An Effective Mercury Ion Adsorbent Based on a Mixed-Matrix Polyvinylidene Fluoride Membrane with Excellent Hydrophilicity and High Mechanical Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterizations
2.2. Fabrication of Mixed-Matrix PVDF Adsorption Membrane
2.3. Adsorption Tests of Hg(II) Removal
2.4. Tests of the Anti-Fouling Ability of Membranes
3. Results
3.1. Characterization
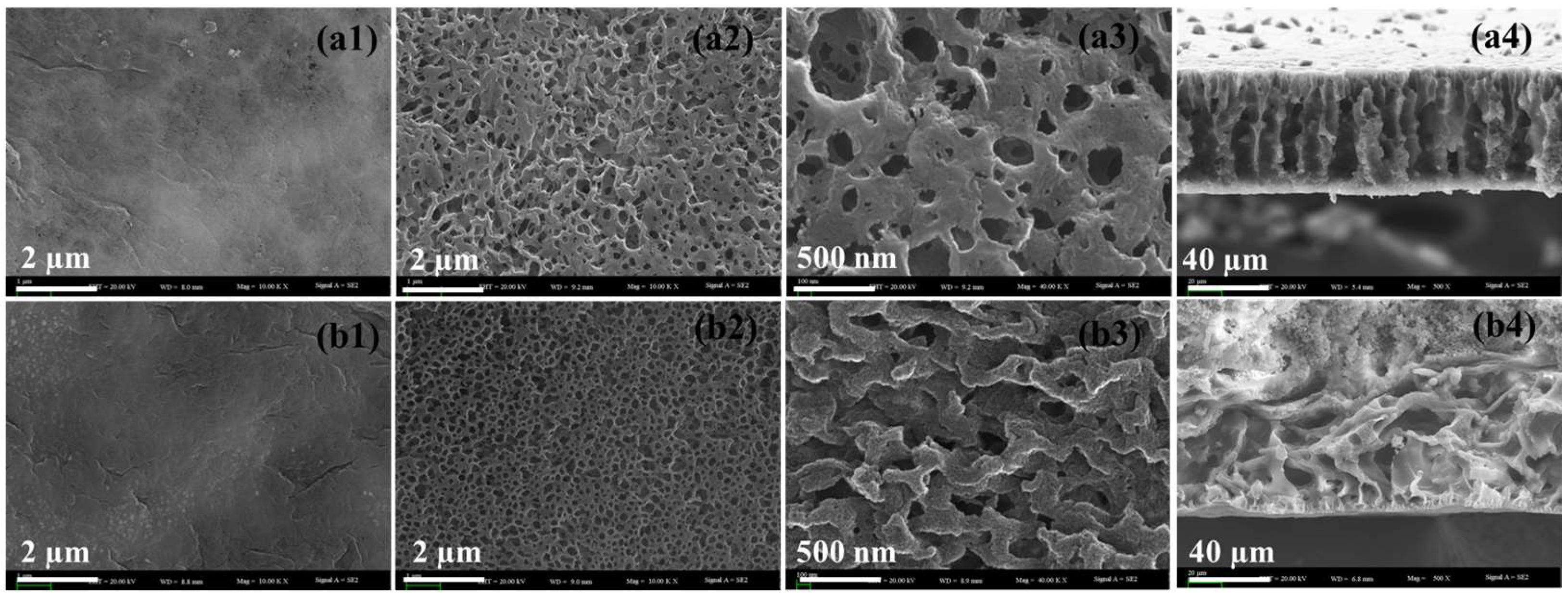
3.1.1. Morphology
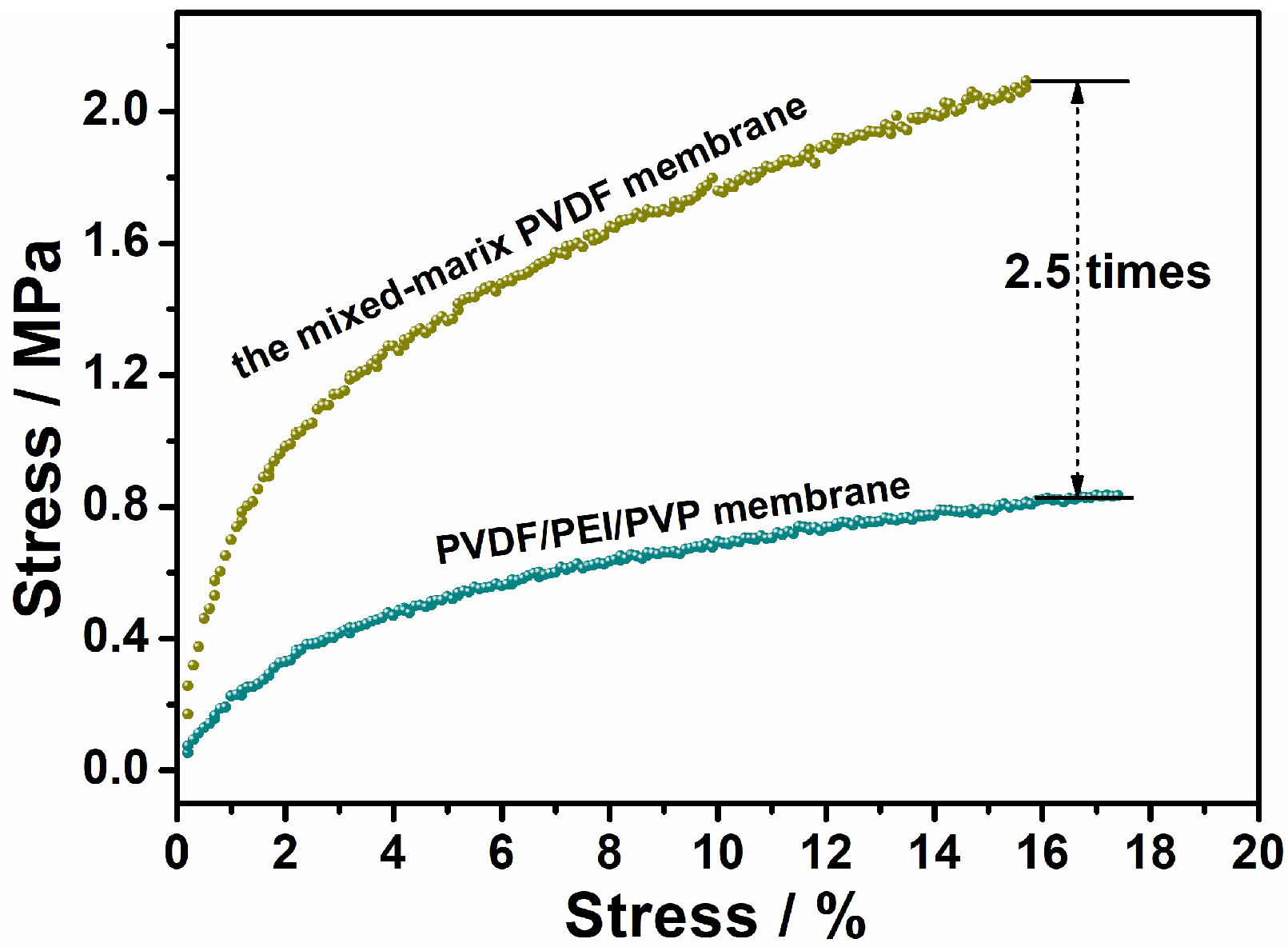
3.1.2. Membrane Hydrophilicity and Mechanical Strength
3.2. Adsorption Studies
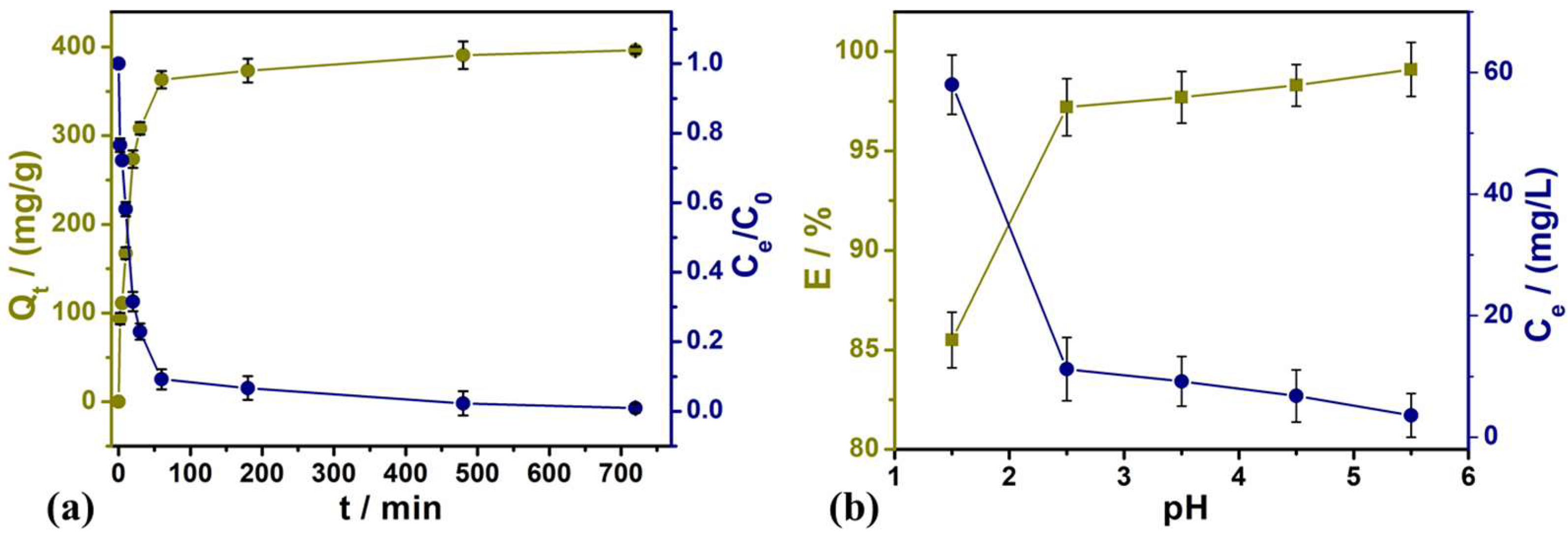
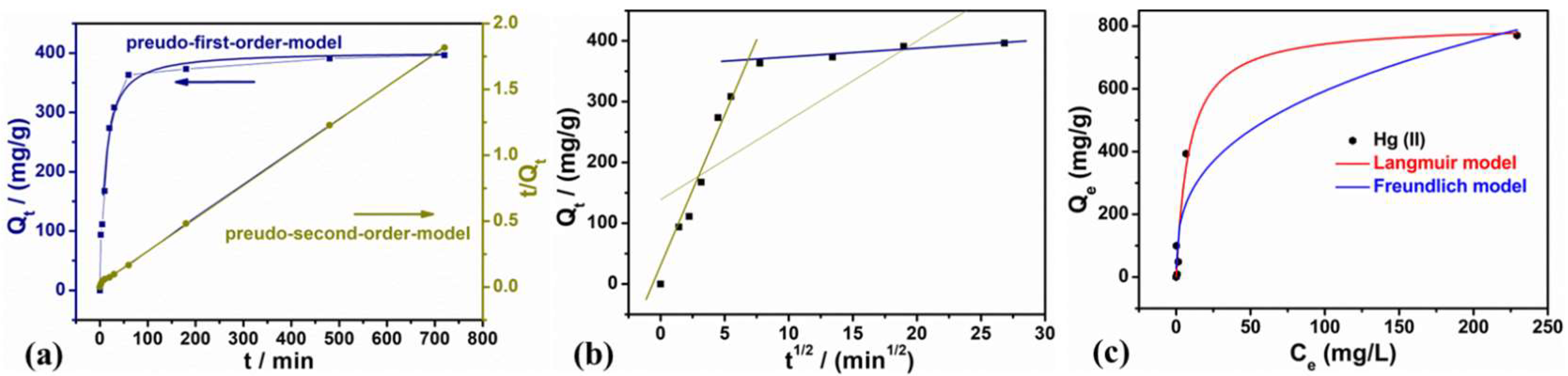
3.2.1. Effects of Contact Time and Solution pH on Adsorption
3.2.2. Effects of Co-Existing Ions and Reusability
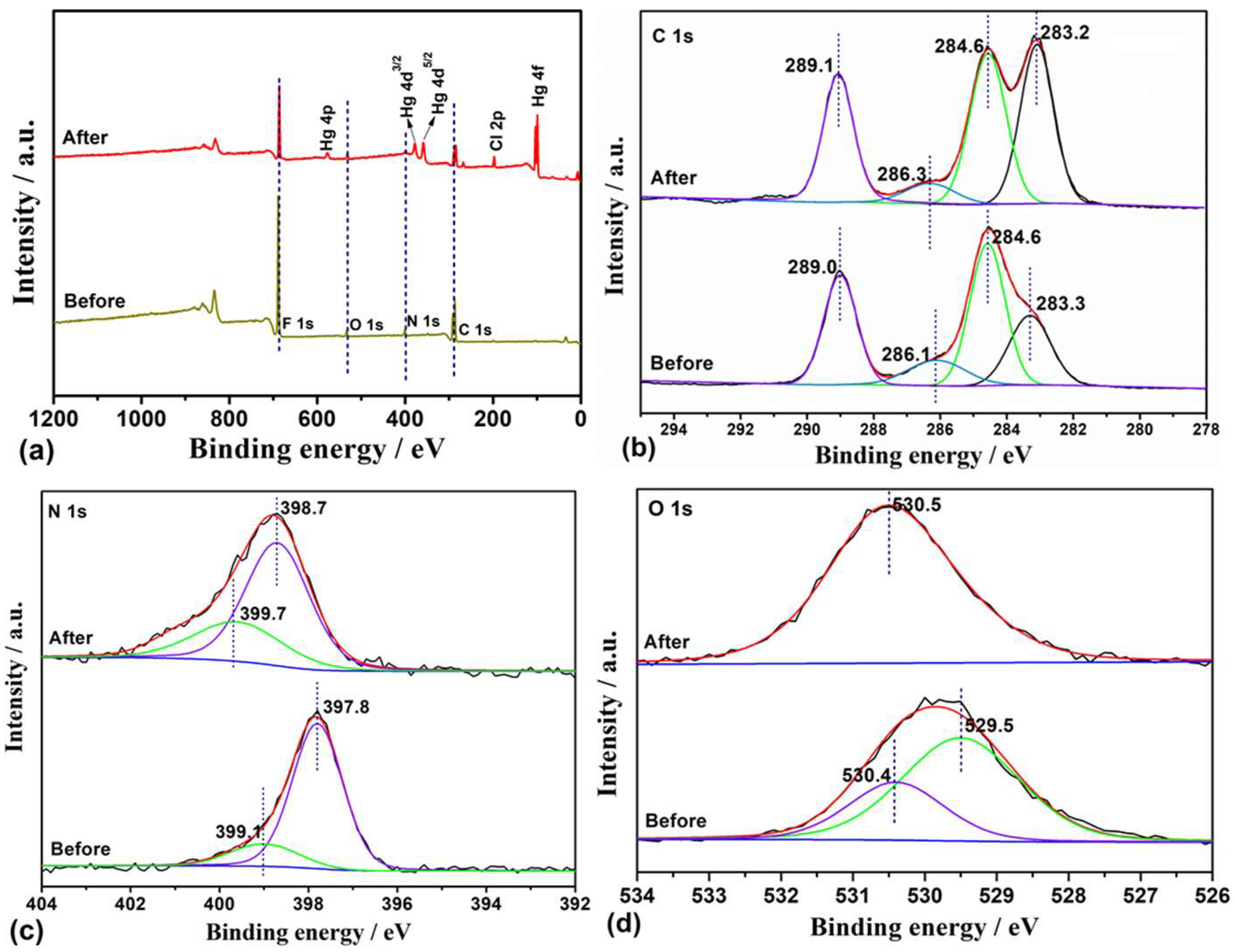
3.3. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Lists of Symbols and Acronyms
| Hg | Mercury |
| PVDF | Polyvinylidene fluoride |
| PEI | Polyethyleneimine |
| PVP | Polyvinylpyrrolidone |
| VT | Vermiculite |
| Qe (mg/g) | Adsorption capacity |
| E | Adsorption efficiency |
| C0 and Ce | Initial and equilibrium concentrations (mg/L) |
| V (L) | Volume of the solution |
| m (g) | Mass of the adsorbent |
| Kd | The distribution coefficient |
| Qm | Theoretical maximum adsorption capacity (mg/g) |
| Ce | Final equilibrium mercury concentration (mg/L) |
| bL | Langmuir constant (L/mg) related to the adsorption strength |
| KF | Freundlich constant related to the adsorption strength (mg/g) (L/mg) |
| nF | Freundlich constant related to the adsorption capacity |
References
- Zhou, Y.C.; Ma, S.; Zhu, W.H.; Shi, Q.Q.; Jiang, H.Q.; Lu, R.; Wu, W.J. Revealing varying relationships between wastewater mercury emissions and economic growth in Chinese cities. Environ. Pollut. 2023, 341, 122944. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Song, X.H.; Guo, Y.Q.; Yang, Q.; Feng, K.S. The determinants of China’s national and regional energy-related mercury emission changes. J. Environ. Manag. 2019, 246, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, J.S.; Chen, G.Q.; Wei, W.D.; Yang, Q.; Yao, M.T.; Shao, J.A.; Zhou, M.; Xia, X.H.; Dong, K.Q.; et al. China’s energy-related mercury emissions: Characteristics, impact of trade and mitigation policies. J. Clean. Prod. 2017, 141, 1259–1266. [Google Scholar] [CrossRef]
- Liu, C.; Peng, J.H.; Liu, J.; Guo, P.; Wang, S.X.; Liu, C.H.; Zhang, L.B. Catalytic removal of mercury from waste carbonaceous catalyst by microwave heating. J. Hazard Mater. 2018, 358, 198–206. [Google Scholar] [CrossRef]
- Li, W.X.; Ju, B.Z.; Zhang, S.F. Preparation of cysteamine-modified cellulose nanocrystal adsorbent for removal of mercury ions from aqueous solutions. Cellulose 2019, 26, 4971–4985. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, Y.; Li, Y.R.; Li, Y.Q.; He, C.Y. Thioether-functionalized covalent organic framework for mercury removal. Colloid Surface A 2024, 681, 132807. [Google Scholar] [CrossRef]
- Tami-Pimiento, L.M.; Joya-Herrera, L.M.; Pérez-Chía, Y.I.; Meléndez, A.M. Selective electrochemical reduction of mercury(II) from a simulated traditional gold mining wastewater contaminated with cyanide and heavy metals. J. Solid State Electrochem. 2023, 363, 192–202. [Google Scholar] [CrossRef]
- Agostino, F.; Bellante, A.; Bonsignore, M.; Core, M.D.; Clarizia, L.; Sabatino, N.; Giaramita, L.; Tranchida, G.; Chiavarini, S.; Sprovieri, M. A chemical remediation technique for a nearly-total removal of arsenic and mercury from contaminated marine sediments. Heliyon 2023, 9, e22633. [Google Scholar] [CrossRef]
- Bessbousse, H.; Rhlalou, T.; Verchère, J.F.; Lebrun, L. Sorption and filtration of Hg(II) ions from aqueous solutions with a membrane containing poly(ethyleneimine) as a complexing polymer. J. Membr. Sci. 2008, 325, 997–1005. [Google Scholar] [CrossRef]
- Zhang, L.P.; Liu, Z.; Zhou, X.L.; Zhang, C.; Cai, Q.W.; Xie, R.; Ju, X.J.; Wang, W.; Faraj, Y.; Chu, L.Y. Novel composite membranes for simultaneous catalytic degradation of organic contaminants and adsorption of heavy metal ions. Sep. Purif. Technol. 2020, 237, 116364. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Meng, J.M.; Xin, Z.Y.; Gong, H.; Li, J.S. Polydopamine-assisted grafting of chitosan on porous poly (L-lactic acid) electrospun membranes for adsorption of heavy metal ions. Int. J. Biol. Macromol. 2021, 167, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard Mater. 2021, 401, 123608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Li, J.; Mu, S.Y.; He, W.; Zhang, D.; Wu, X.; Wan, C.Y.; Zeng, H.H. Efficient removal of mercury ions with MoS2-nanosheet-decorated PVDF composite adsorption membrane. Environ. Pollut. 2021, 268, 115705. [Google Scholar] [CrossRef] [PubMed]
- Pariy, I.O.; Ivanova, A.A.; Shvartsman, V.V.; Lupascu, D.C.; Sukhorukov, G.B.; Surmeneva, M.A.; Surmenev, R.A. Poling and annealing of piezoelectric poly(vinylidene fluoride) micropillar arrays. Mater. Chem. Phys. 2020, 239, 122035. [Google Scholar] [CrossRef]
- Pariy, I.O.; Ivanova, A.A.; Shvartsman, V.V.; Lupascu, D.C.; Sukhorukov, G.B.; Ludwig, T.; Bartasyte, A.; Mathur, S.; Surmeneva, M.A.; Surmenev, R.A. Piezoelectric response in hybrid micropillar arrays of poly(vinylidene fluoride) and reduced graphene oxide. Polymers 2019, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- He, S.J.; Zhai, S.X.; Zhang, C.; Xue, Y.; Yang, W.; Lin, J. Effect of sulfonation degree and PVDF content on the structure and transport properties of SPEEK/PVDF blend membranes. Polymers 2019, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Chen, X.Y.; Luo, J.; Wang, F.; Liu, G.J.; Zhu, H.L.; Guo, Y.H. PVDF grafted Gallic acid to enhance the hydrophilicity and antibacterial properties of PVDF composite membrane. Sep. Purif. Technol. 2021, 259, 118127. [Google Scholar] [CrossRef]
- Munirasu, S.; Banat, F.; Durrani, A.A.; Haija, M.A. Intrinsically superhydrophobic PVDF membrane by phase inversion for membrane distillation. Desalination 2017, 417, 77–86. [Google Scholar] [CrossRef]
- Javadi, M.; Jafarzadeh, Y.; Yegani, R.; Kazemi, S. PVDF membranes embedded with PVP functionalized nanodiamond for pharmaceutical wastewater treatment. Chem. Eng. Res. Des. 2018, 140, 241–250. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.; Ren, L.; Lei, T.; Li, J.; Jin, J.; Luo, S.; Qin, S.; Gao, C.; Lei, T. Properties and preparation of TiO2-HAP@PVDF composite ultrafiltration membranes. Polym. Compos. 2023, 44, 7499–7509. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhu, B.K.; Kong, L.; Xu, Y.Y. Improving hydrophilicity and protein resistance of poly(vinylidene fluoride) membranes by blending with amphiphilic hyperbranched-star polymer. Langmuir 2007, 23, 5779–5786. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, X.; Zhao, Y.; Chen, L. Acryloylmorpholine-grafted PVDF membrane with improved protein fouling resistance. Ind. Eng. Chem. Res. 2013, 52, 18392–18400. [Google Scholar] [CrossRef]
- Ning, L.; Yu, T.; Zhang, J.; Sun, Z.; Zhao, J.; Jian, Z.; Wei, Z. Precisely-controlled modification of PVDF membranes with 3D TiO2/ZnO nanolayer: Enhanced anti-fouling performance by changing hydrophilicity and photocatalysis under visible light irradiation. J. Membrane Sci. 2017, 528, 359–368. [Google Scholar]
- Xiang, S.; Xie, T.; Wang, J.; Fan, W. Improved fouling resistance of poly(vinylidene fluoride) membrane modified with poly(acryloyl morpholine)-based amphiphilic copolymer. Colloid Polym. Sci. 2017, 295, 1211–1221. [Google Scholar]
- Zhang, S.; Shen, J.; Qiu, X.; Weng, D.; Zhu, W. ESR and vibrational spectroscopy study on poly(vinylidene fluoride) membranes with alkaline treatment. J. Power Sources 2006, 153, 234–238. [Google Scholar] [CrossRef]
- Guo, G.B.; Kou, S.S.; An, S.L. Effect factors of the stem grafting rate of the PVDF-g-PSSA membrane modified by the plain sodium silicate (Na4SiO4). Polym. Mater. Sci. Eng. 2010, 26, 93–96. [Google Scholar]
- Estrada-Villegas, G.M.; Bucio, E. Comparative study of grafting a polyampholyte in a fluoropolymer membrane by gamma radiation in one or two-steps. Radiat. Phys. Chem. 2013, 92, 61–65. [Google Scholar] [CrossRef]
- Dong, L.; Liu, X.D.; Xiong, Z.R.; Sheng, D.K.; Lin, C.H.; Zhou, Y.; Yang, Y. Preparation and characterization of functional poly(vinylidene fluoride) (PVDF) membranes with ultraviolet-absorbing property. Appl. Surf. Sci. 2018, 444, 497–504. [Google Scholar] [CrossRef]
- Zeng, H.H.; Wang, L.; Zhang, D.; Wang, C.Y. Efficient capture and detoxification of mercury dichloride from wastewater by a PVDF/PEI adsorption membrane. Chem. Eng. J. 2023, 468, 143621. [Google Scholar] [CrossRef]
- Ike, I.A.; Dumée, L.F.; Groth, A.; Orbell, J.D.; Duke, M. Effects of dope sonication and hydrophilic polymer addition on the properties of low pressure PVDF mixed matrix membranes. J. Membr. Sci. 2017, 540, 200–211. [Google Scholar] [CrossRef]
- Guo, H.H.; Yang, J.; Zhao, W.Q.; Xu, T.; Lin, C.G.; Zhang, J.W.; Zhang, L. Direct formation of amphiphilic crosslinked networks based on PVP as a marine anti-biofouling coating. Chem. Eng. J. 2019, 374, 1353–1363. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Lamponi, S.; Bonechi, C.; Tamasi, G.; Donati, A.; Rossi, C.; Magnani, A. Thixotropic PVA hydrogel enclosing a hydrophilic PVP core as nucleus pulposus substitute. Mat. Sci. Eng.-C 2019, 98, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Wardani, A.K.; Ariono, D.; Subagjo, S.; Wenten, I.G. Fouling tendency of PDA/PVP surface modified PP membrane. Surf. Interfaces 2020, 19, 100464. [Google Scholar] [CrossRef]
- Gu, S.Q.; Wang, L.; Mao, X.Y.; Yang, L.P.; Wang, C.Y. Selective adsorption of Pb(II) from aqueous solution by triethylenetetramine-grafted polyacrylamide/vermiculite. Materials 2018, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhou, S.Y.; Lai, C.Y.; Wang, B.; Li, K. PVDF/palygorskite composite ultrafiltration membranes with enhanced abrasion resistance and flux. J. Membr. Sci. 2015, 495, 91–100. [Google Scholar] [CrossRef]
- Wei, D.Y.; Zhou, S.Y.; Li, M.S.; Xue, A.L.; Zhang, Y.; Zhao, Y.J.; Zhong, J.; Yang, D.W. PVDF/palygorskite composite ultrafiltration membranes: Effects of nanoclay particles on membrane structure and properties. Appl. Clay Sci. 2019, 181, 105171. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, Y.; Bai, Z.; Luque, R.; Xuan, J. Functionalized chitosan biosorbents with ultra-high performance, mechanical strength and tunable selectivity for heavy metals in wastewater treatment. Chem. Eng. J. 2017, 325, 350–359. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 27, 47–52. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.; Lee, D. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Tchinda, A.L.J.; Ngameni, E.; Kenfack, I.T.; Walcarius, A. One-step preparation of thiol-functionalized porous clay heterostructures: Application to Hg(II) binding and characterization of mass transport issues. Chem. Mater. 2009, 21, 4111–4121. [Google Scholar] [CrossRef]
- Bessbousse, H.; Verchère, J.F.; Lebrun, L. Increase in permeate flux by porosity enhancement of a sorptive UF membrane designed for the removal of mercury(II). J. Membr. Sci. 2010, 364, 167–176. [Google Scholar] [CrossRef]
- Gang, X.; Zhao, Y.; Hou, L.; Jian, C.; Tao, M.; Zhang, W. A recyclable phosphinic acid functionalized polyacrylonitrile fiber for selective and efficient removal of Hg2+. Chem. Eng. J. 2017, 325, 533–543. [Google Scholar]
- Tang, H.; Chang, C.Y.; Zhang, L.N. Efficient adsorption of Hg2+ ions on chitin/cellulose composite membranes prepared via environmentally friendly pathway. Chem. Eng. J. 2011, 173, 689–697. [Google Scholar] [CrossRef]
- Rabelo, R.B.; Vieira, R.S.; Luna, F.M.T.; Guibal, E.; Beppu, M.M. Adsorption of copper(II) and mercury(II) ions onto chemically-modified chitosan membranes: Equilibrium and kinetic properties. Adsorpt. Sci. Technol. 2012, 30, 1–21. [Google Scholar] [CrossRef]
- Ballav, N.; Das, R.; Giri, S.; Muliwa, A.M.; Pillay, K. L-cysteine doped polypyrrole (PPy@L-Cyst): A super adsorbent for the rapid removal of Hg2+ and efficient catalytic activity of the spent adsorbent for reuse. Chem. Eng. J. 2018, 345, 621–630. [Google Scholar] [CrossRef]



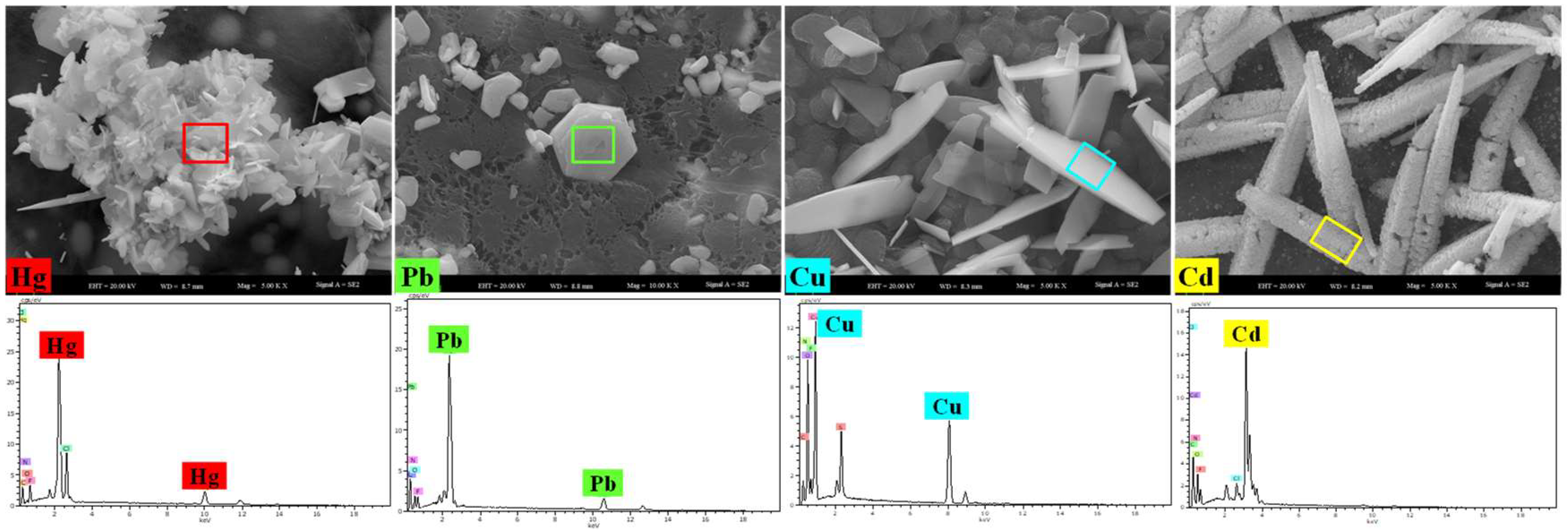
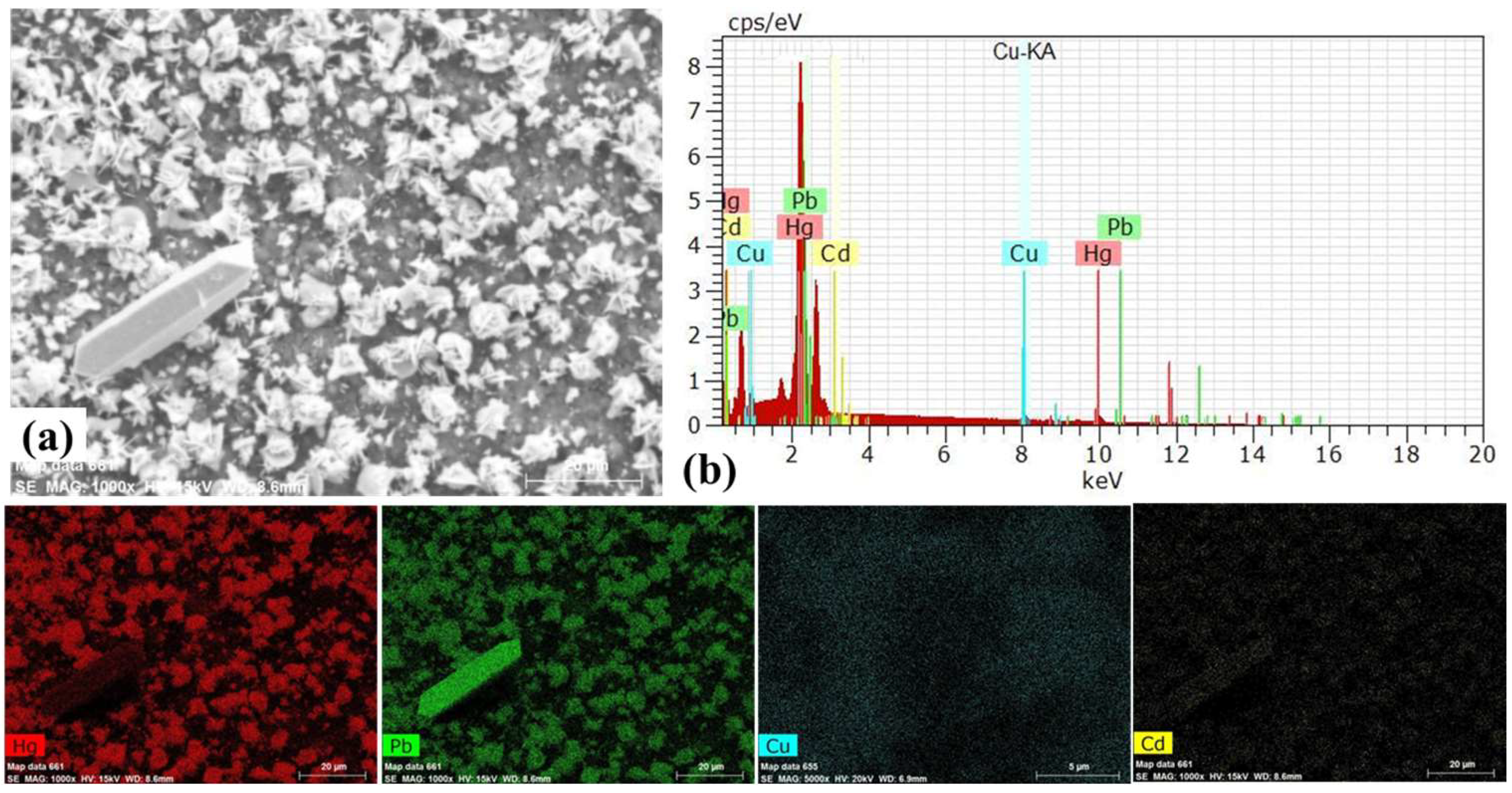
| Ions | C0 (mg/L) | Ce (mg/L) | Qe (mg/g) | E (%) | Kd (mL/g) | KHg/Cd | KHg/Cu | KHg/Pb |
|---|---|---|---|---|---|---|---|---|
| Hg(II) | 400.0 | 126.5 | 273.5 | 68.4% | 2.16 | 1.2 × 105 | 16.6 | 16.6 |
| Cd(II) | 400.0 | 399.993 | 0.007 | 0.002% | 1.8 × 10−5 | |||
| Cu(II) | 400.0 | 352.8 | 47.2 | 11.8% | 0.13 | |||
| Pb(II) | 400.0 | 353.7 | 46.3 | 11.6% | 0.13 |
| Qf (mg/g) | Pseudo-First-Order Model | R2 |
|---|---|---|
| 402.0 | k1 (1/min) 0.0283 | 0.9701 |
| Qf (mg/g) | Pseudo-second-order model | R2 |
| 400.0 | k2 [g/(mg∙min)] 2.8 × 10−4 | 0.9997 |
| θ | Intraparticle diffusion model | R2 |
| 138.5 | kint [mg/(g·min1/2)] 13.04 | 0.3540 |
| Qm (mg/g) | Langmuir Model | R2 |
|---|---|---|
| 807 | bL (L/mg) 0.12 | 0.9620 |
| KF | Freundlich model | R2 |
| 113 | nF 0.34 | 0.8892 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Wu, X.; He, F.; Meng, X.; He, W.; Li, J.; Zhu, G.; Zeng, H.; Wang, C. An Effective Mercury Ion Adsorbent Based on a Mixed-Matrix Polyvinylidene Fluoride Membrane with Excellent Hydrophilicity and High Mechanical Strength. Processes 2024, 12, 30. https://doi.org/10.3390/pr12010030
Cao L, Wu X, He F, Meng X, He W, Li J, Zhu G, Zeng H, Wang C. An Effective Mercury Ion Adsorbent Based on a Mixed-Matrix Polyvinylidene Fluoride Membrane with Excellent Hydrophilicity and High Mechanical Strength. Processes. 2024; 12(1):30. https://doi.org/10.3390/pr12010030
Chicago/Turabian StyleCao, Ling, Xia Wu, Fajun He, Xianfeng Meng, Wei He, Jing Li, Guidan Zhu, Hehua Zeng, and Chuanyi Wang. 2024. "An Effective Mercury Ion Adsorbent Based on a Mixed-Matrix Polyvinylidene Fluoride Membrane with Excellent Hydrophilicity and High Mechanical Strength" Processes 12, no. 1: 30. https://doi.org/10.3390/pr12010030





