Catalysts Based on Nanoscale Iron and Cobalt Immobilized on Polymers for Catalytic Oxidation of Aromatic Hydrocarbons: Synthesis, Physico-Chemical Studies, and Tests of Catalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
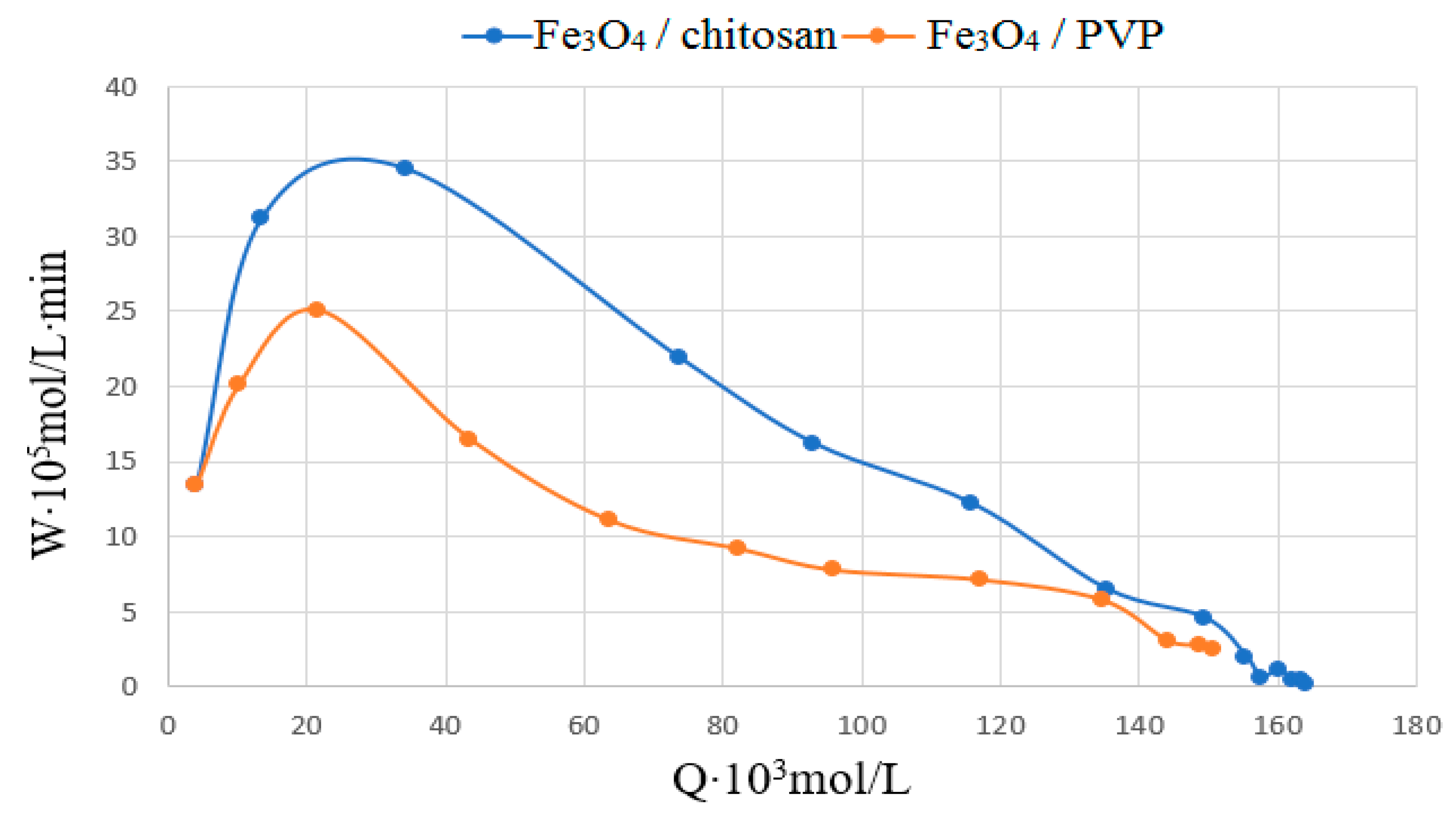
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Portnyagina, N. What Problems Are Experienced by the Kazakhstan Oil Industry and How Can One Get Rid of Them? 19 April 2022. Available online: https://kazpravda.kz/n/kakie-problemy-ispytyvaet-kazahstanskaya-neftyanka-i-kak-mozhno-ot-nih-izbavitsya/ (accessed on 1 July 2023).
- Amaniyazova, G.D. Problems and Prospects for the Use of Hydrocarbon Resources in Kazakhstan. Bull of KazEU. 2012. Available online: https://articlekz.com/article/13950 (accessed on 1 July 2023).
- Datsko, T.; Zelentsov, V.; Dvornikov, D. Advanced Nanotechnology-Based Approaches to Waste Water Purification from Organic Pollutants. IFMBE Proc. 2024, 91, 134–146. [Google Scholar]
- Naĭden, E.P.; Zhuravlev, V.A.; Itin, V.I.; Terekhova, O.G.; Magaeva, A.A.; Ivanov, Y.F. Magnetic properties and structural parameters of nanosized oxide ferrimagnet powders produced by mechanochemical synthesis from salt solutions. Phys. Solid State 2008, 50, 894–900. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Fernández, C.D.J.; Sangregorio, C. Exploring the Magnetic Properties of Cobalt-Ferrite Nanoparticles for the Development of a Rare-Earth-Free Permanent Magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Chithra, M.; Anumol, C.N.; Argish, V.; Sahu, B.N.; Sahoo, S.C. Magnetic properties of co-ferrite nanoparticles prepared by co-precipitation method. J. Mater. Sci. Mater. Electron. 2023, 34, 806. [Google Scholar] [CrossRef]
- Nguyen, T.K.C.; Nguyen, A.T. Structural, optical and magnetic properties of Y-doped CoFe2O4 nanoparticles prepared by a simple co-precipitation method. J. Mater. Sci. Mater. Electron. 2023, 34, 448. [Google Scholar] [CrossRef]
- Kakati, S.; Rendale, M.K.; Mathad, S.N. Synthesis, Characterization, and Applications of CoFe2O4 and M-CoFe2O4 (M = Ni, Zn, Mg, Cd, Cu, RE) Ferrites: A Review. Int. J. Self-Propagating High-Temp. Synth. 2021, 30, 189–2019. [Google Scholar] [CrossRef]
- Cabot, A.; Puntes, V.F.; Shevchenko, E.; Yin, Y.; Balcells, L.; Marcus, M.A.; Hughes, S.M.; Alivisatos, A.P. Vacancy Coalescence during Oxidation of Iron Nanoparticles. J. Am. Chem. Soc. 2007, 129, 10358–10360. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Siva, K.V.; Kumar, A.; Arockiarajan, A. Structural, magnetic and magnetoelectric investigations on CoFe2O4 prepared via various wet chemical synthesis route: A Comparative Study. J. Magn. Magn. Mater. 2021, 535, 168065. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Sashko, N.; Vaitulevich, E.; Yurmazova, T. Synthesis and Properties of Iron-Based Magnetic Nanoparticles. Key Eng. Mater. 2016, 712, 282–287. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Mmelesi, O.K.; Masunga, N.; Kuvarega, A.; Nkambule, T.T.; Mamba, B.B.; Kefeni, K.K. Cobalt ferrite nanoparticles and nanocomposites: Photocatalytic, antimicrobial activity and toxicity in water treatment. Mater. Sci. Semicond. 2021, 123, 105523. [Google Scholar] [CrossRef]
- Sassykova, L.R.; Dossumova, B.T.; Ilmuratova, M.S.; Shakiyeva, T.V.; Baizhomartov, B.B.; Sassykova, A.R.; Zhaxibayeva, Z.M.; Abildin, T.S. Development of nanostructured catalysts for catalytic oxidative water purification from organic impurities, including phenolic compounds. Chim. Tech. Acta 2023, 10, 202310309. [Google Scholar] [CrossRef]
- Alaerts, L.; Wahlen, J.; Jacobs, P.A.; De Vos, D.E. Recent progress in the immobilization of catalysts for selective oxidation in the liquid phase. Chem. Commun. 2008, 15, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Usov, N.A. Expert Opinion: Magnetic Nanoparticles: Theory and Modern Technological Applications. 5 February 2016. Available online: https://habr.com/ru/companies/misis/articles/390127/ (accessed on 1 July 2023).
- Zhang, W.X. Nanoscale iron particles for environmental remediation: An overview. J. Nanopart. Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Yi, D.K.; Lee, S.S.; Ying, J.Y. Synthesis and applications of magnetic nanocomposite catalysts. Chem. Mater. 2006, 18, 2459–2461. [Google Scholar] [CrossRef]
- Taghipour, S.; Hosseini, S.M.; Ataie-Ashtiani, B. Engineering nanomaterials for water and wastewater treatment: Review of classifications, properties and applications. New J. Chem. 2019, 43, 7902–7927. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Stepanov, G.V. Effect of a Low-Frequency Magnetic Field on the Release of Heat by Magnetic Nanoparticles of Different Shapes. Russ. J. Phys. Chem. 2020, 94, 439–444. [Google Scholar] [CrossRef]
- Bolm, C.; Legros, J.; Le Paih, J.; Zani, L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 2004, 104, 6217–6254. [Google Scholar] [CrossRef] [PubMed]
- Chekanova, A.E.; Sorkina, T.A.; Dubov, A.L.; Nikiforov, V.N.; Davydova, G.A.; Selezneva, I.I.; Goodilin, E.A.; Trusov, L.A.; Korolev, V.V.; Aref’ev, I.M.; et al. New environmental nontoxic agents for the preparation of core-shell magnetic nanoparticles. Mendeleev Commun. 2009, 19, 72–74. [Google Scholar] [CrossRef]
- Sassykova, L.R.; Shakiyeva, T.V.; Ilmuratova, M.S.; Muktaly, D.; Zhaxibayeva, Z.M.; Sassykova, A.R.; Baizhomartov, B. Catalysts, magnetic composites for removal of phenol-containing compounds from wastewater. Rasayan J. Chem. 2023, 16, 1605–1612. [Google Scholar] [CrossRef]
- Koksharov, Y.A.; Gubin, S.P.; Taranov, I.V.; Khomutov, G.B.; Gulyaev, Y.V. Magnetic Nanoparticles in Medicine: Progress, Problems, and Advances. J. Commun. Technol. Electron. 2022, 67, 101–116. [Google Scholar] [CrossRef]
- Baranov, D.A.; Gubin, S.P. Magnetic nanoparticles: Advances and problems of chemical synthesis. Radioelektron. Nanosistemy Inf. Tehnol. 2009, 1, 129–147. [Google Scholar]
- Kritika, R.I. Therapeutic applications of magnetic nanoparticles: Recent advances. Mater. Adv. 2022, 3, 7425–7444. [Google Scholar] [CrossRef]
- Tashmukhambetova, Z.K.; Sassykova, L.R.; Aubakirov, Y.A.; Dangaliyeva, A.K.; Kanatbayeva, M.A.; Rustem, A.E. New catalysts for toluene oxidation technology in the liquid phase. Mater. Today Proc. 2020, 31, 529–553. [Google Scholar] [CrossRef]
- Habibi, D.; Faraji, A.R.; Arshadi, M.; Veisi, H.; Gil, A. Manganese nanocatalyst and N-hydroxyphthalimide as an efficient catalytic system for selective oxidation of ethylbenzene, cyclohexene and oximes under aerobic condition. J. Mol. Catal. A Chem. 2014, 382, 41–54. [Google Scholar] [CrossRef]
- Mohammadi-Samani, S.; Miri, R.; Salmanpour, M.; Khalighian, N.; Sotoudeh, S.; Erfani, N. Preparation and assessment of chitosan-coated superparamagnetic Fe3O4 nanoparticles for controlled delivery of methotrexate. Res. Pharm. Sci. 2013, 8, 25–33. [Google Scholar]
- Iorio, E.D.; Colombo, C.; Cheng, Z.; Capitani, G.; Mele, D.; Ventruti, G.; Angelico, R. Characterization of magnetite nanoparticles synthetized from Fe(II)/nitrate solutions for arsenic removal from water. J. Environ. Chem. Eng. 2019, 7, 102986. [Google Scholar] [CrossRef]
- Singh, A.N.; Singh, S.; Suthar, N.; Dubey, V.K. Glutaraldehyde-Activated Chitosan Matrix for Immobilization of a Novel Cysteine Protease, Procerain B. J. Agric. Food Chem. 2011, 59, 6256–6262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Zhu, A.; Yuan, L.; Dai, S. Preparation of Well-Dispersed Superparamagnetic Iron Oxide Nanoparticles in Aqueous Solution with Biocompatible N-Succinyl-O-carboxymethylchitosan. J. Phys. Chem. C 2008, 112, 5432–5438. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Kuznetsov, N.T.; Meshalkin, V.P.; Salerno, M.; Fabiano, B. Systematical analysis of chemical methods in metal nanoparticles synthesis. Theor. Found. Chem. Eng. 2016, 50, 59–66. [Google Scholar] [CrossRef]
- Reverberi, A.; Salerno, M.; Lauciello, S.; Fabiano, B. Synthesis of Copper Nanoparticles in Ethylene Glycol by Chemical Reduction with Vanadium (+2) Salts. Materials 2016, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Magnetic Properties of nanophase cobalt particles synthesized in inversed micelles. J. Appl. Phys. 1994, 76, 6316–6318. [Google Scholar] [CrossRef]
- Chen, K.; Bakuzis, A.F.; Luo, W. Improving surfactant grafting in magnetic colloids. Appl. Surf. Sci. 2006, 252, 6379–6382. [Google Scholar] [CrossRef]
- Pileni, M.P. Reverse micelles: A microreactor. J. Phys. Chem. 1993, 97, 9661–9668. [Google Scholar] [CrossRef]
- Pileni, M.P. Magnetic Fluids: Fabrication, Magnetic Properties, and Organization of Nanocrystals. Adv. Funct. Mater. 2001, 11, 323–336. [Google Scholar] [CrossRef]
- Pileni, M.P. The Role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nat. Mater. 2003, 2, 145–150. [Google Scholar] [CrossRef]
- Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials 2023, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Dossumova, B.T.; Shakiyeva, T.V.; Muktaly, D.; Sassykova, L.R.; Baizhomartov, B.B.; Subramanian, S. Synthesis, Characterization of Magnetic Composites and Testing of Their Activity in Liquid-Phase Oxidation of Phenol with Oxygen. ChemEngineering 2022, 6, 68. [Google Scholar] [CrossRef]
- Narasimharao, K.; Ali, T.T.; Abu-Zied, B.M.; Alfaifi, S.Y. Combustion synthesis of nanocrystalline porous CoFexAl2-xO4 spinels: Structural, textural, magnetic, and electrical properties. Ceram. Int. 2023, 49, 13238–13248. [Google Scholar] [CrossRef]
- Sijo, A.K. Magnetic and structural properties of CoCrxFe2−xO4 spinels prepared by solution self combustion method. Ceram. Int. 2017, 43, 2288–2290. [Google Scholar] [CrossRef]
- Bantz, C.; Koshkina, O.; Lang, T.; Galla, H.-J.; Kirkpatrick, C.J.; Stauber, R.H.; Maskos, M. The surface properties of nanoparticles determine the agglomeration state and the size of the particles under physiological conditions. Beilstein J. Nanotechnol. 2014, 5, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Aluker, N.L.; Lavrentieva, A.L.; Suzdaltseva, Y.M. Direct Optical Research Methods in the Analytics of Phenol. Opt. Spectrosc. 2020, 128, 422–428. [Google Scholar] [CrossRef]
- Vorob’eva, T.V.; Terletskaya, A.V.; Kushchevskaya, N.F. Standardized and unified methods for determining phenols in natural and drinking waters and main trends of their development. J. Water Chem. Technol. 2007, 29, 203–213. [Google Scholar] [CrossRef]
- Li, H.; Wang, N.; Li, H.; Ren, Z.; Ma, W.; Li, J.; Du, Y.; Xu, Q. Polyvinylpyrrolidone-induced size-dependent catalytic behavior of Fe sites on N-doped carbon substrate and mechanism conversion in Fenton-like oxidation reaction. Appl. Catal. B 2024, 341, 123323. [Google Scholar] [CrossRef]
- Sapir, L.; Stanley, C.B.; Harries, D. Properties of Polyvinylpyrrolidone in a Deep Eutectic Solvent. J. Phys. Chem. A 2016, 120, 3253–3259. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Application in Inorganic Chemistry; John Willey & Sons: Hoboken, NJ, USA, 2008; pp. 240–295. [Google Scholar]
- Ryberg, R. Infrared spectroscopy of adsorbed molecules: Some experimental aspects. J. Phys. Colloq. 1983, 44, C10-421–C10-427. [Google Scholar] [CrossRef]
- Semko, L.S.; Storozhuk, L.P.; Khutornoi, S.V.; Abramov, N.V.; Gorbik, P.P. Template synthesis, structure, and properties of magnetically controlled, large surface area Fe3O4/TiO2 adsorbents. Inorg. Mater. 2015, 51, 430–435. [Google Scholar] [CrossRef]
- Paukshtis, E.A.; Yurchenko, E.N. Study of the Acid–Base Properties of Heterogeneous Catalysts by Infrared Spectroscopy. Russ. Chem. Rev. 1983, 52, 242–258. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Smirnov, K.S.; Rzhevskij, A.M.; Mardilovich, P.P. Infrared spectroscopic evidence for the structural OH groups of spinel alumina modifications. Mater. Chem. Phys. 1990, 26, 35–46. [Google Scholar] [CrossRef]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces; John Wiley & Sons: Hoboken, NJ, USA, 2003; 690p, ISBN 978-0-471-98731-4. [Google Scholar]
- Lázár, K.; Nimz, M.; Lietz, G.; Guczi, L. Formation of PdFe alloys on silica supported catalysts. Hyperfine Interact. 1988, 41, 657–660. [Google Scholar] [CrossRef]
- Balcı, S.; Tomul, F. Catalytic wet peroxide oxidation of phenol through mesoporous silica-pillared clays supported iron and/or titanium incorporated catalysts. J. Environ. Manag. 2023, 326 Pt B, 116835. [Google Scholar] [CrossRef]
- Zambrzycki, C.; Shao, R.; Misra, A.; Streb, C.; Herr, U.; Güttel, R. Iron based core-shell structures as versatile materials: Magnetic support and solid catalyst. Catalysts 2021, 11, 72. [Google Scholar] [CrossRef]
- Tomita, K.; Oshima, Y. Stability of manganese oxide in catalytic supercritical water oxidation of phenol. Ind. Eng. Chem. Res. 2004, 43, 7740–7743. [Google Scholar] [CrossRef]

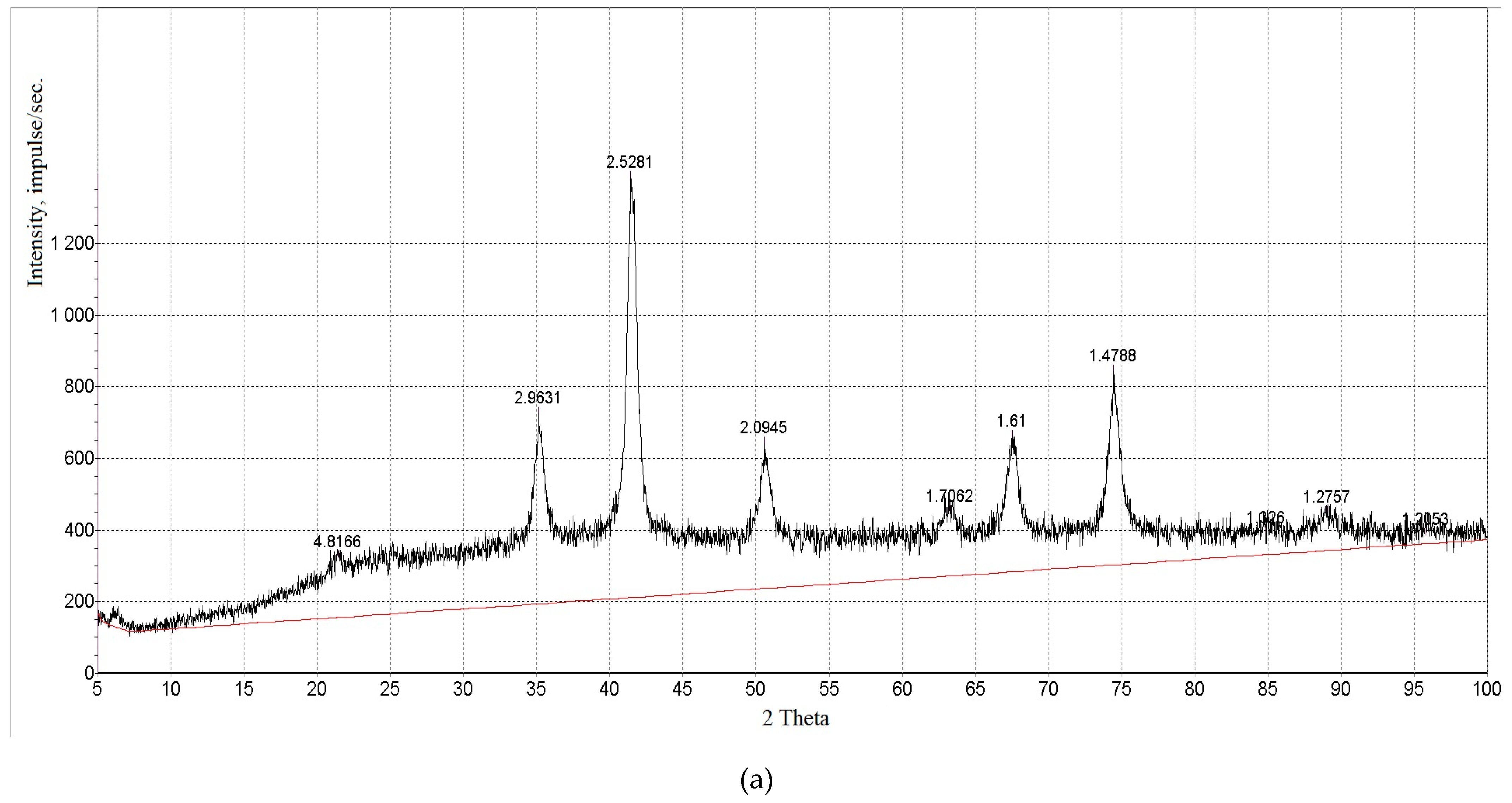

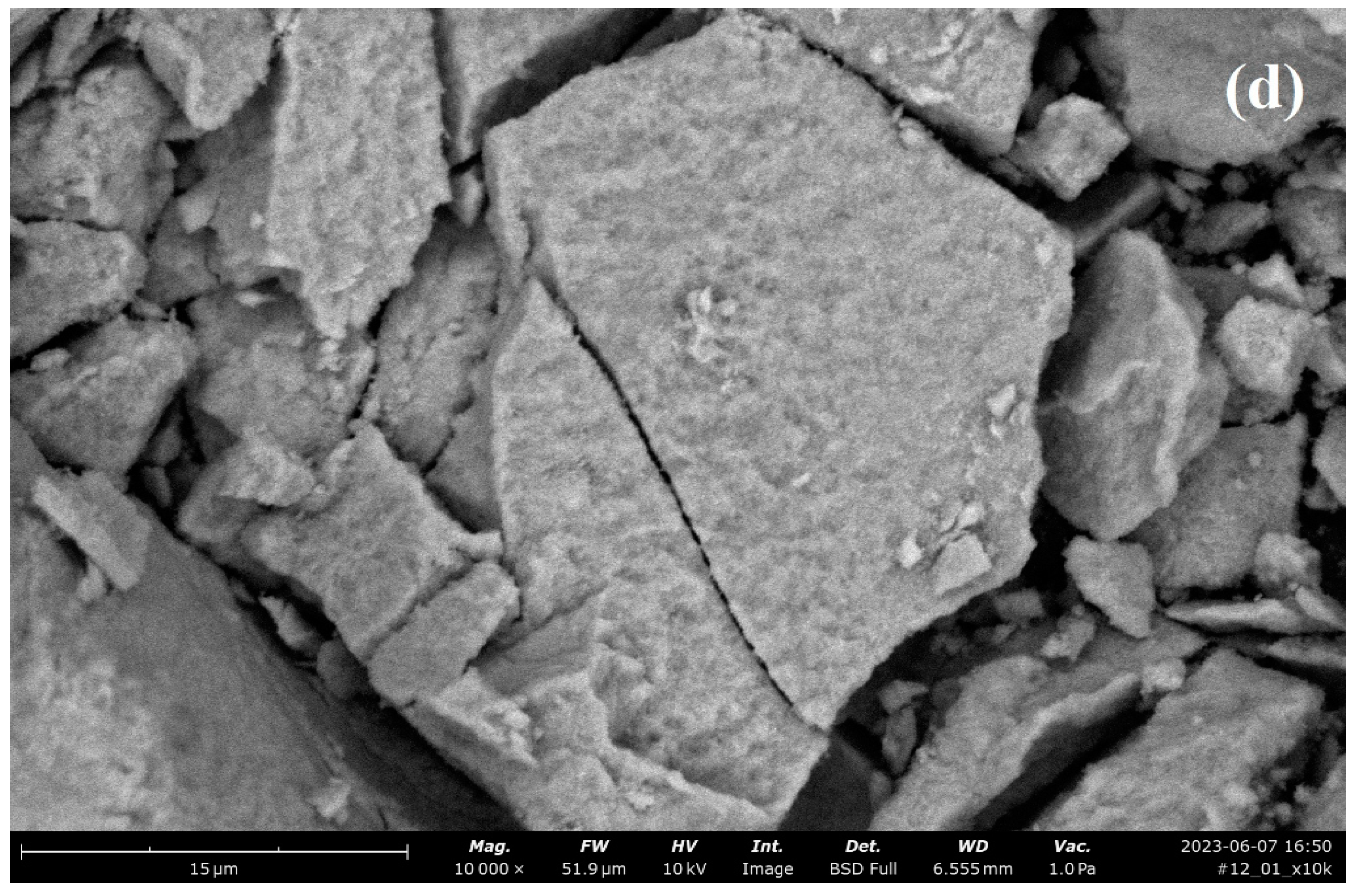

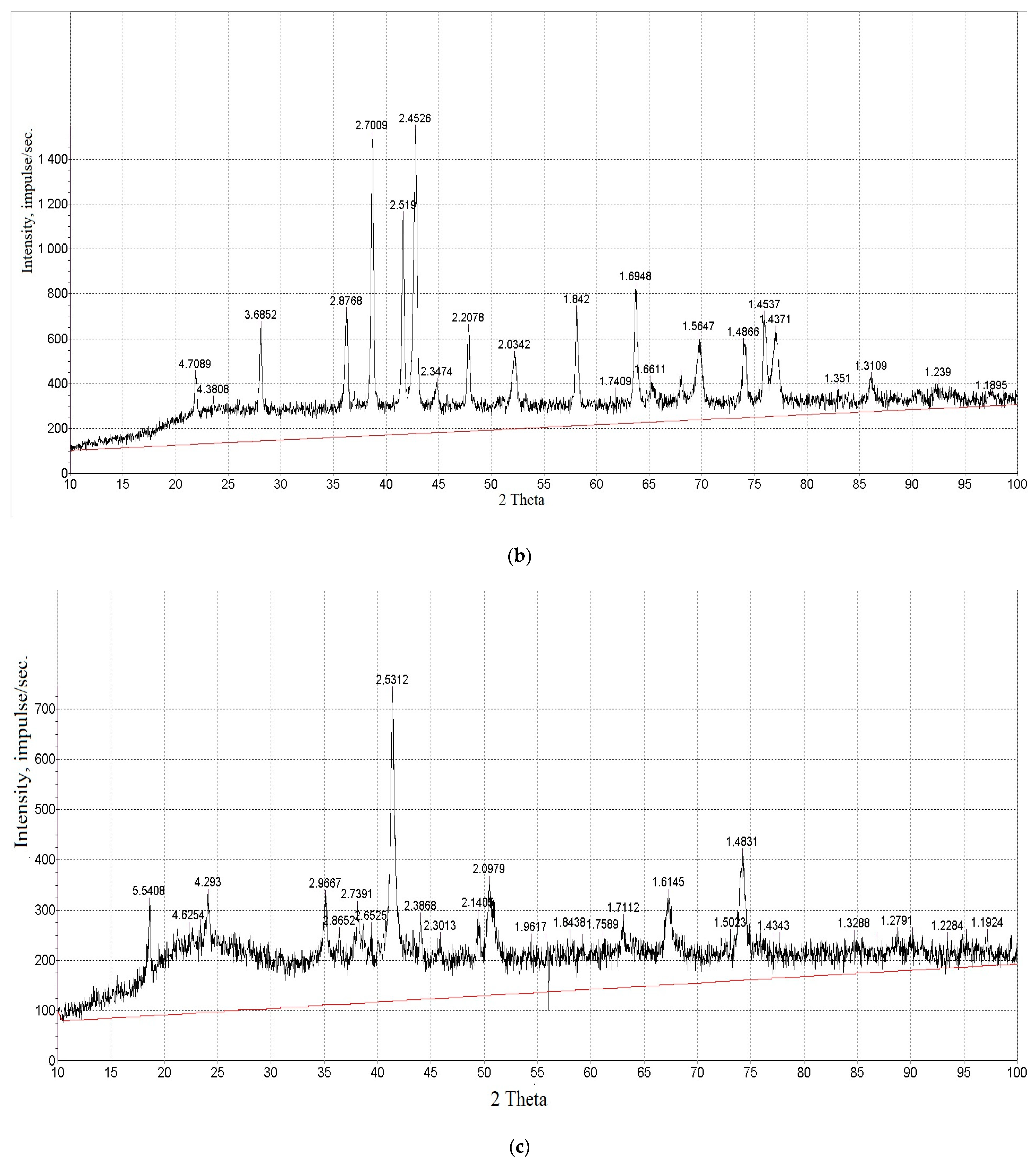
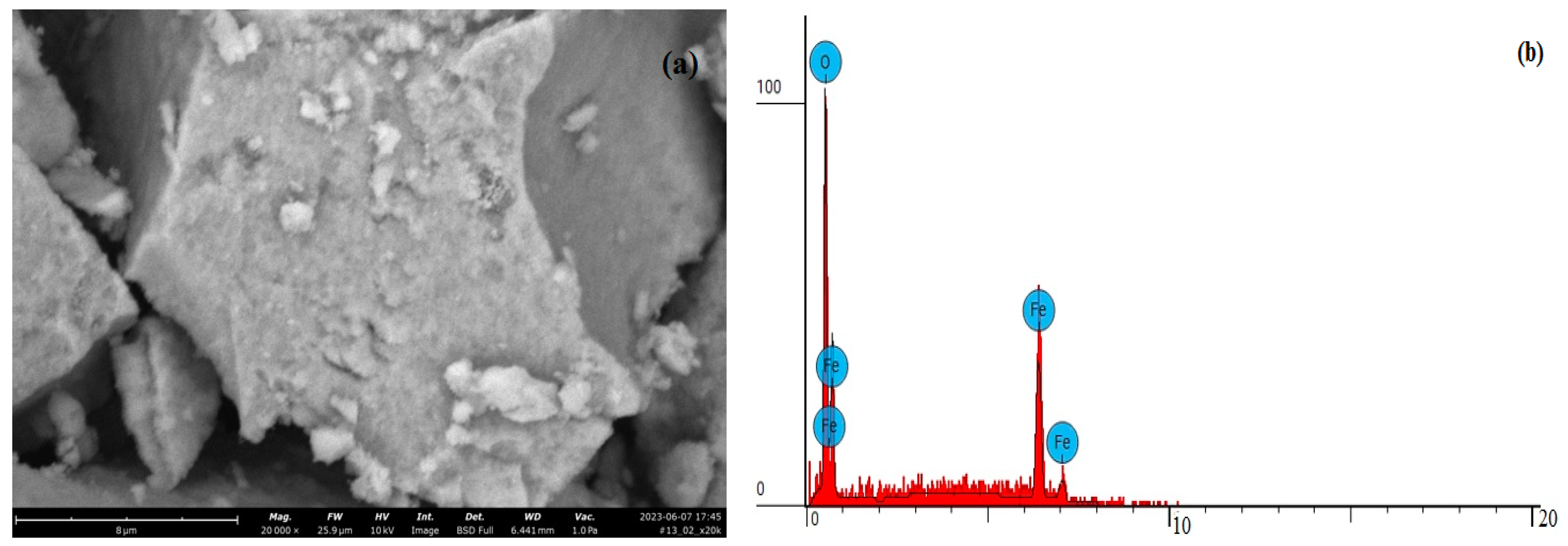
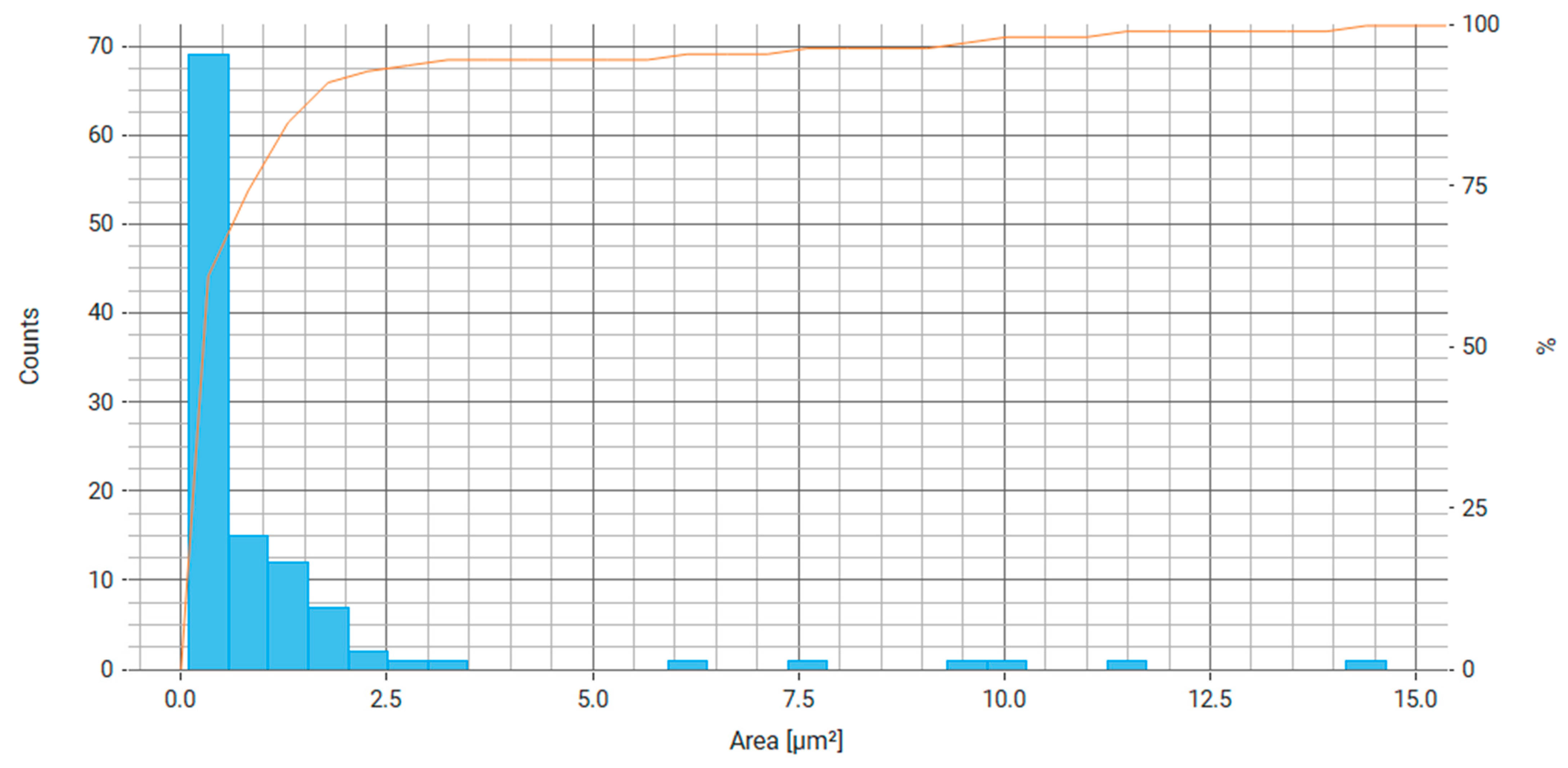
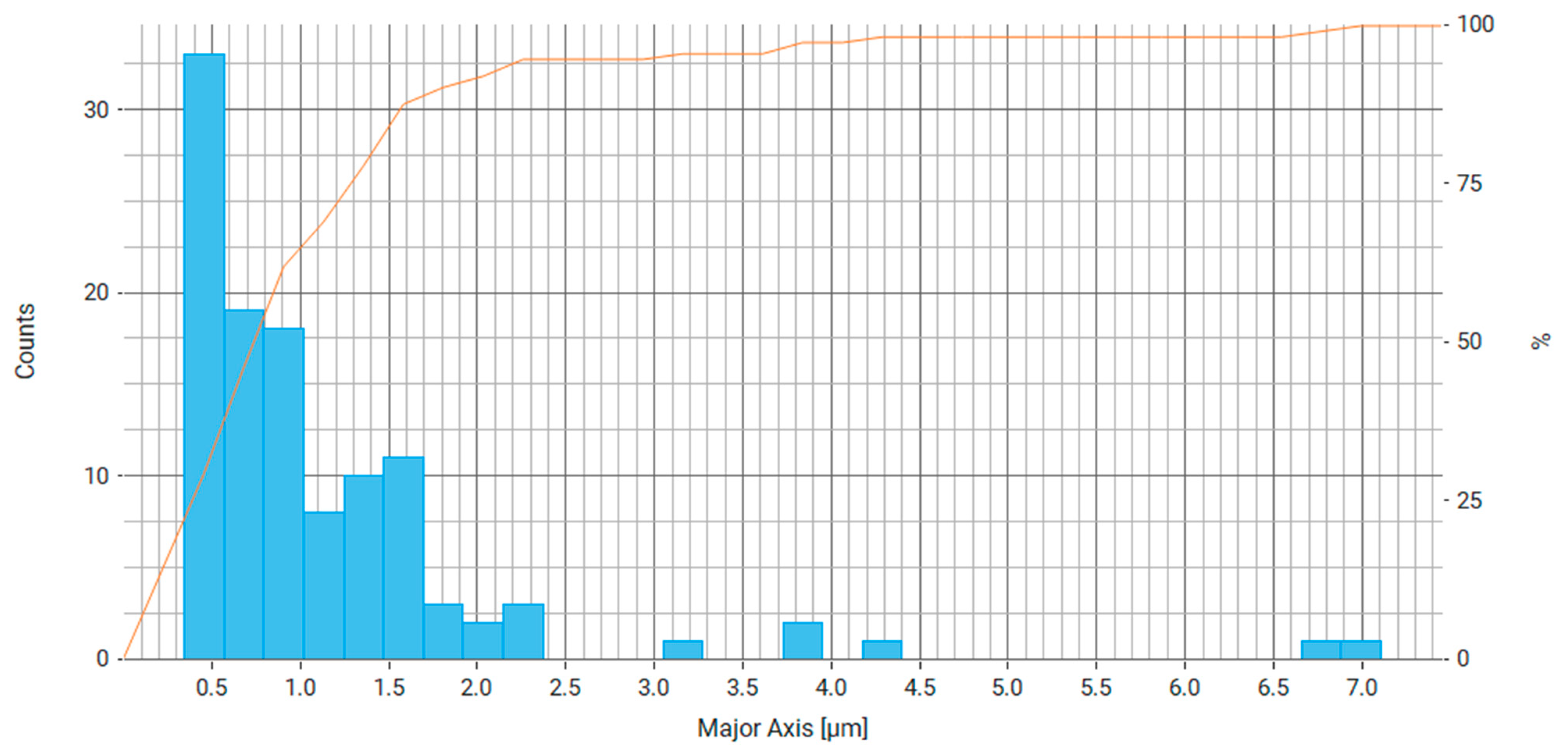

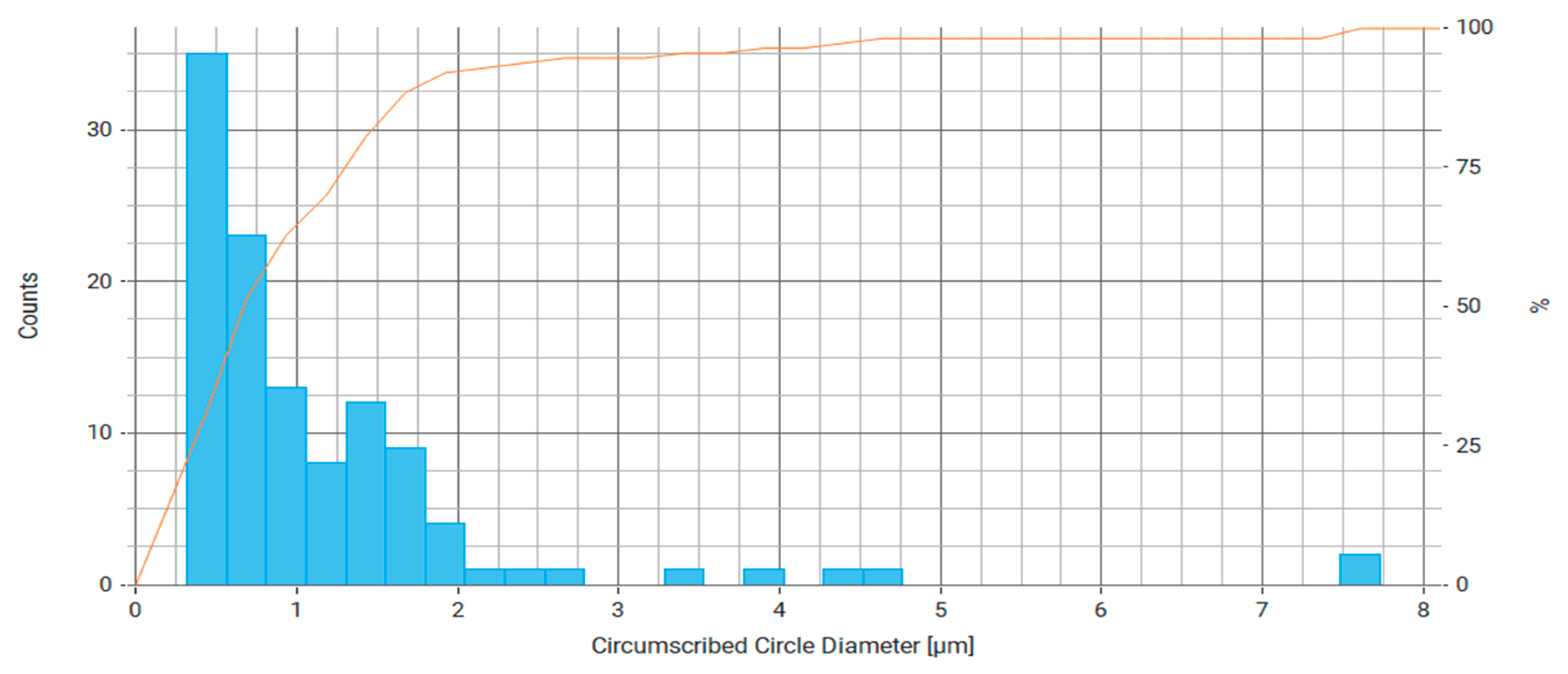
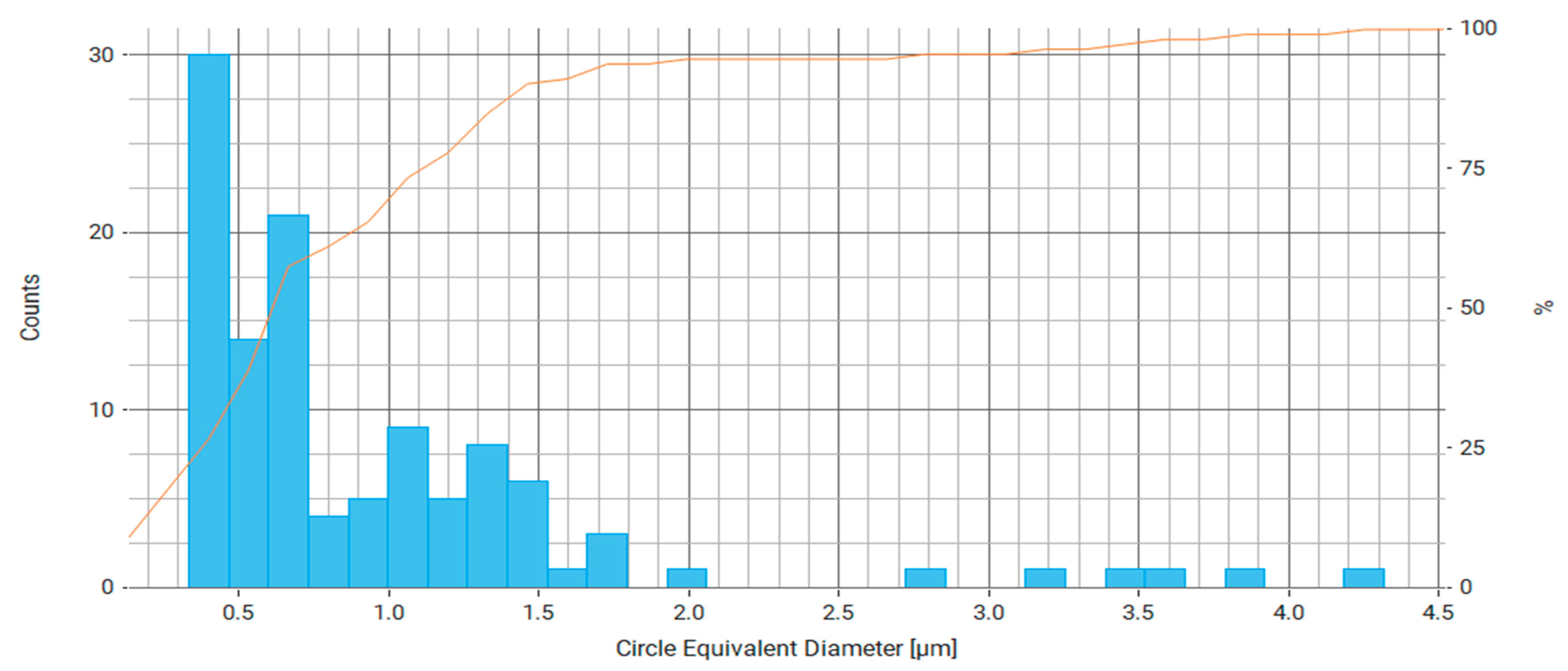
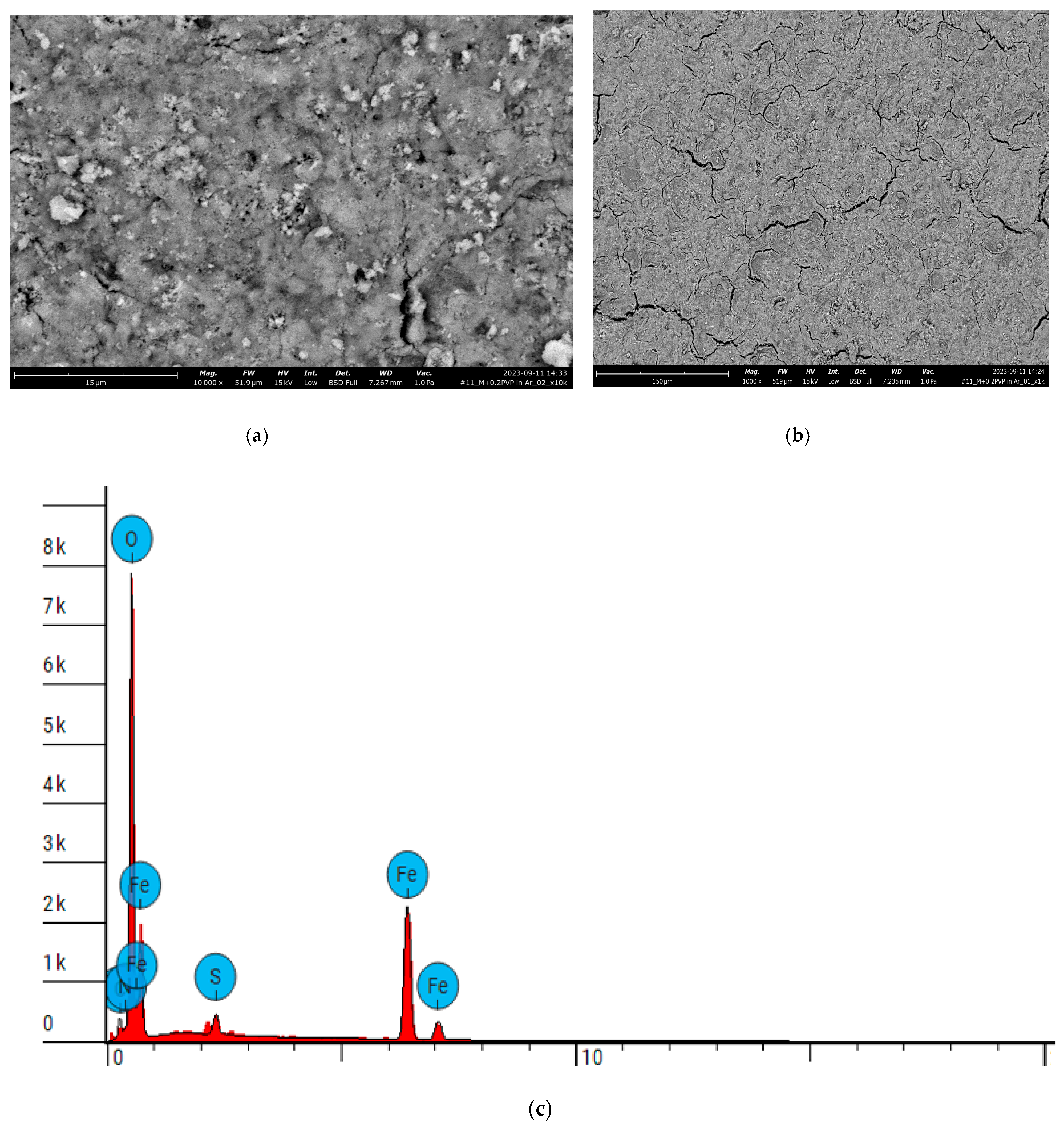


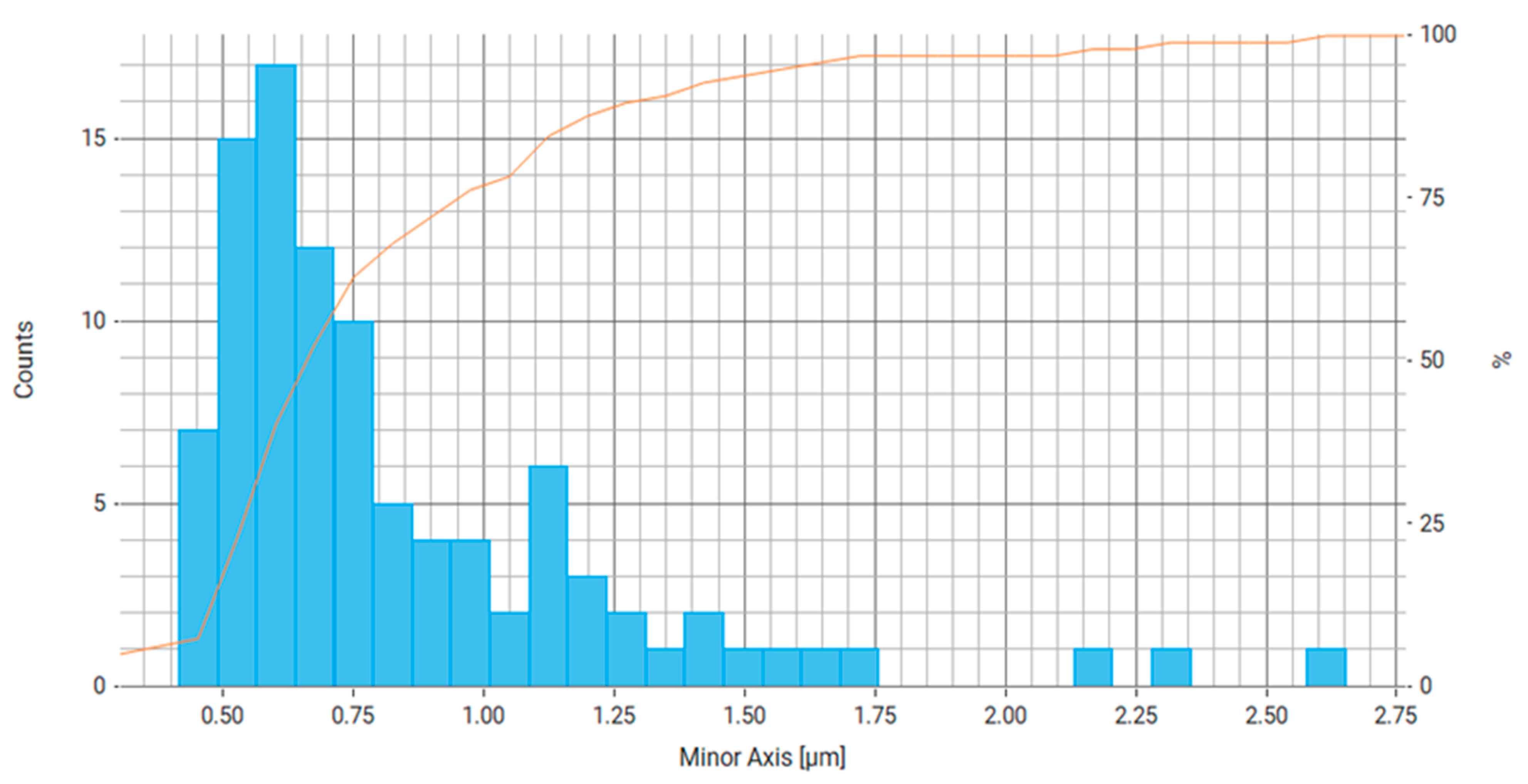
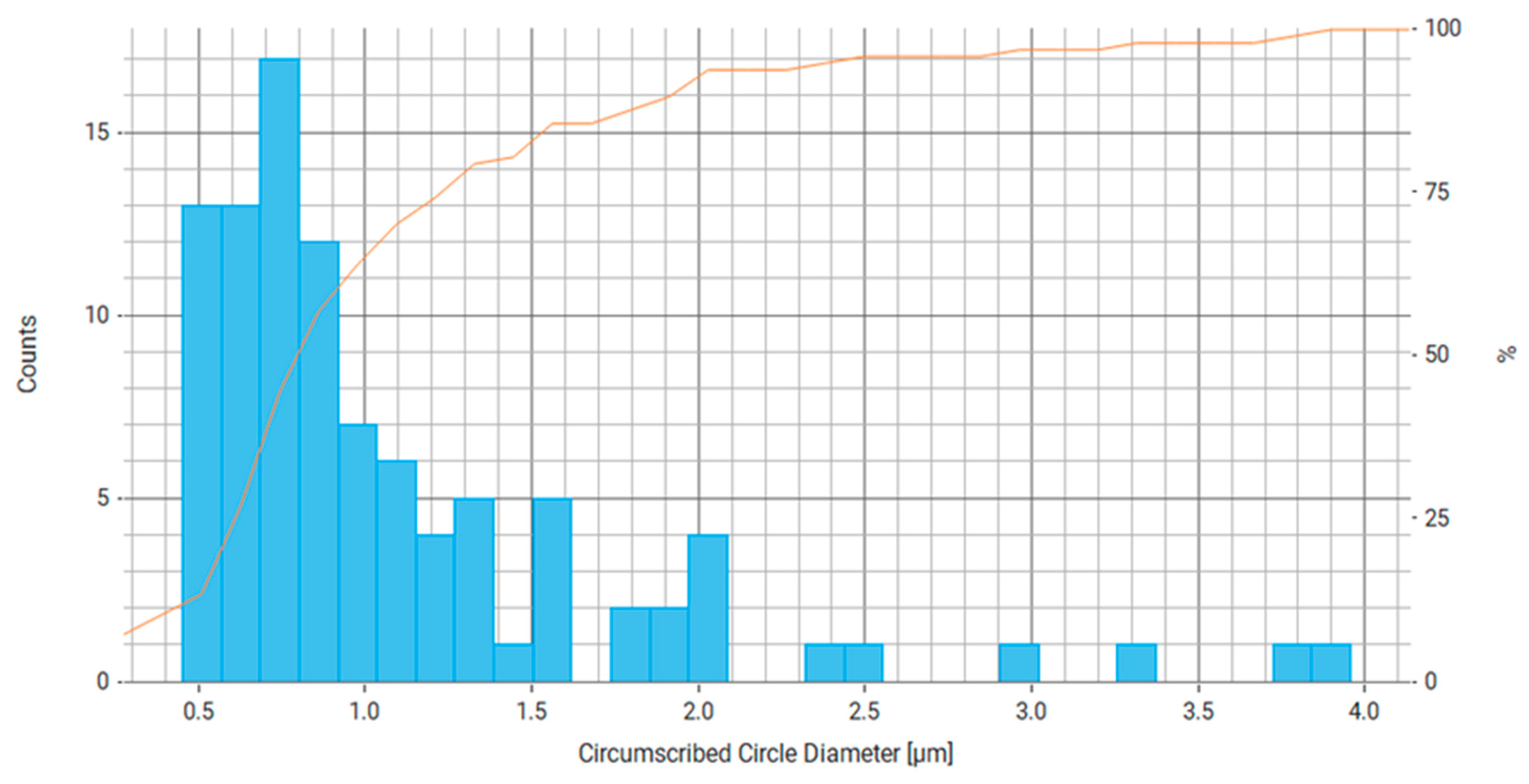
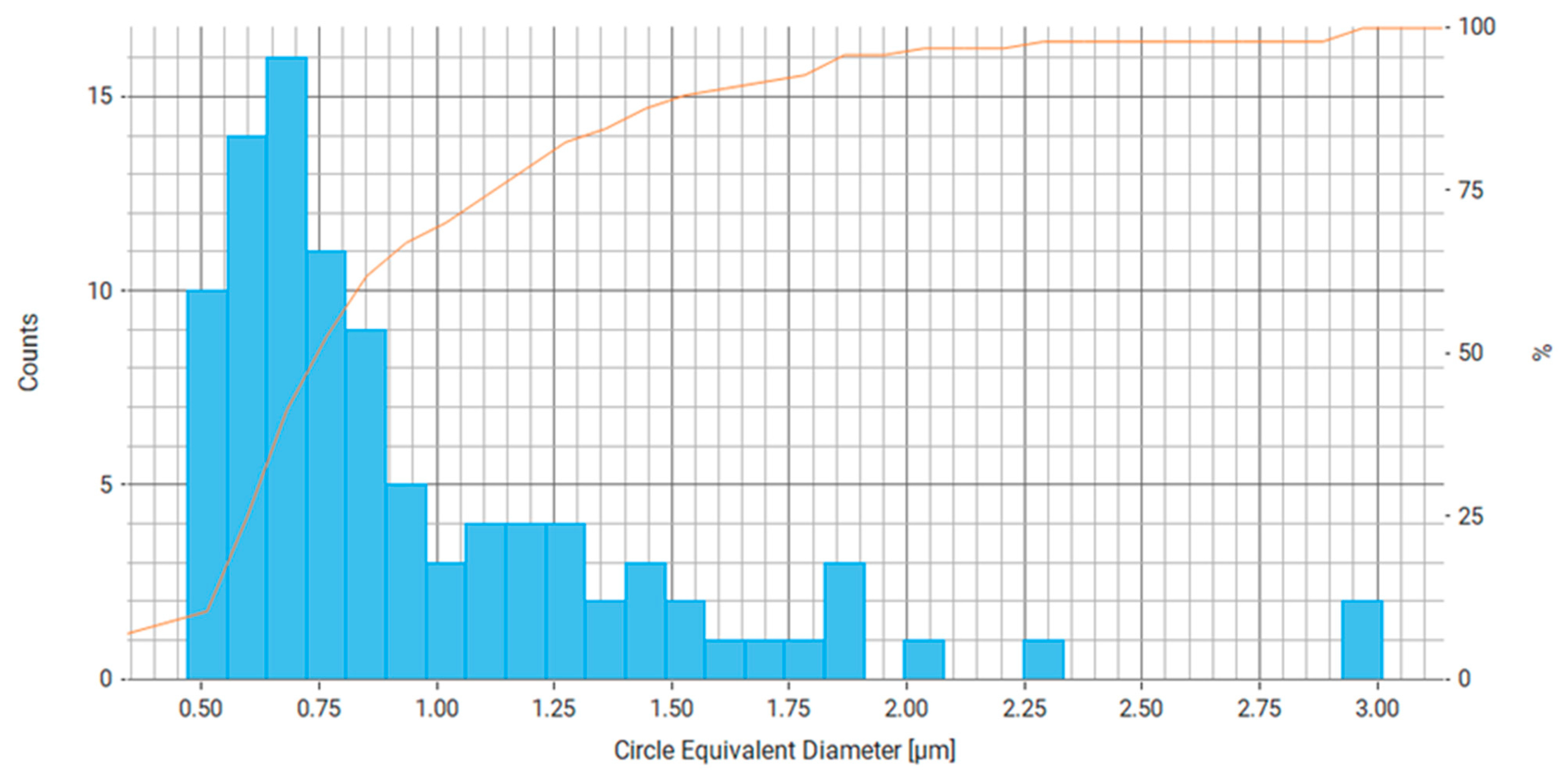
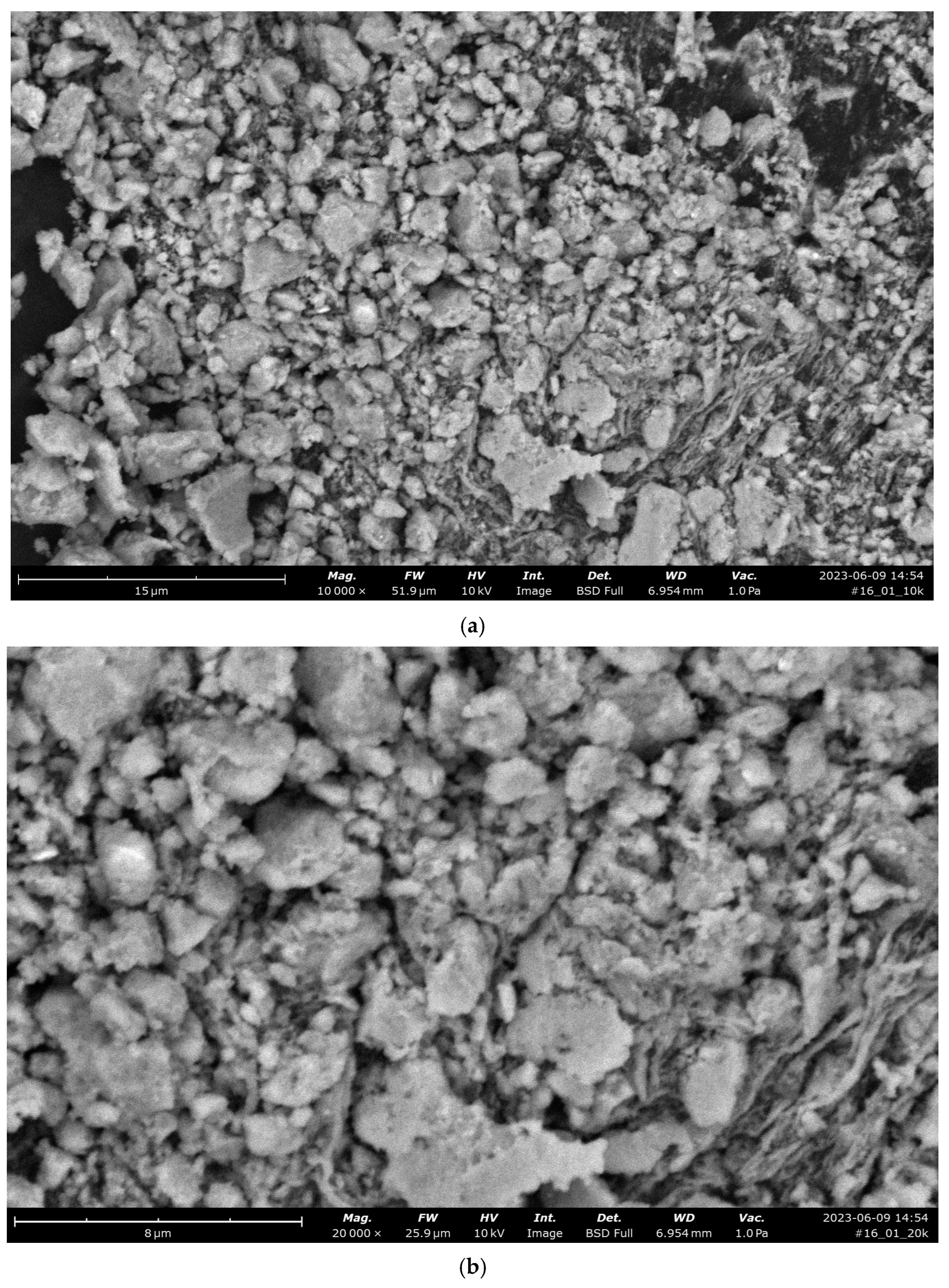
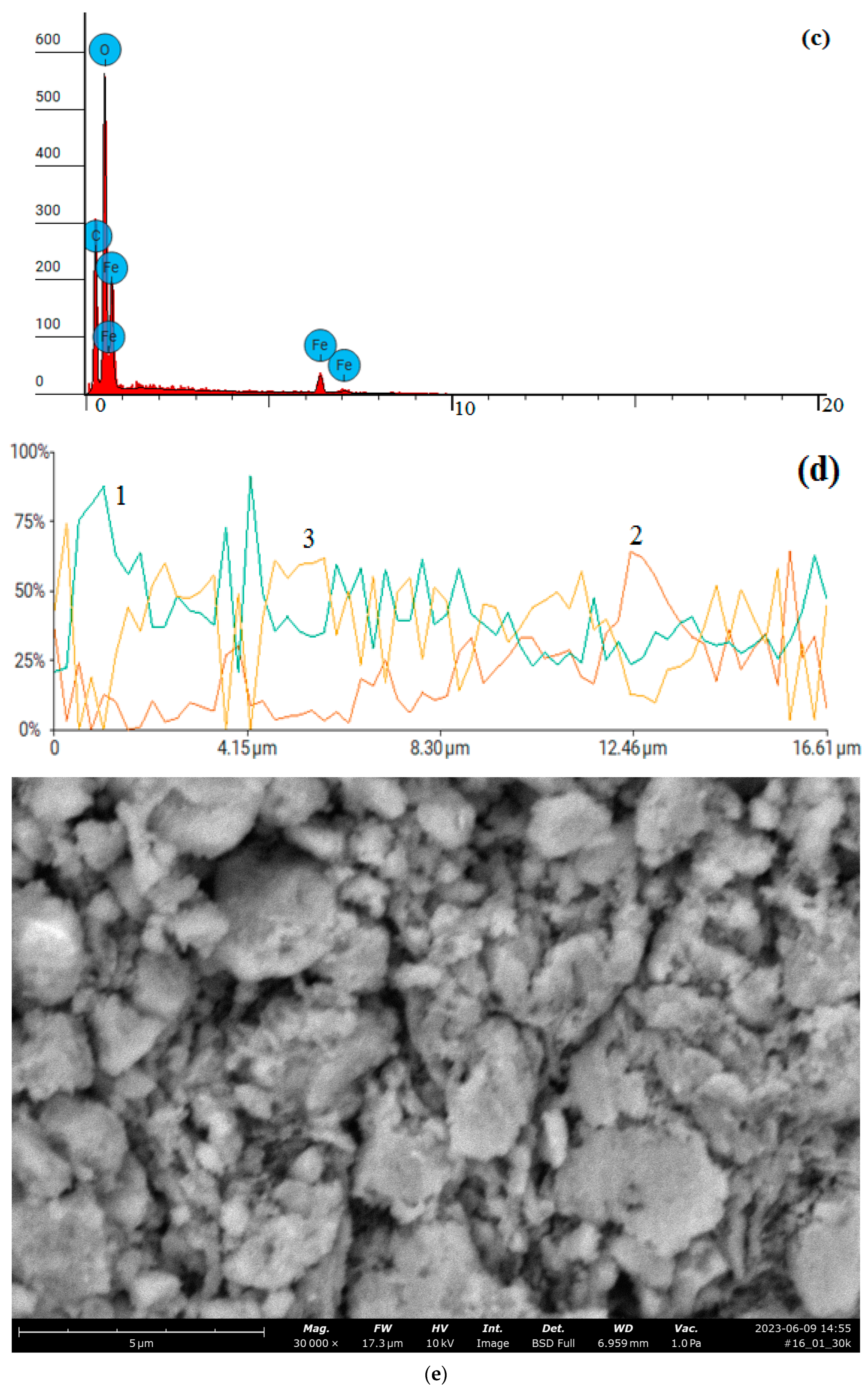
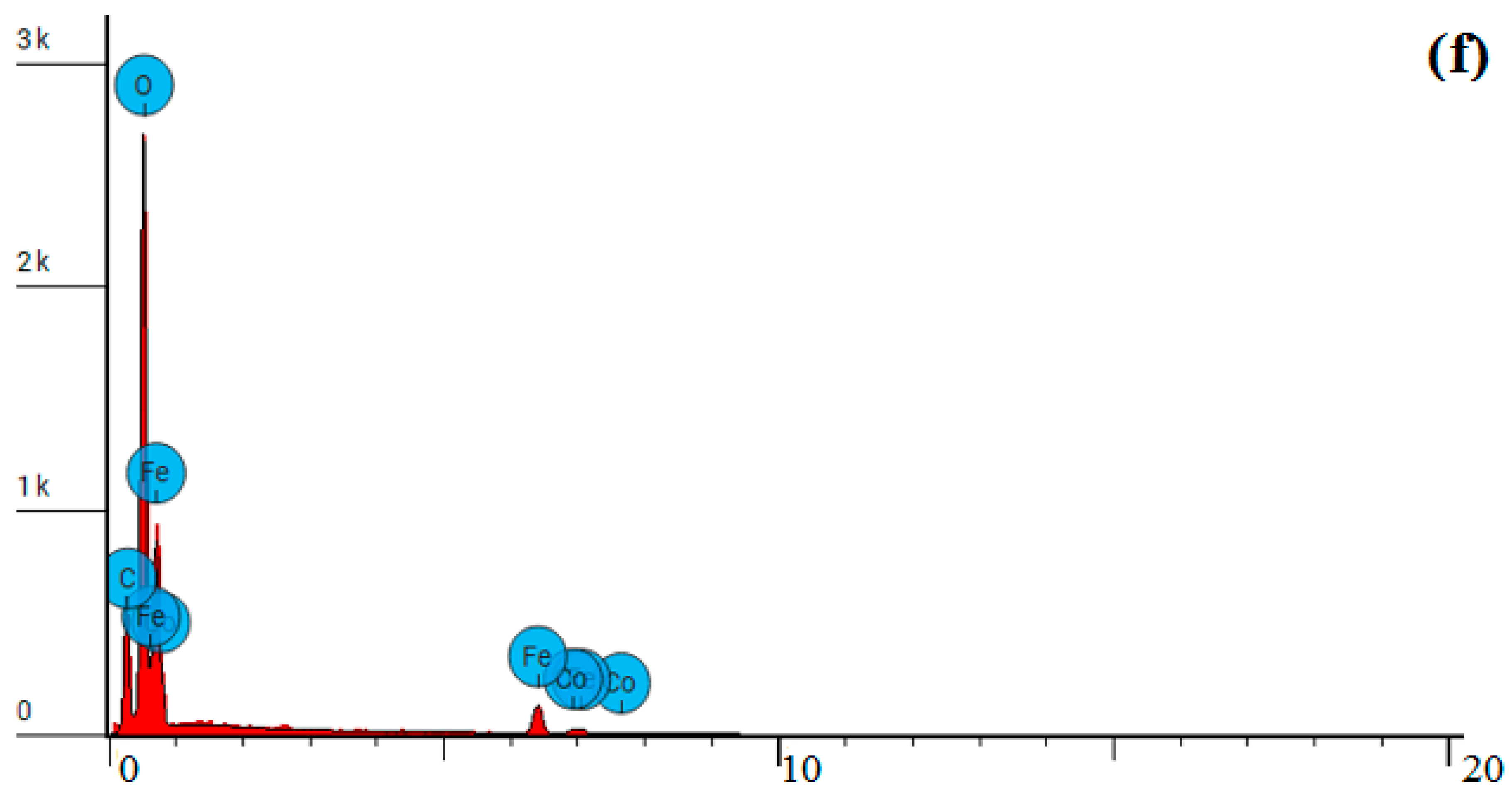

| Property | Average | Median | SD |
|---|---|---|---|
| Area (μm2) | 1.110 | 0.345 | 2.252 |
| Circle Equivalent Diameter (μm) | 0.937 | 0.663 | 0.733 |
| Perimeter (μm) | 3.258 | 2.215 | 3.111 |
| Circumscribed Circle Diameter (μm) | 1.173 | 0.798 | 1.182 |
| Convex Hull Perimeter (μm) | 3.106 | 2.125 | 2.851 |
| Intensity (Mean) | 244.901 | 247.831 | 11.108 |
| Intensity (Std Dev) | 26.955 | 25.902 | 16.998 |
| Major Axis (μm) | 1.147 | 0.841 | 1.064 |
| Minor Axis (μm) | 0.779 | 0.567 | 0.556 |
| Pixel Count | 379.885 | 118.000 | 770.668 |
| Volume By Area (μm3) | 1.739 | 0.152 | 5.785 |
| Aspect Ratio | 0.737 | 0.763 | 0.142 |
| Convexity | 0.967 | 0.968 | 0.021 |
| Circularity | 0.903 | 0.946 | 0.122 |
| Elongation | 0.263 | 0.237 | 0.142 |
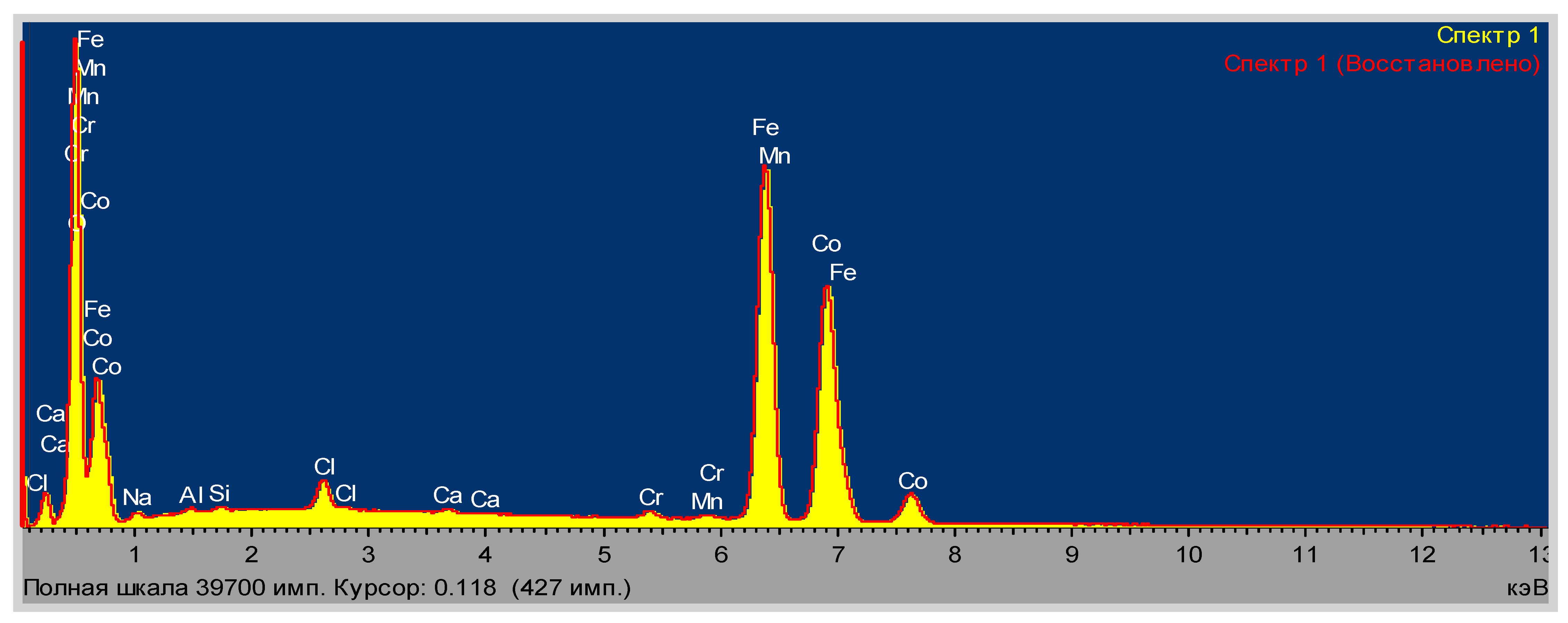
| Element Number | Element | Atomic Concentration | Weight Concentration |
|---|---|---|---|
| 6 | Carbon, C | 13.133 | 6.000 |
| 7 | Nitrogen, N | 0.000 | 0.000 |
| 8 | Oxygen, O | 58.657 | 35.700 |
| 16 | Sulfur, S | 1.803 | 2.200 |
| 26 | Iron, Fe | 26.406 | 56.100 |
| Property | Average | Median | SD |
|---|---|---|---|
| Area (μm2) | 0.918 | 0.483 | 1.167 |
| Circle Equivalent Diameter (μm) | 0.962 | 0.784 | 0.492 |
| Perimeter (μm) | 3.131 | 2.392 | 1.906 |
| Circumscribed Circle Diameter (μm) | 1.100 | 0.863 | 0.684 |
| Convex Hull Perimeter (μm) | 3.026 | 2.340 | 1.772 |
| Intensity (Mean) | 245.001 | 250.962 | 20.374 |
| Intensity (Std Dev) | 23.766 | 17.772 | 22.174 |
| Major Axis (μm) | 1.127 | 0.924 | 0.666 |
| Minor Axis (μm) | 0.836 | 0.689 | 0.405 |
| Pixel Count | 153.876 | 81.000 | 195.708 |
| Volume By Area (μm3) | 0.958 | 0.253 | 2.187 |
| Aspect Ratio | 0.790 | 0.830 | 0.151 |
| Convexity | 0.973 | 0.974 | 0.016 |
| Circularity | 0.949 | 1.000 | 0.099 |
| Elongation | 0.210 | 0.170 | 0.151 |
| Element Number | Element Symbol | Element Name | Atomic Concentration | Weight Concentration |
|---|---|---|---|---|
| 6 | C | Carbon | 24.869 | 10.600 |
| 8 | O | Oxygen | 42.794 | 24.300 |
| 26 | Fe | Iron | 23.157 | 45.900 |
| 27 | Co | Cobalt | 9.180 | 19.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dossumova, B.T.; Sassykova, L.R.; Shakiyeva, T.V.; Ilmuratova, M.S.; Sassykova, A.R.; Batyrbayeva, A.A.; Zhaxibayeva, Z.M.; Dzhatkambayeva, U.N.; Baizhomartov, B.B. Catalysts Based on Nanoscale Iron and Cobalt Immobilized on Polymers for Catalytic Oxidation of Aromatic Hydrocarbons: Synthesis, Physico-Chemical Studies, and Tests of Catalytic Activity. Processes 2024, 12, 29. https://doi.org/10.3390/pr12010029
Dossumova BT, Sassykova LR, Shakiyeva TV, Ilmuratova MS, Sassykova AR, Batyrbayeva AA, Zhaxibayeva ZM, Dzhatkambayeva UN, Baizhomartov BB. Catalysts Based on Nanoscale Iron and Cobalt Immobilized on Polymers for Catalytic Oxidation of Aromatic Hydrocarbons: Synthesis, Physico-Chemical Studies, and Tests of Catalytic Activity. Processes. 2024; 12(1):29. https://doi.org/10.3390/pr12010029
Chicago/Turabian StyleDossumova, Binara T., Larissa R. Sassykova, Tatyana V. Shakiyeva, Madina S. Ilmuratova, Albina R. Sassykova, Aigul A. Batyrbayeva, Zhanar M. Zhaxibayeva, Ulzhan N. Dzhatkambayeva, and Bedelzhan B. Baizhomartov. 2024. "Catalysts Based on Nanoscale Iron and Cobalt Immobilized on Polymers for Catalytic Oxidation of Aromatic Hydrocarbons: Synthesis, Physico-Chemical Studies, and Tests of Catalytic Activity" Processes 12, no. 1: 29. https://doi.org/10.3390/pr12010029






