Design and Characterization of a Melt Electrostatic Precipitator for Advanced Drug Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Spray Drying
2.2.2. Electrostatic Precipitator
2.2.3. Current-Voltage Characteristics
2.2.4. Wall Film Thickness
2.2.5. Dry Precipitation
2.2.6. Wet Precipitation
2.2.7. Product Characterization
3. Results and Discussion
3.1. Design and Construction
3.1.1. Liquid Distributer Design
3.1.2. Electrostatic Precipitator
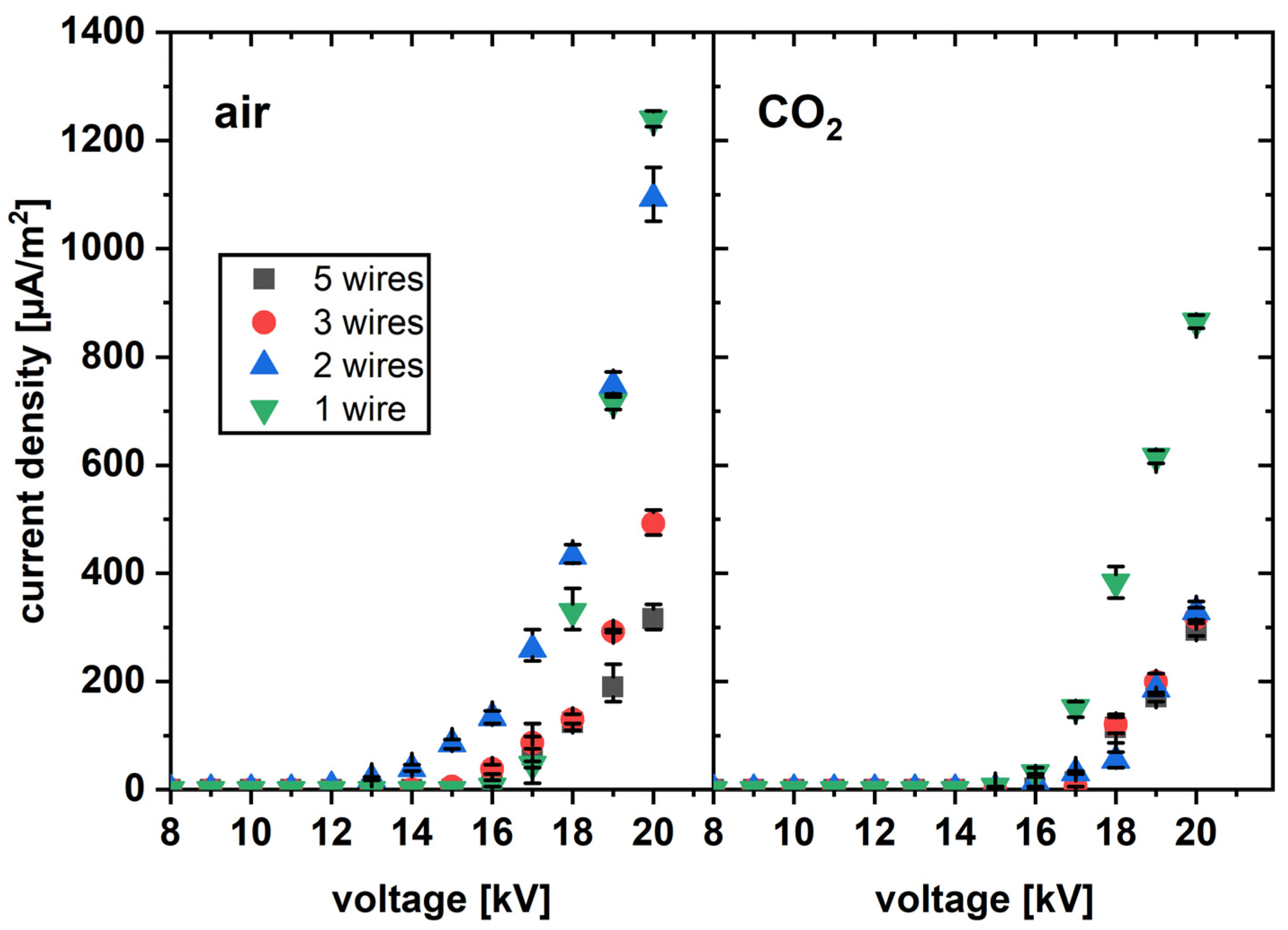
3.2. Current-Voltage Characteristics
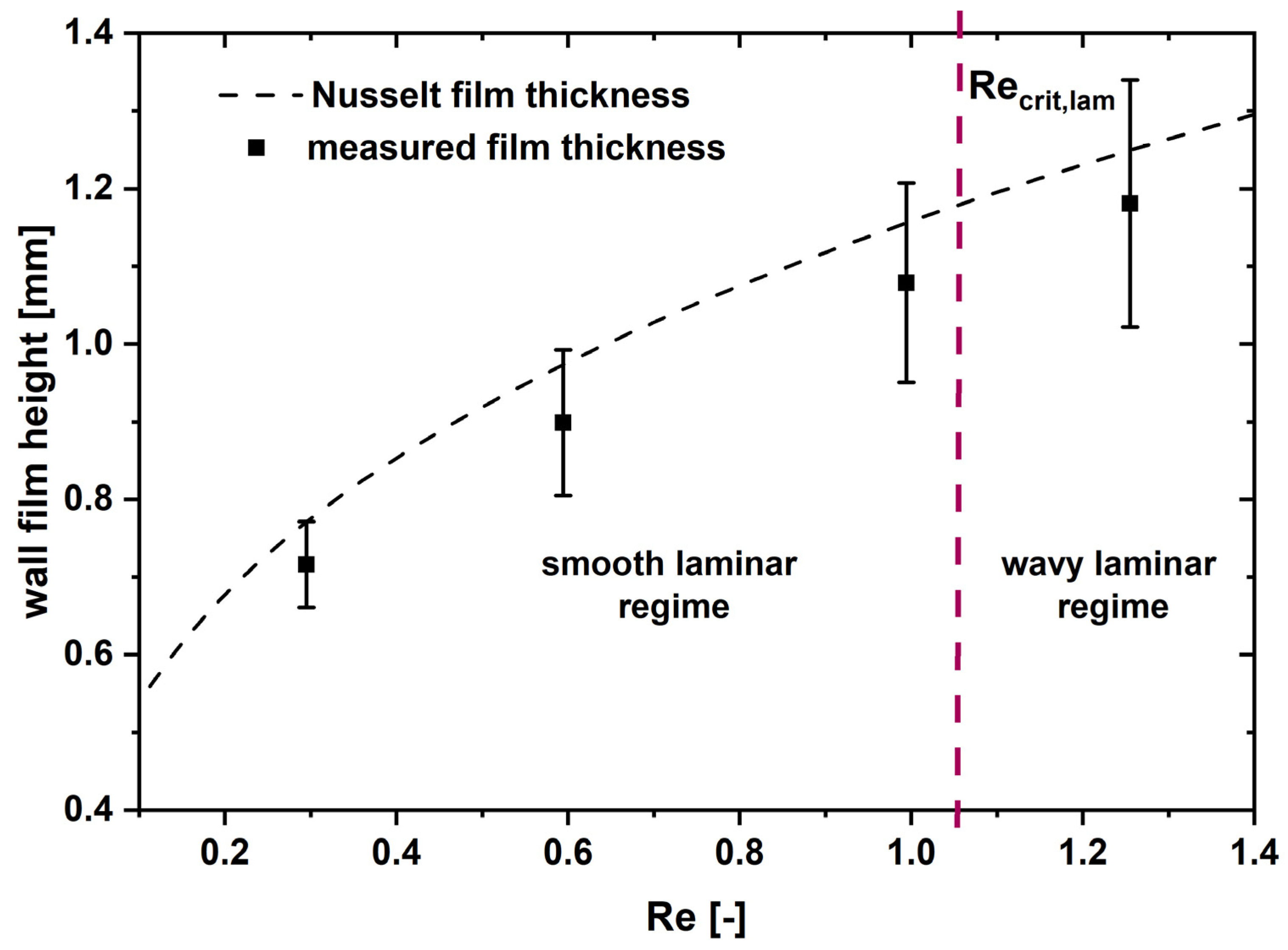
3.3. Wall Film Characterization
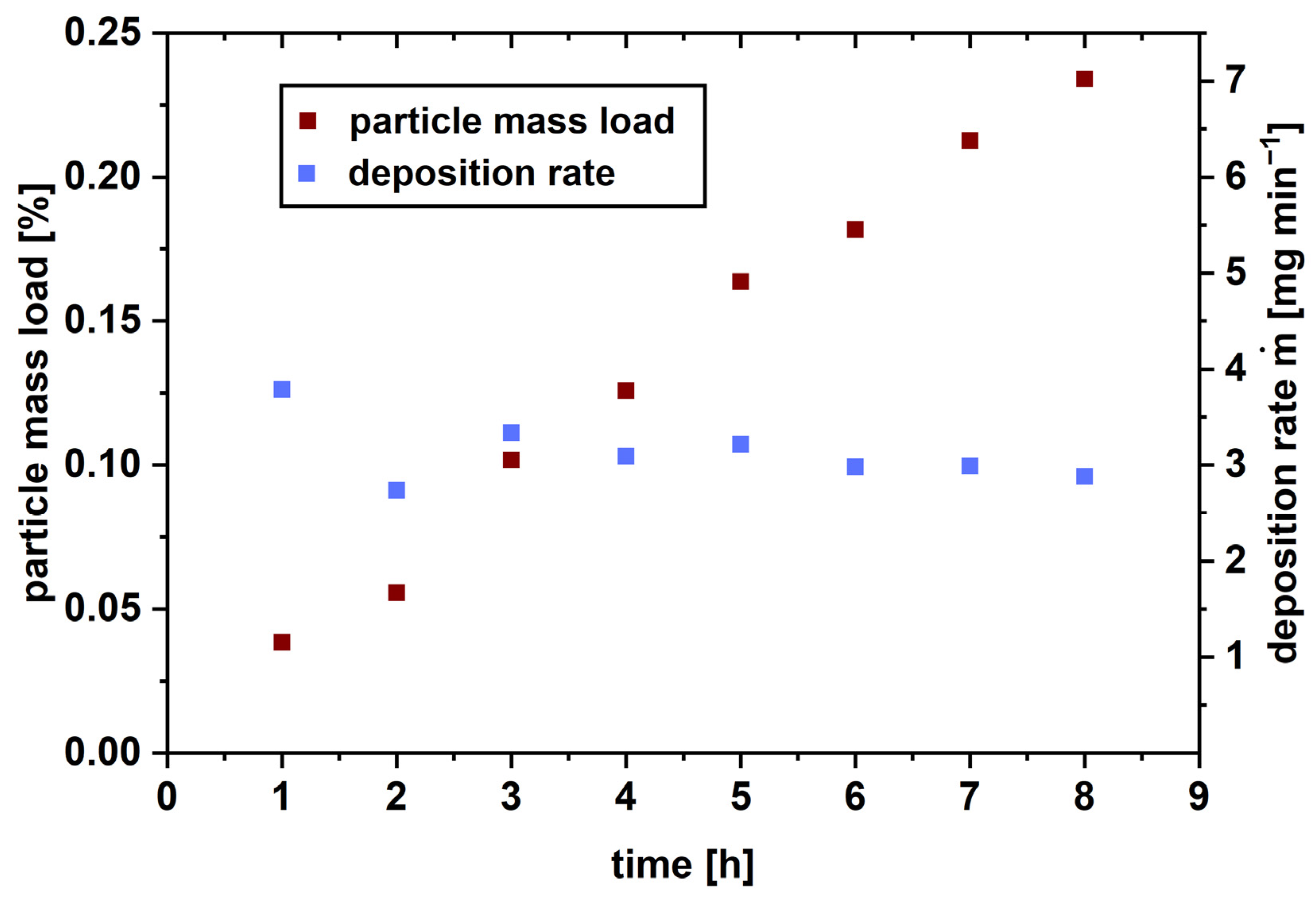
3.4. Particle Deposition on a Hot Surface
3.5. Particle Deposition into a Hot Liquid
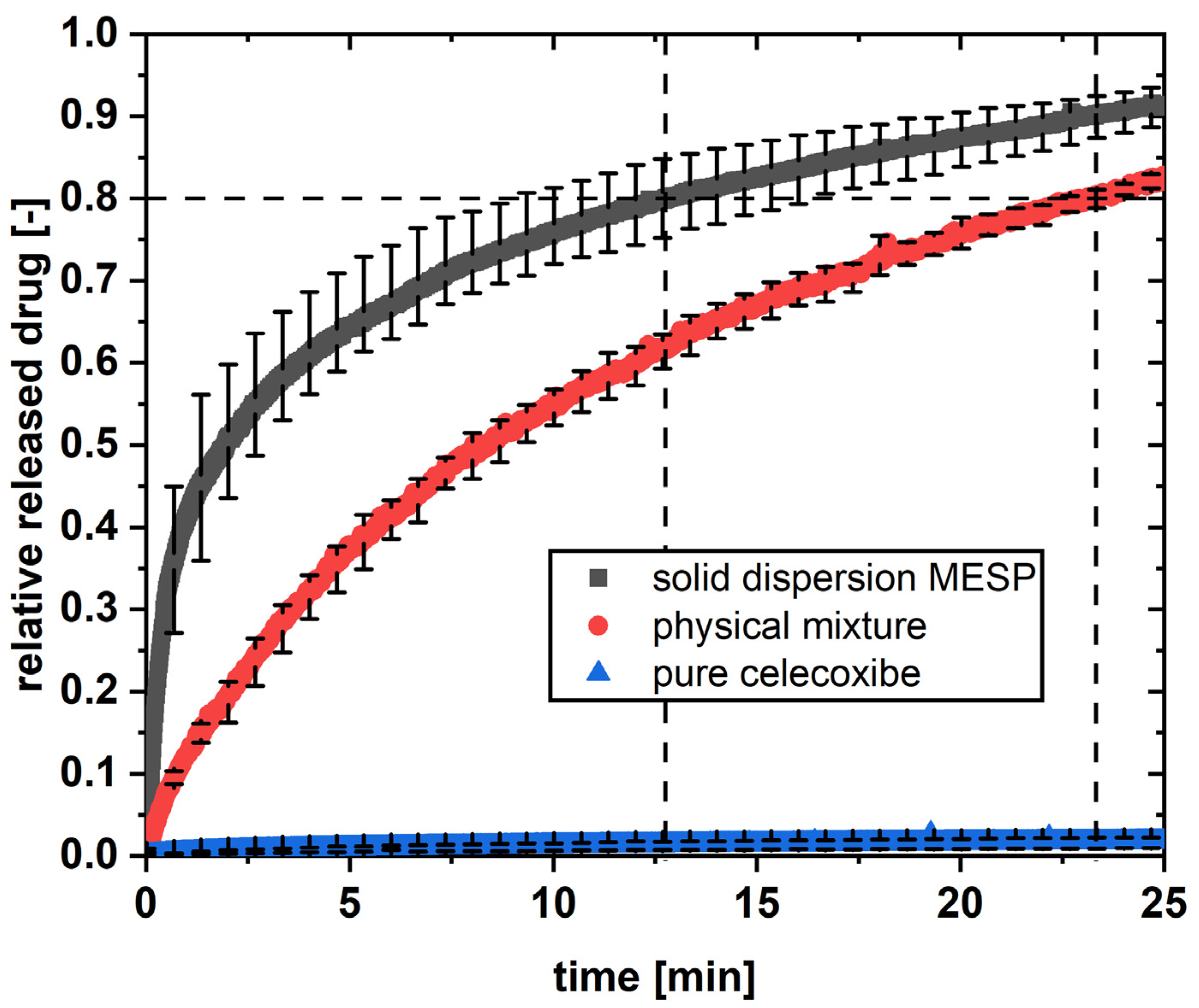
3.6. In Vitro Dissolution
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jaworek, A.; Krupa, A.; Czech, T. Modern electrostatic devices and methods for exhaust gas cleaning: A brief review. J. Electrost. 2007, 65, 133–155. [Google Scholar] [CrossRef]
- Mizuno, A. Electrostatic precipitation. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 615–624. [Google Scholar] [CrossRef]
- Burger, P.; Riebel, U. Formation of highly resistive SiO2 nanoparticle layers from the aerosol by electrostatic precipitation at 200 degrees C: Observations on back corona and nanoparticle layer structure. J. Nanopart. Res. 2021, 23, 165. [Google Scholar] [CrossRef]
- Jaworek, A.; Czech, T.; Rajch, E.; Lackowski, M. Laboratory studies of back-discharge in fly ash. J. Electrost. 2006, 64, 306–317. [Google Scholar] [CrossRef]
- Wang, X.; Chang, J.C.; Xu, C.Y.; Zhang, J.; Wang, P.; Ma, C.Y. Collection and charging characteristics of particles in an electrostatic precipitator with a wet membrane collecting electrode. J. Electrost. 2016, 83, 28–34. [Google Scholar] [CrossRef]
- Chang, J.C.; Wang, P.; Cui, L.; Li, Z.Q.; Ma, C.Y.; Wang, X. Deposition characteristics of dust on wet membrane electrodes. J. Electrost. 2018, 95, 53–60. [Google Scholar] [CrossRef]
- Bayless, D.J.; Alam, M.K.; Radcliff, R.; Caine, J. Membrane-based wet electrostatic precipitation. Fuel Process. Technol. 2004, 85, 781–798. [Google Scholar] [CrossRef]
- Jedrusik, M.; Swierczok, A. The correlation between corona current distribution and collection of fine particles in a laboratory-scale electrostatic precipitator. J. Electrost. 2013, 71, 199–203. [Google Scholar] [CrossRef]
- Xu, C.Y.; Chang, J.C.; Meng, Z.; Wang, X.; Zhang, J.; Cui, L.; Ma, C.Y. Wetting properties and performance test of modified rigid collector in wet electrostatic precipitators. J. Air Waste Manag. Assoc. 2016, 66, 1019–1030. [Google Scholar] [CrossRef]
- Yang, Z.M.; Huang, C.; Ma, X.Q.; Pei, Y.K.; Sun, Y. Wetting properties and performance of modified composite collectors in a membrane-based wet electrostatic precipitator. Open Phys. 2019, 17, 897–904. [Google Scholar] [CrossRef]
- Dey, L.; Venkataraman, C. A Wet Electrostatic Precipitator (WESP) for Soft Nanoparticle Collection. Aerosol Sci. Technol. 2012, 46, 750–759. [Google Scholar] [CrossRef]
- Anderlohr, C.; Schaber, K. Direct Transfer of Gas-Borne Nanoparticles into Liquid Suspensions by Means of a Wet Electrostatic Precipitator. Aerosol Sci. Technol. 2015, 49, 1281–1290. [Google Scholar] [CrossRef]
- Anderlohr, C.; Brachert, L.; Mertens, J.; Schaber, K. Collection and Generation of Sulfuric Acid Aerosols in a Wet Electrostatic Precipitator. Aerosol Sci. Technol. 2015, 49, 144–151. [Google Scholar] [CrossRef]
- Noll, C.G. Temperature dependence of dc corona and charge-carrier entrainment in a gas flow channel. J. Electrost. 2002, 54, 245–270. [Google Scholar] [CrossRef]
- Wang, X.H.; You, C.F. Effects of thermophoresis, vapor, and water film on particle removal of electrostatic precipitator. J. Aerosol Sci. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- You, C.F.; Wang, X.H.; Liu, R.L.; Yang, R.C. Simultaneous effects of electrostatic field and thermophoresis on inhalable particulate matter removal. Powder Technol. 2010, 202, 95–100. [Google Scholar] [CrossRef]
- Peters, M.H.; Cooper, D.W. The Effects of Electrostatic Forces on the Thermophoretic Suppression of Particle Diffusional Deposition onto Hot Surfaces. J. Colloid Interface Sci. 1990, 140, 48–56. [Google Scholar] [CrossRef]
- Tsai, R. A simple approach for evaluating the effect of wall suction and thermophoresis on aerosol particle deposition from a laminar flow over a flat plate. Int. Commun. Heat Mass Transf. 1999, 26, 249–257. [Google Scholar] [CrossRef]
- Tsai, R.; Huang, J.S. Combined effects of thermophoresis and electrophoresis on particle deposition onto a vertical flat plate from mixed convection flow through a porous medium. Chem. Eng. J. 2010, 157, 52–59. [Google Scholar] [CrossRef]
- Chen, C.L.; Chan, K.C. Combined effects of thermophoresis and electrophoresis on particle deposition onto a wavy surface disk. Int. J. Heat Mass Transf. 2008, 51, 2657–2664. [Google Scholar] [CrossRef]
- Opiolka, S.; Schmidt, F.; Fissan, H. Combined Effects of Electrophoresis and Thermophoresis on Particle Deposition onto Flat Surfaces. J. Aerosol Sci. 1994, 25, 665–671. [Google Scholar] [CrossRef]
- Dobrowolski, A.; Pieloth, D.; Wiggers, H.; Thommes, M. Electrostatic Precipitation of Submicron Particles in a Molten Carrier. Pharmaceutics 2019, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Justen, A.; Kurth, C.; Schaldach, G.; Thommes, M. Preparation of Micron and Submicron Particles via Spray Drying and Electrostatic Precipitation. Chem. Eng. Technol. 2023, 46, 343–349. [Google Scholar] [CrossRef]
- Thommes, M.; Ely, D.R.; Carvajal, M.T.; Pinal, R. Improvement of the dissolution rate of poorly soluble drugs by solid crystal suspensions. Mol. Pharm. 2011, 8, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Ziedan, H.; Tlustý, J.; Mizuno, A.; Sayed, A.; Ahmed, A. Corona current-voltage characteristics in wire-duct electrostatic precipitators, Theory versus Experiment. Int. J. Plasma Environ. Sci. Technol. 2010, 4, 154–162. [Google Scholar]
- Kasdi, A. Computation and measurement of corona current density and V-I characteristics in wires-to-plates electrostatic precipitator. J. Electrost. 2016, 81, 1–8. [Google Scholar] [CrossRef]
- El Dein, A.Z.; Usama, K. Experimental and simulation study of V–I characteristics of wire–plate electrostatic precipitators under clean air conditions. Arab. J. Sci. Eng. 2014, 39, 4037–4045. [Google Scholar] [CrossRef]
- Riehle, C. Basic and theoretical operation of ESPs. Appl. Electrost. Precip. 1997, 25–88. [Google Scholar] [CrossRef]
- Andrade, R.G.S.A.; Guerra, V.G. Discharge electrode influence on electrostatic precipitation of nanoparticles. Powder Technol. 2021, 379, 417–427. [Google Scholar] [CrossRef]
- Ning, Z.Y.; Podlinski, J.; Shen, X.J.; Li, S.R.; Wang, S.L.; Han, P.; Yan, K.P. Electrode geometry optimization in wire-plate electrostatic precipitator and its impact on collection efficiency. J. Electrost. 2016, 80, 76–84. [Google Scholar] [CrossRef]
- Schmatloch, V.; Rauch, S. Design and characterisation of an electrostatic precipitator for small heating appliances. J. Electrost. 2005, 63, 85–100. [Google Scholar] [CrossRef]
- Gu, Z.; Xi, X.; Yang, J.; Xu, J. Properties of RE–W cathode and its application in electrostatic precipitation for high temperature gas clean-up. Fuel 2012, 95, 648–654. [Google Scholar] [CrossRef]
- Nusselt, W. Die oberflachenkondensation des wasserdamphes. VDI-Zs 1916, 60, 541. [Google Scholar]
- PubChem Xylitol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Xylitol#section=Other-Identifiers (accessed on 23 June 2023).
- Zheng, F. Thermophoresis of spherical and non-spherical particles: A review of theories and experiments. Adv. Colloid Interface Sci. 2002, 97, 255–278. [Google Scholar] [CrossRef] [PubMed]
- EuropeanPharmacopoeiaCommission. European Pharmacopoeia, 10.8th ed.; European Directorate for the Quality of Medicines: Strasbourg, France, 2022; pp. 326–373. [Google Scholar]
- Michaeli, W. Extrusion Dies for Plastics and Rubber: Design and Engineering Computations, 3rd ed.; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2016; pp. 192–205. [Google Scholar]
- Peek, F. Dielectric Phenomena in High Voltage Engineering, 1st ed.; McGraw-Hill: New York, NY, USA, 1929; pp. 192–237. [Google Scholar]
- Bush, J.R.; Feldman, P.L.; Robinson, M. High temperature, high pressure electrostatic precipitation. J. Air Pollut. Control. Assoc. 1979, 29, 365–371. [Google Scholar] [CrossRef]
- Matts, S.; Öhnfeldt, P. Efficient gas cleaning with SF electrostatic precipitators. Flakten Rev. 1963, 6, 93–110. [Google Scholar]
- Pauthenier, M. The charge on a spherical particle in an ionized field. J. D’physique Radium 1932, 7, 590–613. [Google Scholar] [CrossRef]
- Cochet, R. Lois charge des fines particules (submicroniques) etudes théoriques-controles récents spectre de particules. Coll. Int. Phys. Forces Electrost. Leurs Appl. Cent. Natl. Rech. Sci. Paris 1961, 102, 331–338. [Google Scholar]
- Ishii, K.; Knioshit, M.; Kuroda, H. Dielectric-Constant Measurement on Organic Crystalline Powder. Bull. Chem. Soc. Jpn. 1973, 46, 3385–3391. [Google Scholar] [CrossRef]
- Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. Dielectric Spectroscopy of Small Molecule Pharmaceuticals—Effect of Sample Configuration. J. Pharm. Sci. 2014, 103, 3190–3196. [Google Scholar] [CrossRef]
- Hughes, M.P.; Rosenthal, K.D.; Ran, N.A.; Seifrid, M.; Bazan, G.C.; Nguyen, T.Q. Determining the Dielectric Constants of Organic Photovoltaic Materials Using Impedance Spectroscopy. Adv. Funct. Mater. 2018, 28, 1801542. [Google Scholar] [CrossRef]
- Schmoch, M.; Steiner, D. Handbuch Elektrofilter, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 43–80. [Google Scholar]
- Kapitsa, P.L. Collected Papers, 1st ed.; Pergamon Press: Oxford, UK, 1964; pp. 662–709. [Google Scholar]
- Al-Sibai, F. Experimentelle Untersuchung der Strömungscharakteristik und des Wärmeübergangs bei welligen Rieselfilmen. Cuvillier Verlag: Göttingen, Germany, 2006. [Google Scholar]
- Baehr, H.D.; Stephan, K. Wärme-Und Stoffübertragung, 10th ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Guha, A.; Samanta, S. Effect of thermophoresis and its mathematical models on the transport and deposition of aerosol particles in natural convective flow on vertical and horizontal plates. J. Aerosol Sci. 2014, 77, 85–101. [Google Scholar] [CrossRef]
- Luo, R.; Li, Y.; Zheng, C.H.; Gao, X.; Fan, J.R. Numerical simulation of temperature effect on particles behavior via electrostatic precipitators. Appl. Therm. Eng. 2015, 88, 127–139. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, C.H.; Luo, K.; Gao, X.; Fan, J.R.; Cen, K.F. CFD simulation of high-temperature effect on EHD characteristics in a wire-plate electrostatic precipitator. Chin. J. Chem. Eng. 2015, 23, 633–640. [Google Scholar] [CrossRef]
- Oprandi, A. Fluiddynamik 2, 1st ed.; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2020. [Google Scholar]
- Muehlenfeld, C.; Kann, B.; Windbergs, M.; Thommes, M. Solid dispersion prepared by continuous cogrinding in an air jet mill. J. Pharm. Sci. 2013, 102, 4132–4139. [Google Scholar] [CrossRef]
- Craig, D.Q.M. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int. J. Pharm. 2002, 231, 131–144. [Google Scholar] [CrossRef]
- Cammarn, S.R.; Sakr, A. Predicting dissolution via hydrodynamics: Salicylic acid tablets in flow through cell dissolution. Int. J. Pharm. 2000, 201, 199–209. [Google Scholar] [CrossRef]







| Liquid | Dynamic Viscosity [mPas] | Density [kg/m3] | Surface Tension [mN/m] |
|---|---|---|---|
| glycerol-water mixture 83 wt.% | 90 | 1217 | 54 |
| molten xylitol (120 °C) | 90 | 1311 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Justen, A.; Weltersbach, A.F.; Schaldach, G.; Thommes, M. Design and Characterization of a Melt Electrostatic Precipitator for Advanced Drug Formulations. Processes 2024, 12, 100. https://doi.org/10.3390/pr12010100
Justen A, Weltersbach AF, Schaldach G, Thommes M. Design and Characterization of a Melt Electrostatic Precipitator for Advanced Drug Formulations. Processes. 2024; 12(1):100. https://doi.org/10.3390/pr12010100
Chicago/Turabian StyleJusten, Anna, Alina Faye Weltersbach, Gerhard Schaldach, and Markus Thommes. 2024. "Design and Characterization of a Melt Electrostatic Precipitator for Advanced Drug Formulations" Processes 12, no. 1: 100. https://doi.org/10.3390/pr12010100





