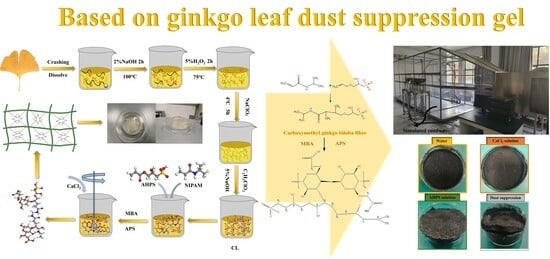
Preparation of Crust Type Dust Suppression Gel Based on Plant Extraction Technology for Ginkgo biloba Leaves: Characterization, Properties, and Function Mechanism
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Reagents
2.2. Experimental Process
2.2.1. Ginkgo biloba Extract Cellulose
2.2.2. C-A-N Dust Suppression Gel
2.3. Response Surface Method Design Experiment to Determine the Optimal Ratio
2.4. Gel Structure Characterization
2.5. Gel Performance Test
2.5.1. Swelling and Water Retention Tests
2.5.2. Dust Suppression Capability
2.5.3. Thermogravimetric Experiments
2.5.4. Cone Calorimetry Experiments
2.5.5. Strength of Consolidation Layer
2.6. Analysis of C-A-N Microscopic Mechanism
3. Experimental Results and Discussion
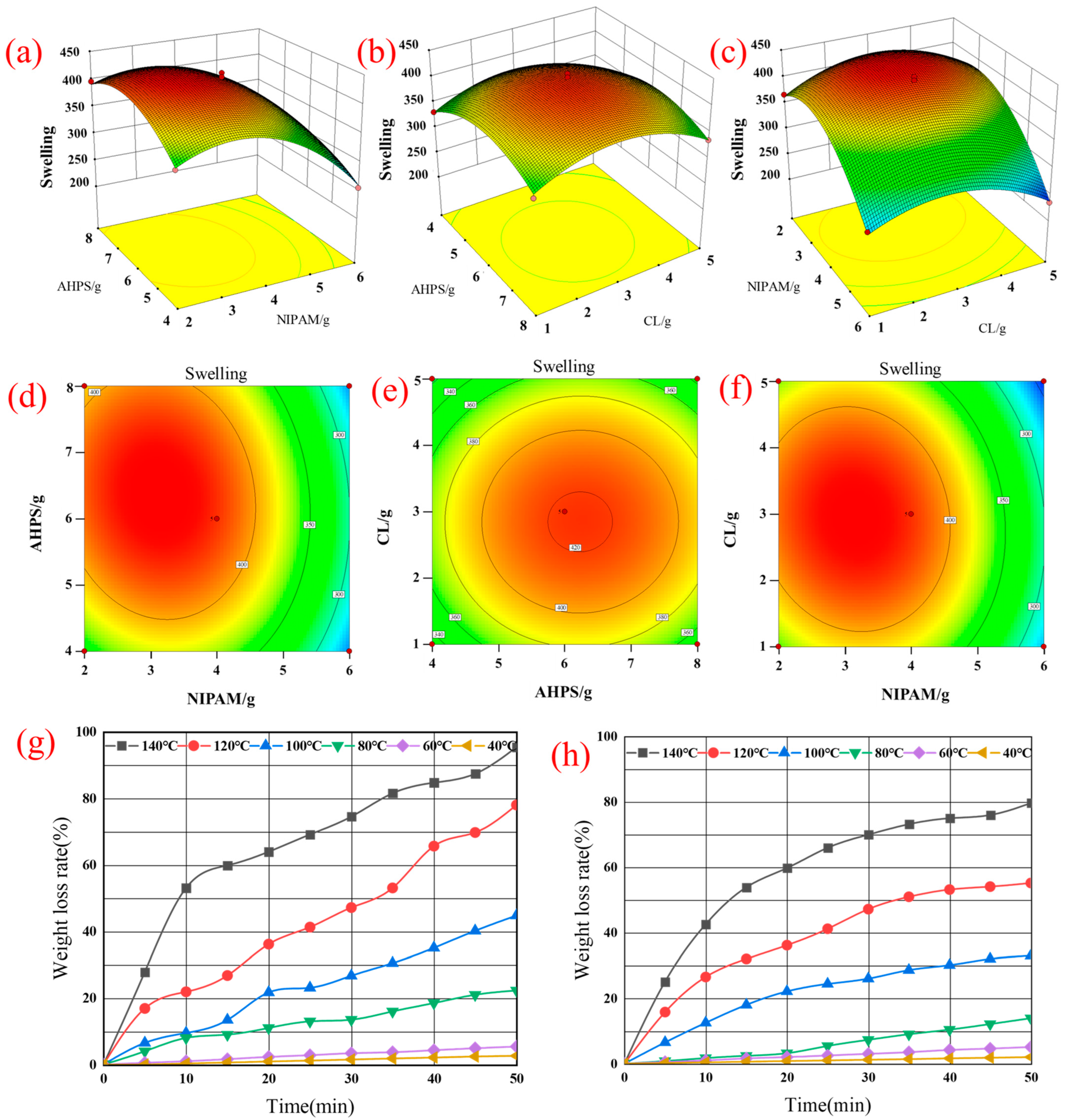
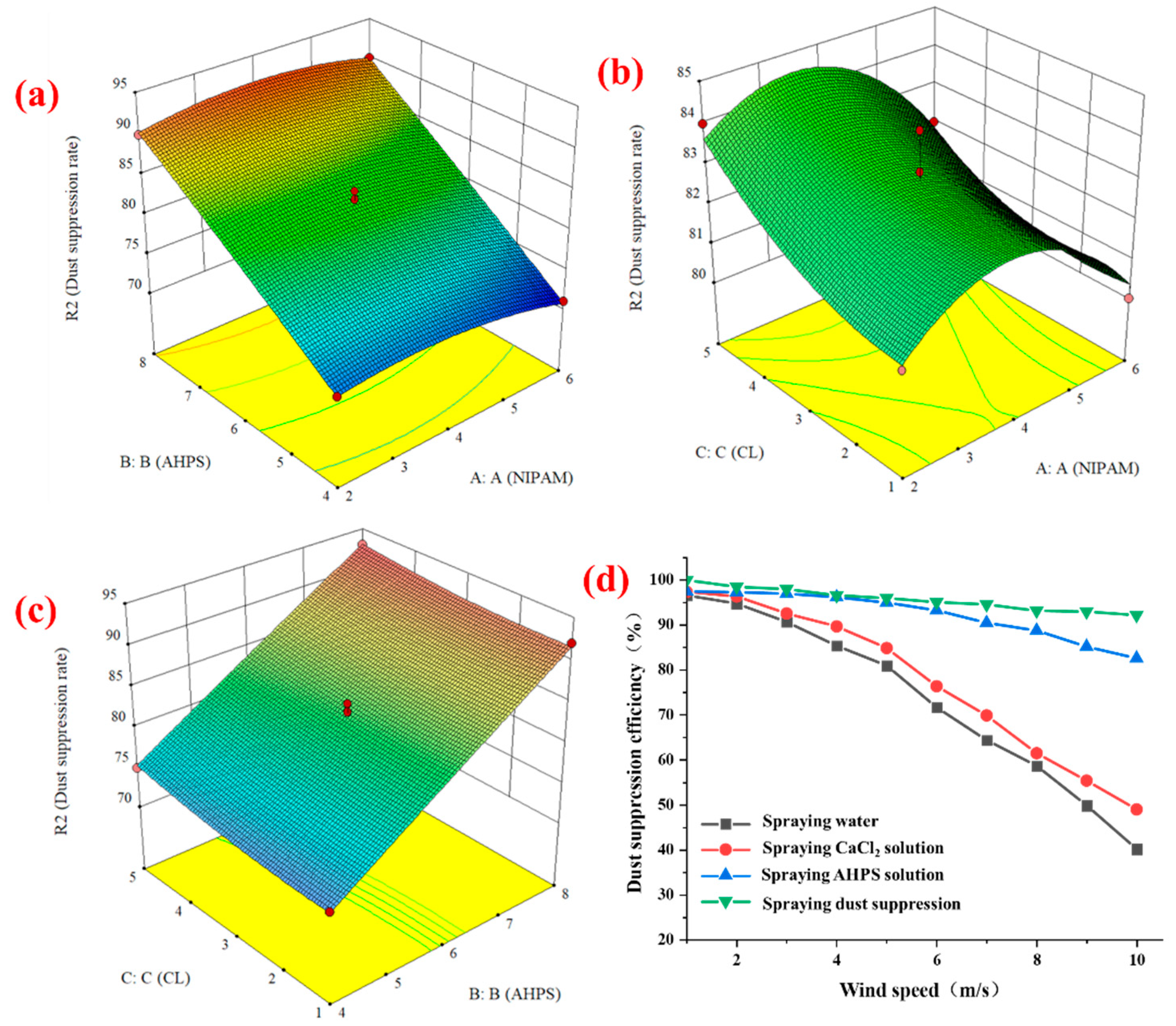
3.1. C-A-N Quadratic Response Surface Regression Analysis
3.2. Characterization Performance Analysis
3.2.1. Fourier-Transform Infrared Spectroscopy Analysis
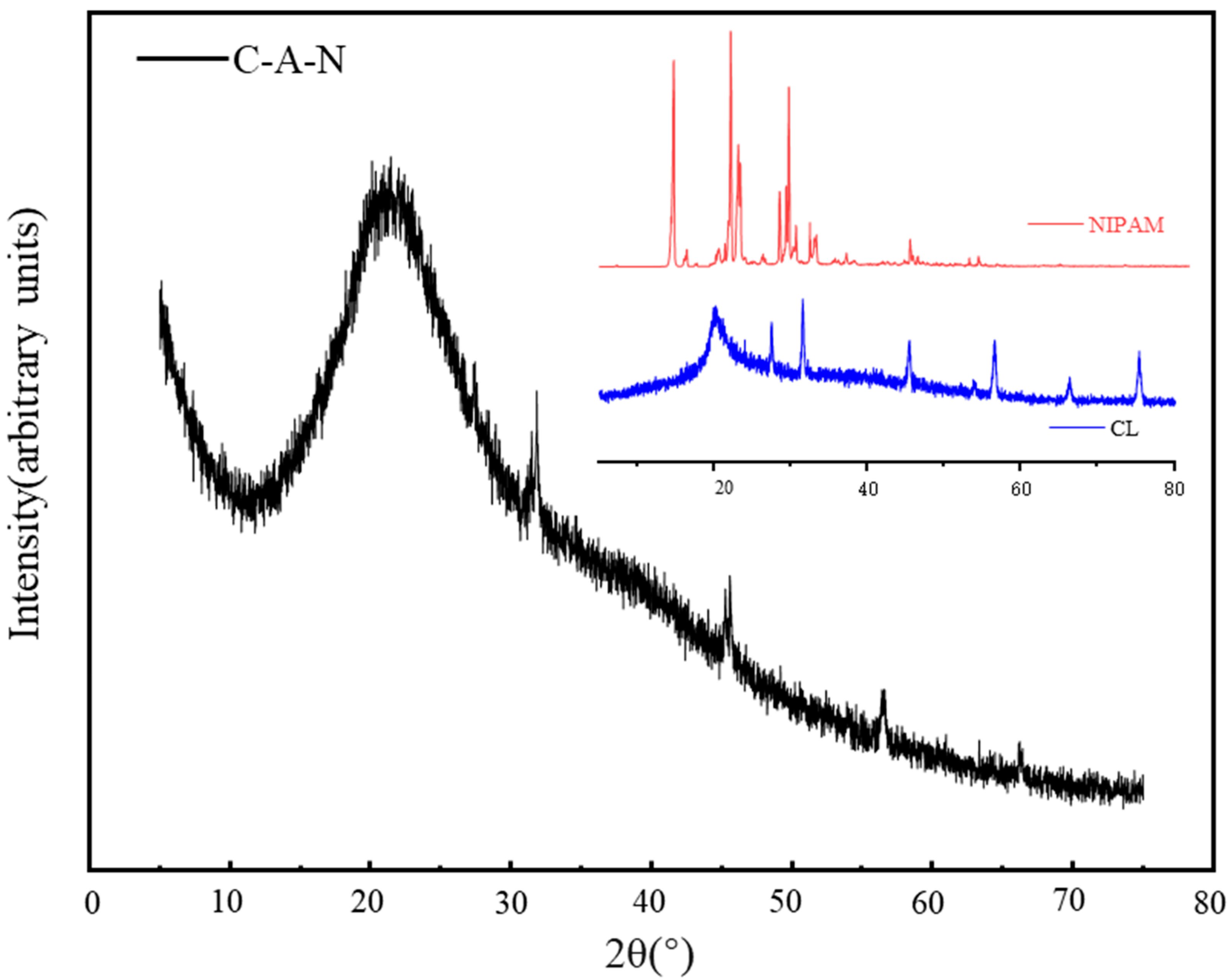
3.2.2. XRD Analysis
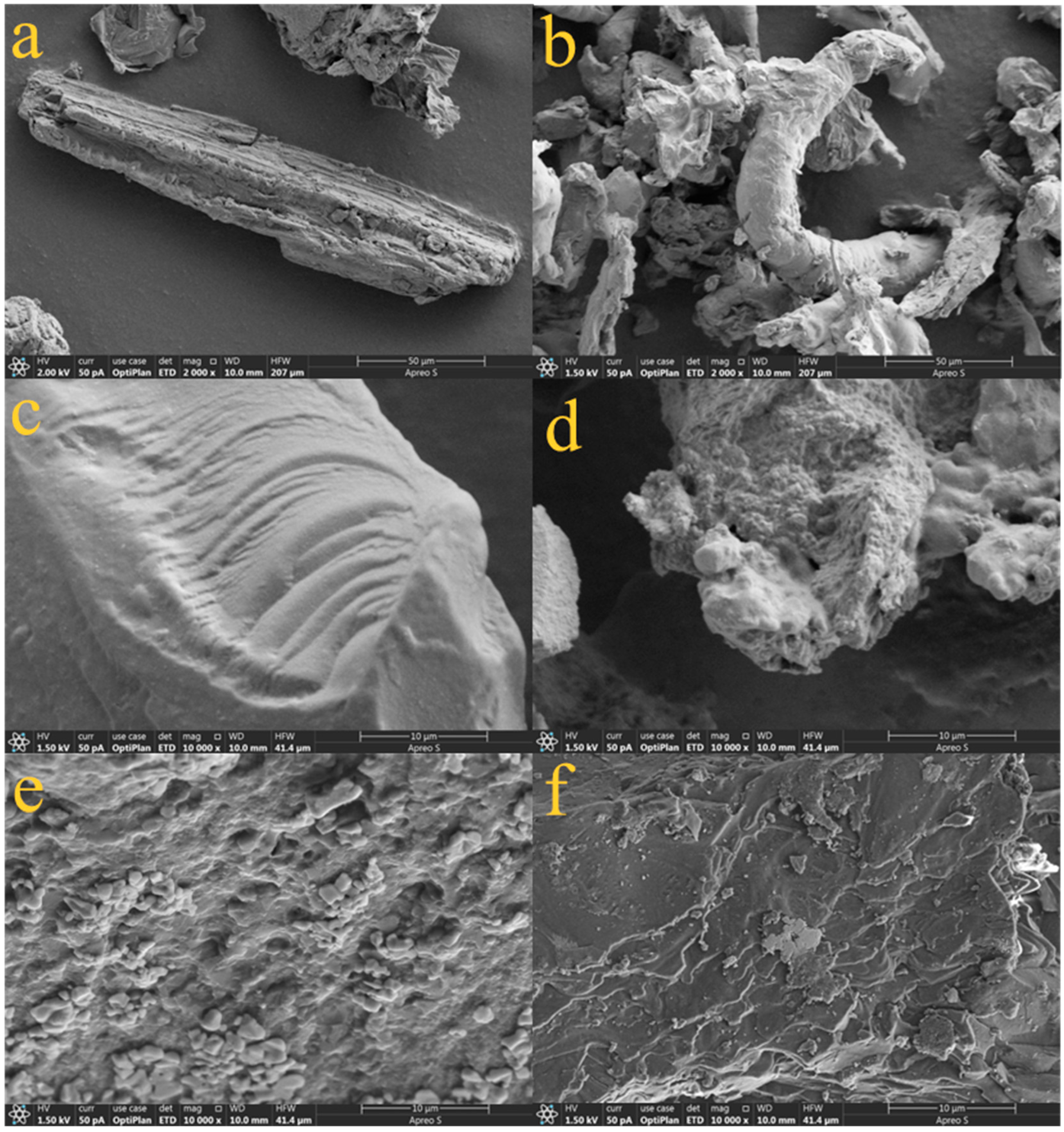
3.2.3. SEM Analysis
3.2.4. Synthesis Mechanism
3.3. Product Performance Test Results and Discussion
3.3.1. Dust Suppression Performance Analysis
3.3.2. Analysis of Dust Suppression Performance
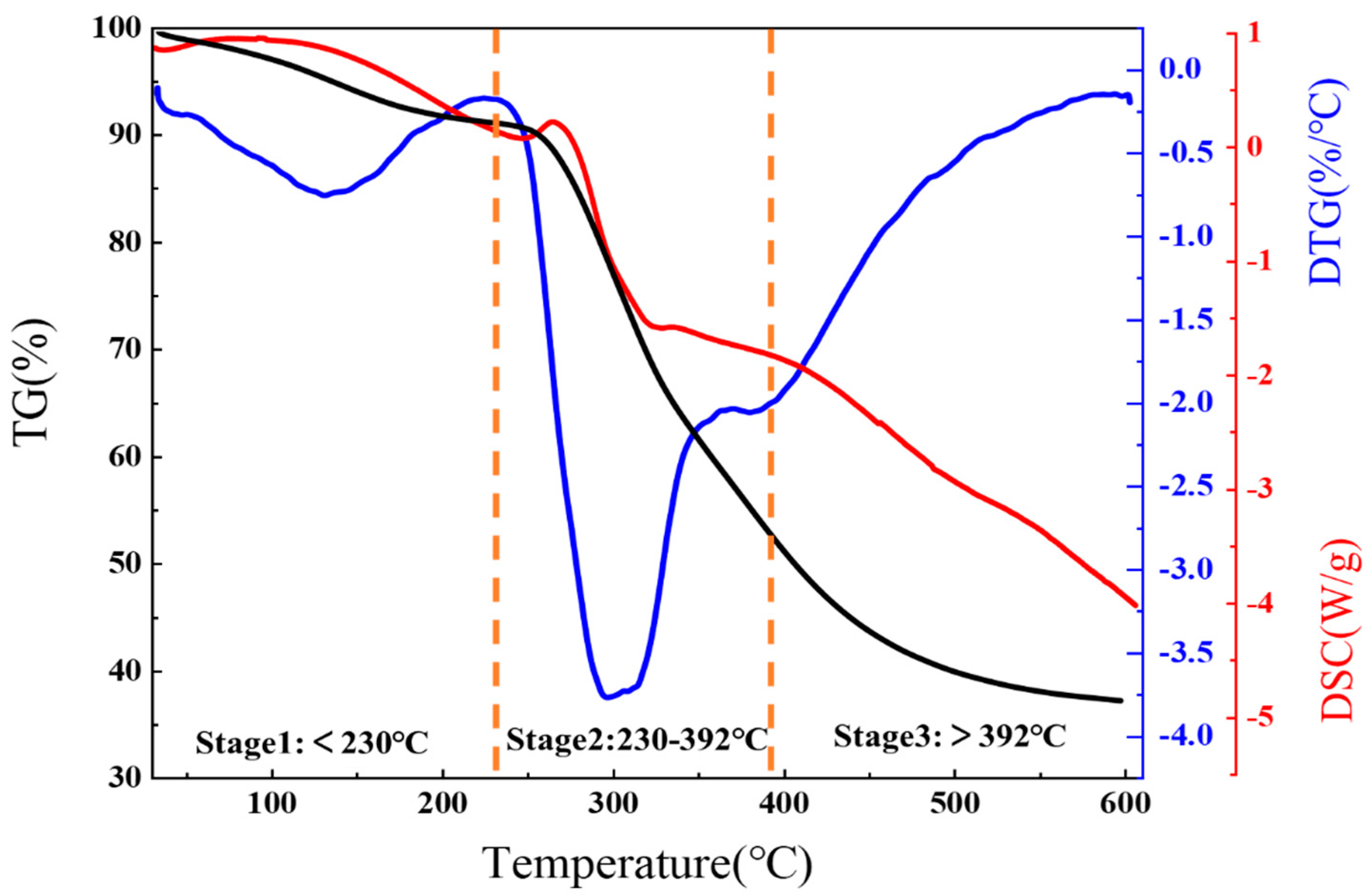
3.3.3. TG-DSC of C-A-N Dust Suppression Gel
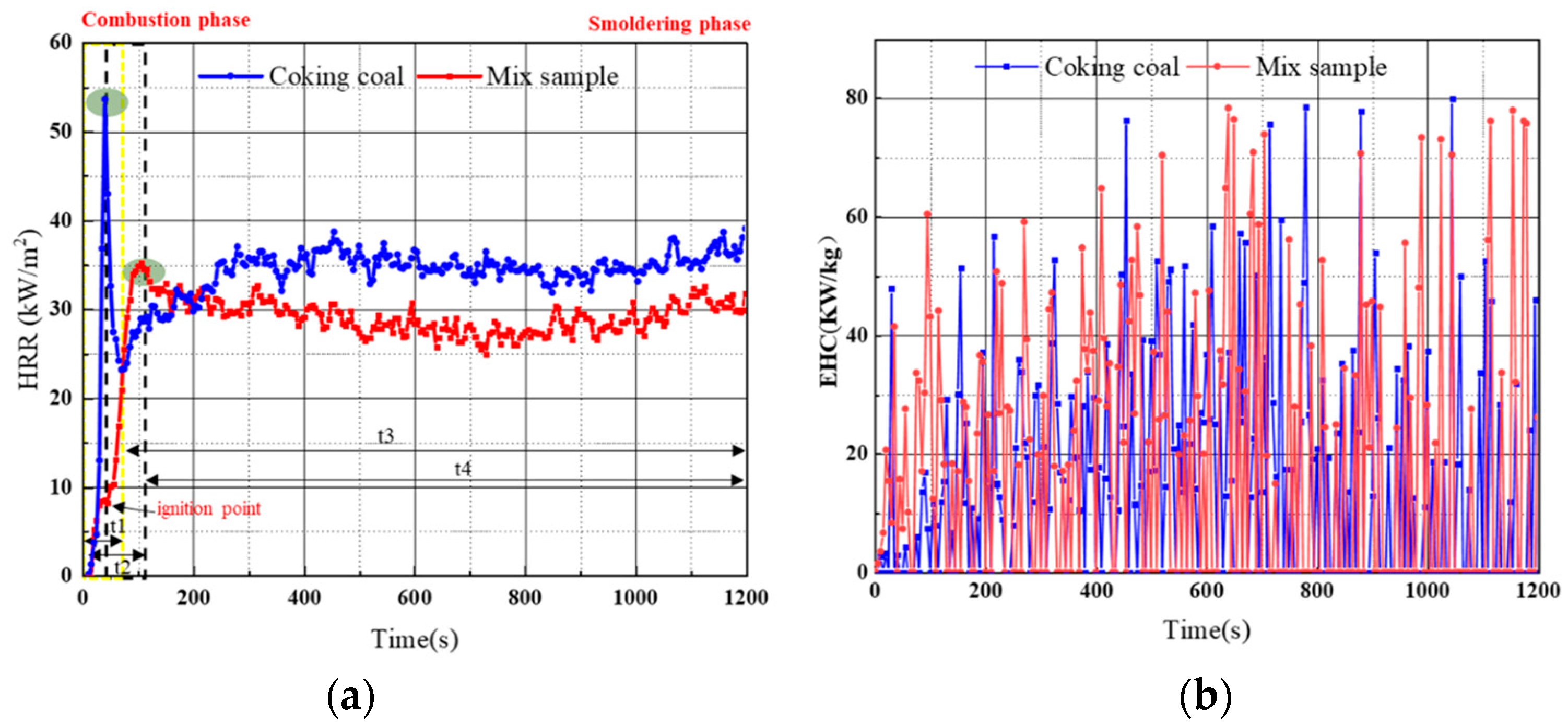
3.3.4. Analyzing Cone Calorimetry
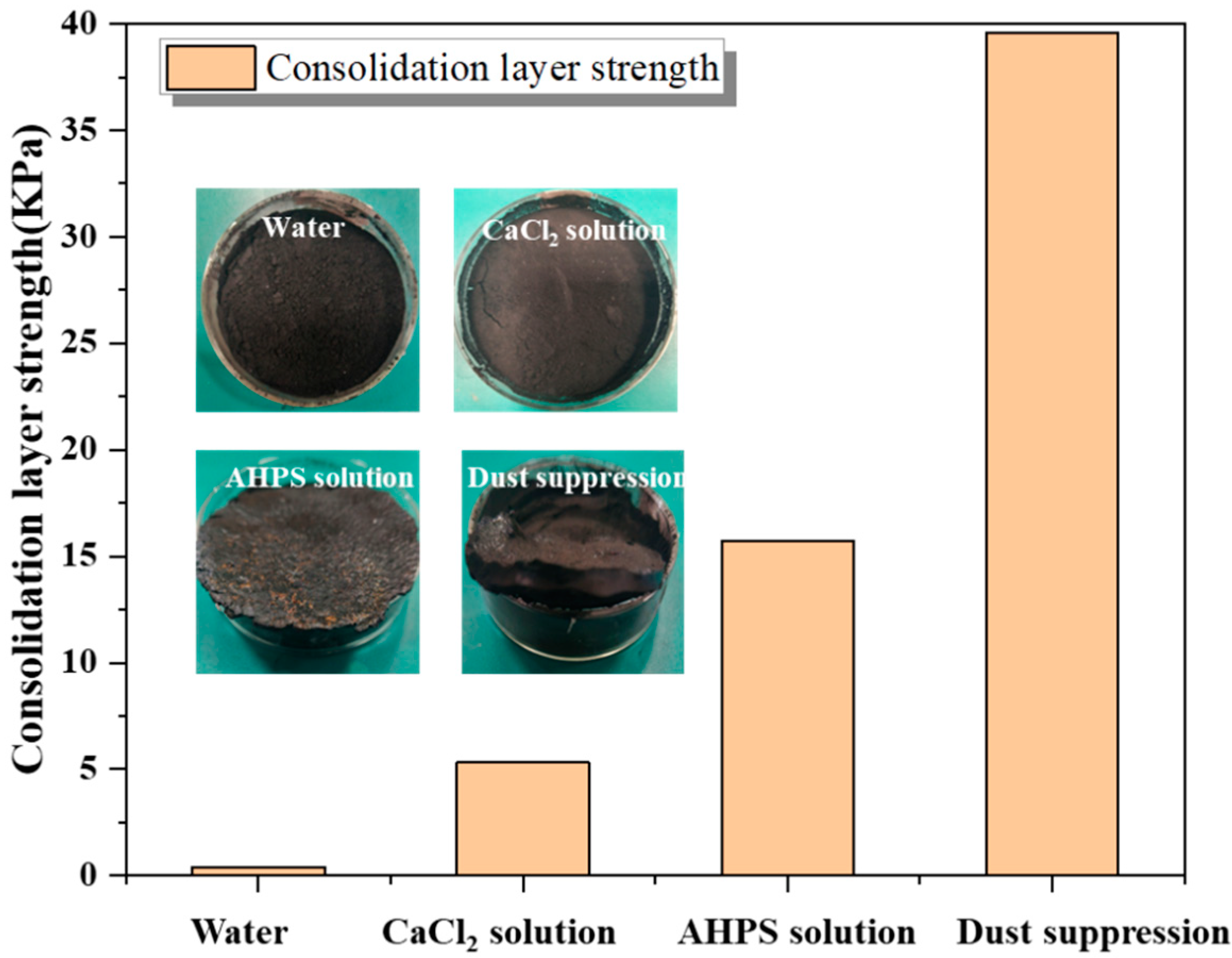
3.3.5. Consolidation Layer Strength
4. Micro Function Mechanism of Dust Suppression Gel
5. Conclusions
- (1)
- Through the response surface regression analysis experiment, the fitting analysis of the best ratio of gel shows that the dust suppression effect is best when CL:AHPS:NIPAM = 4:8:5, the initiator cross-linking agent is 1:5, and the reaction temperature is 60 °C. The dust suppression effect reaches 93% when the wind speed is 10 m/s, indicating the dust suppression efficiency of the product.
- (2)
- FT-IR, XRD, and thermogravimetry experiments proved that C-A-N was successfully prepared, and the synthesis mechanism of gel was analyzed. According to the SEM experiment, the internal structure of Ginkgo biloba leaves in comparison to CL and the gel was analyzed, and it was determined that the product had good thermal stability.
- (3)
- Through cone calorimetry experiments, it was found that the calorific value of coal stabilized at 30 Kw/m2 after adding dust suppression materials and did not decrease the calorific value of coal. The consolidation layer strength of the dust suppression material is as high as 39.6 Kpa, which proves the high crust strength of the product.
- (4)
- Through a molecular dynamics simulation, the interaction between the dust suppressor and coal dust was further revealed from a microscopic perspective. The hydrogen bond and Van der Waals force of the functional groups in the gel system were expounded, and the dust suppression mechanism and wetting effect of the gel were explained. After adding the gel, the thickness of the adsorption layer of coal and water increased from 20 Å to 47 Å, indicating that the gel has a good wetting effect.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.H.; Nie, W.; Yan, J.Y.; Bao, Q.; Wang, H.K.; Jin, H.; Peng, H.T.; Chen, D.W.; Liu, Z.Q.; Liu, Q. Preparation and performance study of a novel polymeric spraying dust suppression agent with enhanced wetting and coagulation properties for coal mine. Powder Technol. 2020, 364, 901–914. [Google Scholar] [CrossRef]
- Yang, X.H.; Yu, H.M.; Wang, Y.H.; Cheng, W.M. Investigation of dust pollution control rules in tunnel excavation based on modularized airflow diverging system. Build. Environ. 2022, 221, 109356. [Google Scholar] [CrossRef]
- Wang, P.F.; Shi, Y.J.; Zhang, L.Y.; Li, Y.J. Effect of structural parameters on atomization characteristics and dust reduction performance of internal-mixing air-assisted atomizer nozzle. Process Saf. Environ. 2019, 128, 316–328. [Google Scholar] [CrossRef]
- Fabiano, B.; Currò, F.; Reverberi, A.P.; Palazzi, E. Coal dust emissions: From environmental control to risk minimization by underground transport. An applicative case-study. Process Saf. Environ. 2014, 92, 150–159. [Google Scholar] [CrossRef]
- Luo, Q.M.; Huang, L.P.; Xue, X.Y.; Chen, Z.S.; Zhou, F.B.; Wei, L.H.; Hua, J.M. Occupational health risk assessment based on dust exposure during earthwork construction. J. Build. Eng. 2021, 44, 103186. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.M.; Zheng, S.Y. Characterization of nano-to-micron sized respirable coal dust: Particle surface alteration and the health impact. J. Hazard. Mater. 2021, 413, 125447. [Google Scholar] [CrossRef]
- Nie, W.; Niu, W.J.; Bao, Q.; Yuan, M.Y.; Zhou, W.W.; Hua, Y.; Yu, F.N.; Liu, C.Y.; Zhang, S.B.; Zhang, X. Study on the combined dust suppression effect of sodium alginate and sodium fatty acid methyl ester sulfonate. Adv. Powder Technol. 2022, 33, 103827. [Google Scholar] [CrossRef]
- Lööw, J.; Nygren, M. Initiatives for increased safety in the Swedish mining industry: Studying 30 years of improved accident rates. Safety Sci. 2019, 117, 437–446. [Google Scholar] [CrossRef]
- Wei, L.J.; Su, M.Q.; Wang, K.; Chen, S.N.; Ju, Y.; Zhao, S.J.; Kong, X.B.; Chu, Y.; Wang, L.X. Suppression effects of ABC powder on explosion characteristics of hybrid CH/polyethylene dust. Fuel 2022, 310, 122159. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.M.; Xu, R.X.; Cheng, W.M.; Ye, Y.X.; Xie, S. Application and characterization of new polymer dust suppression foam in coal mine and tunnel construction space. Constr. Build. Mater. 2023, 397, 132378. [Google Scholar] [CrossRef]
- Niu, W.J.; Nie, W.; Yuan, M.Y.; Bao, Q.; Zhou, W.W.; Yan, J.Y.; Yu, F.N.; Liu, C.Y.; Sun, N.; Xue, Q.Q. Study of the microscopic mechanism of lauryl glucoside wetting coal dust: Environmental pollution prevention and control. J. Hazard. Mater. 2021, 412, 125223. [Google Scholar] [CrossRef]
- Shi, G.Q.; Han, C.; Wang, Y.M.; Wang, H.T. Experimental study on synergistic wetting of a coal dust with dust suppressant compounded with noncationic surfactants and its mechanism analysis. Powder Technol. 2019, 356, 1077–1086. [Google Scholar] [CrossRef]
- Xu, R.X.; Yu, H.M.; Dong, H.; Ye, Y.X.; Xie, S. Preparation and properties of modified starch-based low viscosity and high consolidation foam dust suppressant. J. Hazard. Mater. 2023, 452, 131238. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Chen, X.Y.; Xie, Y.; Wei, X.B.; Liu, W.V. Experimental study on improving performance of dust-suppression foam by magnetization. Colloids Surf. A 2019, 577, 370–377. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.T.; Han, H.; Zhao, X.; Li, X.J.; Wang, Y.X. Experimental study on improving salt resistance of dust suppressing foam with polymers. Fuel 2023, 353, 129036. [Google Scholar] [CrossRef]
- Cheng, J.W.; Zheng, X.R.; Lei, Y.D.; Luo, W.; Wang, Y.; Borowski, M.; Li, X.C.; Song, W.T.; Wang, Z.; Wang, K. A compound binder of coal dust wetting and suppression for coal pile. Process Saf. Environ. 2021, 147, 92–102. [Google Scholar] [CrossRef]
- Wu, M.Y.; Hu, X.M.; Zhang, Q.; Lu, W.; Zhao, Y.Y.; He, Z.L. Study on preparation and properties of environmentally-friendly dust suppressant with semi-interpenetrating network structure. J. Clean. Prod. 2020, 259, 120870. [Google Scholar]
- Yang, X.; Qiao, Z.J.; Miao, Y.Z.; Tian, W.L.; Qing, L.B.; Jiang, L. Self-healing performance of rubber modified asphalt mixtures with microcapsules. J. Shandong Univ. Sci. Technol. (Nat. Sci.) 2023, 42, 68–76. [Google Scholar]
- Wei, X.B.; Wang, H.T.; Xie, Y.; Du, Y.H. An experimental investigation on the effect of carboxymethyl cellulose on morphological characteristics of dust-suppression foam and its mechanism exploration. Process Saf. Environ. 2020, 135, 126–134. [Google Scholar] [CrossRef]
- Zhao, Z.D.; Zhao, Y.Y.; Hu, X.M.; Cheng, W.M.; Hou, J.Y.; Song, C.Y. Preparation and performance analysis of enteromorpha-based environmentally friendly dust suppressant. Powder Technol. 2021, 393, 323–332. [Google Scholar] [CrossRef]
- Yu, X.X.; Zhao, Y.Y.; Feng, Y.; Hu, X.M.; Liu, J.D.; Wang, X.W.; Wu, M.Y.; Dong, H.; Liang, Y.T.; Wang, W.; et al. Synthesis and performance characterization of a road coal dust suppressant with excellent consolidation, adhesion, and weather resistance. Colloids Surf. A 2022, 639, 128334. [Google Scholar] [CrossRef]
- Joshi, G.; Naithani, S.; Varshney, V.K.; Bisht, S.S.; Rana, V.; Gupta, P.K. Synthesis and characterization of carboxymethyl cellulose from office waste paper: A greener approach towards waste management. Waste Manag. 2015, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Nie, W.; Zhang, Y.S.; Wang, H.K.; Zhang, H.H.; Bao, Q.; Yan, J.Y. Development of Environmental Friendly Dust Suppressant Based on the Modification of Soybean Protein Isolate. Processes 2019, 7, 165. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, Y.X.; Wang, Q.; Jiang, B.Y.; Ren, B.; Zhang, X.Y.; Yi, L.X. Wetting-consolidation type dust suppressant based on sugarcane bagasse as an environmental material: Preparation, characterization and dust suppression mechanism. J. Environ. Manag. 2023, 330, 117097. [Google Scholar] [CrossRef]
- Lu, Z.; Lei, Z.; Zafar, M.N. Synthesis and performance characterization of an efficient environmental-friendly Sapindus mukorossi saponins based hybrid coal dust suppressant. J. Clean. Prod. 2021, 306, 127261. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Cui, Y.M.; Shan, Z.H.; Dai, R.; Shi, L.; Chen, H. Fabrication and characterization of a multi-functional and environmentally-friendly starch/organo-bentonite composite liquid dust suppressant. Powder Technol. 2021, 391, 532–543. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Leite, C.M.M.; Lago, R.M. Use of glycerol by-product of biodiesel to produce an efficient dust suppressant. Chem. Eng. J. 2012, 180, 364–369. [Google Scholar] [CrossRef]
- Gao, Z.J.; Ding, Z.Q.; Liu, J.T.; Wang, Z.Y.; Wang, S.; Liu, W.Y. Study on water distribution and migration characteristics of inverted saturated zone and unsaturated zone beneath river. J. Shandong Univ. Sci. Technol. (Nat. Sci.) 2023, 42, 1–10. [Google Scholar]
- Waghmare, N.K.; Khan, S. Extraction and Characterization of Nano-cellulose Fibrils from Indian Sugarcane Bagasse-an Agro Waste. J. Nat. Fibers 2022, 19, 6230–6238. [Google Scholar] [CrossRef]
- Li, Y.Y.; Xiao, G.Q.; Li, F.Z.; Chen, C.Y.; Chen, C.L.; Li, R.L.; Zou, R.; Zhang, M. Response Surface Analysis (RSA) optimization of temperature-resistant gel foam fabrication and performance evaluation. Colloids Surf. A 2022, 655, 130260. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.M.; Sun, G.Z.; Wang, K.; Liu, T.Y.; Wang, J.Q.; Wang, F.S. A study on a Janus-type composite solidified foam and its characteristics for preventing and controlling spontaneous combustion of coal. Energy 2023, 275, 127433. [Google Scholar] [CrossRef]
- Zhang, H.H.; Nie, W.; Wang, H.K.; Bao, Q.; Jin, H.; Liu, Y.H. Preparation and experimental dust suppression performance characterization of a novel guar gum-modification-based environmentally-friendly degradable dust suppressant. Powder Technol. 2018, 339, 314–325. [Google Scholar] [CrossRef]
- Liu, J.G.; Wang, T.Y.; Jin, L.Z.; Li, G.; Wang, S.; Wei, Y.X.; Ou, S.N.; Wang, Y.P.; Xu, J.G.; Lin, M.L.; et al. Suppression Characteristics and Mechanism of Molasses Solution on Coal Dust: A Low-Cost and Environment-Friendly Suppression Method in Coal Mines. Int. J. Environ. Res. Public Health 2022, 19, 16472. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, S.Y.; Hu, B.; Yuan, Z.L.; Wu, H.; Yang, L.J. Evaluating of the performance of a composite wetting dust suppressant on lignite dust. Powder Technol. 2018, 339, 882–893. [Google Scholar] [CrossRef]
- Yan, J.Y.; Nie, W.; Zhang, H.H.; Xiu, Z.H.; Bao, Q.; Wang, H.K.; Jin, H.; Zhou, W.J. Synthesis and performance measurement of a modified polymer dust suppressant. Adv. Powder Technol. 2020, 31, 792–803. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, Y.W.; Xia, Y.C.; Guo, F.Y.; Ding, S.H.; Tan, J.L.; Che, T.; Meng, F.C.; Gui, X.H. Synergistic Adsorption Mechanism of Anionic and Cationic Surfactant Mixtures on Low-Rank Coal Flotation. ACS Omega 2020, 5, 20630–20637. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, L.; Li, H. Study on model construction and optimization of molecular structure. Coal Sci. Technol. 2021, 49, 245–253. [Google Scholar] [CrossRef]
- Bao, Q.; Nie, W.; Liu, C.Q.; Zhang, H.H.; Wang, H.K.; Jin, H.; Yan, J.Y.; Liu, Q. The preparation of a novel hydrogel based on crosslinked polymers for suppressing coal dusts. J. Clean. Prod. 2020, 249, 119343. [Google Scholar] [CrossRef]
- Gao, M.Z.; Li, H.M.; Zhao, Y.; Liu, Y.T.; Zhou, W.Q.; Li, L.M.; Xie, J.; Deng, J. Mechanism of micro-wetting of highly hydrophobic coal dust in underground mining and new wetting agent development. Int. J. Min. Sci. Technol. 2023, 33, 31–46. [Google Scholar] [CrossRef]
- Fan, Y.J.; Zhao, Y.Y.; Hu, X.M.; Cheng, W.M.; Tang, X.L.; Zhu, S.C.; Song, C.Y. Material optimization of microbial dust suppressant nutrient solution based on response surface curve. Powder Technol. 2021, 385, 29–36. [Google Scholar] [CrossRef]
- Wang, Y.; Du, C.F.; Cui, M.M. Formulation Development and Performance Characterization of Ecological Dust Suppressant for Road Surfaces in Cities. Appl. Sci. 2021, 11, 10466. [Google Scholar] [CrossRef]
- Li, W.F.; Wang, H.N.; Li, X.; Liang, Y.N.; Wang, Y.T.; Zhang, H.J. Effect of mixed cationic/anionic surfactants on the low-rank coal wettability by an experimental and molecular dynamics simulation. Fuel 2021, 289, 119886. [Google Scholar] [CrossRef]






| Level | Factor | ||
|---|---|---|---|
| NIPAM/g | AHPS/g | CL/g | |
| Low | 2 | 4 | 1 |
| Middle | 4 | 6 | 3 |
| High | 6 | 8 | 5 |
| Run | Facotr1 | Facotr2 | Facotr3 | Response | Response |
|---|---|---|---|---|---|
| A: NIPAM/g | B: AHPS/g | C: CL/g | Swelling | Dust Suppression Effect | |
| 1 | 4 | 4 | 5 | 324 | 92 |
| 2 | 4 | 6 | 3 | 417 | 83 |
| 3 | 4 | 6 | 3 | 432 | 82 |
| 4 | 4 | 6 | 3 | 425 | 84 |
| 5 | 6 | 6 | 1 | 274 | 80 |
| 6 | 6 | 6 | 5 | 235 | 82 |
| 7 | 4 | 6 | 3 | 421 | 83 |
| 8 | 6 | 8 | 3 | 263 | 90 |
| 9 | 2 | 6 | 5 | 362 | 84 |
| 10 | 4 | 6 | 3 | 413 | 82 |
| 11 | 2 | 4 | 3 | 350 | 73 |
| 12 | 2 | 8 | 3 | 396 | 90 |
| 13 | 4 | 8 | 1 | 342 | 92 |
| 14 | 6 | 4 | 3 | 255 | 71 |
| 15 | 2 | 6 | 1 | 367 | 81 |
| 16 | 4 | 8 | 5 | 336 | 93 |
| 17 | 4 | 4 | 1 | 332 | 73 |
| Source | Sum of | F | Prob | |
|---|---|---|---|---|
| Model | 64,786.18 | 108.67 | <0.0001 | Significant |
| A: NIPAM | 25,088.00 | 378.73 | <0.0001 | |
| B: AHPS | 722.00 | 10.90 | 0.0131 | |
| C: CL | 420.50 | 6.35 | 0.0398 | |
| AB | 361.00 | 5.45 | 0.0523 | |
| AC | 289.00 | 4.36 | 0.0751 | |
| BC | 1.00 | 0.015 | 0.9057 | |
| A2 | 17,680.17 | 266.90 | <0.0001 | |
| B2 | 7009.01 | 105.81 | <0.0001 | |
| C2 | 9420.17 | 142.21 | <0.0001 | |
| Lack of Fit | 248.50 | 1.54 | 0.3346 | Not Significant |
| Source | Sum of | F | Prob | |
|---|---|---|---|---|
| Model | 691.51 | 151.50 | <0.0001 | Significant |
| A: NIPAM | 3.13 | 6.16 | 0.0421 | |
| B: AHPS | 666.13 | 1313.49 | <0.0001 | |
| C: CL | 8.00 | 15.77 | 0.0054 | |
| AB | 1.00 | 1.97 | 0.2030 | |
| AC | 0.25 | 0.49 | 0.5053 | |
| BC | 0.25 | 0.49 | 0.5053 | |
| A2 | 11.46 | 22.60 | 0.0021 | |
| B2 | 0.095 | 0.19 | 0.6786 | |
| C2 | 1.52 | 2.99 | 0.1275 | |
| Lack of Fit | 0.75 | 0.36 | 0.7880 | Not Significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, B.; Zhou, G.; Song, M.; Jiang, B.; Zheng, Y.; Fan, T.; Li, S.; Zhao, J.; Li, H.; Qu, H. Preparation of Crust Type Dust Suppression Gel Based on Plant Extraction Technology for Ginkgo biloba Leaves: Characterization, Properties, and Function Mechanism. Processes 2024, 12, 224. https://doi.org/10.3390/pr12010224
Ren B, Zhou G, Song M, Jiang B, Zheng Y, Fan T, Li S, Zhao J, Li H, Qu H. Preparation of Crust Type Dust Suppression Gel Based on Plant Extraction Technology for Ginkgo biloba Leaves: Characterization, Properties, and Function Mechanism. Processes. 2024; 12(1):224. https://doi.org/10.3390/pr12010224
Chicago/Turabian StyleRen, Bo, Gang Zhou, Mingkun Song, Bingyou Jiang, Yuannan Zheng, Tao Fan, Shuailong Li, Jing Zhao, Haoyang Li, and Hongrui Qu. 2024. "Preparation of Crust Type Dust Suppression Gel Based on Plant Extraction Technology for Ginkgo biloba Leaves: Characterization, Properties, and Function Mechanism" Processes 12, no. 1: 224. https://doi.org/10.3390/pr12010224