Investigation of the Mechanism for Removal of Typical Pathogenic Bacteria from Three-Compartment Septic Tanks under Low Temperature Conditions
Abstract
:1. Introduction
2. Material and Methods
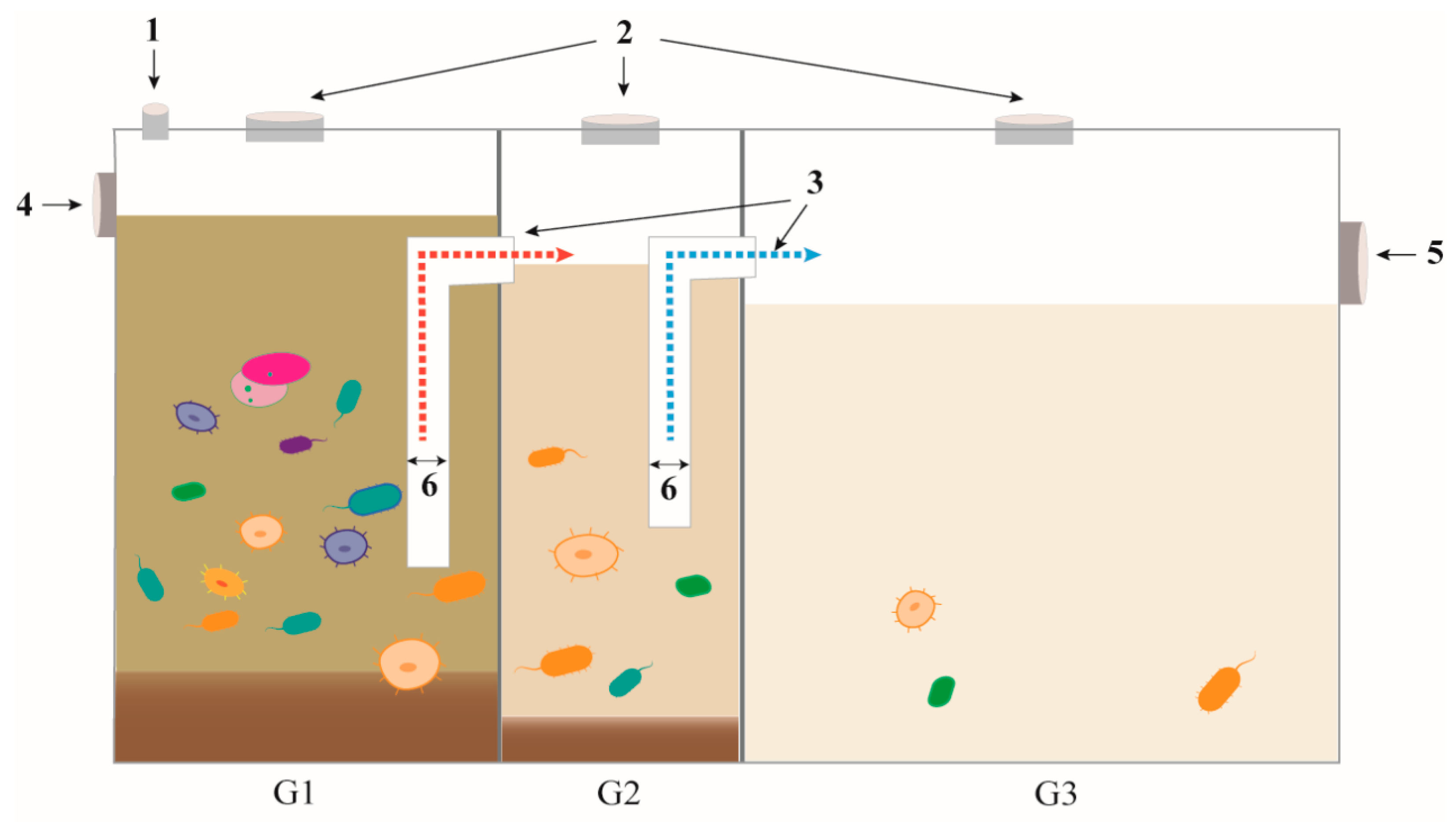
2.1. Laboratory Small Three-Compartment Septic Tanks and the Overall Test Design
2.2. Inflow and Inoculum
2.3. Sample Collection and Physicochemical Parameter Analysis
2.4. RNA Extraction and Real-Time PCR Analysis
2.5. 16S rRNA Gene Sequencing
3. Results and Discussion
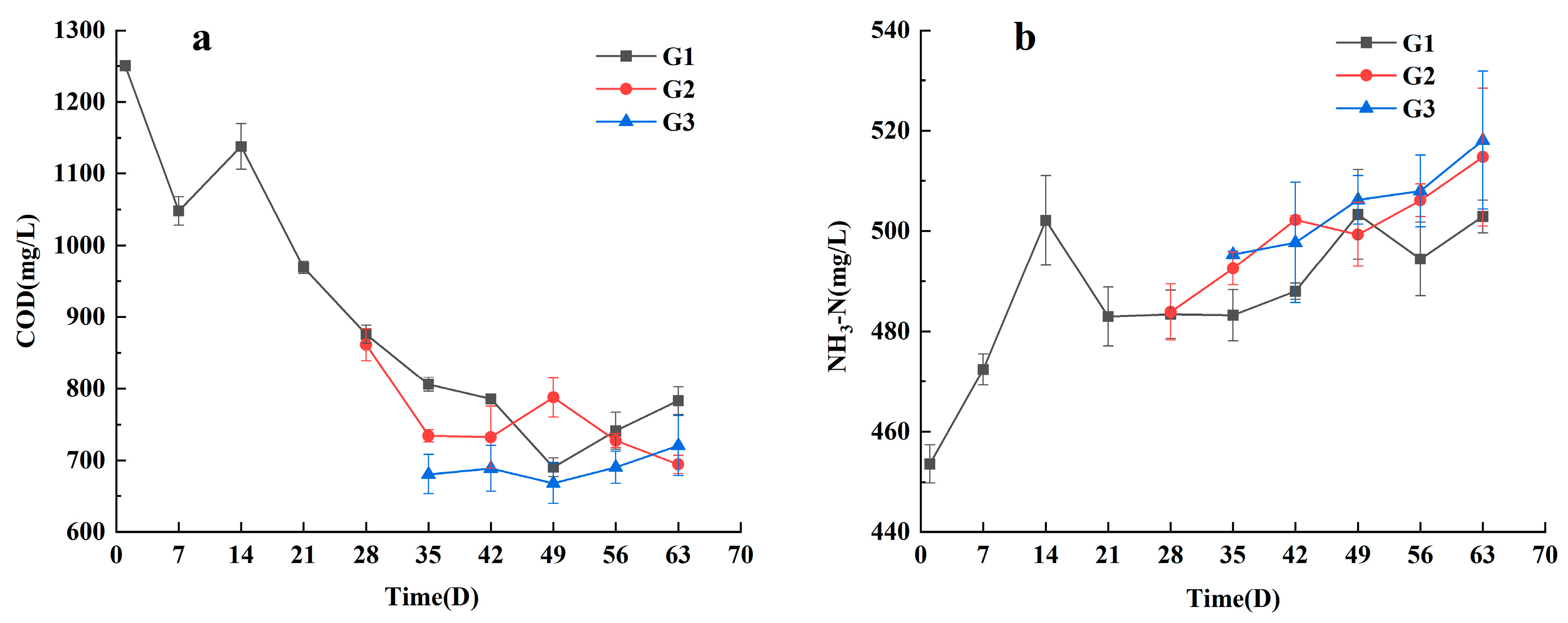
3.1. The Role of VFA in the Early Killing of Pathogenic Bacteria in Septic Tanks
3.2. NH3-N Impact Strategies on Typical Pathogenic Bacteria
3.3. Effects of Microbial Communities
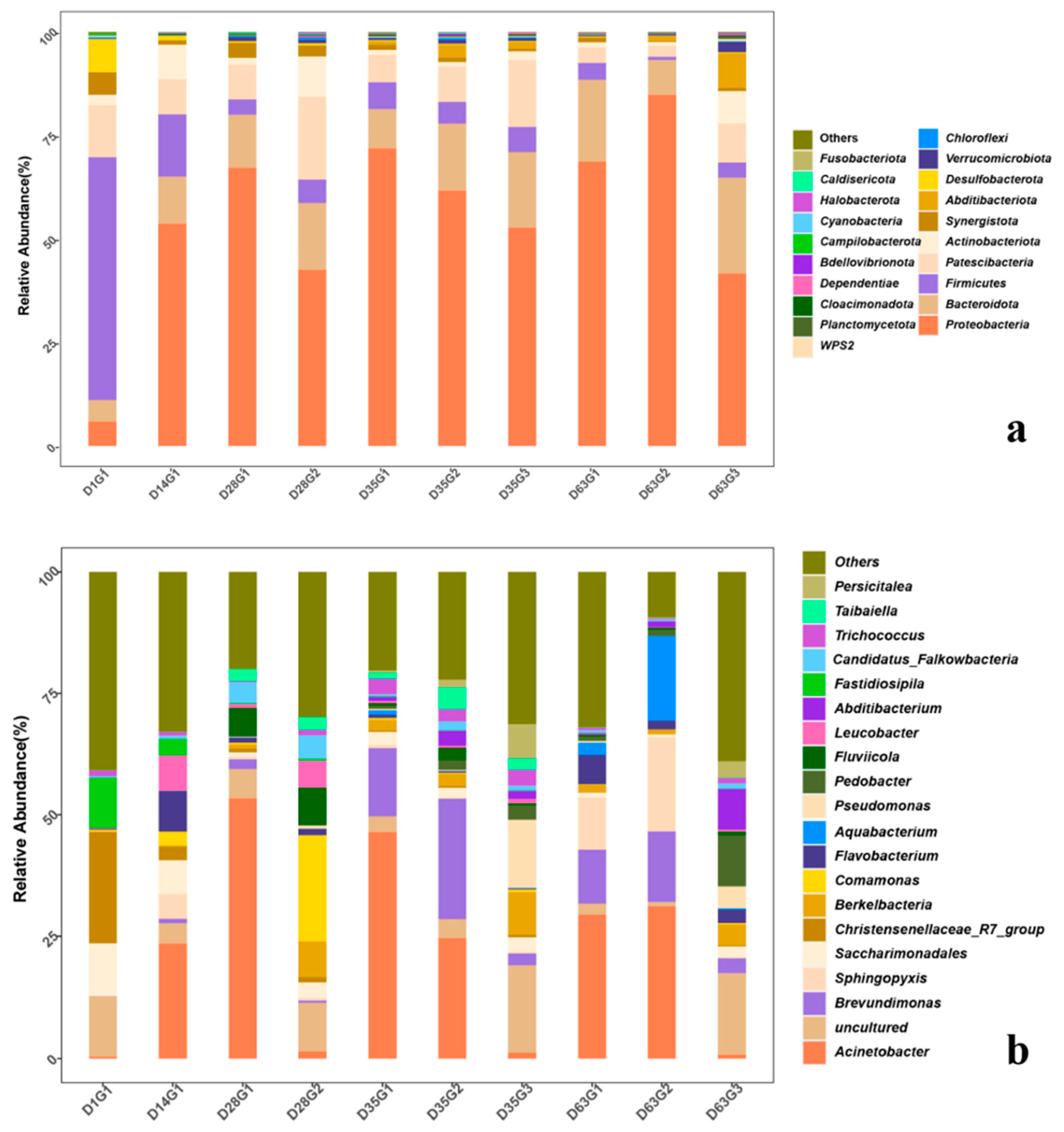
3.3.1. Effects of Changes in Microbial Species
3.3.2. Microbial Diversity Change
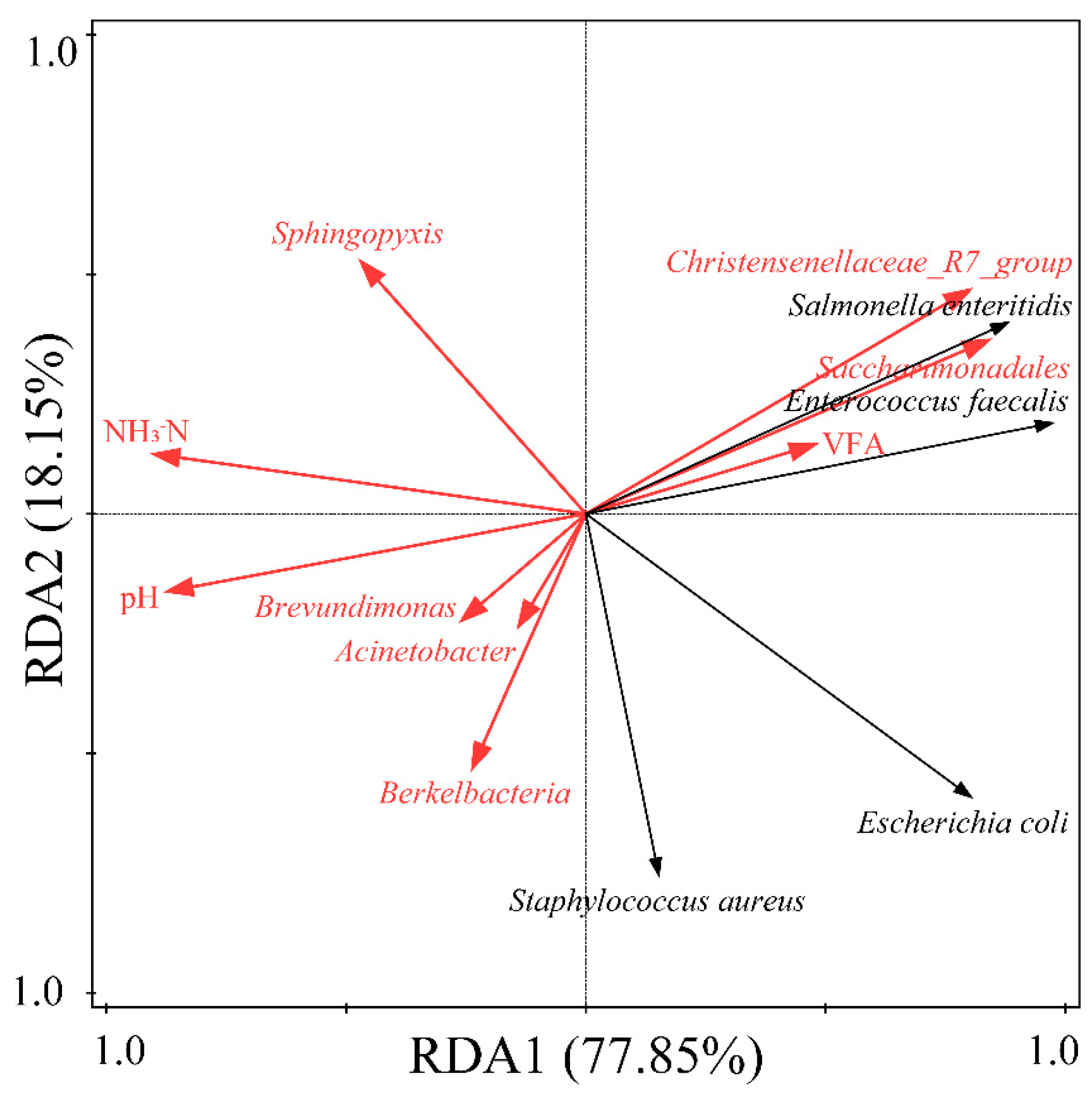
3.3.3. The Interaction between Environmental Factors, Microbial Community, and Typical Pathogen Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; He, P.; Duan, H.; Yang, Z.; Zhang, H.; Lü, F. Leaching risk of antibiotic resistance contamination from organic waste compost in rural areas. Environ. Pollut. 2023, 320, 121108. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhang, J.; Wang, X.G.; Lu, X. Performance of a Tower-Shaped Integrated Ecological Purification Device for Pollutants Removal from Domestic Sewage in Rural Areas. Int. J. Environ. Res. Public Health 2022, 19, 17014. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, B.; Wang, P.; Guo, W.; Li, X.; Gao, H.; Zhang, B.; Chen, J. Revealing factors influencing spatial variation in the quantity and quality of rural domestic sewage discharge across China. Process Saf. Environ. Prot. 2022, 162, 200–210. [Google Scholar] [CrossRef]
- Cao, Y.; Van Loosdrecht, M.C.M.; Daigger, G.T. The bottlenecks and causes, and potential solutions for municipal sewage treatment in China. Water Pract. Technol. 2020, 15, 160–169. [Google Scholar] [CrossRef]
- Ma, D.C.; Chen, H.C.; Feng, Q.G.; Zhang, X.; Wu, D.L.; Feng, J.H.; Cheng, S.K.; Wang, D.B.; Liu, Z.; Zhong, Q.S.; et al. Dissemination of antibiotic resistance genes through fecal sewage treatment facilities to the ecosystem in rural area. J. Environ. Manag. 2023, 333, 117439. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. A review of wastewater irrigation: Environmental implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar] [CrossRef]
- Jiang, T.T.; Chen, X.S. Outcome Impacts Due to Pathogen-Specific Antimicrobial Resistance: A Narrative Review of Published Literature. Int. J. Environ. Res. Public Health 2020, 17, 1395. [Google Scholar] [CrossRef]
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.Y.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hu, C.Q.; Cheng, F.K.; Lu, X.W. Performance of a combined low-consumption biotreatment system with cost-effective ecological treatment technology for rural domestic sewage treatment. J. Water Process Eng. 2023, 51, 103380. [Google Scholar] [CrossRef]
- Bianco, K.; Barreto, C.; Oliveira, S.S.A.; Pinto, L.H.; Albano, R.M.; Miranda, C.C.; Clementino, M.M. Fecal pollution source tracking in waters intended for human supply based on archaeal and bacterial genetic markers. J. Water Health 2015, 13, 985–995. [Google Scholar] [CrossRef]
- Candido, D.; Bolsan, A.C.; Hollas, C.E.; Venturin, B.; Tapparo, D.C.; Bonassa, G.; Antes, F.G.; Steinmetz, R.L.R.; Bortoli, M.; Kunz, A. Integration of swine manure anaerobic digestion and digestate nutrients removal/recovery under a circular economy concept. J. Environ. Manag. 2022, 301, 113825. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Panesar, A.; Rued, S.B.; Olt, C.U. Ecological sanitation: Principles, technologies and project examples for sustainable wastewater and excreta management. Desalination 2009, 248, 392–401. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Z.; Uddin, S.M.N.; Mang, H.-P.; Zhou, X.; Zhang, J.; Zheng, L.; Zhang, L. Toilet revolution in China. J. Environ. Manag. 2018, 216, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Gao, Y.; Xu, Y.; Zhang, C.; Wang, Q.; Wei, X.; Yang, B.; Chen, P.; Li, H.; Li, Q. Application status and model types of three-compartment septic tanks in rural toilet reform in China. J. Agric. Resour. Environ. 2022, 39, 209–219. [Google Scholar] [CrossRef]
- Magri, M.E.; Fidjeland, J.; Jonsson, H.; Albihn, A.; Vinneras, B. Inactivation of adenovirus, reovirus and bacteriophages in fecal sludge by pH and ammonia. Sci. Total Environ. 2015, 520, 213–221. [Google Scholar] [CrossRef]
- Allievi, L.; Colombi, A.; Calcaterra, E.; Ferrari, A. Inactivation of faecal bacteria in sewage sludge by alkaline treatment. Bioresour. Technol. 1994, 49, 25–30. [Google Scholar] [CrossRef]
- Jensen, P.K.M.; Phuc, P.D.; Knudsen, L.G.; Dalsgaard, A.; Konradsen, F. Hygiene versus fertiliser: The use of human excreta in agriculture—A Vietnamese example. Int. J. Hyg. Environ. Health 2008, 211, 432–439. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; van Lierop, L.; Zamalloa, C.; Furlong, C.; Keteman, M.; Hu, B. Study of food waste degradation in a simulated septic tank. Waste Manag. Res. 2019, 37, 1199–1206. [Google Scholar] [CrossRef]
- Lusk, M.G.; Toor, G.S.; Yang, Y.-Y.; Mechtensimer, S.; De, M.; Obreza, T.A. A review of the fate and transport of nitrogen, phosphorus, pathogens, and trace organic chemicals in septic systems. Crit. Rev. Environ. Sci. Technol. 2017, 47, 455–541. [Google Scholar] [CrossRef]
- Ottoson, J.; Stenstrom, T.A. Faecal contamination of greywater and associated microbial risks. Water Res. 2003, 37, 645–655. [Google Scholar] [CrossRef]
- Appling, D.; Habteselassie, M.Y.; Radcliffe, D.; Bradshaw, J.K. Preliminary Study on the Effect of Wastewater Storage in Septic Tank on E. coli Concentration in Summer. Water 2013, 5, 1141–1151. [Google Scholar] [CrossRef]
- Li, Q.X.; Liu, X.N.; Zhang, H.Z.; Peterson, T.C.; Easterling, D.R. Detecting and adjusting temporal inhomogeneity in Chinese mean surface air temperature data. Adv. Atmos. Sci. 2004, 21, 260–268. [Google Scholar] [CrossRef]
- Zeng, H.; Xiao, C.; Chen, X.; Chen, Y.; Dianxiu, Y.E. State of China’s climate in 2019. Atmos. Ocean. Sci. Lett. 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, C.X.; Liu, F.; Chen, P.Z.; Wei, X.C.; Li, H.Y.; Yi, G.; Xu, Y.; Zheng, X.Q. Three-compartment septic tanks as sustainable on-site treatment facilities? Watch out for the potential dissemination of human-associated pathogens and antibiotic resistance. J. Environ. Manag. 2021, 300, 11. [Google Scholar] [CrossRef]
- Eaton, A.; Association, A.H.; Clesceri, L.; Federation, W.E.; Association, A.; Greenberg, A.; Association, A. Standard Methods for the Examination for Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Zhi, S.; Ding, G.; Li, A.; Guo, H.; Shang, Z.; Ding, Y.; Zhang, K. Fate of antibiotic resistance genes during high solid anaerobic digestion with pig manure: Focused on different starting modes. Bioresour. Technol. 2021, 328, 124849. [Google Scholar] [CrossRef] [PubMed]
- Giulietti, A.; Overbergh, L.; Valckx, D.; Decallonne, B.; Bouillon, R.; Mathieu, C. An overview of real-time quantitative PCR: Applications to quantify cytokine gene expression. Methods 2001, 25, 386–401. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Macfarlane, G.T.; Furrie, E.; Fite, A.; Macfarlane, S. Characterisation of intestinal bacteria in infant stools using real-time PCR and northern hybridisation analyses. Fems Microbiol. Ecol. 2005, 54, 77–85. [Google Scholar] [CrossRef]
- Sivaganesan, M.; Aw, T.G.; Briggs, S.; Dreelin, E.; Asian, A.; Dorevitch, S.; Shrestha, A.; Isaacs, N.; Kinzelman, J.; Kleinheinz, G.; et al. Standardized data quality acceptance criteria for a rapid Escherichia coli qPCR method (Draft Method C) for water quality monitoring at recreational beaches. Water Res. 2019, 156, 456–464. [Google Scholar] [CrossRef]
- Sheet, O.H.; Jwher, D.M.; Al-Sanjary, R.A.; Alajami, A.D. Direct detection of Staphylococcus aureus in camel milk in the Nineveh Governorate by using the PCR technique. Iraqi J. Vet. Sci. 2021, 35, 669–672. [Google Scholar] [CrossRef]
- Xiong, D.; Zhou, Y.; Song, L.; Liu, B.W.; Matchawe, C.; Chen, X.; Pelle, R.; Jiao, X.A.; Pan, Z.M. Development of a Duplex TaqMan Real-Time Polymerase Chain Reaction for Accurate Identification and Quantification of Salmonella Enteritidis from Laboratory Samples and Contaminated Chicken Eggs. Foods 2022, 11, 742. [Google Scholar] [CrossRef]
- Lopez-Vazquez, C.; Bandin, I.; Dopazo, C.P. Real-time RT-PCR for detection, identification and absolute quantification of viral haemorrhagic septicaemia virus using different types of standards. Dis. Aquat. Org. 2015, 114, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Rajeevan, M.S.; Ranamukhaarachchi, D.G.; Vernon, S.D.; Unger, E.R. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods 2001, 25, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Bukin, Y.S.; Galachyants, Y.P.; Morozov, I.V.; Bukin, S.V.; Zakharenko, A.S.; Zemskaya, T.I. The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci. Data 2019, 6, 190007. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Castillo, A.; Novoa, L.; Balsa-Castro, C.; Blanco, J.; Mira, A.; Tomas, I. Relationship between periodontitis-associated subgingival microbiota and clinical inflammation by 16S pyrosequencing. J. Clin. Periodontol. 2015, 42, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Ooi, M.L.; Drilling, A.J.; Morales, S.; Fong, S.; Moraitis, S.; Macias-Valle, L.; Vreugde, S.; Psaltis, A.J.; Wormald, P.-J. Safety and Tolerability of Bacteriophage Therapy for Chronic Rhinosinusitis Due to Staphylococcus aureus. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 723–729. [Google Scholar] [CrossRef]
- Jie, W.; Peng, Y.; Ren, N.; Li, B. Volatile fatty acids (VFAs) accumulation and microbial community structure of excess sludge (ES) at different pHs. Bioresour. Technol. 2014, 152, 124–129. [Google Scholar] [CrossRef]
- Salsali, H.R.; Parker, W.J.; Sattar, S.A. Impact of concentration, temperature, and pH on inactivation of Salmonella spp. by volatile fatty acids in anaerobic digestion. Can. J. Microbiol. 2006, 52, 279–286. [Google Scholar] [CrossRef]
- Odey, E.A.; Li, Z.; Zhou, X.; Yan, Y. Locally produced lactic acid bacteria for pathogen inactivation and odor control in fecal sludge. J. Clean. Prod. 2018, 184, 798–805. [Google Scholar] [CrossRef]
- Pratt, S.; Liew, D.; Batstone, D.J.; Werker, A.G.; Morgan-Sagastume, F.; Lant, P.A. Inhibition by fatty acids during fermentation of pre-treated waste activated sludge. J. Biotechnol. 2012, 159, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Mlinar, S.; Weig, A.R.; Freitag, R. Influence of NH3 and NH4+on anaerobic digestion and microbial population structure at increasing total ammonia nitrogen concentrations. Bioresour. Technol. 2022, 361, 127638. [Google Scholar] [CrossRef] [PubMed]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, A.; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sustain. Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Wei, X.; Yang, D.; Yin, X.; Yang, H.; Fang, Y.; Chen, N.; Zhang, H.; Hu, Z. Comparative study of efficiencies of purification of cadmium contaminated irrigation water by different purification systems. Sci. Total Environ. 2024, 907, 167941. [Google Scholar] [CrossRef] [PubMed]
- El Hadj, T.B.; Astals, S.; Gali, A.; Mace, S.; Mata-Alvarez, J. Ammonia influence in anaerobic digestion of OFMSW. Water Sci. Technol. 2009, 59, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Bio/Technol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Park, J.-H.; Yoon, J.-J.; Kumar, G.; Jin, Y.-S.; Kim, S.-H. Effects of acclimation and pH on ammonia inhibition for mesophilic methanogenic microflora. Waste Manag. 2018, 80, 218–223. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Tan, L.; Li, Q.; Liu, W.; Zhang, C.; Gao, Y.; Wei, X.; Gong, Q.; Zheng, X. What role does organic fertilizer actually play in the fate of antibiotic resistome and pathogenic bacteria in planting soil? J. Environ. Manag. 2022, 317, 115382. [Google Scholar] [CrossRef]
- Du, W.; Wang, F.; Fang, S.Y.; Huang, W.X.; Cheng, X.S.; Cao, J.S.; Fang, F.; Wu, Y.; Luo, J.Y. Antimicrobial PCMX facilitates the volatile fatty acids production during sludge anaerobic fermentation: Insights of the interactive principles, microbial metabolic profiles and adaptation mechanisms. Chem. Eng. J. 2022, 446, 137339. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Nie, W.K.; Lin, Y.; Wu, X.; Wu, S.H.; Li, X.; Cheng, J.J.; Yang, C.P. Chitosan-Fe3O4 composites enhance anaerobic digestion of liquor wastewater under acidic stress. Bioresour. Technol. 2023, 377, 128927. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Pang, L.A.; Chatzisymeon, E.; Yang, P. Effects of micron-scale zero valent iron on behaviors of antibiotic resistance genes and pathogens in thermophilic anaerobic digestion of waste activated sludge. Bioresour. Technol. 2023, 376, 128895. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Meng, X.H.; Song, Y.N.; Lv, X.Y.; Sun, Y. Effects of different concentrations of butyrate on microbial community construction and metabolic pathways in anaerobic digestion. Bioresour. Technol. 2023, 377, 128845. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Eyice, O.; Schnurer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef] [PubMed]
- Ariesyady, H.D.; Ito, T.; Okabe, S. Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Res. 2007, 41, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wan, X.; Sun, X.; Cao, Y.; Li, H.; Tian, Y.; Liu, X.; Kang, X.; Wang, Y. Effects of Livestock Manure and Straw Returning to Field on Microbial Community Structure Around Maize Rhizosphere. Biotechnol. Bull. 2020, 36, 137–146. [Google Scholar]
- Hudson, J.; Egan, S. Opportunistic diseases in marine eukaryotes: Could Bacteroidota be the next threat to ocean life? Environ. Microbiol. 2022, 24, 4505–4518. [Google Scholar] [CrossRef]
- Tian, R.M.; Ning, D.L.; He, Z.L.; Zhang, P.; Spencer, S.J.; Gao, S.H.; Shi, W.L.; Wu, L.W.; Zhang, Y.; Yang, Y.F.; et al. Small and mighty: Adaptation of superphylum Patescibacteria to groundwater environment drives their genome simplicity. Microbiome 2020, 8, 51. [Google Scholar] [CrossRef]
- Van der Kolk, J.H.; Endimiani, A.; Graubner, C.; Gerber, V.; Perreten, V. Acinetobacter in veterinary medicine, with an emphasis on Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2019, 16, 59–71. [Google Scholar] [CrossRef]
- Ozgur, O.; Aksaray, N. Acinetobacter Infections and Treatment. Çocuk Enfeksiyon Dergisi/J. Pediatr. Infect. 2014, 8, 28–32. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Yang, C.; Zhang, J.B.; Kalwar, Q.; Liang, Z.Y.; Li, C.; Du, M.; Yan, P.; Long, R.J.; Han, J.L.; et al. Effects of Dietary Energy Levels on Rumen Fermentation, Microbial Diversity, and Feed Efficiency of Yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Li, Y.J.; Chuang, C.H.; Cheng, W.C.; Chen, S.H.; Chen, W.L.; Lin, Y.J.; Lin, C.Y.; Shih, Y.H. A metagenomics study of hexabromocyclododecane degradation with a soil microbial community. J. Hazard. Mater. 2022, 430, 128465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, W.; Li, B.; Qian, S.; Wei, B.; Gong, S.; Wang, J.; Liu, M.; Wei, M. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp. Mol. Med. 2020, 52, 1959–1975. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Z.; Yu, K.Y.; Xiao, Y.; Wang, Y.L.; Xiao, D.; Wang, D.C. Comparative Genomic Analysis Reveals Potential Pathogenicity and Slow-Growth Characteristics of Genus Brevundimonas and Description of Brevundimonas pishanensis sp. nov. Microbiol. Spectr. 2022, 10, e0246821. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Selvakumar, R.; Sathishkumar, M.; Swaminathan, K.; Lakshmanaperumalsamy, P.; Singh, A.; Jain, S.K. Nitrate removal using Brevundimonas diminuta MTCC 8486 from ground water. Water Sci. Technol. 2009, 60, 517–524. [Google Scholar] [CrossRef]
- Sanchez-Osuna, M.; Barbe, J.; Erill, I. Comparative genomics of the DNA damage-inducible network in the Patescibacteria. Environ. Microbiol. 2017, 19, 3465–3474. [Google Scholar] [CrossRef]
- Wang, G.W.; Jin, Z.X.; Wang, X.X.; George, T.S.; Feng, G.; Zhang, L. Simulated root exudates stimulate the abundance of Saccharimonadales to improve the alkaline phosphatase activity in maize rhizosphere. Appl. Soil Ecol. 2022, 170, 104274. [Google Scholar] [CrossRef]

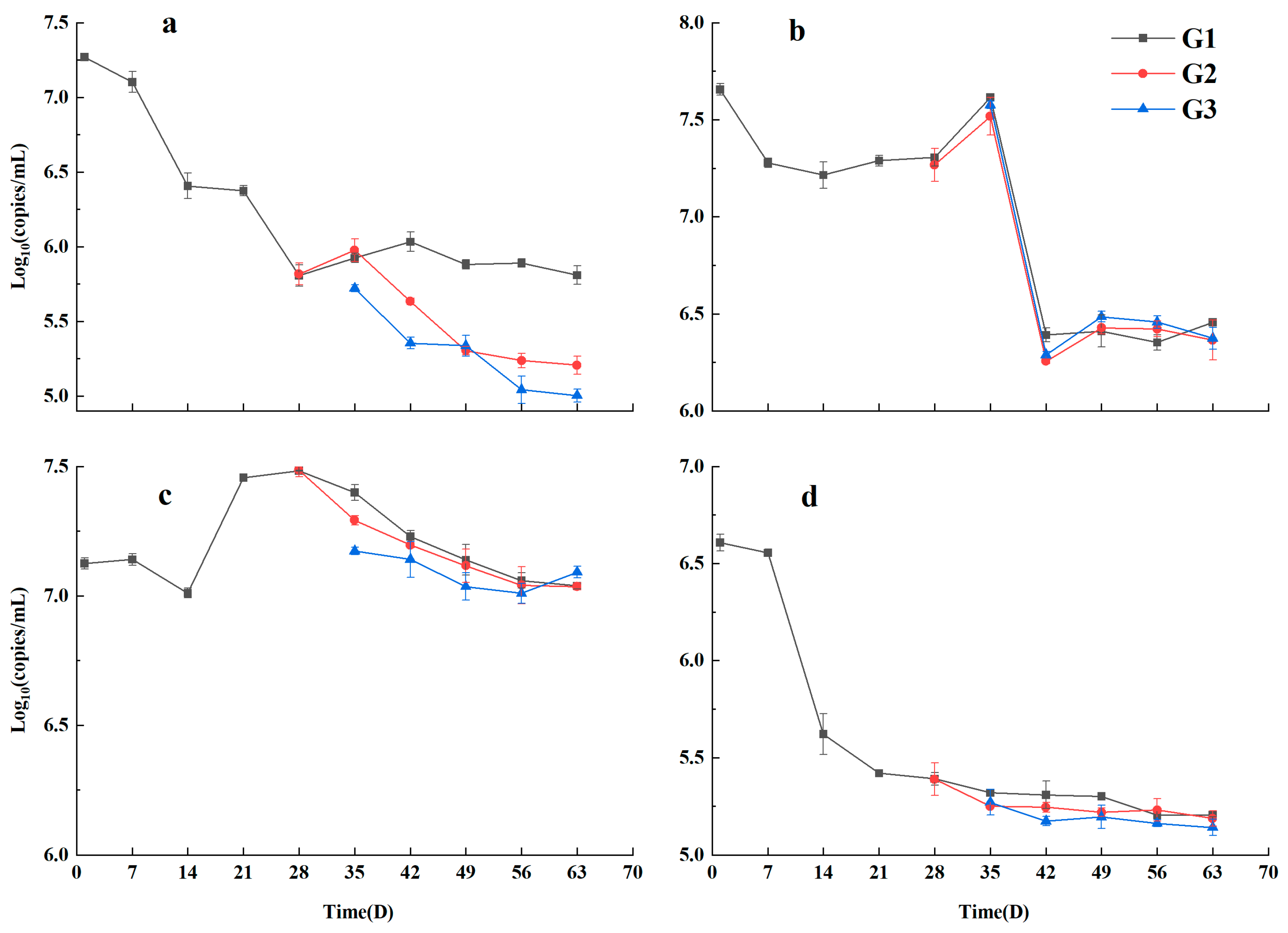

| Enterococcus faecalis | Escherichia coli | Staphylococcus aureus | Salmonella enteritidis |
|---|---|---|---|
| 7.270 ± 0.620 | 7.656 ± 0.443 | 7.125 ± 0.702 | 6.608 ± 0.638 |
| Treatment | Observed_Species | Shannon | Simpson | Chao1 | Goods_Coverage |
|---|---|---|---|---|---|
| D1G1 | 1034.00 | 6.91 | 0.97 | 1160.73 | 1.00 |
| D14G1 | 877.00 | 5.73 | 0.95 | 1212.25 | 0.99 |
| D28G1 | 938.00 | 5.32 | 0.91 | 1207.00 | 0.99 |
| D28G2 | 1006.00 | 6.17 | 0.94 | 1168.35 | 1.00 |
| D35G1 | 771.00 | 4.76 | 0.85 | 1120.96 | 0.99 |
| D35G2 | 743.00 | 5.28 | 0.91 | 942.30 | 1.00 |
| D35G3 | 567.00 | 5.67 | 0.94 | 692.53 | 1.00 |
| D63G1 | 611.90 | 5.00 | 0.93 | 760.76 | 1.00 |
| D63G2 | 471.00 | 3.55 | 0.83 | 688.35 | 1.00 |
| D63G3 | 691.00 | 6.09 | 0.96 | 839.35 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Yang, S.; Huang, J.; Liu, F.; Shen, F. Investigation of the Mechanism for Removal of Typical Pathogenic Bacteria from Three-Compartment Septic Tanks under Low Temperature Conditions. Processes 2024, 12, 87. https://doi.org/10.3390/pr12010087
Cheng S, Yang S, Huang J, Liu F, Shen F. Investigation of the Mechanism for Removal of Typical Pathogenic Bacteria from Three-Compartment Septic Tanks under Low Temperature Conditions. Processes. 2024; 12(1):87. https://doi.org/10.3390/pr12010087
Chicago/Turabian StyleCheng, Shenwei, Shuoxin Yang, Jianyin Huang, Fang Liu, and Feng Shen. 2024. "Investigation of the Mechanism for Removal of Typical Pathogenic Bacteria from Three-Compartment Septic Tanks under Low Temperature Conditions" Processes 12, no. 1: 87. https://doi.org/10.3390/pr12010087
APA StyleCheng, S., Yang, S., Huang, J., Liu, F., & Shen, F. (2024). Investigation of the Mechanism for Removal of Typical Pathogenic Bacteria from Three-Compartment Septic Tanks under Low Temperature Conditions. Processes, 12(1), 87. https://doi.org/10.3390/pr12010087








