Rapid and High-Yield Recovery of Sodium Alginate from Undaria pinnatifida via Microwave-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis Method of Sugars and Sodium Alginate in Undaria pinnatifida
2.3. Effects of Variables on Sodium Alginate Yield Using Microwave-Assisted Extraction
2.4. Experimental Design and Statistical Optimization
2.5. Analytical Methods
2.5.1. HPLC
2.5.2. FT-IR
3. Results and Discussion
3.1. Analysis of Components in Undaria pinnatifida
3.2. Investigation of Variables Affecting Sodium Alginate Microwave-Assisted Extraction
3.3. Optimization of Alkali Extraction Conditions for Undaria pinnatifida Using RSM
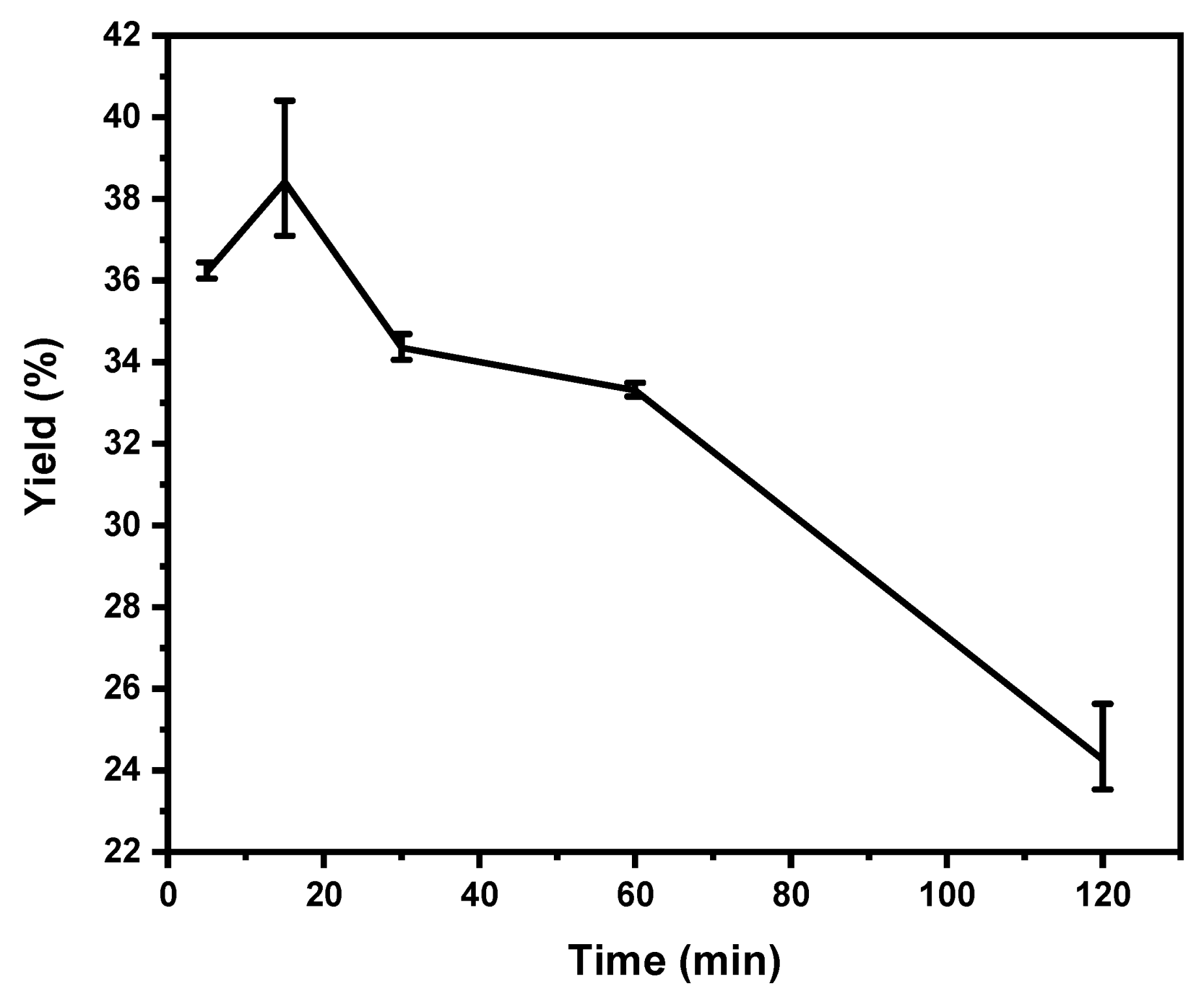
3.4. Effect of Extraction Time on Yield
3.5. FT-IR
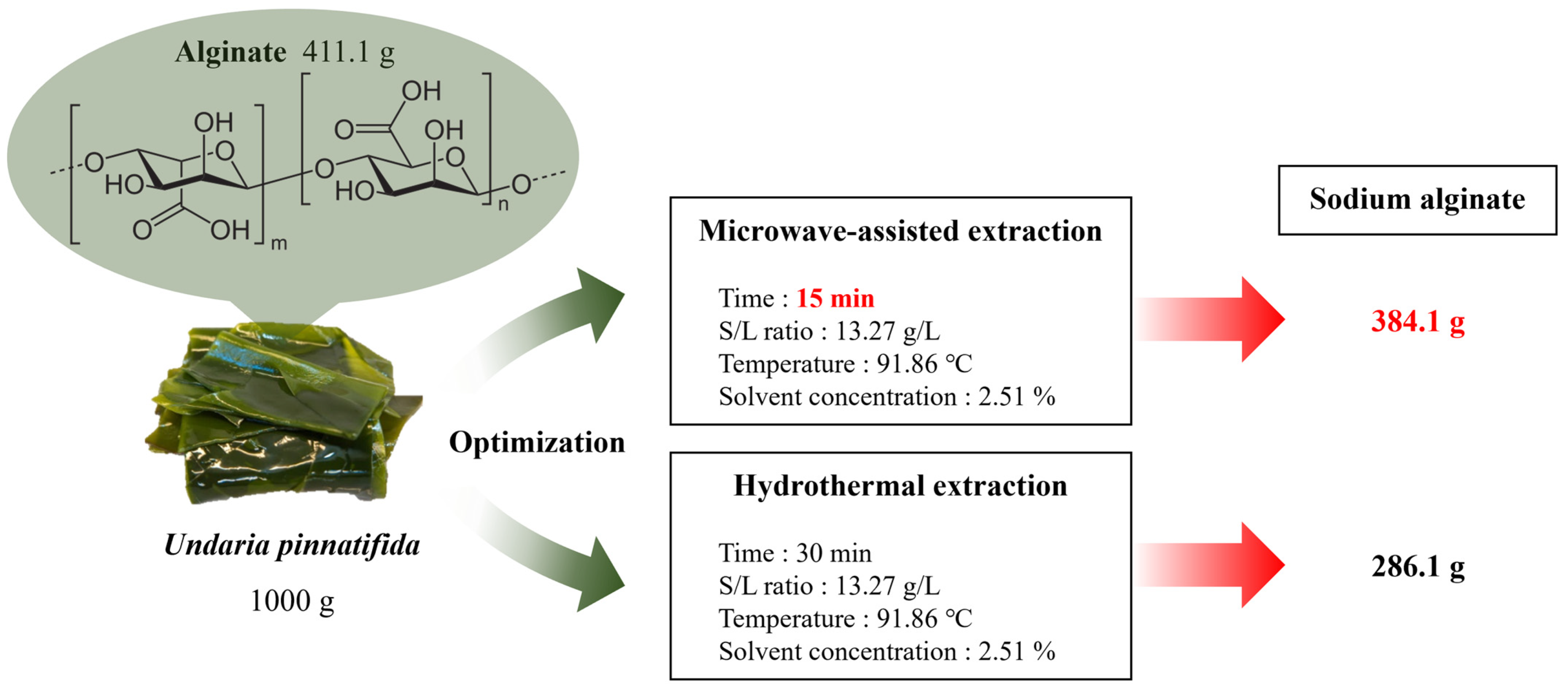
3.6. Evaluation of the Sodium Alginate Extraction Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.S. Strategies to achieve a carbon neutral society: A review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef]
- Rathour, R.K.; Devi, M.; Dahiya, P.; Sharma, N.; Kaushik, N.; Kumari, D.; Kumar, P.; Baadhe, R.R.; Walia, A.; Bhatt, A.K.; et al. Recent Trends, Opportunities and Challenges in Sustainable Management of Rice Straw Waste Biomass for Green Bio-refinery. Energies 2023, 16, 1429. [Google Scholar] [CrossRef]
- Hwang, E.K.; Yotsukura, N.; Pang, S.J.; Su, L.; Shan, T.F. Seaweed breeding programs and progress in eastern Asian countries. Phycologia 2019, 58, 484–495. [Google Scholar] [CrossRef]
- Radulovich, R.; Neori, A.; Valderrama, D.; Reddy, C.R.K.; Cronin, H.; Forster, J. Farming of seaweeds. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 27–59. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture, 2006; Food and Agriculture Organization: Rome, Italy, 2007. [Google Scholar]
- Choi, Y.; Lee, E.C.; Na, Y.; Lee, S.R. Effects of dietary supplementation with fermented and non-fermented brown algae by-products on laying performance, egg quality, and blood profile in laying hens. Asian-Australas. J. Anim. Sci. 2018, 31, 1654–1659. [Google Scholar] [CrossRef]
- Park, J.S.; Shin, S.K.; Wu, H.; Yarish, C.; Yoo, H.I.; Kim, J.K. Evaluation of nutrient bioextraction by seaweed and shellfish aquaculture in Korea. J. World Aquac. Soc. 2021, 52, 1118–1134. [Google Scholar] [CrossRef]
- Wang, L.; Park, Y.-J.; Jeon, Y.-J.; Ryu, B. Bioactivities of the edible brown seaweed, Undaria pinnatifida: A review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustain-ability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as promising natural polymer for pharmaceutical, food, and biomedical applications. Curr. Drug Deliv. 2020, 17, 755–775. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Kohli, K.; Katuwal, S.; Biswas, A.; Sharma, B.K. Effective delignification of lignocellulosic biomass by microwave assisted deep eutectic solvents. Bioresour. Technol. 2020, 303, 122897. [Google Scholar] [CrossRef]
- Mattina, M.I.; Berger, W.I.; Denson, C. Microwave-assisted extraction of taxanes from Taxus biomass. J. Agric. Food Chem. 1997, 45, 4691–4696. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, P.; Lu, C.; Li, S.; Chen, Z.; Wang, X.; Duan, D. Transcriptome sequencing of Saccharina japonica sporophytes during whole developmental periods reveals regulatory networks underlying alginate and mannitol biosynthesis. BMC Genom. 2019, 20, 975. [Google Scholar] [CrossRef] [PubMed]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Optimization of process parameters using D-optimal design for synthesis of ZnO nanoparticles via sol–gel technique. J. Ind. Eng. Chem. 2013, 19, 99–105. [Google Scholar] [CrossRef]
- Mohammed, A.; Rivers, A.; Stuckey, D.C.; Ward, K. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohydr. Polym. 2020, 245, 116419. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Koo, J.-G. Chemical composition and rheological properties of polysaccharides isolated from different parts of brown seaweed Undaria pinnatifida. Korean J. Fish. Aquat. Sci. 2020, 53, 665–671. [Google Scholar] [CrossRef]
- Skriptsova, A.; Khomenko, V.; Isakov, V. Seasonal changes in growth rate, morphology and alginate content in Undaria pin-natifida at the northern limit in the Sea of Japan (Russia). J. Appl. Phycol. 2004, 16, 17–21. [Google Scholar]
- Yoon, M.-O.; Lee, S.-C.; Rhim, J.-W.; Kim, J.-M. Comparison of alginic acid yields and viscosity by different extraction con-ditions from various seaweeds (Laminaria religiosa, Hizikia fusiforme, and Undaria pinnatifida). J. Korean Soc. Food Sci. Nutr. 2004, 33, 747–752. [Google Scholar] [CrossRef]
- Lee, Y.-J. A study on mineral and alginic acid contents by different parts of sea mustards (Undaria pinnatifida). J. Korean Soc. Food Cult. 2004, 19, 691–700. [Google Scholar]
- Lorbeer, A.J.; Lahnstein, J.; Bulone, V.; Nguyen, T.; Zhang, W. Multiple-response optimization of the acidic treatment of the brown alga Ecklonia radiata for the sequential extraction of fucoidan and alginate. Bioresour. Technol. 2015, 197, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Holdt, S.L.; De Francisci, D.; Alvarado-Morales, M.; Mishra, H.N.; Angelidaki, I. Extraction of alginate from Sargassum muticum: Process optimization and study of its functional activities. J. Appl. Phycol. 2016, 28, 3625–3634. [Google Scholar] [CrossRef]
- Sugiono, S.; Masruri, M.; Estiasih, T.; Widjanarko, S.B. Optimization of extrusion-assisted extraction parameters and charac-terization of alginate from brown algae (Sargassum cristaefolium). J. Food Sci. Technol. 2019, 56, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carmona, G.; McHugh, D.J.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E. Pilot plant scale extraction of alginate from Macrocystis pyrifera. 1. Effect of pre-extraction treatments on yield and quality of alginate. J. Appl. Phycol. 1998, 10, 507–513. [Google Scholar] [CrossRef]
- Torres, M.; Cortizo, A.; Oberti, T.; Fernández, J. Characterization of commercial and algae (Undaria pinnatifida) extracted sodium alginate for future application in bone tissue engineering. Environ. Sci. 2016, 24, 1–13. [Google Scholar]
- Fang, S.-E.; Perera, R. Damage identification by response surface based model updating using D-optimal design. Mech. Syst. Signal Process. 2011, 25, 717–733. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Experimental Designs Using ANOVA; Thomson/Brooks/Cole: Belmont, CA, USA, 2007; Volume 724. [Google Scholar]
- Chauhan, B.; Gupta, R. Application of statistical experimental design for optimization of alkaline protease production from Bacillus sp. RGR-14. Process Biochem. 2004, 39, 2115–2122. [Google Scholar] [CrossRef]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.Y.; Ward, K. Mul-tistage extraction and purification of waste Sargassum natans to produce sodium alginate: An optimization approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef]
- Liudvinaviciute, D.; Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Dagys, L. Formation and characteristics of alginate and anthocyanin complexes. Int. J. Biol. Macromol. 2020, 164, 726–734. [Google Scholar] [CrossRef]
- Morais, S. Ultrasonic- and microwave-assisted extraction and modification of algal components. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 585–605. [Google Scholar]
- Faidi, A.; Stumbé, J.F.; Safta, F.; Sfar, S. Implementation of response surface methodology for the optimization of the extraction of sodium alginate from Padina pavonica brown algae. J. Food Meas. Charact. 2022, 16, 4457–4469. [Google Scholar] [CrossRef]
- Gomez, C.G.; Perez Lambrecht, M.V.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction-purification con-ditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Effects of extraction methods on molecular characteristics, antioxidant properties and immunomodulation of alginates from Sargassum angustifolium. Int. J. Biol. Macromol. 2017, 101, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Florez-Fernandez, N.; Dominguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Torabi, P.; Hamdami, N.; Keramat, J. Microwave-assisted extraction of sodium alginate from brown macroalgae Nizimuddinia zanardini, optimization and physicochemical properties. Sep. Sci. Technol. 2021, 57, 872–885. [Google Scholar] [CrossRef]



| Factor | Units | Symbol | Low Level | High Level |
|---|---|---|---|---|
| Solid/liquid ratio (x1) | g/L | x1 | 10 | 30 |
| Extraction temperature (x2) | °C | x2 | 50 | 100 |
| Extraction solvent concentration (x3) | % (w/v) | x3 | 1 | 5 |
| Algae | Monosaccharides/Polysaccharide Contents (%) | |||
|---|---|---|---|---|
| Glucose | XMG | Arabinose | Sodium Alginate | |
| Undaria pinnatifida | 4.9 | 3.0 | - | 41.11 |
| Sodium Alginate Yield (%) | |||||
|---|---|---|---|---|---|
| Solid/liquid ratio | 20 | 60 | 100 | ||
| Extraction temperature | |||||
| 50 | 33.57 | 22.03 | 13.82 | ||
| Extraction solvent concentration: 1% | |||||
| Extraction Temperature | 50 | 70 | 90 | ||
| Extraction solvent concentration | |||||
| 1 | 33.57 | 36.48 | 37.40 | ||
| Solid/liquid ratio: 20 g/L | |||||
| Extraction solvent Concentration | 1 | 5 | 10 | ||
| Solid/liquid ratio | |||||
| 20 | 33.57 | 25.14 | 12.49 | ||
| Extraction temperature: 50 °C | |||||
| Run | Extraction Parameters | Yield (%) | ||
|---|---|---|---|---|
| x1: Solid/Liquid Ratio (g/L) | x2: Extraction Temperature (°C) | x3: Extraction Solvent Concentration (% (w/v)) | ||
| 1 | 18 | 50 | 3 | 29.91 |
| 2 | 10 | 100 | 1 | 36.02 |
| 3 | 30 | 50 | 5 | 21.12 |
| 4 | 30 | 80 | 3 | 34.73 |
| 5 | 30 | 50 | 1 | 27.35 |
| 6 | 18 | 50 | 3 | 29.73 |
| 7 | 30 | 100 | 5 | 36.26 |
| 8 | 28 | 75 | 5 | 34.58 |
| 9 | 10 | 100 | 4 | 37.66 |
| 10 | 23 | 100 | 1 | 34.99 |
| 11 | 30 | 50 | 1 | 27.23 |
| 12 | 18 | 80 | 3 | 35.54 |
| 13 | 10 | 50 | 5 | 24.80 |
| 14 | 10 | 67 | 1 | 36.03 |
| 15 | 30 | 100 | 5 | 34.93 |
| 16 | 23 | 69 | 1 | 33.22 |
| 17 | 10 | 50 | 5 | 24.26 |
| 18 | 18 | 80 | 5 | 34.82 |
| 19 | 28 | 55 | 3 | 27.28 |
| 20 | 10 | 67 | 1 | 36.01 |
| Extraction time: 5 min | ||||
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 448.90 | 9 | 49.88 | 54.29 | <0.0001 |
| x1: S/L | 20.62 | 1 | 20.62 | 22.45 | 0.0008 |
| x2: Temperature | 202.60 | 1 | 202.60 | 220.51 | <0.0001 |
| x3: Concentration | 11.82 | 1 | 11.82 | 12.87 | 0.0050 |
| x1x2 | 2.02 | 1 | 2.02 | 2.20 | 0.1690 |
| x1x3 | 2.03 | 1 | 2.03 | 2.21 | 0.1677 |
| x2x3 | 30.41 | 1 | 30.41 | 33.10 | 0.0002 |
| x12 | 1.22 | 1 | 1.22 | 1.32 | 0.2766 |
| x22 | 27.06 | 1 | 27.06 | 29.45 | 0.0003 |
| x32 | 5.14 | 1 | 5.14 | 5.59 | 0.0396 |
| Residual | 9.19 | 10 | 0.92 | ||
| Lack of Fit | 8.13 | 5 | 1.63 | 7.68 | 0.0215 |
| Pure Error | 1.06 | 5 | 0.21 | ||
| Cor Total | 458.09 | 19 |
| Factor | Actual Level | ||
|---|---|---|---|
| Solid/liquid ratio (x1) | 13.27 g/L | ||
| Extraction temperature (x2) | 91.86 °C | ||
| Extraction solvent concentration (x3) | 2.51% (w/v) | ||
| Response | Predicted | Experimental | |
| 37.79% | 36.21% | ||
| Algae | Solvent and the Others | Extraction Condition | Extraction Time | Yield | Reference |
|---|---|---|---|---|---|
| Sargassum | Pretreatment, 3.75% alkali, 12.63 mL/g, 80 °C | Hydrothermal extraction | 6 h | 20.76% | [17] |
| Sargassum muticum | Pretreatment, 3% alkali, 86 °C, Precipitated with 93% EtOH | Hydrothermal extraction | 3 h | 13.57% | [24] |
| Padina pavonica | Pretreatment, 4% alkali, 50 mL/g, 50 °C | Hydrothermal extraction | 5 h | 36.5% | [34] |
| Ecklonia radiata | Pretreatment, 0.2 M alkali, 45 °C | Hydrothermal extraction | 2 h | 45% | [23] |
| Macrocystis pyrifera | Pretreatment, 1 N alkali, 25 mL/g, 60 °C | Hydrothermal extraction | 2 h | 34% | [35] |
| Sargassum cristaefolium | Pretreatment, pH 10.3 alkali, 31.1 g/L | Extrusion system (2.95 rpm) | 6.8 min | 34.96% | [25] |
| Sargassum angustifolium | Pretreatment, 5% alcalase, pH 8, 50 °C, 3% alkali, pH 11, 65 °C | Enzyme-assisted extraction | 24 h (alcalase)/3 h (alkali) | 3.50% | [36] |
| Sargassum muticum | 20 mL/g, 25 °C | Ultrasound-assisted extraction (150 W) | 30 min | 15% | [37] |
| Nizimuddinia zanardini | Pretreatment, 29 mL/g, 67 °C | Microwave-assisted extraction (400 W) | 19 min | 31.39% | [38] |
| Undaria pinnatifida | 2.51% alkali, 13.27 g/L, 92 °C | Microwave-assisted extraction | 15 min | 38.41% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, H.-B.; Lee, K.H.; Yoo, H.Y.; Park, C.; Lim, J.-M.; Lee, J.H. Rapid and High-Yield Recovery of Sodium Alginate from Undaria pinnatifida via Microwave-Assisted Extraction. Processes 2024, 12, 208. https://doi.org/10.3390/pr12010208
Nam H-B, Lee KH, Yoo HY, Park C, Lim J-M, Lee JH. Rapid and High-Yield Recovery of Sodium Alginate from Undaria pinnatifida via Microwave-Assisted Extraction. Processes. 2024; 12(1):208. https://doi.org/10.3390/pr12010208
Chicago/Turabian StyleNam, Hyeon-Bin, Kang Hyun Lee, Hah Young Yoo, Chulhwan Park, Jong-Min Lim, and Ja Hyun Lee. 2024. "Rapid and High-Yield Recovery of Sodium Alginate from Undaria pinnatifida via Microwave-Assisted Extraction" Processes 12, no. 1: 208. https://doi.org/10.3390/pr12010208
APA StyleNam, H.-B., Lee, K. H., Yoo, H. Y., Park, C., Lim, J.-M., & Lee, J. H. (2024). Rapid and High-Yield Recovery of Sodium Alginate from Undaria pinnatifida via Microwave-Assisted Extraction. Processes, 12(1), 208. https://doi.org/10.3390/pr12010208










