Effects of Si Content on the Growth of Oxide Layers in Carbon Steels during the Heating Process
Abstract
:1. Introduction
2. Experimental Materials and Methods
3. Results and Discussion
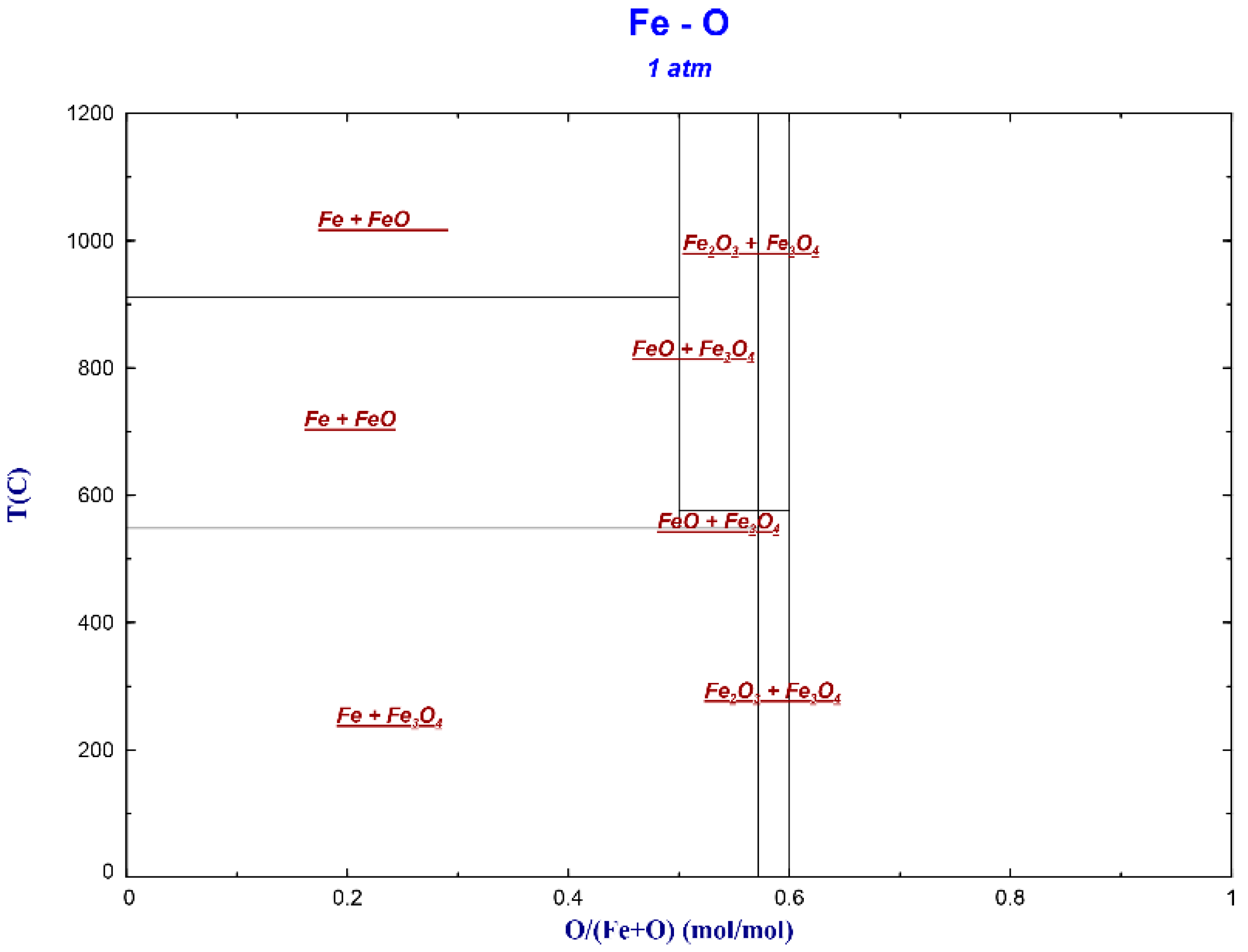
3.1. Fe-O Transformation Laws
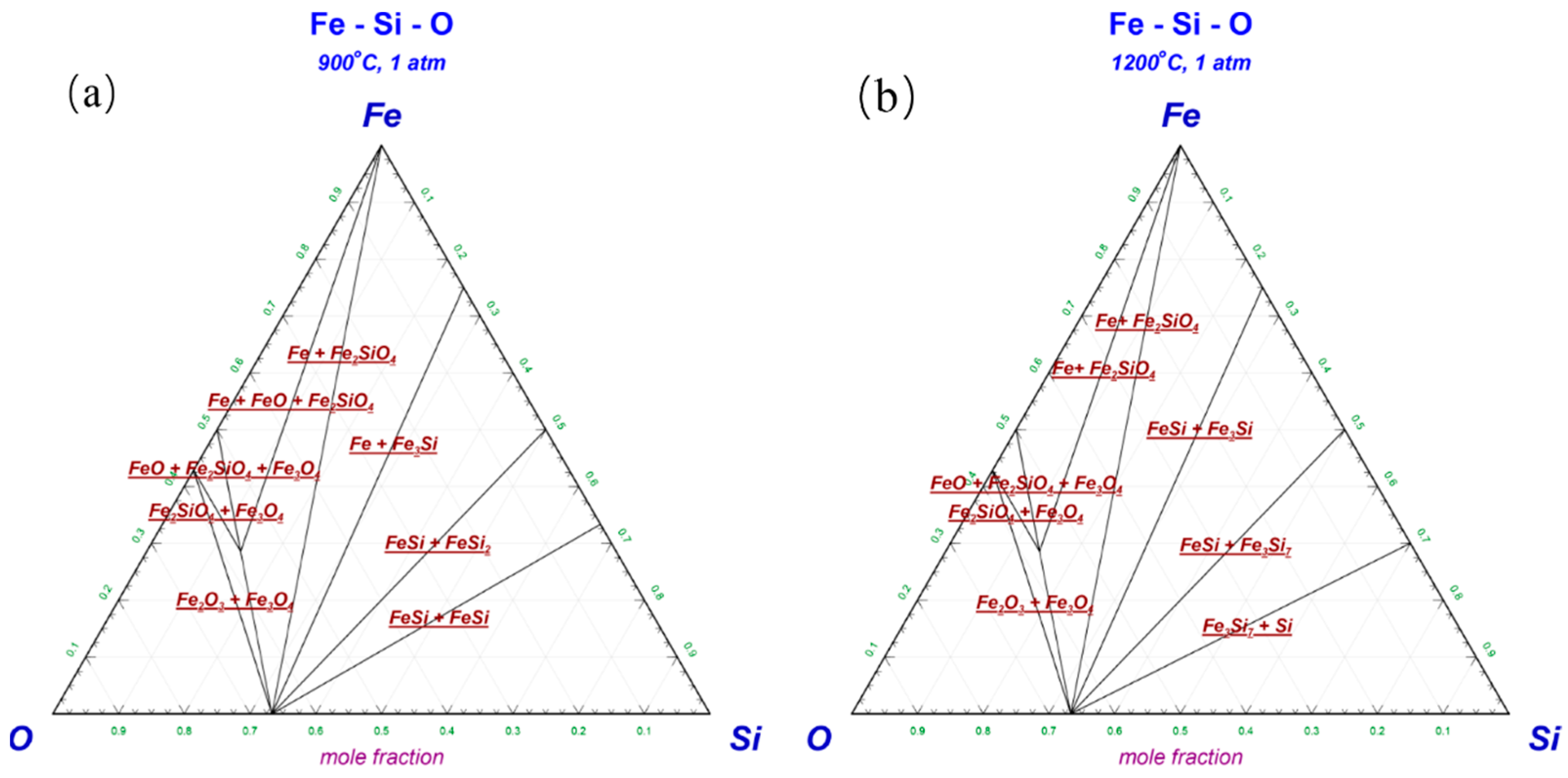
3.2. Fe-Si-O Phase Diagram and Transformation Laws
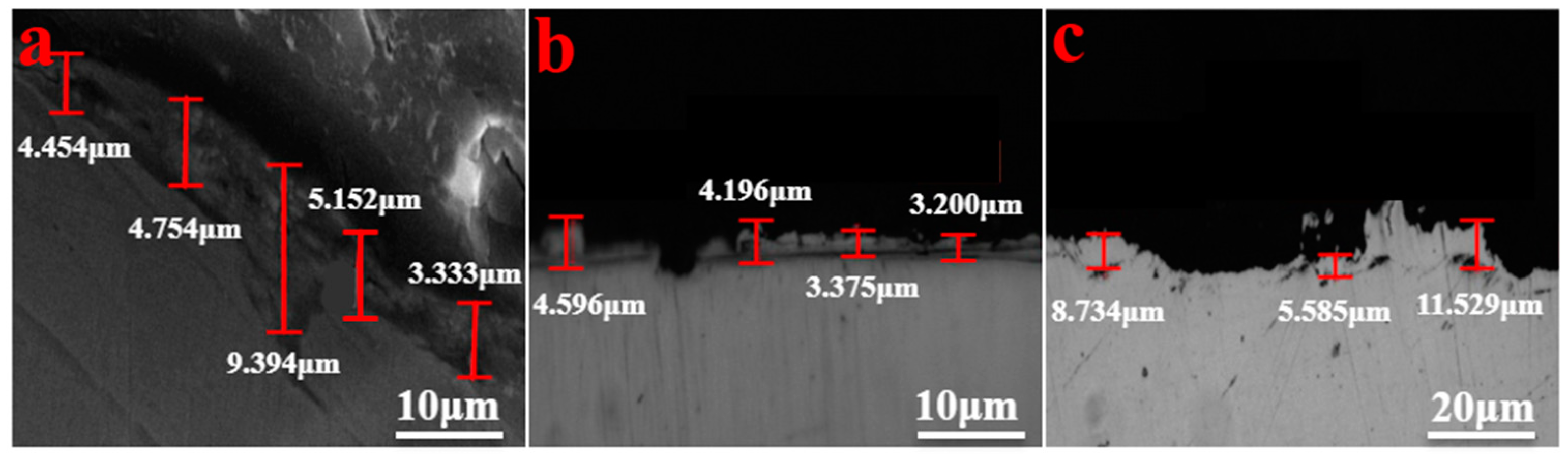
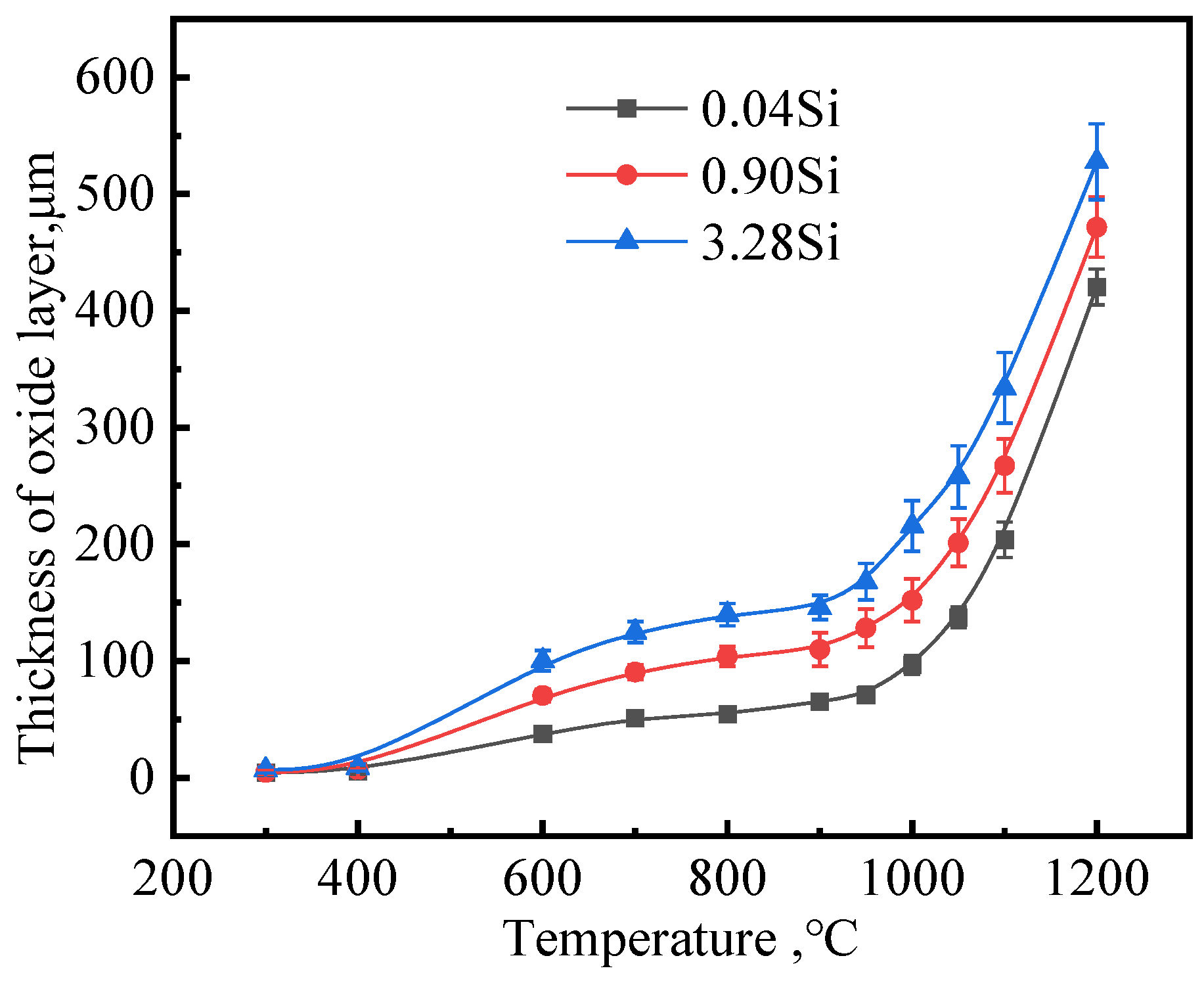
3.3. Effect of Si Content on Oxidation Behavior
3.4. Kinetic Analysis of Si Oxidation Behavior
4. Conclusions
- (1)
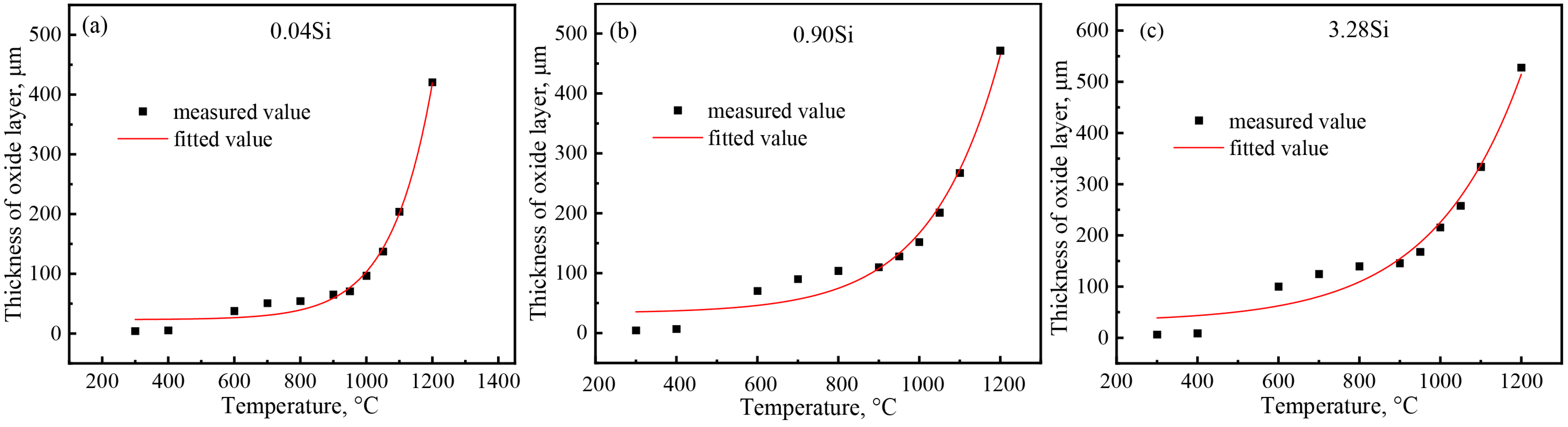
- By selecting three steel grades with large differences in silicon content, the influence of silicon content on the growth law of the oxidized layer during the heating process of carbon steel was analyzed. When the heating temperature is below 500 °C, the oxidation degree of the steel is not obvious, and the oxidized layer is extremely thin. When the temperature is between 500 °C and 900 °C, the thickness of the oxidized layer begins to increase, and when it exceeds 900 °C, the thickness of the oxidized layer increases sharply. At 1200 °C, the thicknesses of the oxidized layers of the three steels are similar.
- (2)
- The kinetic model of the thickness of the oxidized layer of the three tested steel plates and the heating temperature were obtained, and the growth curve of the thickness of the oxidized layer showed a parabolic growth law. The fitting value of 0.04Si steel is most consistent with the measured value, followed by 0.9Si steel and 3.2Si steel.
- (3)
- For steel with a higher silicon content, the iron olivine layer formed on the surface can effectively prevent the reaction between Fe and O, slowing down the increase in thickness of the oxidized layer. When the heating temperature exceeds the melting point of iron olivine, the inhibition effect of iron olivine decreases, and the thickness of the oxidized layer increases rapidly.
- (4)
- The morphology of iron olivine, phase transformation stress during heating, cooling shrinkage, and preparation methods will cause cracks in the oxidized layer, or even peeling off.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Cha, X.; Chen, L. Control techniques of oxide scales in hot-rolled heavy and medium plates. J. Northeast. Univ. 2011, 32, 960–963. [Google Scholar]
- Cao, G.; Wang, C.; Jiang, Y.; Li, S.; Gao, X.; Liu, Z. Effect of Cr on Isothermal Structure Transformation of Iron Oxide Scale. Steel Res. Int. 2022, 94, 2200512. [Google Scholar] [CrossRef]
- Picqué, B.; Bouchard, P.-O.; Montmitonnet, P.; Picard, M. Mechanical behaviour of iron oxide scale: Experimental and numerical study. Wear 2006, 260, 231–242. [Google Scholar] [CrossRef]
- Hidaka, Y.; Anraku, T.; Otsuka, N. Deformation of iron oxides upon tensile tests at 600–1250 C. Oxid. Met. 2003, 59, 97–113. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- He, Y.-Q.; Jia, T.; Liu, X.-J.; Cao, G.-M.; Liu, Z.-Y.; Li, J. Hot-dip Galvanizing of Carbon Steel after Cold Rolling with Oxide Scale and Hydrogen Descaling. J. Iron Steel Res. Int. 2014, 21, 222–226. [Google Scholar] [CrossRef]
- Bolt, P.H. Understanding the properties of oxide scales on hot rolled steel strip. Steel Res. Int. 2004, 75, 399–404. [Google Scholar] [CrossRef]
- Li, Z.-F.; Wang, H.; Gao, Y.; Cao, G.-M.; Liu, Z.-Y. Reduction Behavior of Oxide Scales Containing Different Structures on the Surface of Hot-Rolled Steel Strip in Gas Mixture H2/Ar at 400–625 °C. Steel Res. Int. 2020, 91, 1900333. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Y.F.; Yang, Z.Z.; Rao, J.P.; Hong, X. Analyzing and control of surface inclusion defect of hot-rolled coil. Adv. Mater. Res. 2013, 753, 230–235. [Google Scholar] [CrossRef]
- Cao, G.; Li, Z.; Wang, H.; Liu, Z. Research process on key technology of hot-dip galvanizing technology of hot-rolled strip based on direct reduction annealing without acid-pickling. J. Iron Steel Res. 2022, 34, 9. [Google Scholar]
- Sun, B.; Cheng, L.; You, H.-G.; Cao, G.-M. Microstructure Transformation Behavior of Hot-Rolled Steel Oxide Scale during the Preheating Process. Steel Res. Int. 2021, 92, 2000422. [Google Scholar] [CrossRef]
- Takeda, M.; Onishi, T.; Nakakubo, S.; Fujimoto, S. Physical properties of iron-oxide scales on Si-containing steels at high temperature. Mater. Trans. 2009, 50, 2242–2246. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, S.; Guo, X.; Guan, J.; Wang, G.; Liu, J. Formation and Elimination of Red Scale on Hot Rolled Strip. Iron Steel 2006, 41, 7. [Google Scholar]
- Cao, G.-M.; Wu, T.-Z.; Xu, R.; Li, Z.-F.; Wang, F.-X.; Liu, Z.-Y. Effects of Coiling Temperature and Cooling Condition on Transformation Behavior of Tertiary Oxide Scale. J. Iron Steel Res. Int. 2015, 22, 892–896. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, C.-H.; Lin, S.; Chen, C.; Tsai, W. Effects of Si and its content on the scale formation on hot-rolled steel strips. Mater. Chem. Phys. 2008, 112, 566–571. [Google Scholar] [CrossRef]
- Sun, B.; You, H.G.; Hao, M.X. Kinetics of high temperature oxidation of 0.09C-0.5Mn-0.22/1.9Si steel. Kang T’ieh/Iron Steel 2018, 53, 53–56. [Google Scholar] [CrossRef]
- Cao, G.M.; Shan, W.C.; Liu, X.J.; Wang, C.Y. High temperature oxidation behavior of Fe-2.2%Si steel in different atmosphere. Iron Steel 2022, 57, 132–142. [Google Scholar] [CrossRef]
- Suárez, L.; Schneider, J.; Houbaert, Y. Effect of Si on High-Temperature Oxidation of Steel during Hot Rolling. Defect Diffus. Forum 2008, 273–276, 655–660. [Google Scholar] [CrossRef]
- Wang, J.; Wan, S.; Yi, G.; Jiang, J.; Cheng, Q.; Shan, Y.; Wang, W. Influence of spinning temperature on microstructure and mechanical descaling property of oxide layer of 82B wire rod. Trans. Mater. Heat Treat. 2023, 44, 109–117. [Google Scholar]
- Himmel, L.; Mehl, R.; Birchenall, C.E. Self-diffusion of iron in iron oxides and the Wagner theory of oxidation. Jom 1953, 5, 827–843. [Google Scholar] [CrossRef]
- Martin, D.; Guinea, D.M.; García-Alegre, M.C.; Villanueva, E.; Guinea, D. Multi-modal defect detection of residual oxide scale on a cold stainless steel strip. Mach. Vis. Appl. 2010, 21, 653–666. [Google Scholar] [CrossRef]
- Deng, G.Y.; Tieu, A.K.; Si, L.Y.; Su, L.H.; Lu, C.; Wang, H.; Liu, M.; Zhu, H.T.; Liu, X.H. Influence of cold rolling reduction on the deformation behaviour and crystallographic orientation development. Comput. Mater. Sci. 2014, 81, 2–9. [Google Scholar] [CrossRef]
- Li, R.; Ye, D.; Xu, Z.; Yin, C.; Xu, H.; Zhou, H.; Yi, J.; Chen, Y.; Pan, J. Nondestructive Evaluation of Thermal Barrier Coatings Thickness Using Terahertz Time-Domain Spectroscopy Combined with Hybrid Machine Learning Approaches. Coatings 2022, 12, 1875. [Google Scholar] [CrossRef]



| Sample | C | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|---|
| 0.04Si | 0.07 | 0.04 | 0.27 | 0.0091 | 0.0027 | Bar. |
| 0.90Si | 0.07 | 0.90 | 0.28 | 0.0214 | 0.0085 | Bar. |
| 3.28Si | 0.08 | 3.28 | 0.29 | 0.0106 | 0.0055 | Bar. |
| Sample | A1 | A2 | A3 | A4 |
|---|---|---|---|---|
| 0.04Si | 23.26300 | 2,440,894.75392 | 2291.66558 | 0.00347 |
| 0.90Si | 33.16924 | 5,229,486.46421 | 2809.67759 | 0.00254 |
| 3.28Si | 30.69415 | 8,305,158.01963 | 3344.35849 | 0.00197 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Chen, Y.; Wu, X.; Jiang, Y.; Fan, P. Effects of Si Content on the Growth of Oxide Layers in Carbon Steels during the Heating Process. Processes 2024, 12, 88. https://doi.org/10.3390/pr12010088
Wang Q, Chen Y, Wu X, Jiang Y, Fan P. Effects of Si Content on the Growth of Oxide Layers in Carbon Steels during the Heating Process. Processes. 2024; 12(1):88. https://doi.org/10.3390/pr12010088
Chicago/Turabian StyleWang, Qingxia, Yongli Chen, Xin Wu, Yueyue Jiang, and Peigeng Fan. 2024. "Effects of Si Content on the Growth of Oxide Layers in Carbon Steels during the Heating Process" Processes 12, no. 1: 88. https://doi.org/10.3390/pr12010088
APA StyleWang, Q., Chen, Y., Wu, X., Jiang, Y., & Fan, P. (2024). Effects of Si Content on the Growth of Oxide Layers in Carbon Steels during the Heating Process. Processes, 12(1), 88. https://doi.org/10.3390/pr12010088







