. Am. Ceram. Soc. 1992, 75, 180–188. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.J.; Lü, B.; Wang, C.G.; Liu, Y.X.; Geng, G.L.; Li, L. Easy synthesis of TiC nanocrystallite. J. Cryst. Growth 2004, 264, 316–319. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.J.; Lü, B.; Wang, C.G.; Liu, Y.X.; Geng, G.L.; Li, L. Low temperature induced synthesis of TiN nanocrystals. Inorg. Chem. 2004, 43, 3558–3560. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.Q.; Song, M.; Zhu, Q.S.; Hu, C.Q.; Yang, Y.F.; Lv, P.P.; Zhao, H.D.; Yun, F. Facile synthesis of high-melting point spherical TiC and TiN powders at low temperature. J. Am. Ceram. Soc. 2019, 103, 889–898. [Google Scholar] [CrossRef]
- Alexandrescu, R.; Borsella, E.; Bottl, S.; Cesile, M.C.; Martelli, S.; Giorgi, R.; Turtu, S. Synthesis of TiC and SiC/TiC nanocrystalline powders by gas-phase laser-induced reaction. J. Mater. Sci. 1997, 32, 5629–5635. [Google Scholar] [CrossRef]
- Koc, R.; Folmer, J.S. Carbothermal synthesis of titanium carbide using ultrafine titania powders. J. Mater. Sci. 1997, 32, 3101–3111. [Google Scholar] [CrossRef]
- Ali, M.; Basu, P. Mechanochemical synthesis of nano-structured TiC from TiO2 powders. J. Alloys Compd. 2010, 500, 220–223. [Google Scholar] [CrossRef]
- Welham, N.J.; Willis, P.E. Formation of TiN/TiC-Fe composites from ilmenite (FeTiO3) concentrate. Metall. Mater. Trans. B 1998, 29, 1077–1083. [Google Scholar] [CrossRef]
- Anacleto, N.; Ostrovski, O.; Ganguly, S. Reduction of Manganese Ores by Methane-containing Gas. Trans. Iron Steel Inst. Jpn. 2004, 44, 1615–1622. [Google Scholar] [CrossRef]
- Anacleto, N.; Ostrovski, O. Solid-state reduction of chromium oxide by methane-containing gas. Metall. Mater. Trans. B 2004, 35, 609–615. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, D.; Fan, G.Q.; Sun, H.B.; Dang, J. Thermodynamic and experimental study on the reduction and carburisation of TiO2 through gas-solid reaction. Int. J. Energy Res. 2019, 43, 4551. [Google Scholar] [CrossRef]
- Fan, G.Q.; Hou, Y.L.; Huang, D.J.; Dang, J.; Zhang, R.; Xiang, J.Y.; Lv, X.W.; Ding, X.M. Synthesis of Ti(C, N, O) ceramic from rutile at low temperature by CH4-H2-N2 gas mixture. Int. J. Refract. Met. Hard Mater. 2021, 101, 105659. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Ostrovski, O. Reduction of titania by methane-hydrogen-argon gas mixture. Metall. Mater. Trans. B 2000, 31, 129–139. [Google Scholar] [CrossRef]
- Welham, N.J.; Williams, J.S. Carbothermic reduction of llmenite (FeTiO3) and rutile (TiO2). Metall. Mater. Trans. B 1999, 30, 1075–1081. [Google Scholar] [CrossRef]
- Qing, J. Fundamental Study on Carburisation and Nitridation of Vanadium-Titanium Magnetite in the Process of Coal-Based Direct Reduction Followed by Magnetic Separation. Ph.D. Thesis, Chongqing University, Chongqing, China, 2020. [Google Scholar]
- Cao, Z.M.; Xie, W.; Jung, I.H.; Du, G.W.; Qiao, Z.Y. Critical evaluation and thermodynamic optimization of the Ti-C-O system and its applications to Carbothermic TiO2 reduction process. Metall. Mater. Trans. B 2015, 46, 1782–1801. [Google Scholar] [CrossRef]
- Wang, K.F.; Sun, G.D.; Wu, Y.D.; Zhang, G.H. Fabrication of ultrafine and high-purity tungsten carbide powders via a carbothermic reduction–carburization process. J. Alloys Compd. 2023, 784, 362–369. [Google Scholar] [CrossRef]
- Subasinghe, H.C.S.; Ratnayake, A.S. Effect of different carbon sources on the conversion of ilmenite into synthetic rutile via ball milling induced carbothermic reduction. J. Alloys Compd. 2023, 954, 170086. [Google Scholar] [CrossRef]
- Long, W.; Gao, F.; Wang, D. Forming thermodynamics, structure, and electrical conductivity of TiCxOy compounds fabricated through the carbothermal reduction process. J. Alloys Compd. 2022, 892, 16220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |




| Temperature (°C) | Time (h) | TiC (wt%) | TTi (wt%) |
|---|---|---|---|
| 1400 | 2 | 11.02 | 48 |
| 1400 | 4 | 15.57 | 51 |
| 1400 | 6 | 20.74 | 52 |
| 1400 | 8 | 23.62 | 53 |
| 1400 | 10 | 23.91 | 53 |
| 1500 | 2 | 36 | 56 |
| 1500 | 4 | 50 | 61 |
| 1500 | 6 | 56 | 63 |
| 1500 | 8 | 62 | 65 |
| 1500 | 10 | 64 | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, T.; Tian, F.; Hu, T. Preparation of Titanium Carbide by Carburisation of Titanium Dioxide. Processes 2024, 12, 102. https://doi.org/10.3390/pr12010102
Lv T, Tian F, Hu T. Preparation of Titanium Carbide by Carburisation of Titanium Dioxide. Processes. 2024; 12(1):102. https://doi.org/10.3390/pr12010102
Chicago/Turabian StyleLv, Tingting, Fang Tian, and Tu Hu. 2024. "Preparation of Titanium Carbide by Carburisation of Titanium Dioxide" Processes 12, no. 1: 102. https://doi.org/10.3390/pr12010102
APA StyleLv, T., Tian, F., & Hu, T. (2024). Preparation of Titanium Carbide by Carburisation of Titanium Dioxide. Processes, 12(1), 102. https://doi.org/10.3390/pr12010102





