Novel Betulin-1,2,4-Triazole Derivatives Promote In Vitro Dose-Dependent Anticancer Cytotoxicity
Abstract
:1. Introduction
2. Results
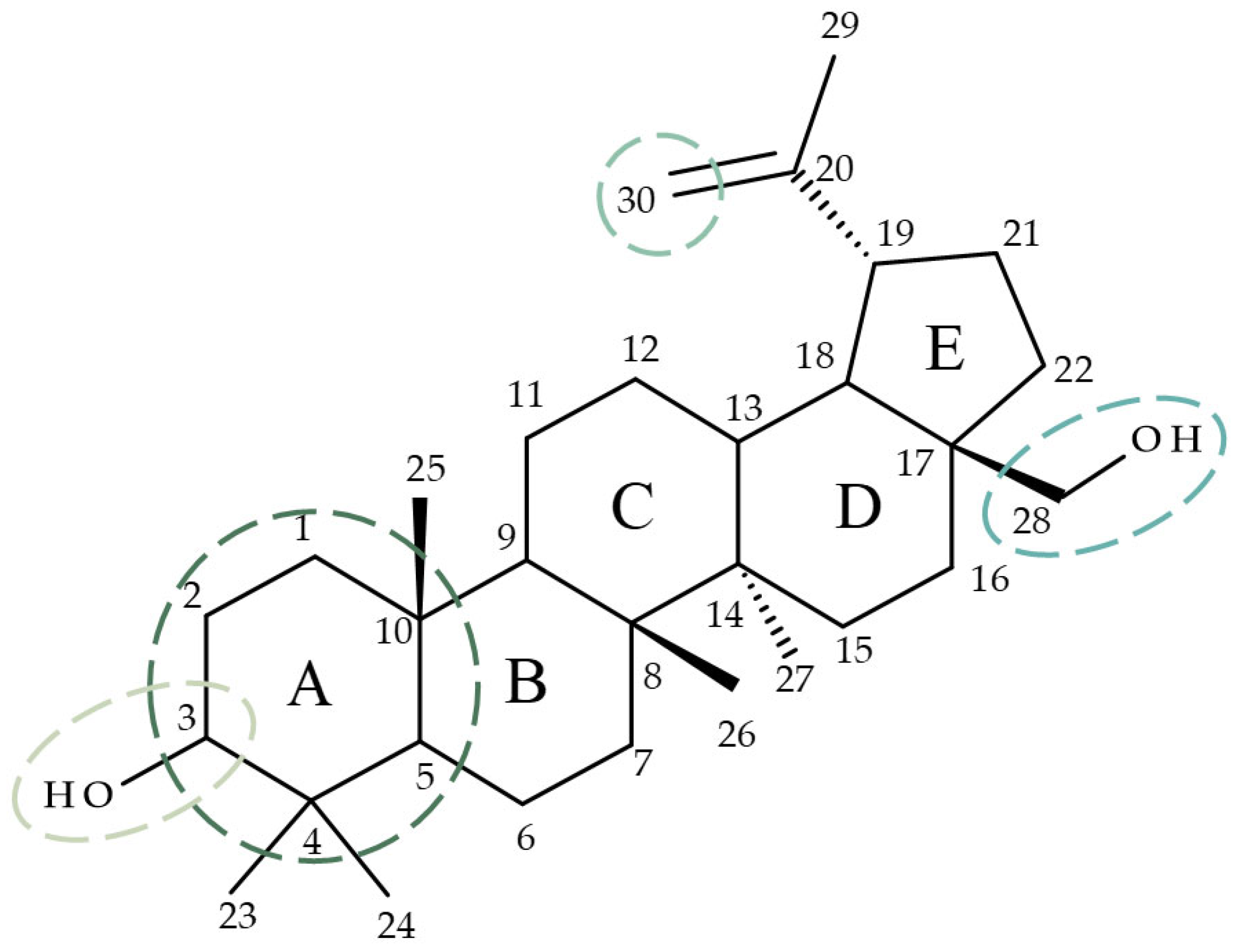
2.1. Chemistry
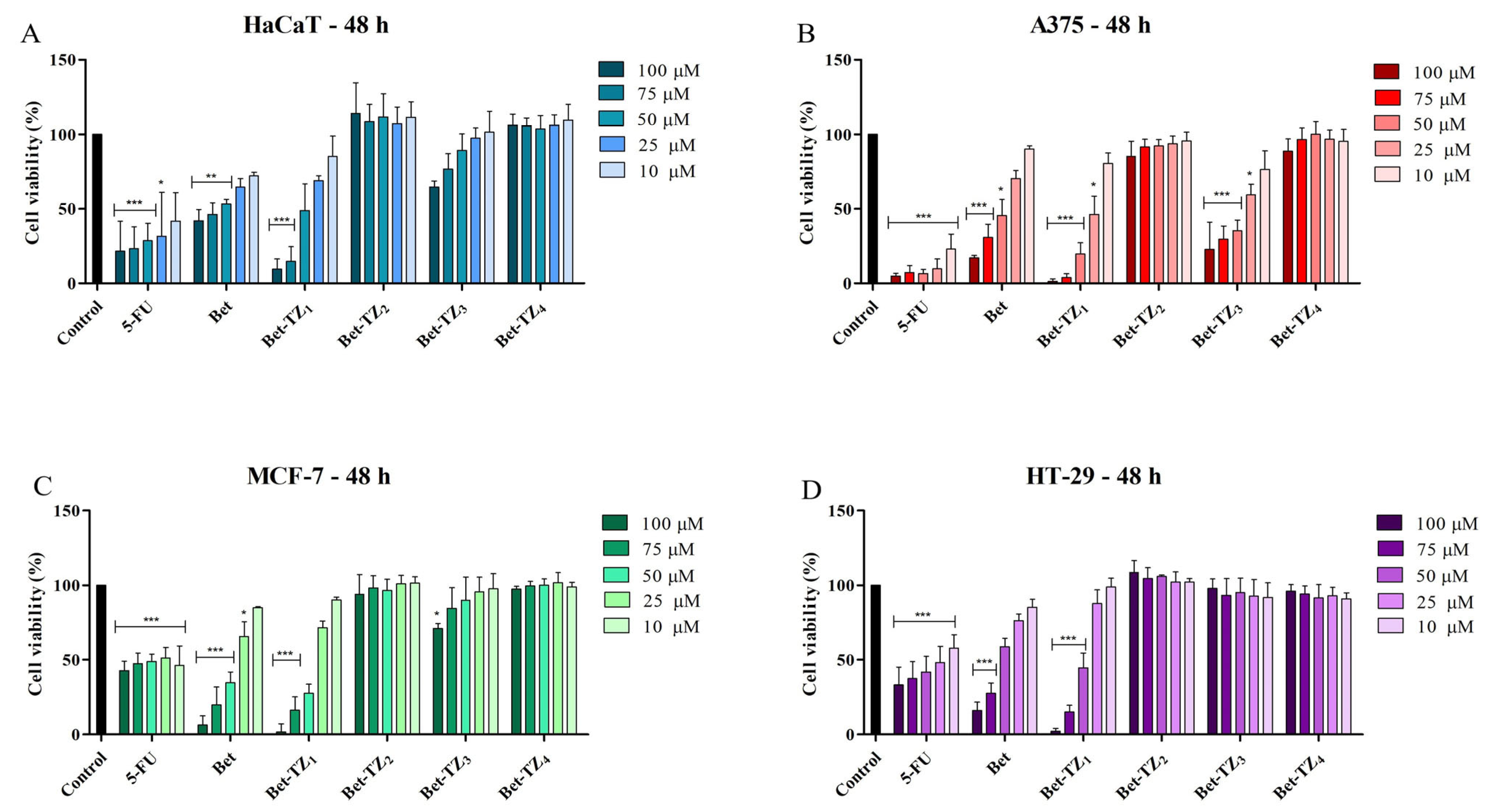
2.2. Evaluation of Diacetylbetulin Derivatives Cytotoxic Effect
2.3. Diacetylbetulin Derivatives Effect on Cell Morphology
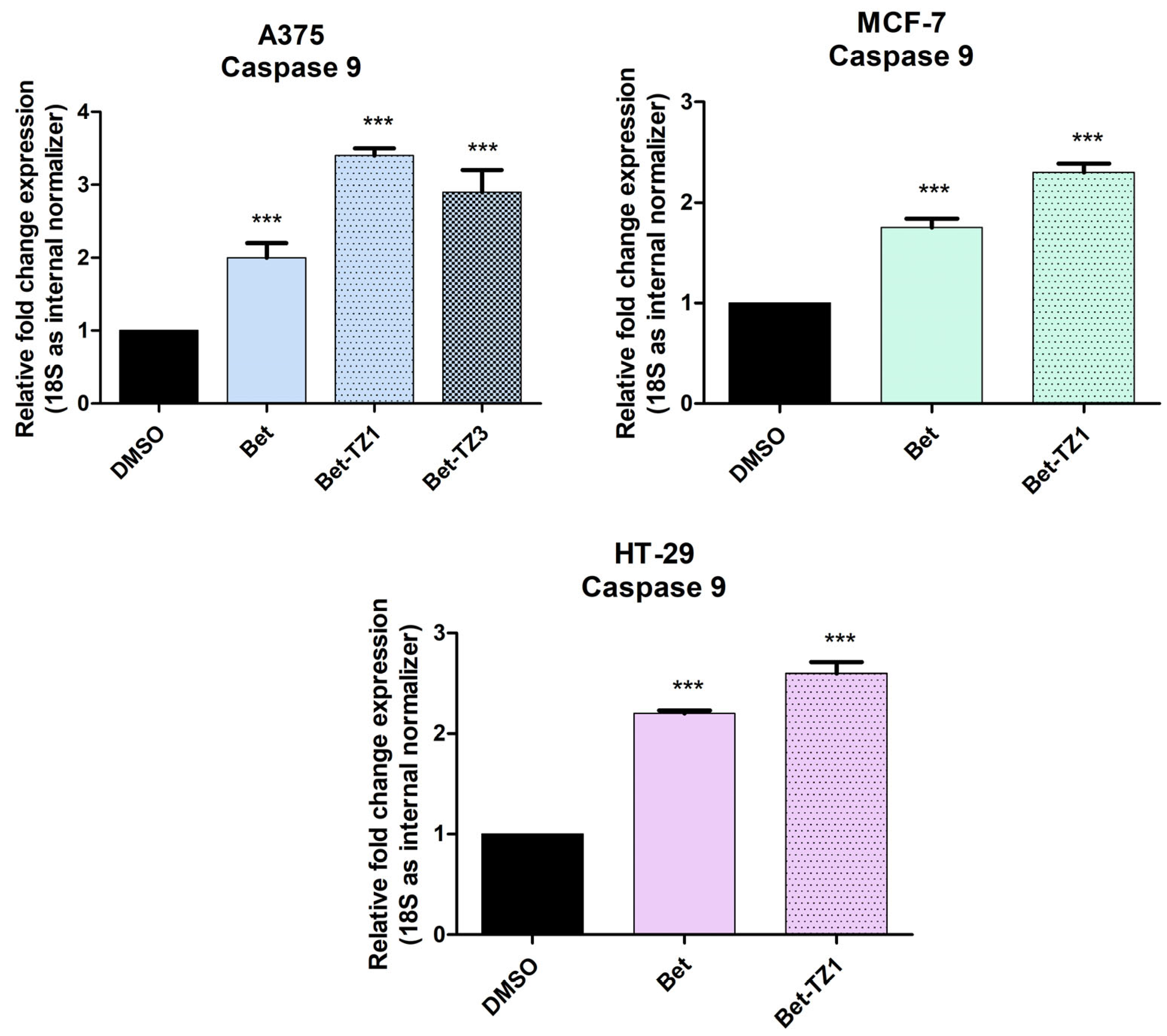
2.4. Real-Time PCR Quantification of Apoptotic Markers
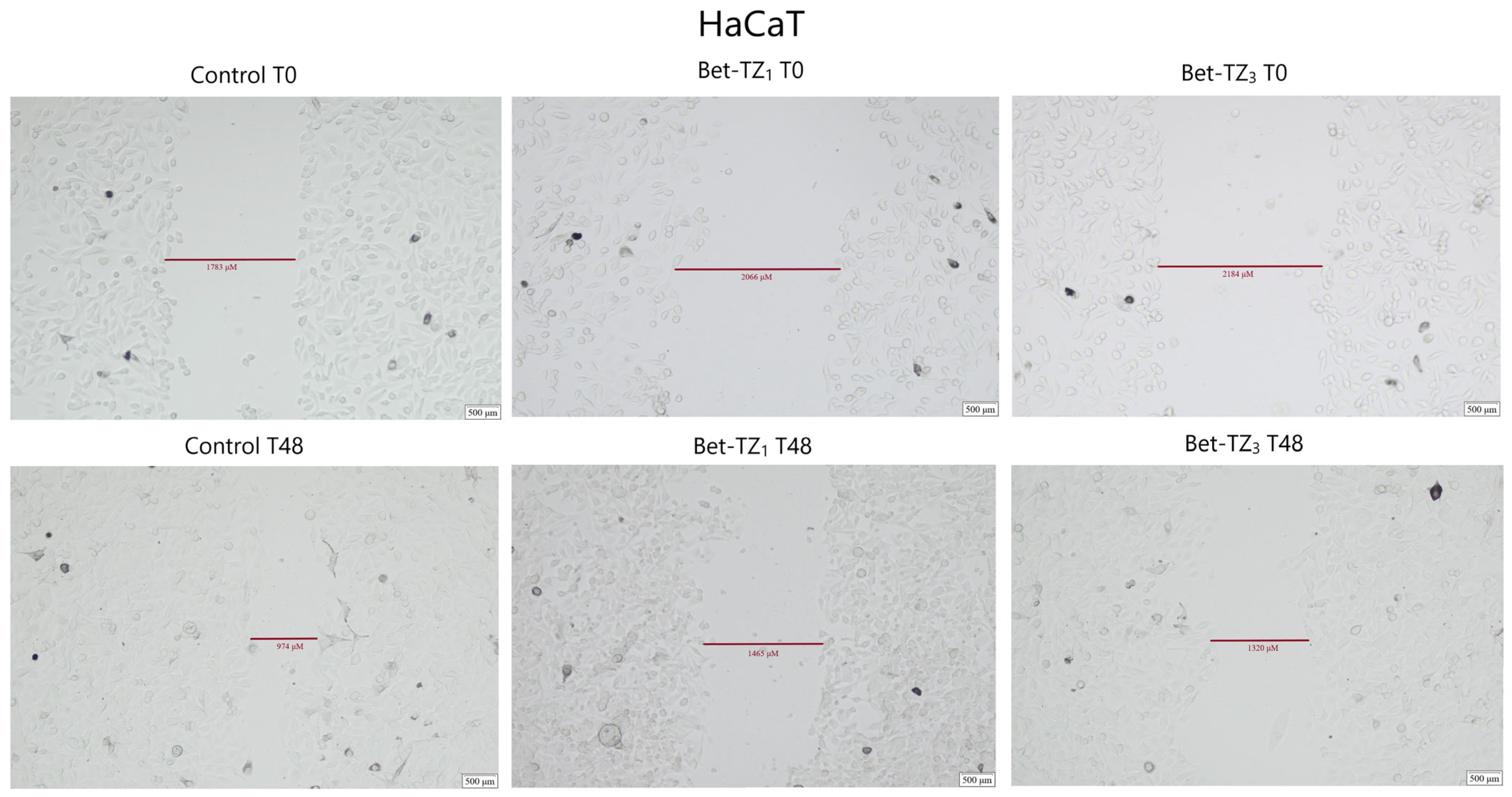
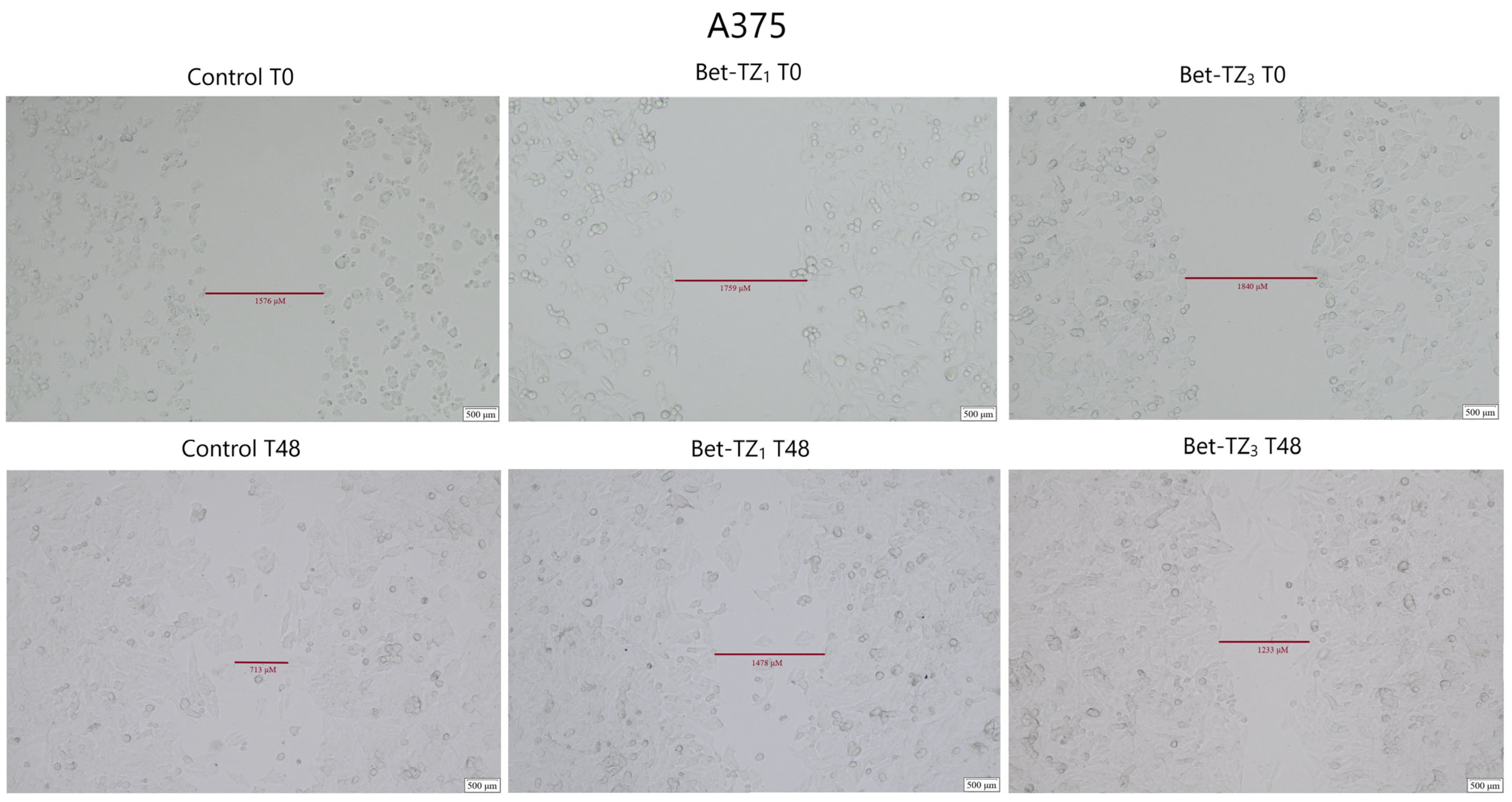
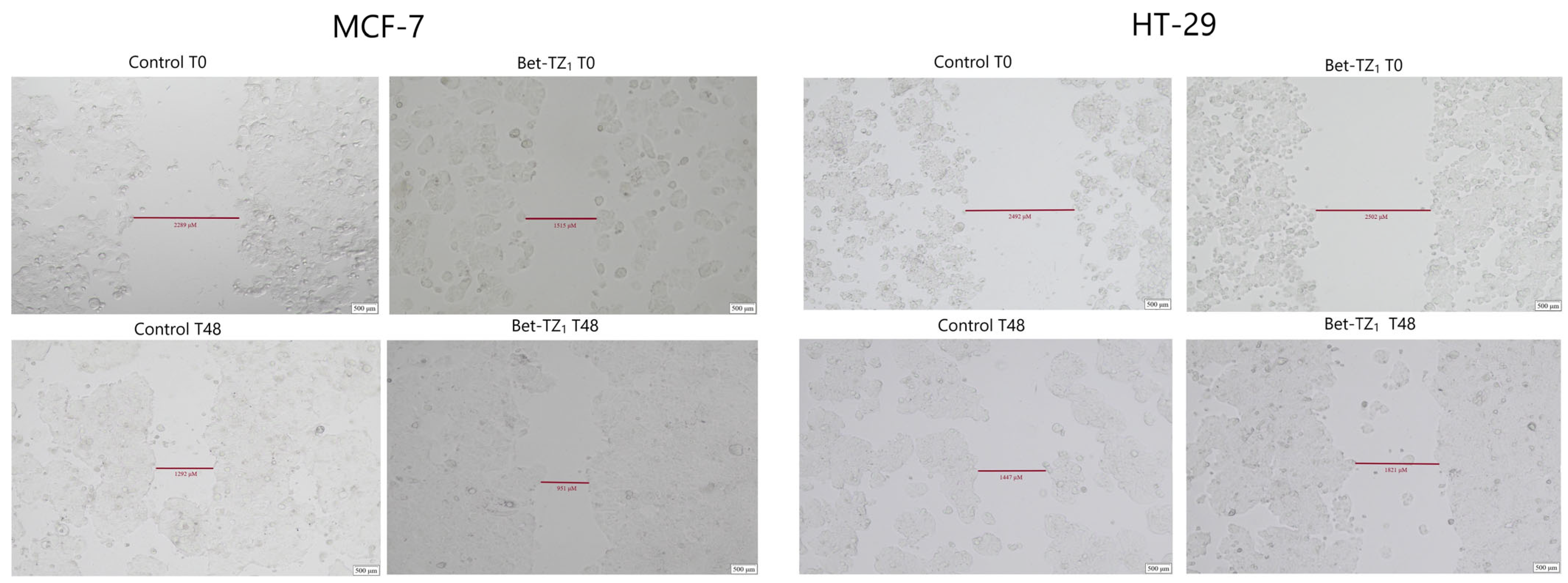
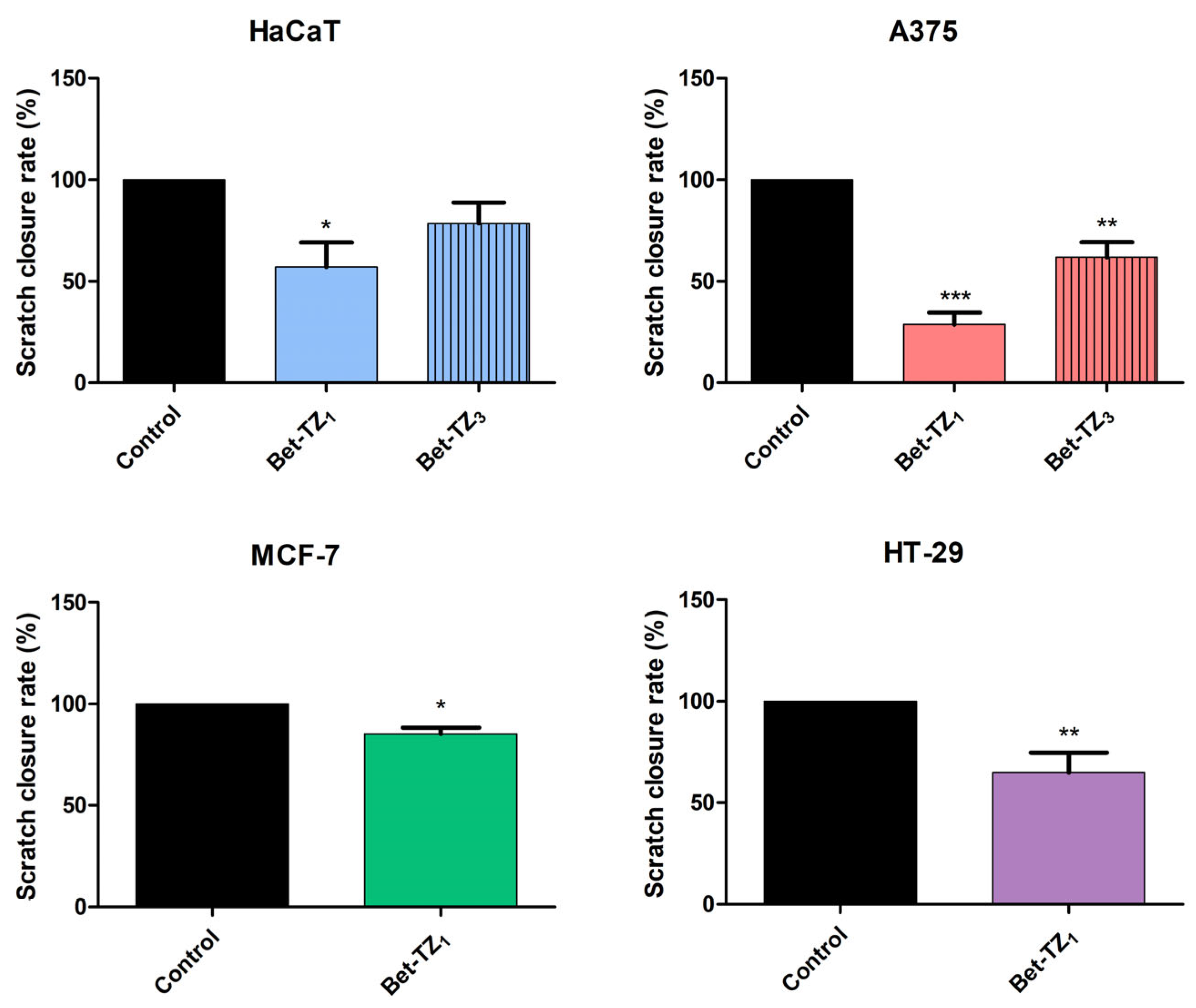
2.5. Scratch Assay
2.6. HET−CAM Test
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Instruments
4.1.2. Synthesis Procedure for Br-Bet
4.1.3. Synthesis Procedure for TZ1
4.1.4. Synthesis Procedure for TZ2-4
4.1.5. Synthesis Procedure for Bet-TZ1-4
4.2. Biological Assessment
4.2.1. Cell Culture
4.2.2. Cell Viability Assessment
4.2.3. Immunofluorescence Assay—Morphological Assessment of Apoptotic Cells
4.2.4. Real-Time PCR Quantification of Apoptotic Markers
4.2.5. Scratch Assay
4.2.6. Statistical Analysis
4.3. HET−CAM Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maitra, U.; Stephen, C.; Ciesla, L.M. Drug discovery from natural products—Old problems and novel solutions for the treatment of neurodegenerative diseases. J. Pharm. Biomed. Anal. 2022, 210, 114553. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Islam, S.U.; Alghamdi, A.A.A.; Kamran, M.; Ahsan, H.; Lee, Y.S. Phytochemicals as Chemo-Preventive Agents and Signaling Molecule Modulators: Current Role in Cancer Therapeutics and Inflammation. Int. J. Mol. Sci. 2022, 23, 15765. [Google Scholar] [CrossRef] [PubMed]
- Andor, B.; Tischer, A.; Berceanu-Vaduva, D.; Lazureanu, V.; Cheveresan, A.; Poenaru, M. Antimicrobial activity and cytotoxic effect on gingival cells of silver nanoparticles obtained by biosynthesis. Rev. Chim. 2019, 70, 781–783. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Demets, O.V.; Takibayeva, A.T.; Kassenov, R.Z.; Aliyeva, M.R. Methods of Betulin Extraction from Birch Bark. Molecules 2022, 27, 3621. [Google Scholar] [CrossRef]
- Özdemir, Z.; Rybková, M.; Vlk, M.; Šaman, D.; Rárová, L.; Wimmer, Z. Synthesis and Pharmacological Effects of Diosgenin–Betulinic Acid Conjugates. Molecules 2020, 25, 3546. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sak, K.; Gupta, D.S.; Kaur, G.; Aggarwal, D.; Chaturvedi Parashar, N.; Choudhary, R.; Yerer, M.B.; Kaur, J.; Kumar, M.; et al. Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments. Plants 2021, 10, 2663. [Google Scholar] [CrossRef]
- John, R.; Dalal, B.; Shankarkumar, A.; Devarajan, P.V. Innovative Betulin Nanosuspension exhibits enhanced anticancer activity in a Triple Negative Breast Cancer Cell line and Zebrafish angiogenesis model. Int. J. Pharm. 2021, 600, 120511. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E.; Boryczka, S. Chromatographic and Computational Screening of Lipophilicity and Pharmacokinetics of Newly Synthesized Betulin-1,4-quinone Hybrids. Processes 2021, 9, 376. [Google Scholar] [CrossRef]
- Grymel, M.; Zawojak, M.; Adamek, J. Triphenylphosphonium Analogues of Betulin and Betulinic Acid with Biological Activity: A Comprehensive Review. J. Nat. Prod. 2019, 82, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Kuczynska, K.; Cmoch, P.; Rárová, L.; Oklešťková, J.; Korda, A.; Pakulski, Z.; Strnad, M. Influence of intramolecular hydrogen bonds on regioselectivity of glycosylation. Synthesis of lupane-type saponins bearing the OSW-1 saponin disaccharide unit and its isomers. Carbohydr. Res. 2016, 423, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Lugiņina, J.; Linden, M.; Bazulis, M.; Kumpiņš, V.; Mishnev, A.; Popov, S.A.; Golubeva, T.S.; Waldvogel, S.R.; Shults, E.E.; Turks, M. Electrosynthesis of Stable Betulin-Derived Nitrile Oxides and their Application in Synthesis of Cytostatic Lupane-Type Triterpenoid-Isoxazole Conjugates. Eur. J. Org. Chem. 2021, 2021, 2557–2577. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Markelova, N.Y.; Penkov, N.V.; Shakurova, E.R.; Belosludtsev, K.N.; Parfenova, L.V. New quaternized pyridinium derivatives of betulin: Synthesis and evaluation of membranotropic properties on liposomes, pro- and eukaryotic cells, and isolated mitochondria. Chem. Biol. Interact. 2021, 349, 109678. [Google Scholar] [CrossRef]
- Grishko, V.V.; Tolmacheva, I.A.; Nebogatikov, V.O.; Galaiko, N.V.; Nazarov, A.V.; Dmitriev, M.V.; Ivshina, I.B. Preparation of novel ring-A fused azole derivatives of betulin and evaluation of their cytotoxicity. Eur. J. Med. Chem. 2017, 125, 629–639. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Strzelecka, M.; Świątek, P. 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals 2021, 14, 224. [Google Scholar] [CrossRef]
- Malik, M.S.; Ahmed, S.A.; Althagafi, I.I.; Ansari, M.A.; Kamal, A. Application of triazoles as bioisosteres and linkers in the development of microtubule targeting agents. RSC Med. Chem. 2020, 11, 327–348. [Google Scholar] [CrossRef]
- Lengerli, D.; Ibis, K.; Nural, Y.; Banoglu, E. The 1,2,3-triazole ‘all-in-one’ ring system in drug discovery: A good bioisostere, a good pharmacophore, a good linker, and a versatile synthetic tool. Expert Opin. Drug Discov. 2022, 17, 1209–1236. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.M.; Matin, P.; Rahman, M.R.; Ben Hadda, T.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and Their Derivatives: Chemistry, Synthesis, and Therapeutic Applications. Front. Mol. Biosci. 2022, 9, 864286. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile. Molecules 2017, 22, 1876. [Google Scholar] [CrossRef] [PubMed]
- Mioc, M.; Soica, C.; Bercean, V.; Avram, S.; Balan-Porcarasu, M.; Coricovac, D.; Ghiulai, R.; Muntean, D.; Andrica, F.; Dehelean, C.; et al. Design, synthesis and pharmaco-toxicological assessment of 5-mercapto-1,2,4-triazole derivatives with antibacterial and antiproliferative activity. Int. J. Oncol. 2017, 50, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.M.C.; Silva, G.N.; da Rocha Pitta, I.; Melo Rêgo, M.J.B.; Gnoato, S.C.B.; da Rocha Pitta, M.G. Novel betulin derivatives inhibit IFN-γ and modulates COX-2 expression. Nat. Prod. Res. 2020, 34, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Bodrikov, I.V.; Kurskii, Y.A.; Chiyanov, A.A.; Subbotin, A.Y. Electrophilic Substitution of Hydrogen in Betulin and Diacetylbetulin. Russ. J. Org. Chem. 2018, 54, 131–138. [Google Scholar] [CrossRef]
- Phalgune, U.D.; Vanka, K.; Rajamohanan, P.R. GIAO/DFT studies on 1,2,4-triazole-5-thiones and their propargyl derivatives. Magn. Reson. Chem. 2013, 51, 767–774. [Google Scholar] [CrossRef]
- Chaudhary, P.M.; Chavan, S.R.; Kavitha, M.; Maybhate, S.P.; Deshpande, S.R.; Likhite, A.P.; Rajamohanan, P.R. Structural elucidation of propargylated products of 3-substituted-1,2,4-triazole-5-thiols by NMR techniques. Magn. Reson. Chem. 2008, 46, 1168–1174. [Google Scholar] [CrossRef]
- Luepke, N.P.; Kemper, F.H. The HET-CAM test: An alternative to the draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Szoka, Ł.; Isidorov, V.; Nazaruk, J.; Stocki, M.; Siergiejczyk, L. Cytotoxicity of Triterpene Seco-Acids from Betula pubescens Buds. Molecules 2019, 24, 4060. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brahmbhatt, J.G.; Pandya, P.A.; Daraji, D.G.; Patel, H.D.; Rawal, R.M.; Baran, S.K. Design, synthesis and biological evaluation of novel 5-(4-chlorophenyl)-4-phenyl-4H-1,2,4-triazole-3-thiols as an anticancer agent. J. Mol. Struct. 2021, 1231, 130000. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Marciniec, K.; Chrobak, E.; Bębenek, E.; Boryczka, S. Lipophilicity, Pharmacokinetic Properties, and Molecular Docking Study on SARS-CoV-2 Target for Betulin Triazole Derivatives with Attached 1,4-Quinone. Pharmaceutics 2021, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Sidova, V.; Zoufaly, P.; Pokorny, J.; Dzubak, P.; Hajduch, M.; Popa, I.; Urban, M. Cytotoxic conjugates of betulinic acid and substituted triazoles prepared by Huisgen Cycloaddition from 30-azidoderivatives. PLoS ONE 2017, 12, e0171621. [Google Scholar] [CrossRef] [PubMed]
- Dangroo, N.A.; Singh, J.; Rath, S.K.; Gupta, N.; Qayum, A.; Singh, S.; Sangwan, P.L. A convergent synthesis of novel alkyne–azide cycloaddition congeners of betulinic acid as potent cytotoxic agent. Steroids 2017, 123, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kuczynska, K.; Bończak, B.; Rárová, L.; Kvasnicová, M.; Strnad, M.; Pakulski, Z.; Cmoch, P.; Fiałkowski, M. Synthesis and cytotoxic activity of 1,2,3-triazoles derived from 2,3-seco-dihydrobetulin via a click chemistry approach. J. Mol. Struct. 2022, 1250, 131751. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Liebman, J.F. The annular tautomerism of imidazoles and pyrazoles: The possible existence of nonaromatic forms. Struct. Chem. 2006, 17, 439–444. [Google Scholar] [CrossRef]
- Claramunt, R.M.; López, C.; Angeles García, M.; Dolores Otero, M.; Rosario Torres, M.; Pinilla, E.; Alarcón, S.H.; Alkorta, I.; Elguero, J. The structure of halogeno-1,2,4-triazoles in the solid state and in solution. New J. Chem. 2001, 25, 1061–1068. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Hall, C.D.; El-Gendy, B.E.-D.M.; Draghici, B. Tautomerism in drug discovery. J. Comput. Aided. Mol. Des. 2010, 24, 475–484. [Google Scholar] [CrossRef]
- Larina, L.I. Tautomerism and Structure of Azoles. Adv. Heterocycl. Chem. 2018, 124, 233–321. [Google Scholar]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [PubMed]
- Shi, W.; Tang, N.; Yan, W.-D. Synthesis and cytotoxicity of triterpenoids derived from betulin and betulinic acid via click chemistry. J. Asian Nat. Prod. Res. 2015, 17, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, W.; Zhang, B.; Li, C.; Zhang, X.; Wang, Q.; Wang, Y.; Zhou, Q.; Li, X.; Shen, X.L. Central role of TRAP1 in the ameliorative effect of oleanolic acid on the mitochondrial-mediated and endoplasmic reticulum stress-excitated apoptosis induced by ochratoxin A. Toxicology 2021, 450, 152681. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Dutta, D.; Mukherjee, S.; Das, S.; Maiti, N.C.; Das, P.; Chowdhury, C. Synthesis and biological evaluation of a novel betulinic acid derivative as an inducer of apoptosis in human colon carcinoma cells (HT-29). Eur. J. Med. Chem. 2015, 102, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective assessment of changes in nuclear morphology and cell distribution following induction of apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Nistor, G.; Mioc, M.; Mioc, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Prodea, A.; Milan, A.; Ghiulai, R.; Semenescu, A.; Dehelean, C.; et al. The C30-Modulation of Betulinic Acid Using 1,2,4-Triazole: A Promising Strategy for Increasing Its Antimelanoma Cytotoxic Potential. Molecules 2022, 27, 7807. [Google Scholar] [CrossRef] [PubMed]
- Nistor, G.; Mioc, A.; Mioc, M.; Balan-Porcarasu, M.; Ghiulai, R.; Racoviceanu, R.; Avram, Ș.; Prodea, A.; Semenescu, A.; Milan, A.; et al. Novel Semisynthetic Betulinic Acid−Triazole Hybrids with In Vitro Antiproliferative Potential. Processes 2022, 11, 101. [Google Scholar] [CrossRef]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Juszczak, M.; Grabarska, A.; Sifringer, M.; Kaczor, J.; Kandefer-Szerszeń, M. Betulin Elicits Anti-Cancer Effects in Tumour Primary Cultures and Cell Lines In Vitro. Basic Clin. Pharmacol. Toxicol. 2009, 105, 425–432. [Google Scholar] [CrossRef]
- Pfarr, K.; Danciu, C.; Arlt, O.; Neske, C.; Dehelean, C.; Pfeilschifter, J.M.; Radeke, H.H. Simultaneous and Dose Dependent Melanoma Cytotoxic and Immune Stimulatory Activity of Betulin. PLoS ONE 2015, 10, e0118802. [Google Scholar] [CrossRef]
- Zehra, B.; Ahmed, A.; Sarwar, R.; Khan, A.; Farooq, U.; Abid Ali, S.; Al-Harrasi, A. Apoptotic and antimetastatic activities of betulin isolated from Quercus incana against non-small cell lung cancer cells. Cancer Manag. Res. 2019, 11, 1667–1683. [Google Scholar] [CrossRef]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.-G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the Cytochrome c–initiated Caspase Cascade: Hierarchical Activation of Caspases-2, -3, -6, -7, -8, and -10 in a Caspase-9–dependent Manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Tian, T. MCF-7 cells lack the expression of Caspase-3. Int. J. Biol. Macromol. 2023, 231, 123310. [Google Scholar] [CrossRef] [PubMed]
- Orchel, A.; Chodurek, E.; Jaworska-Kik, M.; Paduszyński, P.; Kaps, A.; Chrobak, E.; Bębenek, E.; Boryczka, S.; Borkowska, P.; Kasperczyk, J. Anticancer Activity of the Acetylenic Derivative of Betulin Phosphate Involves Induction of Necrotic-Like Death in Breast Cancer Cells In Vitro. Molecules 2021, 26, 615. [Google Scholar] [CrossRef] [PubMed]
- Pęcak, P.; Świtalska, M.; Chrobak, E.; Boryczka, G.; Bębenek, E. Betulin Acid Ester Derivatives Inhibit Cancer Cell Growth by Inducing Apoptosis through Caspase Cascade Activation: A Comprehensive In Vitro and In Silico Study. Int. J. Mol. Sci. 2022, 24, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Xiao, M.; Lin, H.; Luo, J.; Wang, T. Novel betulin derivative induces anti-proliferative activity by G2/M phase cell cycle arrest and apoptosis in Huh7 cells. Oncol. Lett. 2018, 15, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Entschladen, F.; Drell, T.L.; Lang, K.; Joseph, J.; Zaenker, K.S. Tumour-cell migration, invasion, and metastasis: Navigation by neurotransmitters. Lancet Oncol. 2004, 5, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Härmä, V.; Haavikko, R.; Virtanen, J.; Ahonen, I.; Schukov, H.-P.; Alakurtti, S.; Purev, E.; Rischer, H.; Yli-Kauhaluoma, J.; Moreira, V.M.; et al. Optimization of Invasion-Specific Effects of Betulin Derivatives on Prostate Cancer Cells through Lead Development. PLoS ONE 2015, 10, e0126111. [Google Scholar] [CrossRef]
- Bache, M.; Bernhardt, S.; Passin, S.; Wichmann, H.; Hein, A.; Zschornak, M.P.; Kappler, M.; Taubert, H.; Paschke, R.; Vordermark, D. Betulinic Acid Derivatives NVX-207 and B10 for Treatment of Glioblastoma—An in Vitro Study of Cytotoxicity and Radiosensitization. Int. J. Mol. Sci. 2014, 15, 19777–19790. [Google Scholar] [CrossRef]
- Winter, G.; Koch, A.B.F.; Löffler, J.; Jelezko, F.; Lindén, M.; Li, H.; Abaei, A.; Zuo, Z.; Beer, A.J.; Rasche, V. In vivo PET/MRI Imaging of the Chorioallantoic Membrane. Front. Phys. 2020, 8, 151. [Google Scholar] [CrossRef]
- de Araujo Lowndes Viera, L.M.; Silva, R.S.; da Silva, C.C.; Presgrave, O.A.F.; Boas, M.H.S.V. Comparison of the different protocols of the Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM) by evaluating the eye irritation potential of surfactants. Toxicol. Vitr. 2022, 78, 105255. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Creţu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef] [PubMed]
- Frew, Q.; Rennekampff, H.-O.; Dziewulski, P.; Moiemen, N.; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, I.A.; Shelepen’kina, L.N.; Vikharev, Y.B.; Anikina, L.V.; Grishko, V.V.; Tolstikov, A.G. Synthesis and biological activity of S-containing betulin derivatives. Chem. Nat. Compd. 2005, 41, 701–705. [Google Scholar] [CrossRef]
- Uzenkova, N.V.; Petrenko, N.I.; Shakirov, M.M.; Shul’ts, E.E.; Tolstikov, G.A. Synthesis of 30-amino derivatives of lupane triterpenoids. Chem. Nat. Compd. 2005, 41, 692–700. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Wei, X.; Wu, X.; Chen, G.; Cao, G.; Shen, X.; Zhang, X.; Tang, Q.; Liang, G.; et al. Synthesis and biological evaluation of novel thiazolidinone derivatives as potential anti-inflammatory agents. Eur. J. Med. Chem. 2013, 64, 292–301. [Google Scholar] [CrossRef]
- Ainsworth, C. 1,2,4-TRIAZOLE. Org. Synth. 1960, 40, 99. [Google Scholar] [CrossRef]
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). ICCVAM-Recommended Test Method Protocol: Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Test Method; National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 2010.
- Maghiari, A.L.; Coricovac, D.; Pinzaru, I.A.; Macașoi, I.G.; Marcovici, I.; Simu, S.; Navolan, D.; Dehelean, C. High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells. Nutrients 2020, 12, 3600. [Google Scholar] [CrossRef]
- Guercio, B.J.; Zhang, S.; Niedzwiecki, D.; Li, Y.; Babic, A.; Morales-Oyarvide, V.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; et al. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance). PLoS ONE 2018, 13, e0199244. [Google Scholar] [CrossRef]

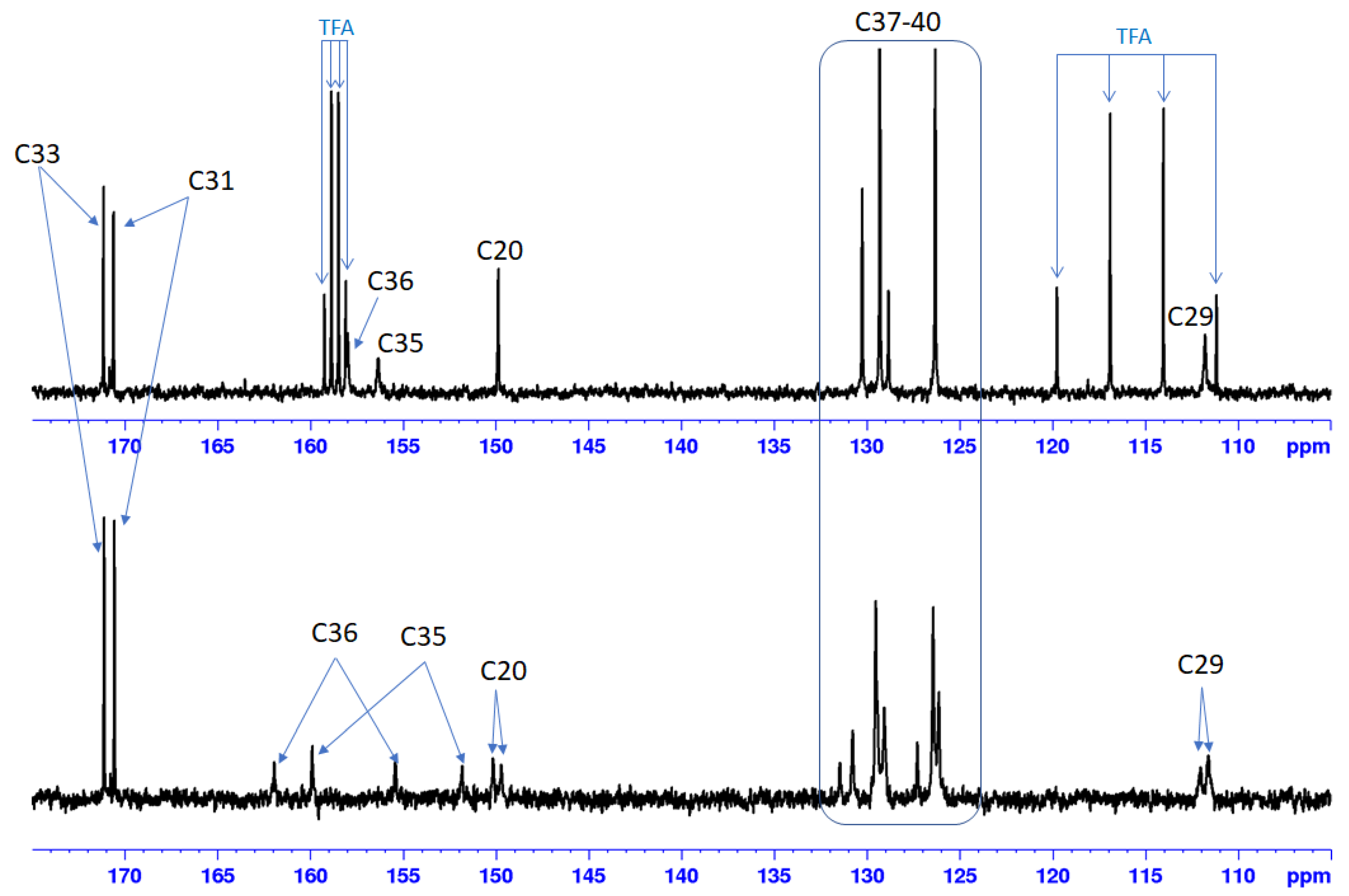





| Compound | Bet-TZ1 | Bet-TZ2 | Bet-TZ3 | Bet-TZ4 |
|---|---|---|---|---|
| Tautomer percentage | 25% | 19% | 41% | 26% |
| 75% | 81% | 59% | 74% |
| 5-FU | Bet | Bet-TZ1 | Bet-TZ3 | |
|---|---|---|---|---|
| 48 h | 48 h | 48 h | 48 h | |
| HaCaT | 15.25 | 60.75 | 42.52 | >100 |
| A375 | 1.06 | 46.19 | 22.41 | 34.34 |
| MCF-7 | 38.01 | 37.29 | 33.52 | >100 |
| HT-29 | 29.80 | 55.67 | 46.92 | >100 |
| Samples | IF | Effect |
|---|---|---|
| H2O | 0 | No irritation |
| SDS | 16.29 ± 0.23 | Severe irritation |
| Bet | 0 | No irritation |
| Bet-TZ1 | 0 | No irritation |
| Bet-TZ2 | 0 | No irritation |
| Bet-TZ3 | 0 | No irritation |
| Bet-TZ4 | 0 | No irritation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodea, A.; Milan, A.; Mioc, M.; Mioc, A.; Oprean, C.; Racoviceanu, R.; Negrea-Ghiulai, R.; Mardale, G.; Avram, Ș.; Balan-Porcărașu, M.; et al. Novel Betulin-1,2,4-Triazole Derivatives Promote In Vitro Dose-Dependent Anticancer Cytotoxicity. Processes 2024, 12, 24. https://doi.org/10.3390/pr12010024
Prodea A, Milan A, Mioc M, Mioc A, Oprean C, Racoviceanu R, Negrea-Ghiulai R, Mardale G, Avram Ș, Balan-Porcărașu M, et al. Novel Betulin-1,2,4-Triazole Derivatives Promote In Vitro Dose-Dependent Anticancer Cytotoxicity. Processes. 2024; 12(1):24. https://doi.org/10.3390/pr12010024
Chicago/Turabian StyleProdea, Alexandra, Andreea Milan, Marius Mioc, Alexandra Mioc, Camelia Oprean, Roxana Racoviceanu, Roxana Negrea-Ghiulai, Gabriel Mardale, Ștefana Avram, Mihaela Balan-Porcărașu, and et al. 2024. "Novel Betulin-1,2,4-Triazole Derivatives Promote In Vitro Dose-Dependent Anticancer Cytotoxicity" Processes 12, no. 1: 24. https://doi.org/10.3390/pr12010024





.jpg)

