Role of Natural Phytoconstituents as a Potential Bioenhancer of Anti-Cancer and Anti-Microbial Agents: Spotlight on the Mechanism of Action, Clinical Studies and Patents
Abstract
:1. Introduction
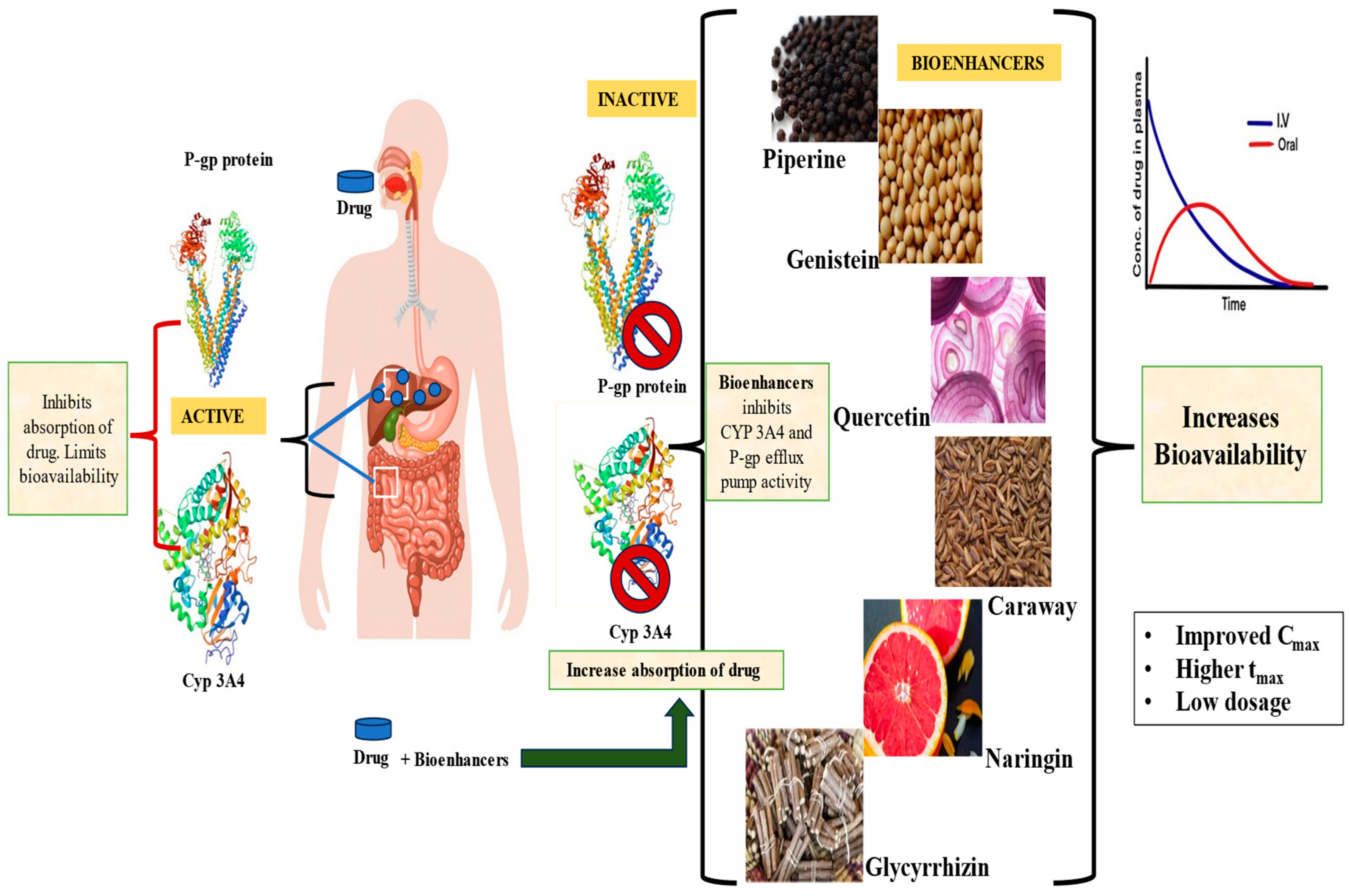
2. Enhanced Efficiency of Therapeutic Agents: Role of Selective Bioenhancers
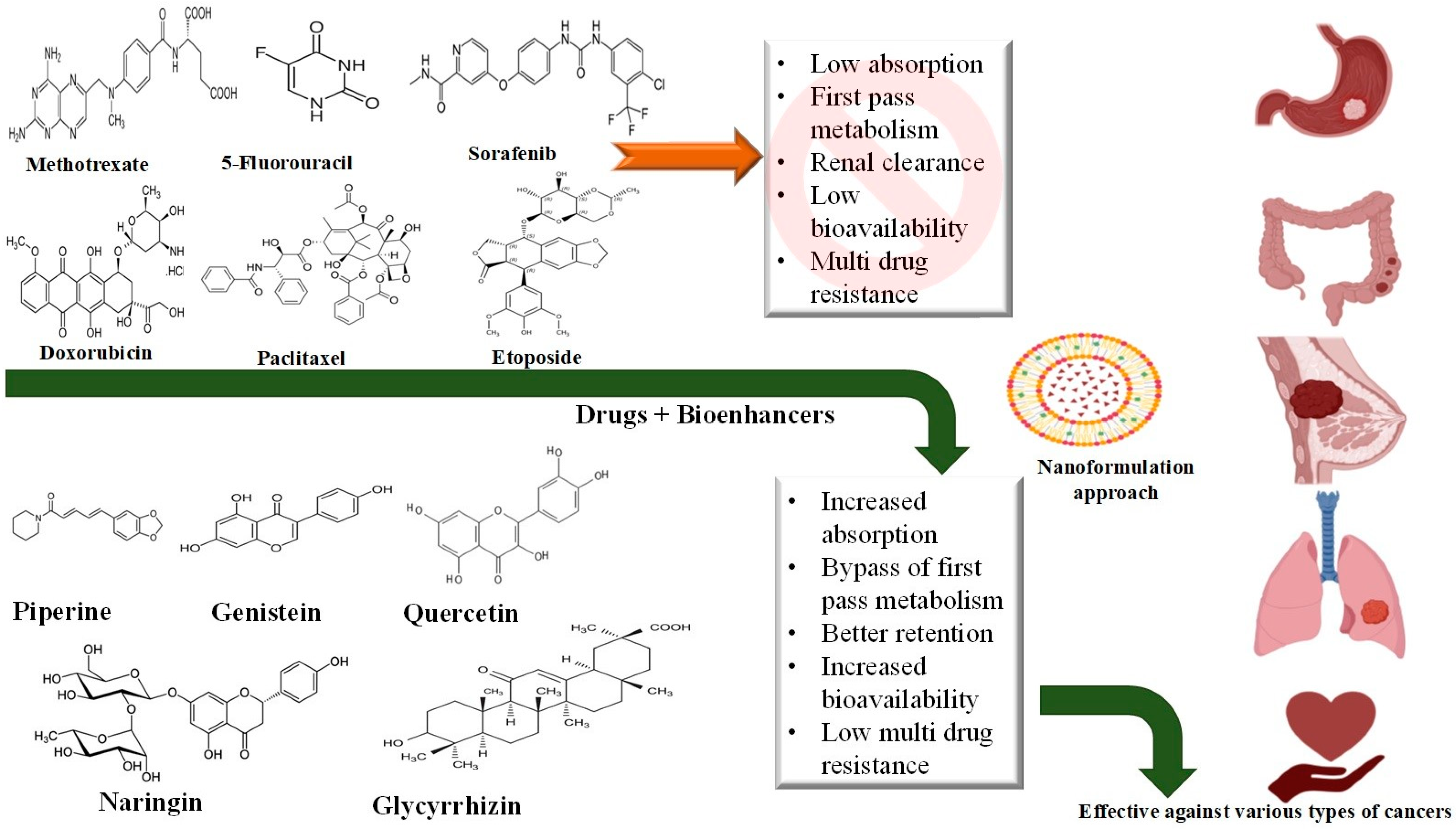
2.1. Anti-Cancer Agents
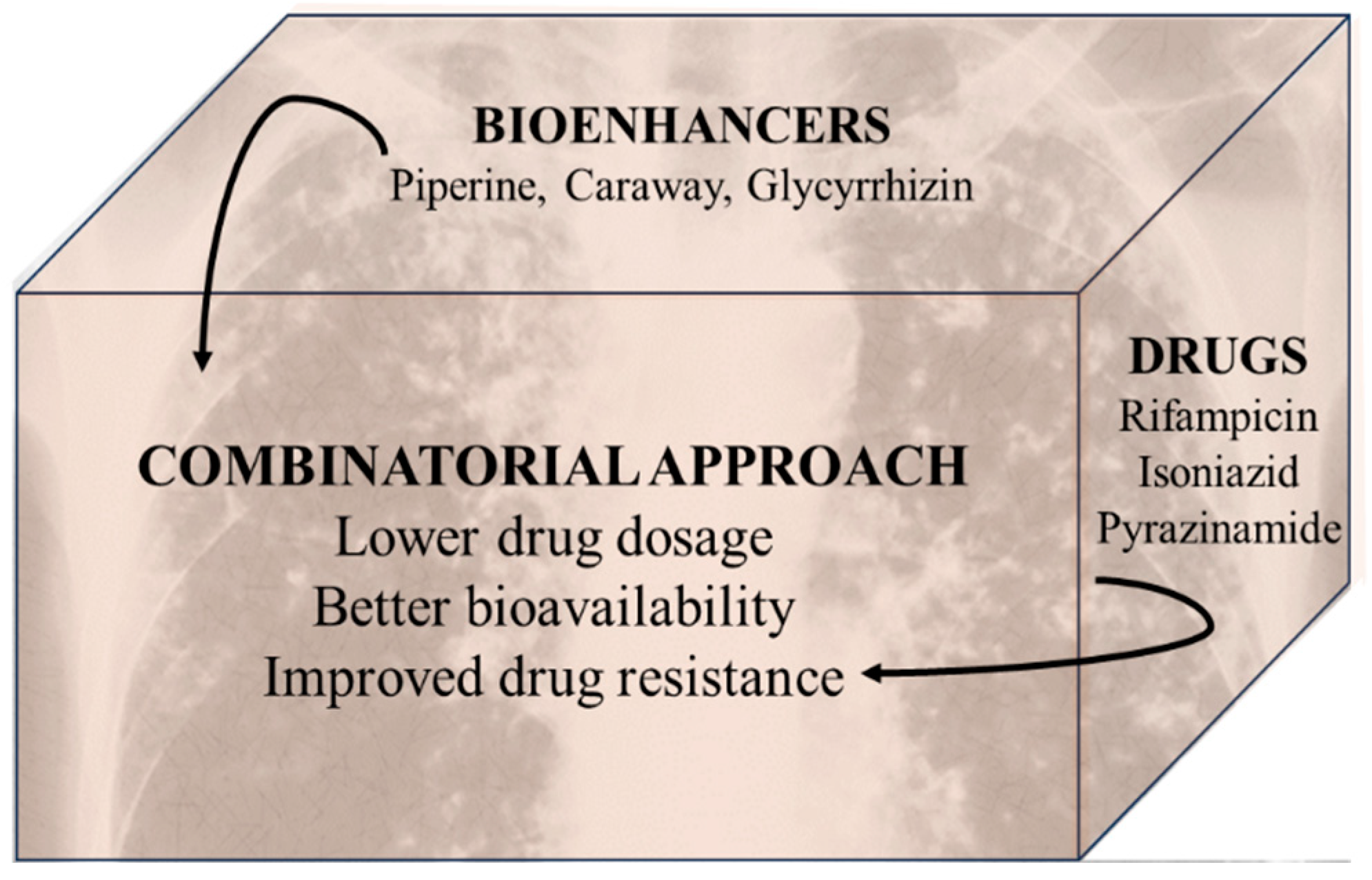
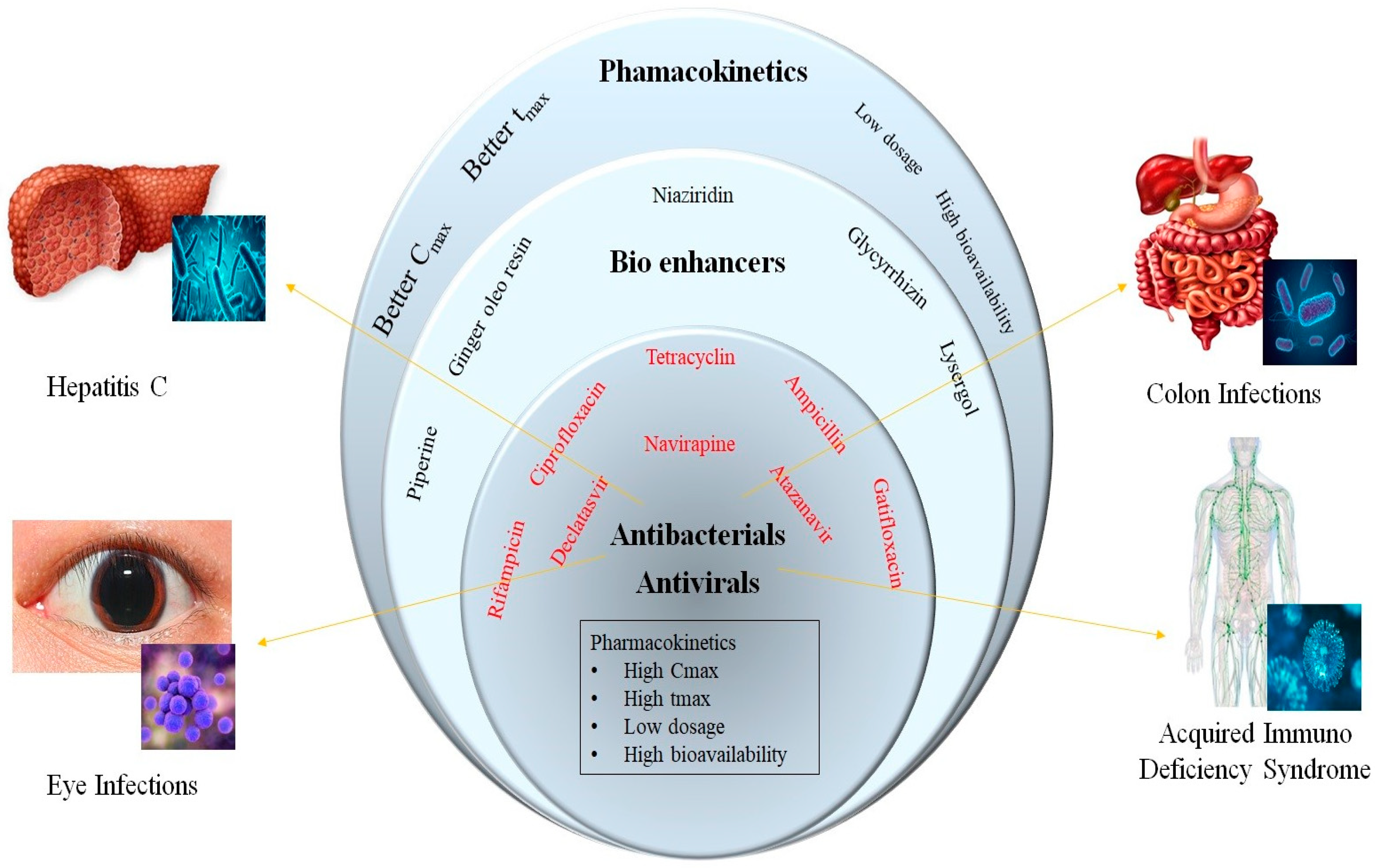
2.2. Anti-Microbial Agents
2.2.1. Anti-Tubercular Agents
2.2.2. Miscellaneous Agents
3. Mechanism of Action of Bioenhancers
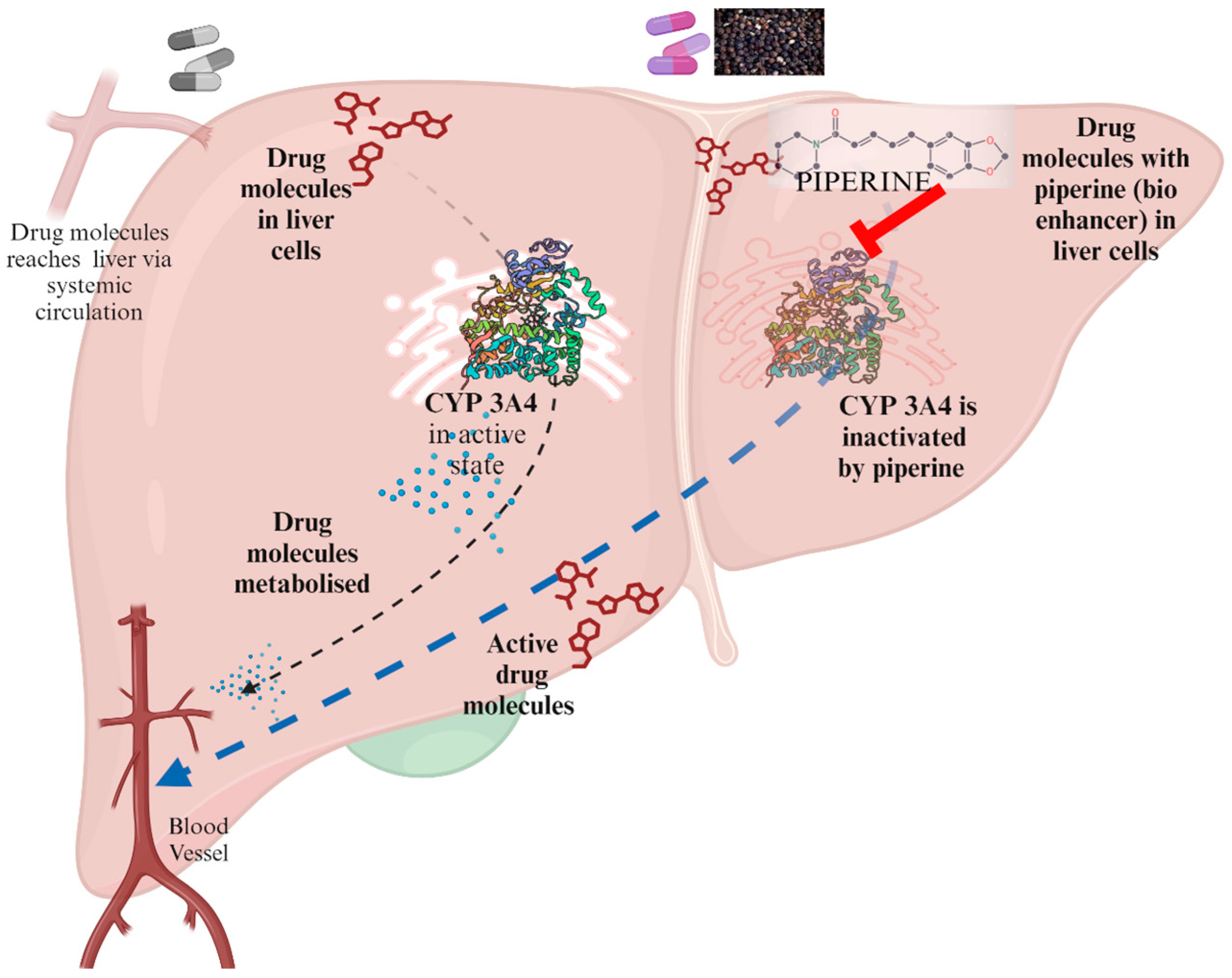
3.1. Bypassing the Metabolism by Liver Enzymes
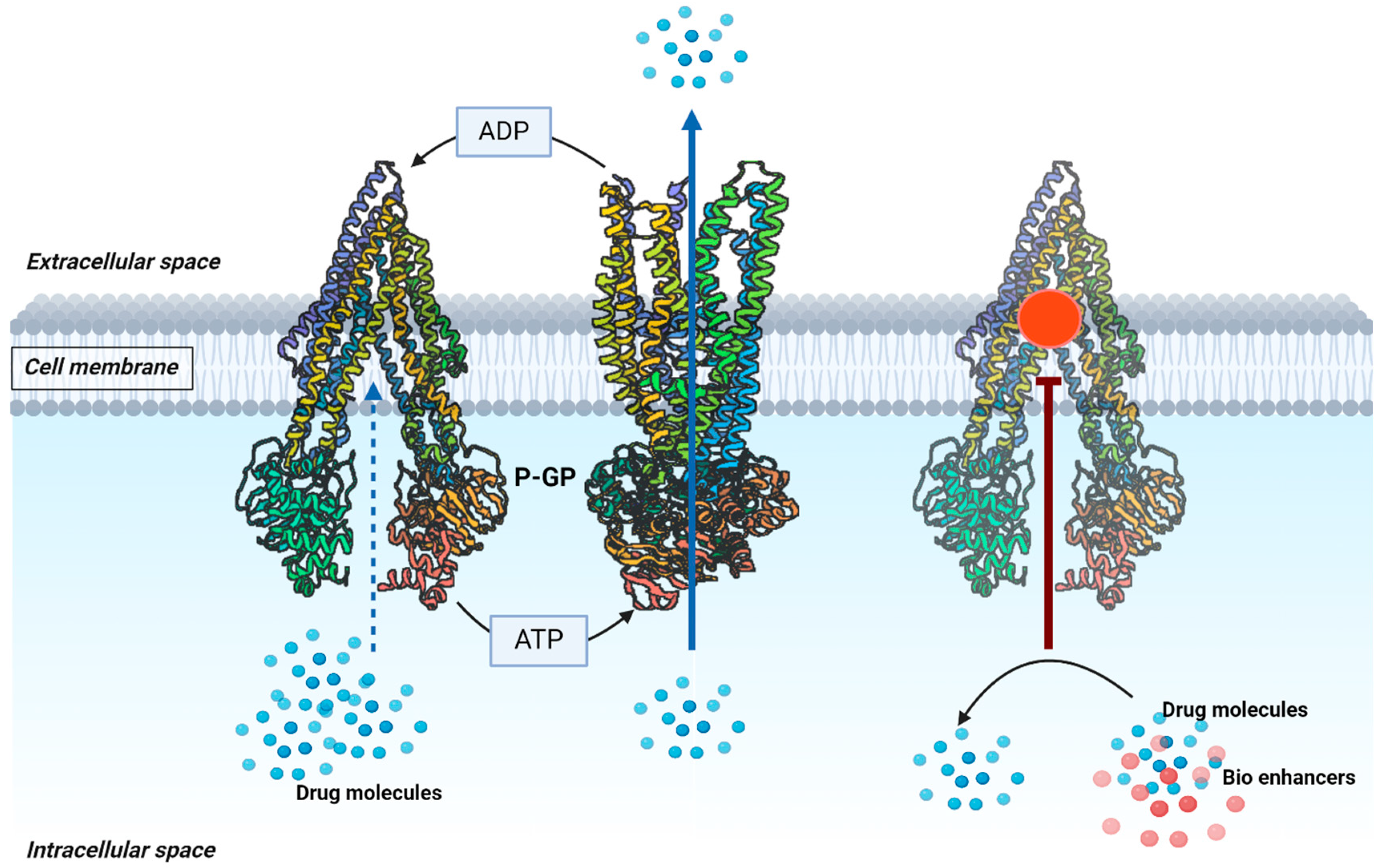
3.2. Inhibiting the P-gp Efflux Pump
3.3. Miscellaneous Mechanisms
4. Natural Bioenhancers in Novel Drug Delivery Formulations
5. Clinical Studies to Assess the Safety and Effectiveness of Bioenhancers
6. Patent Review
7. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGS-cyr 61 | Gastric adenocarcinoma cysteine-rich angiogenic inducer 61 |
| ATP | Adenosine triphosphate |
| AUC | Area under the curve |
| B. subtilis | Bacillus subtilis |
| C max | Maximum serum concentration |
| C. carvi | Carum carvi |
| C. cyminum | Cuminum cyminum |
| Caco2 | Cancer coli-2 cells |
| Carum carvi L. | Carum carvi Linnaeus |
| COVID | Coronavirus disease 2019 |
| CYP | Cytochrome |
| DNA | Deoxy ribonucleic acid |
| DNMT3B | DNA methyltransferase 3B |
| E. coli | Escherichia coli |
| EP | European patent |
| FDC | Fixed-dose combination |
| FOLFOX | Folinic acid, fluorouracil and oxaliplatin |
| FU | 5-Fluorouracil |
| G. glabra | Glycyrrhiza glabra |
| GI | Gastrointestinal tract |
| H37Rv | Mycobacterium tuberculosis strain H37 rough virulent |
| HDAC3 | Histone deacetylase 3 |
| HepG2 | Hepatoblastoma cell line |
| HIV/AIDS | Human immunodeficiency virus/acquired immunodeficiency syndrome |
| I | Isoniazid |
| IC50 | Half-maximal inhibitory concentration |
| KB | KERATIN-forming tumor cell line HeLa. |
| KB-V1 | Multidrug-resistant human KB carcinoma cells |
| MCF | Michigan Cancer Foundation-7 |
| MDA-MB-231 | M.D. Anderson metastasis breast cancer |
| MDCK | Madin–Darby canine kidney |
| MDCK-MDR1 | Madin–Darby canine kidney cells transfected with either the multidrug resistance gene-1 |
| MDP | Methylenedioxyphenyl ring |
| MDR | Multidrug resistance |
| Mentha spicata L. | Mentha spicata Linnaeus |
| MIC | Minimum inhibitory concentration |
| MTX | Methotrexate |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| NCT | National clinical trial |
| P | Pyrazinamide |
| P450 | Cytochrome P450 |
| Papp | Apparent permeability coefficient |
| PGLA | Poly lactic-co-glycolic acid |
| P-gp | P-glycoprotein |
| R | Rifampicin |
| RT-qPCR | Quantitative reverse-transcription polymerase chain reaction |
| STP | Sorafenib with thymoquinone and piperine |
| SW480 | Cells from the large intestine of a Dukes C colorectal cancer patient |
| t max | Maximum time |
| UDP-GT | Uridine 5′-diphospho-glucuronosyltransferase |
| US | United States |
| WHO | World Health Organization |
| μM | Micromolar |
References
- Ahamad, J. A Comprehensive Review on Natural Bioenhancers. Int. Res. J. Pharm. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Bhardwaj, R.K.; Glaeser, H.; Becquemont, L.; Klotz, U.; Gupta, S.K.; Fromm, M.F. Piperine, a Major Constituent of Black Pepper, Inhibits Human P-Glycoprotein and CYP3A4. J. Pharmacol. Exp. Ther. 2002, 302, 645–650. [Google Scholar] [CrossRef]
- Thorat, S.S.; Gujar, K.N.; Karale, C.K. Bioenhancers from Mother Nature: An Overview. Future J. Pharm. Sci. 2023, 9, 20. [Google Scholar] [CrossRef]
- Jhanwar, B.; Gupta, S. Biopotentiation Using Herbs: Novel Technique for PoorBioavailable Drugs. Int. J. PharmTech Res. 2014, 6, 443–454. [Google Scholar]
- Drabu, S.; Khatri, S.; Babu, S.; Lohani, P. Use of Herbal Bioenhancers to Increase the Bioavailability of Drugs. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 107–119. [Google Scholar]
- Randhawa, G.; Kullar, J. Rajkumar Bioenhancers from Mother Nature and Their Applicability in Modern Medicine. Int. J. Appl. Basic Med. Res. 2011, 1, 5. [Google Scholar] [CrossRef]
- Global Cancer Burden Growing, Amidst Mounting Need for Services. World Health Organization. 2024. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 7 July 2024).
- Fleck, A. TB Is the World’s Second Deadliest Infectious Disease; Statista: London, UK, 2023. [Google Scholar]
- Nishat, A.; Thaiseen, S.; Rajesh, B.; Sama, V. A Strategy to Enhance Bioavailability of Drug Candidates: Natural Bioenhancers. SunText Rev. Pharm. Sci. 2021, 1, 10–14. [Google Scholar] [CrossRef]
- Breedveld, P.; Beijnen, J.H.; Schellens, J.H.M. Use of P-Glycoprotein and BCRP Inhibitors to Improve Oral Bioavailability and CNS Penetration of Anticancer Drugs. Trends Pharmacol. Sci. 2006, 27, 17–24. [Google Scholar] [CrossRef]
- Kang, M.J.; Cho, J.Y.; Shim, B.H.; Kim, D.K.; Lee, J. Bioavailability Enhancing Activities of Natural Compounds from Medicinal Plants. J. Med. Plant Res. 2009, 3, 1204–1211. [Google Scholar]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and Pharmacological Aspects of Piperine as a Potential Molecule for Disease Prevention and Management: Evidence from Clinical Trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef]
- Qazi, G.N.; Bedi, K.L.; Johri, R.K.; Tikoo, M.K.; Tikoo, A.K.; Sharma, S.C.; Abdullah, S.T.; Suri, O.P.; Gupta, B.D.; Suri, K.A.; et al. Bioavailability/Bioefficacy Enhancing Activity of Cuminum cyminum and Extracts and Fractions Thereof. U.S. Patent US007514105B2, 7 April 2009. [Google Scholar]
- El-Shehawy, A.A.; Elmetwalli, A.; El-Far, A.H.; Mosallam SA, E.R.; Salama, A.F.; Babalghith, A.O.; Mahmoud, M.A.; Mohany, H.; Gaber, M.; El-Sewedy, T. Thymoquinone, Piperine, and Sorafenib Combinations Attenuate Liver and Breast Cancers Progression: Epigenetic and Molecular Docking Approaches. BMC Complement. Med. Ther. 2023, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.; Golovine, K.; Canter, D.; Kutikov, A.; Simhan, J.; Corlew, M.M.; Uzzo, R.G.; Kolenko, V.M. Co-Administration of Piperine and Docetaxel Results in Improved Anti-Tumor Efficacy via Inhibition of CYP3A4 Activity. Prostate 2011, 72, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mittal, V. Pawan Jalwal Role of Herbal Bioenhancers (Piperine and Curcumin) on the Oral Bioavailability of Tamoxifen Using Experimental Rats. J. Pharmacogn. Phytochem. 2015, 4, 133–136. [Google Scholar]
- Najar, I.A.; Sharma, S.C.; Singh, G.D.; Koul, S.; Gupta, P.N.; Javed, S.; Johri, R.K. Involvement of P-Glycoprotein and CYP 3A4 in the Enhancement of Etoposide Bioavailability by a Piperine Analogue. Chem.-Biol. Interact. 2011, 190, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Choi, J.-S. Effects of Quercetin on the Pharmacokinetics of Etoposide after Oral or Intravenous Administration of Etoposide in Rats. PubMed 2009, 29, 1411–1415. [Google Scholar]
- Choi, J.-S.; Piao, Y.-J.; Kang, K.W. Effects of Quercetin on the Bioavailability of Doxorubicin in Rats: Role of CYP3A4 and P-Gp Inhibition by Quercetin. Arch. Pharmacal Res. 2011, 34, 607–613. [Google Scholar] [CrossRef]
- Dudhatra, G.B.; Mody, S.K.; Awale, M.M.; Patel, H.B.; Modi, C.M.; Kumar, A.; Kamani, D.R.; Chauhan, B.N. A Comprehensive Review on Pharmacotherapeutics of Herbal Bioenhancers. Sci. World J. 2012, 2012, 637953. [Google Scholar] [CrossRef]
- Lim, S.-C.; Choi, J.-S. Effects of Naringin on the Pharmacokinetics of Intravenous Paclitaxel in Rats. Biopharm. Drug Dispos. 2006, 27, 443–447. [Google Scholar] [CrossRef]
- Li, X.; Yun, J.; Choi, J. Effects of Morin on the Pharmacokinetics of Etoposide in Rats. Biopharm. Drug Dispos. 2007, 28, 151–156. [Google Scholar] [CrossRef]
- Limtrakul, P.; Khantamat, O.; Pintha, K. Inhibition of P-Glycoprotein Function and Expression by Kaempferol and Quercetin. J. Chemother. 2005, 17, 86–95. [Google Scholar] [CrossRef]
- Hyun, H.; Moon, J.; Cho, S. Quercetin Suppresses CYR61-Mediated Multidrug Resistance in Human Gastric Adenocarcinoma AGS Cells. Molecules 2018, 23, 209. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.L.; Tolcher, A.; O’Shaughnessy, J.A.; Denicoff, A.M.; Noone, M.; Ognibene, F.P.; Cowan, K.H.; Balis, F.M. Effect of R-Verapamil on the Pharmacokinetics of Paclitaxel in Women with Breast Cancer. J. Clin. Oncol. 1995, 13, 2039–2042. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Al Balushi, K.; Francis, A.P.; Khan, S.A. Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review. Pharmaceutics 2024, 16, 761. [Google Scholar] [CrossRef] [PubMed]
- Gade, J.; Jain, B.; Rawat, R.; Prashant Sharma, P.; Gupta, P. An Effective Nanoparticles for Drug Delivery System. Mater. Today Proc. 2021, 51, A1–A7. [Google Scholar] [CrossRef]
- Khanuja SP, S.; Kumar, S.; Arya, J.S.; Shasany, A.K.; Singh, M.; Awasthi, S.; Gupta, S.C.; Darokar, M.P.; Rahman, L.U. Composition Comprising Pharmaceutical/Nutraceutical Agent and a Bioenhancer Obtained from Glycyrrhizaglabra. U.S. Patent US006979471B1, 27 December 2005. [Google Scholar]
- Khajuria, V.; Tandon, V.; Arora, E.; Choudhary, N.; Gillani, Z. Effect of Carum Carvi, a Herbal Bioenhancer on Pharmacokinetics of Antitubercular Drugs: A Study in Healthy Human Volunteers. Perspect. Clin. Res. 2014, 5, 80. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, A.; Gupta, N.; Tarke, C. Role of Bioenhancers in Tuberculosis. Int. J. Health Sci. Res. 2016, 6, 307–313. [Google Scholar]
- Nageswari, A.D.; Rajanandh, M.G.; Uday, M.K.R.A.; Nasreen, R.J.; Pujitha, R.R.; Prathiksha, G. Effect of Rifampin with Bio-Enhancer in the Treatment of Newly Diagnosed Sputum Positive Pulmonary Tuberculosis Patients: A Double-Center Study. J. Clin. Tuberc. Other Mycobact. Dis. 2018, 12, 73–77. [Google Scholar] [CrossRef]
- Bokolia, N.P.; Bag, K.; Sarkar, B.; Jhawar, R.; Chatterji, D.; Jayaraman, N.; Ghosh, A. A Novel Vitamin c Analog Acts as a Potent Bio-Enhancer to Augment the Activities of Anti-Tuberculosis Drugs against Mycobacterium Tuberculosis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Khanuja, S.P.S.; Kumar, S.; Arya, J.S.; Shasany, A.K.; Singh, M.; Awasthi, S. Composition Comprising Pharmaceutical/Nutraceutical Agent and a Bioenhancer Obtained from Glycyrrhizaglabra. U.S. Patent US20060057234A1, 16 March 2006. [Google Scholar]
- Kasibhatta, R.; Naidu, M.U.R. Influence of Piperine on the Pharmacokinetics of Nevirapine under Fasting Conditions. Drugs R D 2007, 8, 383–391. [Google Scholar] [CrossRef]
- Prakash, S.; Kherde, P.; Rangari, V. Bioenhancement Effect of Piperine and Ginger Oleo Resin on the Bioavailability of Atazanvir. Int. J. Pharm. Pharm. Sci./Int. J. Pharm. Pharm. Sci. 2015, 7, 241–245. [Google Scholar]
- Magotra, A.; Kotwal, P.; Bhatt, S.; Dogra, A.; Singh, G.; Nandi, U. Impact of Concomitantly Administered Curcumin on Pharmacokinetics of Daclatasvir in Mice under the Frame of Herb-Drug Interaction. Indian J. Pharm. Educ. 2018, 52, s11–s15. [Google Scholar] [CrossRef]
- Janakiraman, K.; Manavalan, R. Studies on Effect of Piperine on Oral Bioavailability of Ampicillin and Norfloxacin. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, B.S.; Yogesh, P.V. Influence of Co-Administration of Piperine on Pharmacokinetic Profile of Ciprofloxacin. Indian Drugs 2002, 39, 166–168. [Google Scholar]
- Patel, S.; Devada, S.; Patel, H.; Patel, N.; Bhavsar, S.; Thaker, A. Influence of Co-Administration of Piperine on Pharmacokinetic Profile of Gatifloxacin in Layer Birds. Glob. Vet. 2011, 7, 427–432. [Google Scholar]
- Khanuja, S.; Arya, J.; Srivastava, S.; Shasany, A.; Kumar, T.S.; Darokar, M.; Kumar, S. Antibioticpharmaceutical Composition with Lysergol as Bioenhancerand Method of Treatment. U.S. Patent US20070060604A1, 15 March 2007. [Google Scholar]
- Patil, S.; Dash, R.P.; Anandjiwala, S.; Nivsarkar, M. Simultaneous Quantification of Berberine and Lysergol by HPLC-UV: Evidence that Lysergol Enhances the Oral Bioavailability of Berberine in Rats. Biomed. Chromatogr. 2011, 26, 1170–1175. [Google Scholar] [CrossRef]
- Tatiraju, D.V.; Bagade, V.B.; Karambelkar, P.J.; Jadhav, V.M.; Kadam, V. Natural Bioenhancers: An Overview. J. Pharmacogn. Phytochem. 2013, 2, 55–60. [Google Scholar]
- Bailey, D.G.; Dresser, G.; Arnold, J.M.O. Grapefruit–Medication Interactions: Forbidden Fruit or Avoidable Consequences? Can. Med. Assoc. 2013, 185, 309–316. [Google Scholar] [CrossRef]
- Cui, T.; Wang, Q.; Tian, X.; Zhang, K.; Peng, Y.; Zheng, J. Piperine Is a Mechanism-Based Inactivator of CYP3A. Drug Metab. Dispos. 2019, 48, 123–134. [Google Scholar] [CrossRef]
- Ho, P.-C.; Saville, D.J. Sompon Wanwimolruk Inhibition of Human CYP3A4 Activity by Grapefruit Flavonoids, Furanocoumarins and Related Compounds. PubMed 2001, 4, 217–227. [Google Scholar]
- Kondža, M.; Bojić, M.; Tomić, I.; Maleš, Ž.; Rezić, V.; Ćavar, I. Characterization of the CYP3A4 Enzyme Inhibition Potential of Selected Flavonoids. Molecules 2021, 26, 3018. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Kuffa, K.; Needs, P.W.; Kroon, P.A.; Telbisz, Á.; Özvegy-Laczka, C.; Poór, M. Inhibitory Effects of Quercetin and Its Main Methyl, Sulfate, and Glucuronic Acid Conjugates on Cytochrome P450 Enzymes, and on OATP, BCRP and MRP2 Transporters. Nutrients 2020, 12, 2306. [Google Scholar] [CrossRef] [PubMed]
- Östlund, J.; Zlabek, V.; Zamaratskaia, G. In Vitro Inhibition of Human CYP2E1 and CYP3A by Quercetin and Myricetin in Hepatic Microsomes Is Not Gender Dependent. Toxicology 2017, 381, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-F.; Qiu, J.; Zhou, Z.-W.; He, Z.; Zhang, X.; Zhu, S. Estimation of the Binding Modes with Important Human Cytochrome P450 Enzymes, Drug Interaction Potential, Pharmacokinetics, and Hepatotoxicity of Ginger Components Using Molecular Docking, Computational, and Pharmacokinetic Modeling Studies. Drug Des. Dev. Ther. 2015, 9, 841–866. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, P.; Yue, Q.; Li, J.; Chu, R.; Zhang, W.; Wang, H. Pungent Ginger Components Modulates Human Cytochrome P450 Enzymes in Vitro. Acta Pharmacol. Sin. 2013, 34, 1237–1242. [Google Scholar] [CrossRef]
- Albassam, A.A.; Ahad, A.; Alsultan, A.; Al-Jenoobi, F.I. Inhibition of Cytochrome P450 Enzymes by Thymoquinone in Human Liver Microsomes. Saudi Pharm. J. 2018, 26, 673–677. [Google Scholar] [CrossRef]
- Alam, A.; Kowal, J.; Broude, E.; Roninson, I.; Locher, K.P. Structural Insight into Substrate and Inhibitor Discrimination by Human P-Glycoprotein. Science 2019, 363, 753–756. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Yin, Y.; Zhang, J.; Su, W.; White, A.M.; Jiang, B.; Xu, J.; Zhang, Y.; Stewart, S.; et al. Carbon Nano-Onion-Mediated Dual Targeting of P-Selectin and P-Glycoprotein to Overcome Cancer Drug Resistance. Nat. Commun. 2021, 12, 312. [Google Scholar] [CrossRef]
- Kim, Y.; Chen, J. Molecular Structure of Human P-Glycoprotein in the ATP-Bound, Outward-Facing Conformation. Science 2018, 359, 915–919. [Google Scholar] [CrossRef]
- Crowe, A.; Tan, A.M. Oral and Inhaled Corticosteroids: Differences in P-Glycoprotein (ABCB1) Mediated Efflux. Toxicol. Appl. Pharmacol. 2012, 260, 294–302. [Google Scholar] [CrossRef]
- Rather, R.A.; Bhagat, M. Cancer Chemoprevention and Piperine: Molecular Mechanisms and Therapeutic Opportunities. Front. Cell Dev. Biol. 2018, 6, 10. [Google Scholar] [CrossRef]
- Syed, S.B.; Arya, H.; Fu, I.-H.; Yeh, T.-K.; Periyasamy, L.; Hsieh, H.-P.; Coumar, M.S. Targeting P-Glycoprotein: Investigation of Piperine Analogs for Overcoming Drug Resistance in Cancer. Sci. Rep. 2017, 7, 7972. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xiao, Y.; Zheng, L.; Xu, M.; Jiang, X.; Wang, L. Enhancing Paclitaxel Efficacy with Piperine-Paclitaxel Albumin Nanoparticles in Multidrug-Resistant Triple-Negative Breast Cancer by Inhibiting P-Glycoprotein. Pharmaceutics 2023, 15, 2703. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Tripathi, J.; Rai, N. An Appraisal of the Bioavailability Enhancers in Ayurveda in the Light of Recent Pharmacological Advances. AYU (Int. Q. J. Res. Ayurveda) 2016, 37, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, Y.; Yang, T.; Huang, X.; Zhou, J.; Yang, C.; Zhou, J.; Liu, Y.; Shi, S. Inhibitory Effects of Quercetin and Its Major Metabolite Quercetin-3-O-β-D-Glucoside on Human UDP-Glucuronosyltransferase 1A Isoforms by Liquid Chromatography-Tandem Mass Spectrometry. Exp. Ther. Med. 2021, 22, 842. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, Z.; Wang, C.; Meng, Q.; Huo, X.; Liu, Q.; Sun, H.; Sun, P.; Yang, X.; Ma, X. P-Gp, MRP2 and OAT1/OAT3 Mediate the Drug-Drug Interaction between Resveratrol and Methotrexate. Toxicol. Appl. Pharmacol. 2016, 306, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bajad, S.; Bedi, K.L.; Singla, A.K.; Johri, R.K. Piperine Inhibits Gastric Emptying and Gastrointestinal Transit in Rats and Mice. Planta Medica 2001, 67, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Khan, I.A.; Koul, S.; Koul, J.L.; Taneja, S.C.; Ali, I.; Ali, F.; Sharma, S.; Mirza, Z.M.; Kumar, M.; et al. Novel Structural Analogues of Piperine as Inhibitors of the NorA Efflux Pump of Staphylococcus Aureus. J. Antimicrob. Chemother. 2008, 61, 1270–1276. [Google Scholar] [CrossRef]
- Ray, L.; Karthik, R.; Srivastava, V.; Singh, S.P.; Pant, A.B.; Goyal, N.; Gupta, K.C. Efficient Antileishmanial Activity of Amphotericin B and Piperine Entrapped in Enteric Coated Guar Gum Nanoparticles. Drug Deliv. Transl. Res. 2020, 11, 118–130. [Google Scholar] [CrossRef]
- Kumar, V.; Chaudhary, H.; Kamboj, A. Development and Evaluation of Isradipine via Rutin-Loaded Coated Solid–Lipid Nanoparticles. Interv. Med. Appl. Sci. 2018, 10, 236–246. [Google Scholar] [CrossRef]
- Rathee, P.; Kamboj, A.; Sidhu, S. Enhanced Oral Bioavailability of Nisoldipine-Piperine-Loaded Poly-Lactic-Co-Glycolic Acid Nanoparticles. Nanotechnol. Rev. 2017, 6, 517–526. [Google Scholar] [CrossRef]
- Pingale, P.L.; Pandharinath, R.R.; Shrotriya, P.G. Study of Herbal Bioenhancers on Various Characteristics of Isoniazid and Rifampicin Microspheres. Int. J. Infect. Dis. 2014, 21, 235. [Google Scholar] [CrossRef]
- Gao, T.H.; Liao, W.; Lin, L.T.; Zhu, Z.P.; Lu, M.G.; Fu, C.M.; Xie, T. Curcumae rhizoma and its major constituents against hepatobiliary disease: Pharmacotherapeutic properties and potential clinical applications. Phytomedicine 2022, 102, 154090. [Google Scholar] [CrossRef] [PubMed]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.-M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Curcumin Supplementation in Cervical Cancer. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06080841 (accessed on 24 June 2024).
- Curcumin and Piperine in Reducing Inflammation for Ureteral Stent-Induced Symptoms in Patients with Cancer. Available online: https://clinicaltrials.gov/study/NCT02598726 (accessed on 24 June 2024).
- Curcumin to Improve Inflammation and Symptoms in Patients with Clonal Cytopenia of Undetermined Significance, Low Risk Myelodysplastic Syndrome, and Myeloproliferative Neoplasms. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06063486 (accessed on 24 June 2024).
- Effect of Quercetin in Prevention and Treatment of Oral Mucositis. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01732393 (accessed on 24 June 2024).
- Therapeutic Efficacy of Quercetin Versus Its Encapsulated Nanoparticle on Tongue Squamous Cell Carcinoma Cell Line. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05456022 (accessed on 24 June 2024).
- Effect of Quercetin on Green Tea Polyphenol Uptake in Prostate Tissue from Patients with Prostate Cancer Undergoing Surgery. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01912820 (accessed on 24 June 2024).
- Prostate Cancer Prevention Trial with Quercetin and Genistein (QUERGEN). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01538316 (accessed on 24 June 2024).
- Dasatinib Combined with Quercetin to Reverse Chemo Resistance in Triple Negative Breast Cancer. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06355037 (accessed on 24 June 2024).
- Genistein in Treatment of Metastatic Colorectal Cancer. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01985763 (accessed on 24 June 2024).
- Phase I/II a Study of Decitabine in Combination with Genistein in Pediatric Relapsed or Refractory Malignancies. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02499861 (accessed on 24 June 2024).
- Genistein and Interleukin-2 in Treating Patients with Metastatic Melanoma or Kidney Cancer. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT00276835 (accessed on 24 June 2024).
- Soy Isoflavone in Combination with Radiation Therapy and Cisplatin in SCC of the Head and Neck. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02075112 (accessed on 24 June 2024).
- Genistein, Gemcitabine, and Erlotinib in Treating Patients with Locally Advanced or Metastatic Pancreatic Cancer. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT00376948 (accessed on 24 June 2024).
- Phase II Study of Isoflavone G-2535 (Genistein) in Patients with Bladder Cancer. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT00118040 (accessed on 24 June 2024).
- Prokinetic Effect of Selected Nutraceuticals. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06157034 (accessed on 24 June 2024).
- Majeed, M.; Badmaev, V.; Rajendran, R. Use of Piperine to Increase the Bioavailability of Nutritional Compounds. U.S. Patent USOO5536506A, 28 April 1996. [Google Scholar]
- Qazi, G.N.; Tikoo, L.; Gupta, A.K.; Ganju, K.; Gupta, D.K.; Jaggi, B.S. Bioavailability Enhancing Activity of Zingiber Officinale Linn and Its Extracts/Fractions Thereof. European Patent EP1465646A1, 12 December 2002. [Google Scholar]
- Khanuja, S.P.S.; Arya, J.S.; Tiruppadiripuliyur, R.S.K.; Saikia, D.; Kaur, H.; Singh, M.; Gupta, S.C.; Shasany, A.K.; Darokar, M.P.; Srivastava, S.K.; et al. Nitrile Glycoside Useful as a Bioenhancer of Drugs and Nutrients, Process of Its Isolation from Moringa Oleifera. U.S. Patent US 6858588 B2, 31 March 2003. [Google Scholar]
- Majeed, M.; Badmaev, V.; Rajendran, R. Use of Piperine as a Bioavailability Enhancer. U.S. Patent US5972382, 26 October 1999. [Google Scholar]
- Majeed, M.; Badmaev, V.; Rajendran, R.; Prakash, S.; Natarajan, S. Method of Increased Boavailability of Nutrients and Pharmaceutical Preparations with Tetrahydropperine and its Analogues and Dervatives. U.S. Patent US6849645, 1 February 2005. [Google Scholar]
- Khanuja, S.P.S.; Kumar, S.; Shasany, A.K.; Arya, J.S.; Darokar, M.P.; Singh, M.; Sinha, P.; Awasthi, S.; Gupta, S.C.; Gupta, V.K.; et al. Pharmaceutical Composition Containing Cow Urine Distillate and an Antibiotic. U.S. Patent US 6410059B1, 25 June 2002. [Google Scholar]
- Qazi, G.N.; Bedi, K.; Johri, R.; Tikoo, M.; Tikoo, A.; Sharma, S.; Abdullah, T.; Suri, O.; Gupta, B.; Suri, K.; et al. Bioavailability Enhancing Activity of Carum Carvi Extracts and Fractions Thereof. U.S. Patent US20070020347A1, 25 January 2007. [Google Scholar]
- Kapil, R.S.; Zutshi, U.; Bedi, K.L.; Singh, G.; Johri, R.K.; Dhar, S.K.; Kaul, J.L.; Sharma, S.C.; Pahwa, G.S.; Kapoor, N.; et al. Pharmaceutical Compositions Containing Piperine and an Antituberculosis or Antileprosydrug. European Patent EP 0650728B1, 3 May 1995. [Google Scholar]
- Benny, A. Composition to Enhance the Bioavailability of Curcumin. United. U.S. Patent US 9861677B2, 9 January 2018. [Google Scholar]
- Gokaraju, G.R.; Gokaraju, R.R.; D’Souza, C.; Frank, E. Bio-Availability/Bio-Efficacy Enhancing Activity of Stevia Rebaudiana and Extracts and Fractions and Compounds Thereof. U.S. Patent US20100112101A1, 6 May 2010. [Google Scholar]
| S. No. | Bioenhancer | Bioenhancement of Drugs/Nutrients | Patent Number |
|---|---|---|---|
| 1 | Piperine | Essential nutrients and supplements | US 5536506 [85] |
| 2 | Gingerin Piperine Gingerin + Piperine | Several drugs, especially anti-cancer and anti-infective agents/nutrients | EP 1465646A1 [86] |
| 3 | Niaziridin | Antibiotics | US 6858588 B2 [87] |
| 4 | Glycyrrhizin | Antibiotics | US 6979471B1 [28] |
| 5 | Piperine | Nutritional compounds | US 5972382 [88] |
| 6 | Piperine and its derivatives | Essential nutrients and supplements | US 6849645 [89] |
| 7 | Cow urine distillate | Antibiotics | US 6410059B1 [90] |
| 8 | C. Carvi | - | US 0020347A1 [91] |
| 9 | Piperine | Anti-TB drug | EP 0650728B1 [92] |
| 10 | Cumin | - | US 007514105B2 [13] |
| 11 | Essential oil | Curcumin | US 9861677B2 [93] |
| 12 | Lysergol (Rivea corymbosa) | Antibiotics | US 20070060604A1 [40] |
| 13 | Stevia rebaudiana | - | US 20100112101A1 [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Akhtar, M.J.; Khan, S.A. Role of Natural Phytoconstituents as a Potential Bioenhancer of Anti-Cancer and Anti-Microbial Agents: Spotlight on the Mechanism of Action, Clinical Studies and Patents. Processes 2024, 12, 2060. https://doi.org/10.3390/pr12102060
Unnikrishnan Meenakshi D, Narde GK, Ahuja A, Akhtar MJ, Khan SA. Role of Natural Phytoconstituents as a Potential Bioenhancer of Anti-Cancer and Anti-Microbial Agents: Spotlight on the Mechanism of Action, Clinical Studies and Patents. Processes. 2024; 12(10):2060. https://doi.org/10.3390/pr12102060
Chicago/Turabian StyleUnnikrishnan Meenakshi, Dhanalekshmi, Gurpreet Kaur Narde, Alka Ahuja, Md Jawaid Akhtar, and Shah Alam Khan. 2024. "Role of Natural Phytoconstituents as a Potential Bioenhancer of Anti-Cancer and Anti-Microbial Agents: Spotlight on the Mechanism of Action, Clinical Studies and Patents" Processes 12, no. 10: 2060. https://doi.org/10.3390/pr12102060








