Experimental Study on Chrome Tanned Leather Shavings Modification—Properties and Prospective for Future Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Waste Material (CTLS)
2.2. Modification of Waste Material
2.3. Testing of Physical and Chemical Parameters
- mc is the mass of the sample together with the measuring cylinder [g],
- m is the mass of the measuring cylinder [g], and
- vc represents the volume of sample in the measuring cylinder [cm3].
2.4. FTIR Analysis
2.5. Phytotoxicity Test
2.5.1. Experimental Design in Soil
2.5.2. Biological Analysis
2.6. Statistical Analysis
3. Results and Discussion
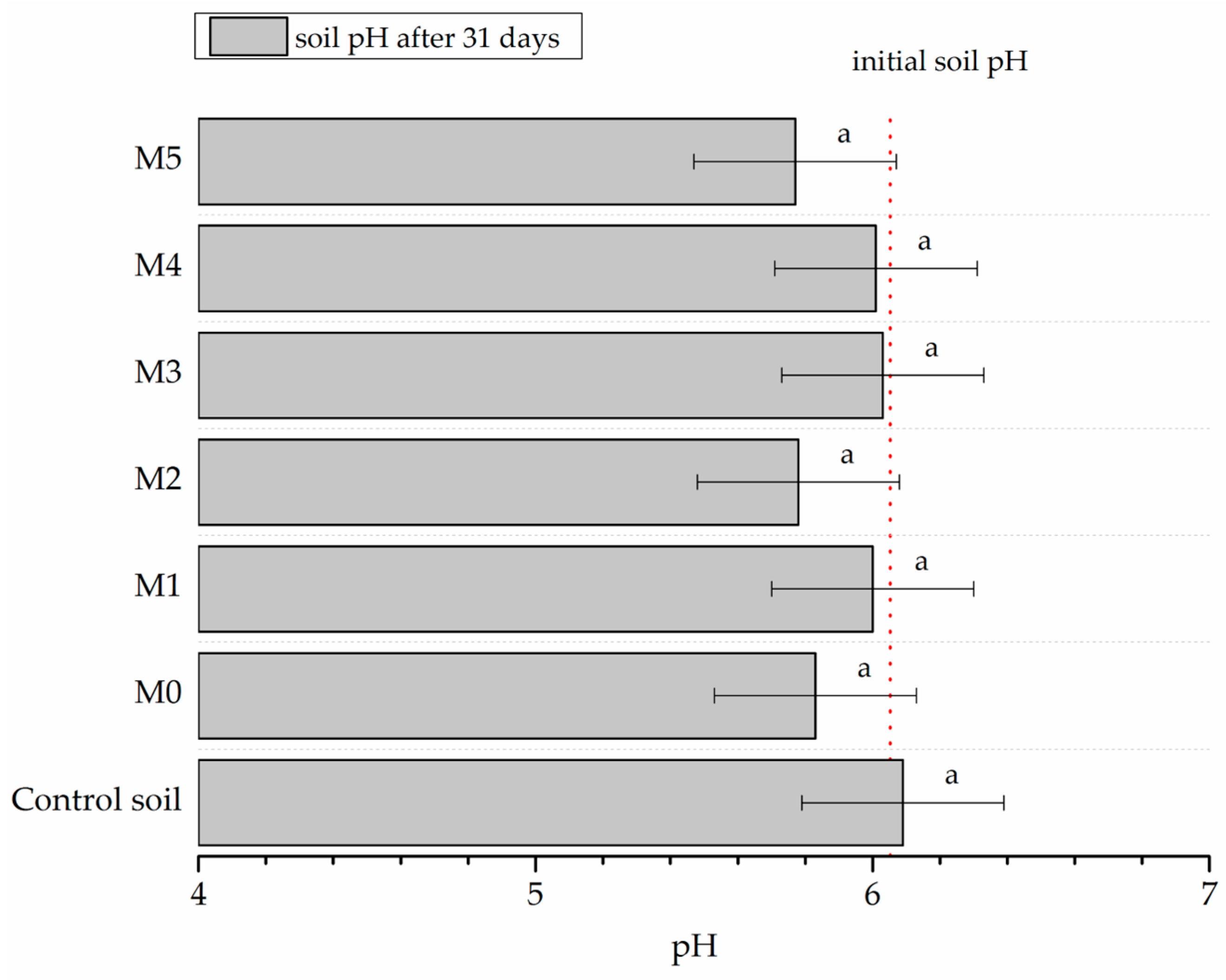
3.1. Testing of Physical and Chemical Parameters
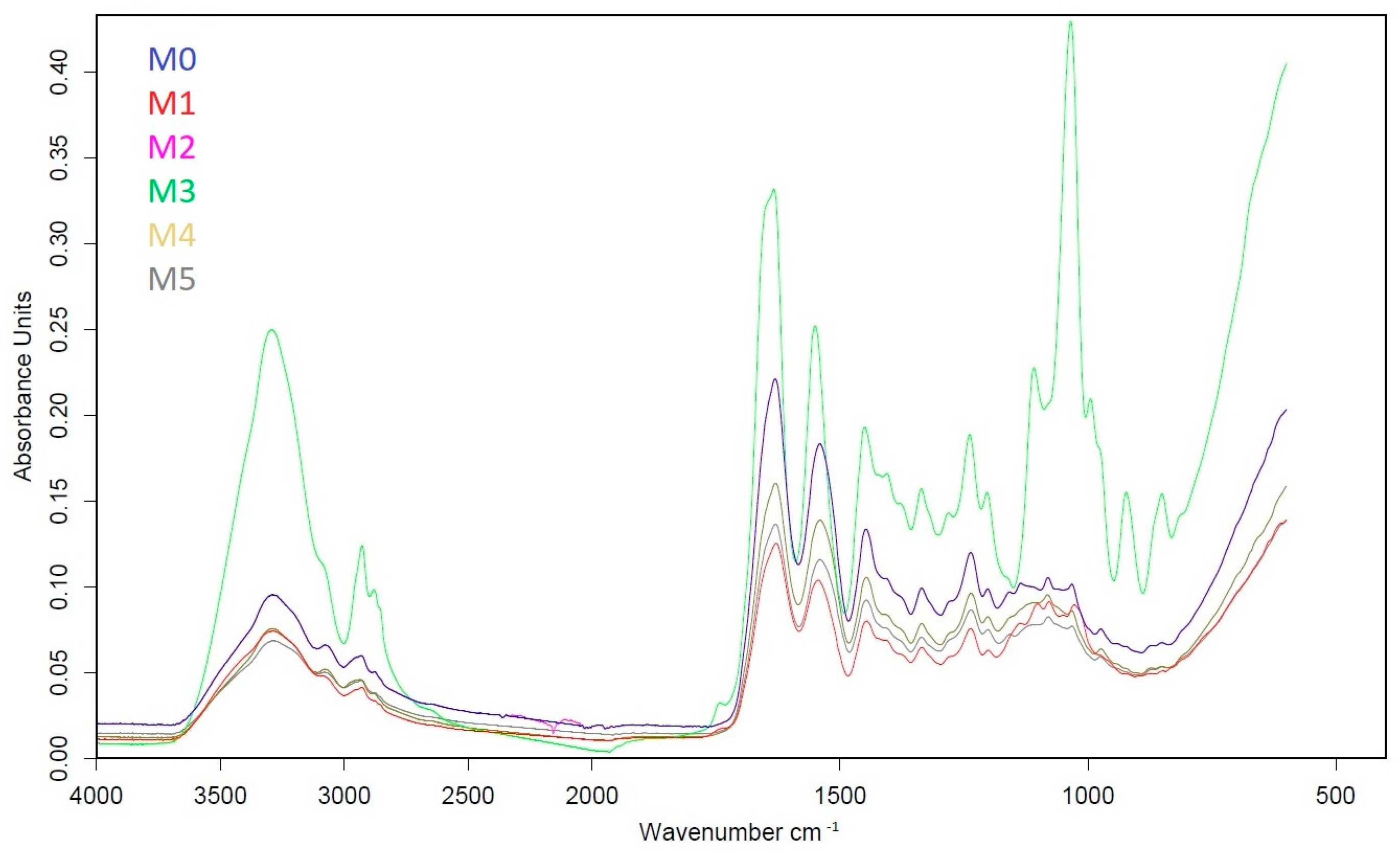
3.2. FTIR Analysis
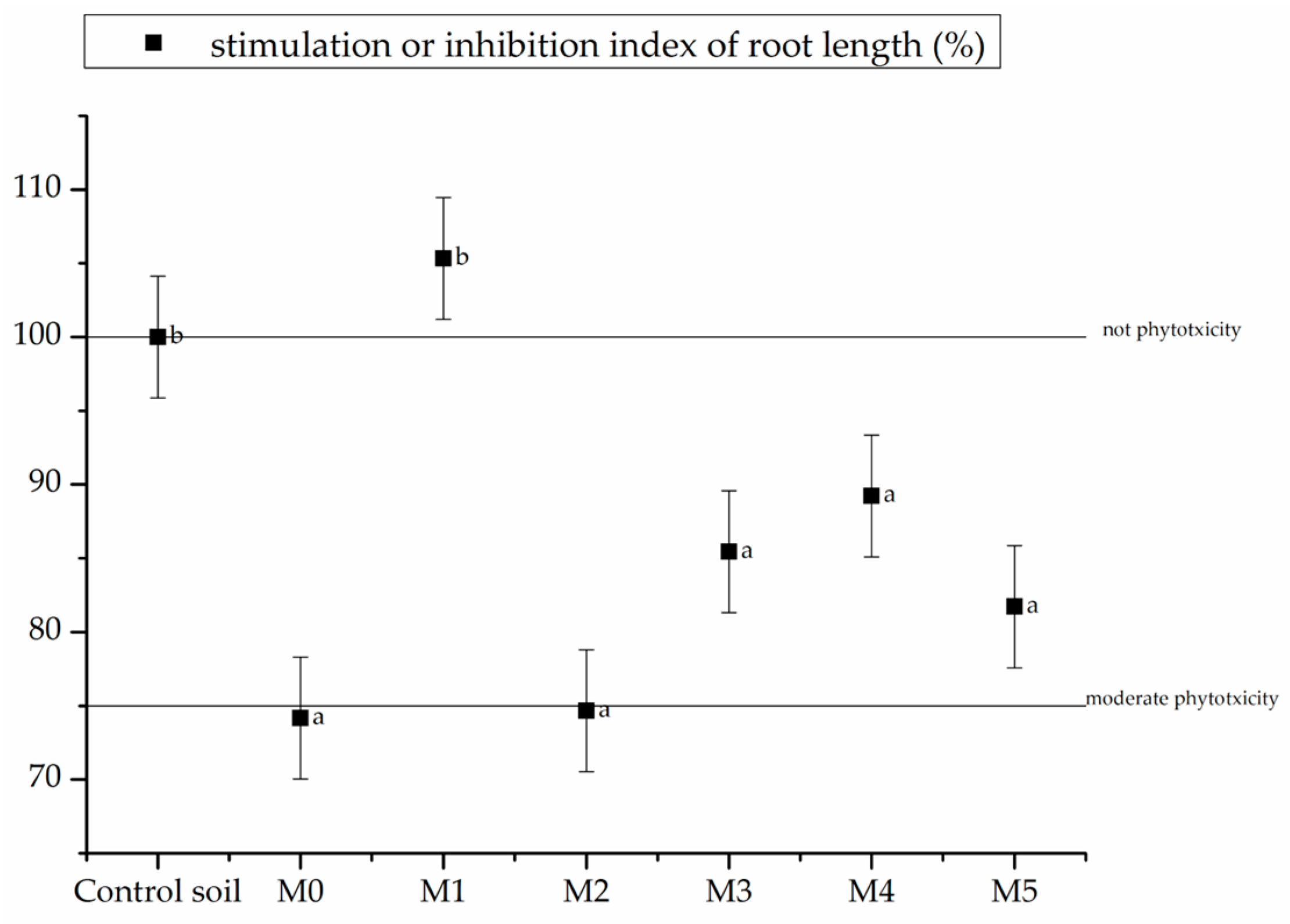
3.3. Phytotoxicity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sivaram, N.M.; Barik, D. Chapter 5—Toxic Waste From Leather Industries. In Energy from Toxic Organic Waste for Heat and Power Generation; Debabrata, B., Ed.; Woodhead Publishing: Duxford, UK, 2019; pp. 55–67. [Google Scholar] [CrossRef]
- Li, Y.; Guo, R.; Lu, W.; Zhu, D. Research progress on resource utilization of leather solid waste. J. Leather Sci. Eng. 2019, 1, 6. [Google Scholar] [CrossRef]
- Quadery, A.H.; Uddin, M.T.; Chowdhury, M.J.; Deb, A.K. Extraction of polypeptide solution from tannery solid waste (chrome shavings) and its application as poultry feed. IOSR J. Appl. Chem. 2016, 9, 32–35. [Google Scholar]
- Onukak, I.E.; Mohammed-Dabo, I.A.; Ameh, A.O.; Okoduwa, S.I.; Fasanya, O.O. Production and Characterization of Biomass Briquettes from Tannery Solid Waste. Recycling 2017, 2, 17. [Google Scholar] [CrossRef]
- Ławińska, K.; Lasoń-Rydel, M.; Gendaszewska, D.; Grzesiak, E.; Sieczyńska, K.; Gaidau, C.; Epure, D.G.; Obraniak, A. Coating of Seeds with Collagen Hydrolysates from Leather Waste. Fibres Text. East. Eur. 2019, 27, 59–64. [Google Scholar] [CrossRef]
- Parisi, M.; Nanni, A.; Colonna, M. Recycling of Chrome-Tanned Leather and Its Utilization as Polymeric Materials and in Polymer-Based Composites. Rev. Polym. 2021, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Gendaszewska, D.; Lasoń-Rydel, M.; Ławińska, K.; Grzesiak, E.; Pipiak, P. Characteristics of collagen preparations from leather wastes by the high pressure liquid chromatography method. Fibres Text. East. Eur. 2021, 5, 75–79. [Google Scholar] [CrossRef]
- Kantarli, I.C.; Yanik, J. Activated carbon from leather shaving wastes and its application in removal of toxic materials. J. Hazard. Mater. 2010, 179, 348–356. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Ławińska, K.; Modrzewski, R.; Obraniak, A. Comparison of Granulation Methods for Tannery Shavings. Fibres Text. East. Eur. 2020, 5, 119–123. [Google Scholar] [CrossRef]
- Velusamy, M.; Chakali, B.; Ganesan, S.; Tinwala, F.; Venkatachalam, S.S. Investigation on pyrolysis and incineration of chrome-tanned solid waste from tanneries for effective treatment and disposal: An experimental study. Environ. Sci. Pollut. Res. 2020, 27, 29778–29790. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, C.; Peng, Q.; Zaman, F.; Zhang, H.; Jin, Z.; Wang, A.; Wang, W.; Huang, Y. Pyrolysis kinetics behavior of solid leather wastes. Waste Manag. 2019, 100, 122–127. [Google Scholar] [CrossRef]
- Santos, R.J.; Agostini, D.L.; Cabrera, F.; Budemberg, E.R. Recycling leather waste: Preparing and studying on the microstructure, mechanical, and rheological properties of leather waste/rubber composite. Polym. Compos. 2014, 36, 12. [Google Scholar] [CrossRef]
- Saikia, P.; Goswami, T.; Dutta, D.; Dutta, N.K.; Sengupta, P.; Neog, D. Development of a flexible composite from leather industry waste and evaluation of their physico-chemical properties. Clean Technol. Environ. Policy 2017, 19, 2171–2178. [Google Scholar] [CrossRef]
- Milu, M.S.; Hashem, M.A.; Payel, S.; Hasan, M.A. Leather buffing dust in brick production: Solid waste management in tanneries. Case Stud. Constr. Mater. 2022, 17, e01625. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Mikula, K.; Witek-Krowiak, A.; Izydorczyk, G.; Kuligowski, K.; Bandrów, P.; Kułażyński, M. Progress in sustainable technologies of leather wastes valorization as solutions for the circular economy. J. Clean. Prod. 2021, 313, 127902. [Google Scholar] [CrossRef]
- Ma, J.; Li, T.; Liu, Y.; Cai, T.; Wei, Y.; Dong, W.; Chen, H. Rice husk derived double network hydrogel as efficient adsorbent for Pb(II), Cu(II) and Cd(II) removal in individual and multicomponent systems. Bioresour. Technol. 2019, 290, 121793. [Google Scholar] [CrossRef]
- Yunoki, S.; Suzuki, T.; Takai, M. Stabilization of low denaturation temperature collagen from fish by physical cross-linking methods. J. Biosci. Bioeng. 2006, 96, 575–577. [Google Scholar] [CrossRef]
- Dhayal, S.K.; Gruppen, H.; de Vries, R.; Wierenga, P.A. Controlled formation of protein nanoparticles by enzymatic cross-linking of α-lactalbumin with horseradish peroxidase. Food Hydrocoll. 2014, 36, 53–59. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Jiang, Q.; Reddy, N.; Zhang, S.; Roscioli, N.; Yang, Y. Water-stable electrospun collagen fibers from a non-toxic solvent and crosslinking system. J. Biomed. Mater. Res. 2013, 101, 1237–1247. [Google Scholar] [CrossRef]
- Sionkowska, J.; Skopinska-Wisniewska, M.; Gawron, J.; Kozlowska, A.; Planecka, A. Chemical and thermal cross-linking of collagen and elastin hydrolysates. Int. J. Biol. Macromol. 2010, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Manjari, M.S.; Kavati, P.A.; Chellappa, M.; Chellan, R. Highly biocompatible novel polyphenol cross-linked collagen scaffold for potential tissue engineering applications. React. Funct. Polym. 2020, 153, 104630. [Google Scholar] [CrossRef]
- Ofoegbu, O.; Umah, N.; Akor, E. The use of natural cross linkers in molecularly imprinted polymer technology-past, present and future. J. Chem. Soc. Niger. 2021, 46, 129–140. [Google Scholar]
- Kaczmarek, B.; Mazur, O. Collagen-Based Materials Modified by Phenolic Acids—A Review. Materials 2020, 13, 3641. [Google Scholar] [CrossRef]
- Beauchamp, R.O.; St Clair, M.B.; Fennell, T.R.; Clarke, D.O.; Morgan, K.T.; Kari, F.W. A critical review of the toxicology of glutaraldehyde. Crit. Rev. Toxicol. 1992, 22, 143–174. [Google Scholar] [CrossRef] [PubMed]
- Damink, L.H.O.; Dijkstra, P.J.; Van Luyn, M.J.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef]
- Tropini, V.; Lens, J.P.; Mulder, W.J.; Silvestre, F. Wheat gluten films cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and N-hydroxysuccinimide. Ind. Crop. Prod. 2004, 20, 281–289. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Langmaier, F.; Mokrejs, P.; Kolomaznik, K.; Mladek, M. Plasticizing collagen hydrolysate with glycerol and low-molecular weight poly(ethylene glycols). Thermochim. Acta 2008, 469, 52–58. [Google Scholar] [CrossRef]
- Wu, L.; Shao, H.; Fang, Z.; Zhao, Y.; Cao, C.Y.; Li, Q. Mechanism and Effects of Polyphenol Derivatives for Modifying Collagen. ACS Biomater. Sci. Eng. 2019, 5, 4272–4284. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Markiewicz, E.; Kołodziejczak, A. Preparation and characterization of tannic acid cross-linked scaffolds made of chitosan, collagen and hyaluronic acid. Eng. Biomater. 2016, 19, 21–27. (In Polish) [Google Scholar]
- Goel, H.; Gupta, N.; Santhiya, D.; Dey, N.; Bohidar, H.B.; Bhattacharya, A. Bioactivity reinforced surface patch bound collagen-pectin hydrogel. Int. J. Biol. Macromol. 2021, 174, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sheardown, H. Crosslinking of collagen with dendrimers. J. Biomed. Mater. Res. 2005, 75, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Bello, J.; Bello, H.R. Chemical modification and cross-linking of proteins by impurities in glycerol. Arch. Biochem. Biophys. 1976, 172, 608–610. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 17072-2:2019; Leather—Chemical Determination of Metal Content—Part 2: Total Metal Content. Polish Committee for Standardization: Warszawa, Poland, 2019.
- PN-EN ISO 17075-1:2017-05; Leather. Chemical Determination of Chromium(VI) Content in Leather. Part1: Colorimetric Method. Polish Committee for Standardization: Warszawa, Poland, 2017.
- ISO 18763:2016; Soil Quality—Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. ISO: Geneva, Switzerland, 2016.
- Ławinska, K. Production of Agglomerates, Composite Materials, and Seed Coatings from Tannery Waste as New Methods for Its Management. Materials 2021, 14, 6695. [Google Scholar] [CrossRef] [PubMed]
- Pati, A.; Chaudhary, R.; Subramani, S. A review on management of chrome-tanned leather shavings: A holistic paradigm to combat the environmental issues. Environ. Sci. Pollut. Res. 2014, 21, 11266–11282. [Google Scholar] [CrossRef] [PubMed]
- Litwińczuk-Mammadova, A.; Cieślik-Boczula, K.; Rospenk, M. FTIR-ATR and fluorescence studies of protein-lipid systems. Wiadomości Chem. 2017, 71, 109–132. (In Polish) [Google Scholar]
- Mehta, M.; Naffa, R.; Maidment, C.; Holmes, G.; Waterland, M. Raman and atr-ftir spectroscopy towards classification of wet blue bovine leather using ratiometric and chemometric analysis. J. Leather Sci. Eng. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Boskey, A.; Pleshko Camacho, N. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 2007, 28, 2465–2478. [Google Scholar] [CrossRef]
- Jayakumar, G.C.; Usharani, N.; Kawakami, K.; Rao, J.R.; Naira, B.U. Studies on the physico-chemical characteristics of collagen–pectin composites. RSC Adv. 2014, 4, 63840–63849. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K. Modification of collagen and chitosan mixtures by the addition of tannic acid. J. Mol. Liq. 2014, 199, 318–323. [Google Scholar] [CrossRef]
- Narasimman, P.; Sethuraman, P. An overview on the fundamentals of pectin. Int. J. Approx. Reason. 2016, 4, 1855–1860. [Google Scholar] [CrossRef]
- Mise, K.; Koyama, Y.; Matsumoto, A.; Fujita, K.; Kunito, T.; Senoo, K.; Otsuka, S. Pectin drives microbial phosphorus solubilization in soil: Evidence from isolation-based and community-scale approaches. Eur. J. Soil Biol. 2020, 97, 103169. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Famielec, S. Environmental effects of tannery waste incineration in a tunnel furnace system. Proc. ECOpole 2015, 9, 441–450. [Google Scholar] [CrossRef]
- Usha, R.; Thirumalachari, R. Effect of crosslinking agents (basic chromium sulfate and formaldehyde) on the thermal and thermomechanical stability of rat tail tendon collagen fibre. Thermochim. Acta 2000, 356, 59–66. [Google Scholar] [CrossRef]
- Campa, M.F.; Techtmann, S.M.; Gibson, C.M.; Zhu, X.; Patterson, M.; Garcia de Matos Amaral, A.; Ulrich, N.J.; Campagna, S.R.; Grant, C.J.; Lamendella, R.; et al. Impacts of Glutaraldehyde on Microbial Community Structure and Degradation Potential in Streams Impacted by Hydraulic Fracturing. Environ. Sci. Technol. 2018, 52, 5989–5999. [Google Scholar] [CrossRef]
- Wieczorek, D.; Kwapisz, E.; Marchut-Mikołajczyk, O.; Bielecki, S. Biotechnologia Phytotests as tools for monitoring the bioremediation process of soil contaminated with diesel oil. Journal of Biotechnology. Comput. Biol. Bionanotechnol. 2013, 93, 431–439. [Google Scholar] [CrossRef]
- Batool, M.; El-Badri, A.M.; Wang, C.; Mohamed, I.A.A.; Wang, Z.; Khatab, A.; Bashir, F.; Xu, Z.; Wang, J.; Kuai, J.; et al. The role of storage reserves and their mobilization during seed germination under drought stress conditions of rapeseed cultivars with high and low oil contents. Crop. Environ. 2022, 1, 231–240. [Google Scholar] [CrossRef]
- Wieczorek, D.; Gendaszewska, D.; Miśkiewicz, K.; Słubik, A.; Ławińska, K. Biotransformation of protein-rich waste by Yarrowia lipolytica IPS21 to high-value products—Amino acid supernatants. Microbiol. Spectr. 2023, 14, 0274923. [Google Scholar] [CrossRef] [PubMed]
- Barrow, N.J.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
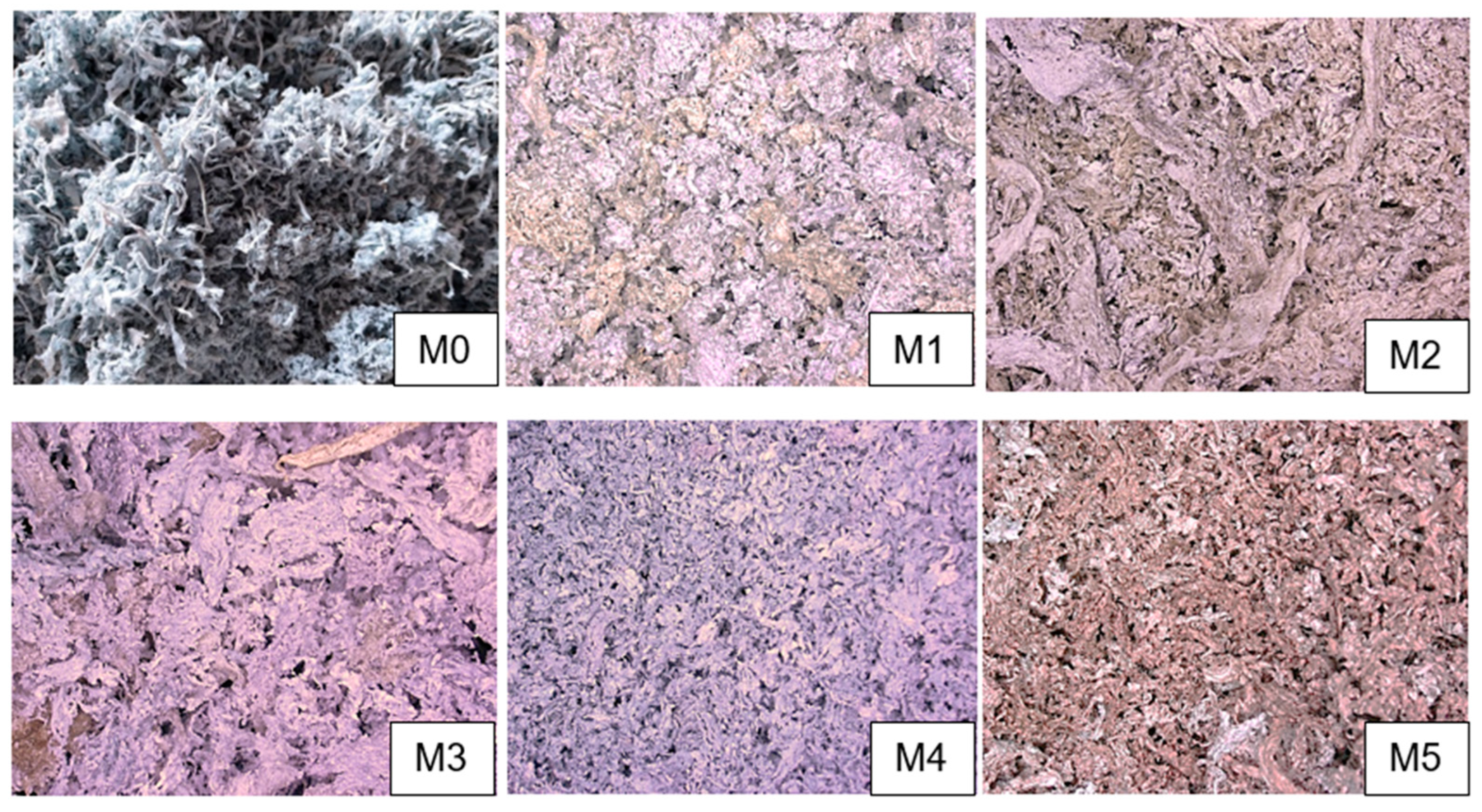
| Acronym of Sample | Raw Material | Type of Additives | Physical State of Additives | pH of Additives | Additives [%] |
|---|---|---|---|---|---|
| M0 | CTLS | - | - | - | 0 |
| M1 | Pectin | powder | 4.00 | 11 | |
| M2 | Glutaraldehyde | liquid | 3.70 | 5 | |
| M3 | Glycerol | liquid | 5.00 | 50 | |
| M4 | EDC * | powder | 4.00 | 4 | |
| M5 | Tannin | powder | 2.80 | 2 |
| Acronym of Sample | Bulk Density [g cm−3] | pH [-] | Size of Fiber [mm] | Tensile Strenght [N/mm2] |
|---|---|---|---|---|
| M0 | 0.15 ± 0.02 a | 3.86 ± 0.23 a | 70 ± 20 a | 0.34 ± 0.03 b |
| M1 | 0.52 ± 0.03 d | 3.83 ± 0.20 a | 40 ± 10 a | 0.46 ± 0.02 c |
| M2 | 0.17 ± 0.01 a | 3.33 ± 0.12 a | 50 ± 10 a | 0.83 ± 0.04 d |
| M3 | 0.39 ± 0.04 c | 4.16 ± 0.17 a | 50 ± 10 a | 0.03 ± 0.01 a |
| M4 | 0.15 ± 0.03 a | 3.89 ± 0.20 a | 40 ± 15 a | 0.78 ± 0.08 d |
| M5 | 0.20 ± 0.02 b | 4.25 ± 0.13 a | 60 ± 10 a | 0.04 ± 0.01 a |
| Metal | Sample | |||||
|---|---|---|---|---|---|---|
| M0 | M1 | M2 | M3 | M4 | M5 | |
| Ca | 1019.78 a | 2111.37 b | 971.92 a | 739.24 a | 871.59 a | 1297.21 a |
| Cr | 24,844.92 b | 17,650.54 a | 19,739.65 a | 18,540.24 a | 21,054.03 a | 24,494.17 b |
| Cr (VI) | un * | un * | un * | un * | un * | un * |
| Fe | 564.24 b | 813.69 c | 380.3 a | 295.12 a | 374.20 a | 812.18 c |
| K | 34.45 a | 50.61 a | 41.14 a | 14.38 b | 33.11 a | 110.10 c |
| Mg | 754.44 b | 409.49 a | 1036.33 c | 405.08 a | 863.81 b | 808.65 b |
| Na | 10,651.40 a | 19,813.52 b | 13,889.17 b | 1070.88 b | 8355.70 a | 12,028.4 b |
| P | 333.65 d | 199.39 b | 282.51 c | 114.10 a | 180.14 b | 337.62 d |
| S | 13,825.62 a | 11,309.49 a | 15,400.63 a | 12,234.42 a | 14,956.20 a | 14,456.39 a |
| Cd | un * | un * | un * | un * | un * | un * |
| Hg | un * | un * | un * | un * | un * | un * |
| Pb | un * | un * | un * | un * | un * | un * |
| Amide Band Symbol | MO | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|---|
| A | 3291 | 3292 | 3293 | 3293 | 3293 | 3286 |
| B | 2932 | 2930 | 2927 | 2928 | 2937 | 2931 |
| I | 1631 | 1628 | 1631 | 1634 | 1630 | 1630 |
| II | 1540 | 1544 | 1542 | 1551 | 1540 | 1541 |
| III | 1236 | 1237 | 1236 | 1238 | 1235 | 1236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gendaszewska, D.; Pipiak, P.; Wieczorek, D.; Sieczyńska, K. Experimental Study on Chrome Tanned Leather Shavings Modification—Properties and Prospective for Future Application. Processes 2024, 12, 228. https://doi.org/10.3390/pr12010228
Gendaszewska D, Pipiak P, Wieczorek D, Sieczyńska K. Experimental Study on Chrome Tanned Leather Shavings Modification—Properties and Prospective for Future Application. Processes. 2024; 12(1):228. https://doi.org/10.3390/pr12010228
Chicago/Turabian StyleGendaszewska, Dorota, Paulina Pipiak, Dorota Wieczorek, and Katarzyna Sieczyńska. 2024. "Experimental Study on Chrome Tanned Leather Shavings Modification—Properties and Prospective for Future Application" Processes 12, no. 1: 228. https://doi.org/10.3390/pr12010228








