Evaluation of Polyphenol Profile from Citrus Peel Obtained by Natural Deep Eutectic Solvent/Ultrasound Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Citrus Peel
2.2. Drying, Grinding, and Sieving of Citrus Peel
2.3. Preparation of Natural Deep Eutectic Solvent Based on Choline Chloride
2.4. Polyphenol Extraction from Citrus Peels Using NADES by Ultrasound Bath
2.5. Determination of Polyphenol Profile of Citrus Peel Extracts
2.6. Evaluation of Total Polyphenol Content in Citrus Peel Extracts
2.7. Antioxidant Capacity Assessment of Citrus Peel Extract
2.8. Statistical Analysis
3. Results
3.1. Polyphenol Profile from Citrus Peel Extracts
3.2. TPC and Antioxidant Capacity on Citrus Peel Extracts
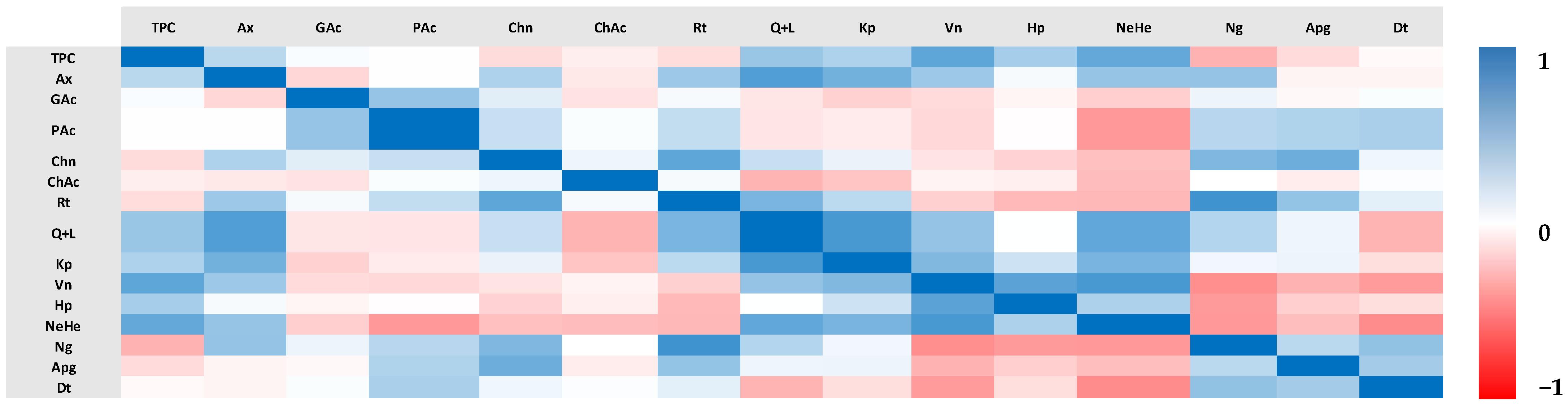
3.3. Linear and Pearson Correlation
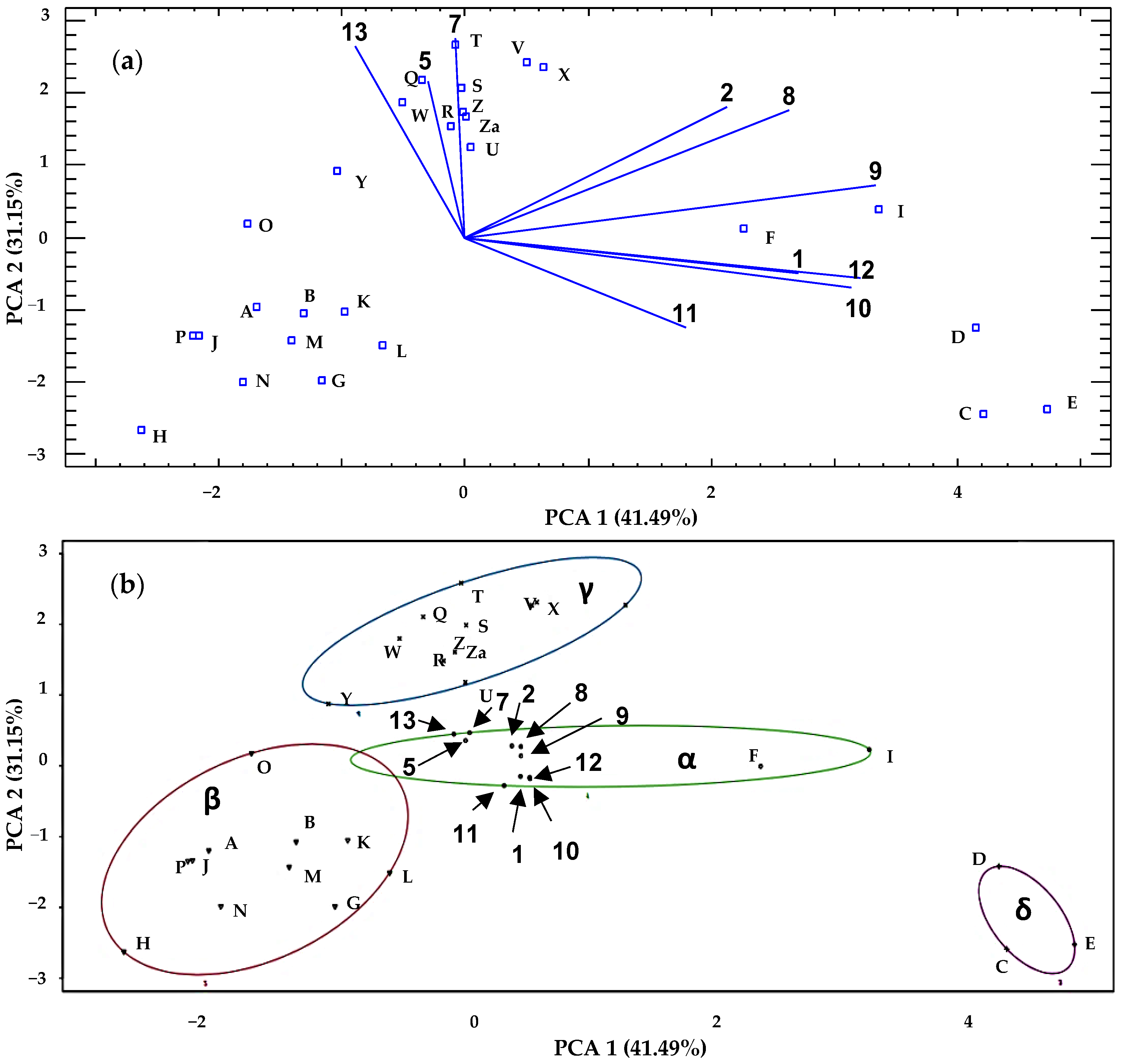
3.4. Principal Analysis Component
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United States Department of Agriculture (USDA): Foreign Agriculture Service. Citrus: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 20 June 2024).
- Kuma, V.; Kaur, R.; Aggarwal, P.; Singh, G. Underutilized citrus species: An insight of their nutraceutical potential and importance for the development of functional food. Sci. Hortic. 2024, 296, 110909. [Google Scholar] [CrossRef]
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 20 June 2024).
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Lagha-Benamrouche, S.; Madani, K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind. Crops Prod. 2013, 50, 723–730. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.C.; Vuong, Q.V. Impact of different solvents on the recovery of bioactive compounds and antioxidant properties from lemon (Citrus limon L.) pomace waste. Food Sci. Biotechnol. 2016, 25, 971–977. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Malik, M.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Tecnhol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin:A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Mihoubi Boudhrioua, N.; Ghoul, M. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod. Process. 2015, 96, 161–170. [Google Scholar] [CrossRef]
- Roy, J.; Azamthulla, M.; Mukkerjee, D. Hesperidin and Diosmin-A novel Drugs. Int. J. Pharm. Res. Technol. 2020, 10, 25–33. [Google Scholar]
- Hajialyani, M.; Farzaei, M.H.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a neuroprotective agent: A review of animal and clinical evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.J.; Huynh, T.K.; Yang, C.S.; Hu, D.W.; Shen, Y.C.; Tu, C.Y.; Wu, Y.C.; Tang, C.H.; Huang, W.C.; Chen, Y.; et al. Hesperidin is a potential inhibitor against SARS-CoV-2 infection. Nutrients 2021, 13, 2800. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Comparative Study on the Extraction and Quantification of Polyphenols from Citrus Peels using Maceration and Ultrasonic Technique. Curr. Res. Nutr. Food Sci. J. 2019, 7, 678–685. [Google Scholar] [CrossRef]
- Hilali, S.; Fabiano-Tixier, A.; Ruiz, K.; Hejjaj, A.; Nouh, F.A.; Idlimam, A.; Bily, A.; Mandi, L.; Chemat, F. Green Extraction of Essential Oils, Polyphenols, and Pectins from Orange Peel Employing Solar Energy: Toward a Zero-Waste Biorefinery. ACS Sustain. Chem. Eng. 2019, 7, 11815–11822. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.M.; Hayyan, A.; Hayyan, M.; Mirghani, M.E.S.; Salleh, H.M.; Rashid, S.N.; Ngoh, G.C.; Liew, S.Q.; Nor, M.R.M.; bin Mohd Yusoff, M.Y.Z.; et al. Natural Deep Eutectic Solvent-Assisted Pectin Extraction from Pomelo Peel Using Sonoreactor: Experimental Optimization Approach. Processes 2019, 7, 416. [Google Scholar] [CrossRef]
- Molnar, M.; Gašo-Sokač, D.; Komar, M.; Jakovljević Kovač, M.; Bušić, V. Potential of Deep Eutectic Solvents in the Extraction of Organic Compounds from Food Industry By-Products and Agro-Industrial Waste. Separations 2024, 11, 35. [Google Scholar] [CrossRef]
- Wu, K.; Ren, J.; Wang, Q.; Nuerjiang, M.; Xia, X.; Bian, C. Research Progress on the Preparation and Action Mechanism of Natural Deep Eutectic Solvents and Their Application in Food. Foods 2022, 11, 3528. [Google Scholar] [CrossRef]
- Sailau, Z.; Almas, N.; Aldongarov, A.; Toshtay, K. Studying the Formation of Choline Chloride- and Glucose-Based Natural Deep Eutectic Solvent at the Molecular Level. J. Mol. Model. 2022, 28, 235. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.D.; Oney-Montalvo, J.E.; Rodríguez-Buenfil, I.M. Phytochemical characterization of by-products of habanero pepper grown in two different types of soils from Yucatán, Mexico. Plants 2021, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Betanzos, K.A.; Oney-Montalvo, J.E.; Cauich-Rodríguez, J.V.; González-Ávila, M.; Scampicchio, M.; Morozova, K.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Antioxidant Capacity, Vitamin C and Polyphenol Profile Evaluation of a Capsicum chinense By-Product Extract Obtained by Ultrasound Using Eutectic Solvent. Plants 2022, 11, 2060. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Betanzos, K.A.; Cauich-Rodríguez, J.V.; González-Ávila, M.; Scampicchio, M.; Morozova, K.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Natural Deep Eutectic Solvent Optimization to Obtain an Extract Rich in Polyphenols from Capsicum chinense Leaves Using an Ultrasonic Probe. Processes 2023, 11, 1729. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 152–178. [Google Scholar]
- Covarrubias C., A.; Jesús, P.V.; Hugo, E.A.; Teresa, A.T.; Ulises, G.C.; Neith, P. Antioxidant capacity and UPLC–PDA ESI–MS polyphenolic profile of Citrus aurantium extracts obtained by ultrasound assisted extraction. J. Food Sci. Technol. 2018, 55, 5106–5114. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yu, H.; Guo, S.; Chen, D. Deep eutectic solvent as a green solvent for enhanced extraction of narirutin, naringin, hesperidin and neohesperidin from Aurantii Fructus. Phytochem. Anal. 2019, 30, 156–163. [Google Scholar] [CrossRef]
- Rosiak, N.; Wdowiak, K.; Tykarska, E.; Cielecka-Piontek, J. Amorphous Solid Dispersion of Hesperidin with Polymer Excipients for Enhanced Apparent Solubility as a More Effective Approach to the Treatment of Civilization Diseases. Int. J. Mol. Sci. 2022, 23, 15198. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Zhang, Z.; Yang, C.; Tian, Y. Quasi-MSn identification of flavanone 7-glycoside isomers in Da Chengqi Tang by high performance liquid chromatography-tandem mass spectrometry. Chin. Med. 2009, 4, 15. [Google Scholar] [CrossRef]
- Liu, F.; Han, S.; Ni, Y. Isolation and purification of four flavanones from peel of Citrus changshanensis. J. Food Process. Preserv. 2017, 41, e13278. [Google Scholar] [CrossRef]
- MacIkova, P.; Halouzka, V.; Hrbac, J.; Bartak, P.; Skopalova, J. Electrochemical behavior and determination of rutin on modified carbon paste electrodes. Sci. World J. 2012, 1, 394756. [Google Scholar] [CrossRef]
- Zang, Y.Y.; Yang, X.; Chen, Z.G.; Wu, T. One-pot preparation of quercetin using natural deep eutectic solvents. Process Biochem. 2020, 89, 193–198. [Google Scholar] [CrossRef]
- Kunaedi, A.; Indawati, I.; Karlina, N.; Fadhillah, Z.; Cantika, C.D.; Khulfiah, A.A. Study on the optimization of mulberry leaf extract by macerating ethanol and microwave assisted extraction method (MAE) with natural deep eutectic solvents (NADES). J. Farm. Sains Dan Prakt. 2023, 9, 11–19. [Google Scholar] [CrossRef]
- Edrisi, S.; Bakhshi, H. Separation of polyphenolic compounds from Citrus aurantium L. peel by deep eutectic solvents and their recovery using a new DES-based aqueous two-phase system. J. Mol. Liq. 2024, 402, 124790. [Google Scholar] [CrossRef]
- Mulia, K.; Muhammad, F.; Krisanti, E. Extraction of vitexin from binahong (Anredera cordifolia (Ten.) Steenis) leaves using betaine—1,4 butanediol natural deep eutectic solvent (NADES). AIP Conf. Proc. 2017, 1823, 020018. [Google Scholar] [CrossRef]
- Ranjha, A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a potential technique for extraction of phytoconstituents: A systematic review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Olfa, T.; Gargouri, M.; Akrouti, A.; Brits, M.; Gargouri, M.; ben Ameur, R.; Pieters, L.; Foubert, K.; Magné, C.; Soussi, A.; et al. A comparative study of phytochemical investigation and antioxidative activities of six citrus peel species. Flavour Fragr. J. 2021, 36, 564–575. [Google Scholar] [CrossRef]
- García, B.F.; Torres, A.; Macías, F.A. Synergy and other interactions between polymethoxyflavones from citrus byproducts. Molecules 2015, 20, 20079–20106. [Google Scholar] [CrossRef]
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros, G.; Viuda-Martos, M. Valorization of citrus co-products: Recovery of bioactive compounds and application in meat and meat products. Plants 2021, 10, 1069. [Google Scholar] [CrossRef]
- di Majo, D.; Giammanco, M.; la Guardia, M.; Tripoli, E.; Giammanco, S.; Finotti, E. Flavanones in Citrus fruit: Structure-antioxidant activity relationships. Food Res. Int. 2005, 38, 1161–1166. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A. The pharmacological potential of catechin. Indian J. Biochem. Biophys. 2020, 57, 505–511. Available online: https://api.semanticscholar.org/CorpusID:226365739 (accessed on 15 June 2024).



| #EXP | Encoded Values | Actual Values | Response Variable ** | ||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | MR | WA | CPe * | ||
| 1 | −1 | −1 | −1 | 1:0.5 | 50 | C. aurantium | Y1 |
| 2 | 0 | −1 | −1 | 1:1 | 50 | C. aurantium | Y2 |
| 3 | 1 | −1 | −1 | 1:2 | 50 | C. aurantium | Y3 |
| 4 | −1 | 0 | −1 | 1:0.5 | 60 | C. aurantium | Y4 |
| 5 | 0 | 0 | −1 | 1:1 | 60 | C. aurantium | Y5 |
| 6 | 1 | 0 | −1 | 1:2 | 60 | C. aurantium | Y6 |
| 7 | −1 | 1 | −1 | 1:0.5 | 70 | C. aurantium | Y7 |
| 8 | 0 | 1 | −1 | 1:1 | 70 | C. aurantium | Y8 |
| 9 | 1 | 1 | −1 | 1:2 | 70 | C. aurantium | Y9 |
| 10 | −1 | −1 | 0 | 1:0.5 | 50 | C. sinensis | Y10 |
| 11 | 0 | −1 | 0 | 1:1 | 50 | C. sinensis | Y11 |
| 12 | 1 | −1 | 0 | 1:2 | 50 | C. sinensis | Y12 |
| 13 | −1 | 0 | 0 | 1:0.5 | 60 | C. sinensis | Y13 |
| 14 | 0 | 0 | 0 | 1:1 | 60 | C. sinensis | Y14 |
| 15 | 1 | 0 | 0 | 1:2 | 60 | C. sinensis | Y15 |
| 16 | −1 | 1 | 0 | 1:0.5 | 70 | C. sinensis | Y16 |
| 17 | 0 | 1 | 0 | 1:1 | 70 | C. sinensis | Y17 |
| 18 | 1 | 1 | 0 | 1:2 | 70 | C. sinensis | Y18 |
| 19 | −1 | −1 | 1 | 1:0.5 | 50 | C. limon | Y19 |
| 20 | 0 | −1 | 1 | 1:1 | 50 | C. limon | Y20 |
| 21 | 1 | −1 | 1 | 1:2 | 50 | C. limon | Y21 |
| 22 | −1 | 0 | 1 | 1:0.5 | 60 | C. limon | Y22 |
| 23 | 0 | 0 | 1 | 1:1 | 60 | C. limon | Y23 |
| 24 | 1 | 0 | 1 | 1:2 | 60 | C. limon | Y24 |
| 25 | −1 | 1 | 1 | 1:0.5 | 70 | C. limon | Y25 |
| 26 | 0 | 1 | 1 | 1:1 | 70 | C. limon | Y26 |
| 27 | 1 | 1 | 1 | 1:2 | 70 | C. limon | Y27 |
| Individual Polyphenol | Main Factors and Interactions | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | AB | AC | BC | ABC | |
| Gallic acid | 0.0103 | 0.1122 | 0.2965 | 0.0533 | 0.2024 | 1.0000 | 1.0000 |
| Protocatechuic acid | 0.5460 | 0.6303 | 0.0003 | 0.1288 | 0.5473 | 0.6643 | 0.3163 |
| Chlorogenic acid | 0.4602 | 0.5858 | 0.3388 | 0.0188 | 0.2426 | 0.5544 | 0.4694 |
| Catechin | 0.2820 | 0.0428 | <0.0001 | 0.4860 | 0.7561 | 0.402 | 0.8375 |
| Rutin | 0.1186 | 0.0495 | <0.0001 | 0.1930 | 0.9539 | 0.4421 | 0.5229 |
| Quercetin + Luteolin | 0.0450 | 0.4300 | 0.1299 | 0.1040 | 0.1298 | 0.6507 | 0.7122 |
| Kaempferol | 0.0027 | 0.4617 | 0.623 | 0.2498 | 0.1655 | 0.854 | 0.8114 |
| Vanillin | 0.3814 | 0.4521 | 0.0124 | 0.3908 | 0.998 | 0.5348 | 0.0877 |
| Hesperidin | 0.0893 | 0.0224 | 0.0544 | 0.0458 | 0.0762 | 0.0462 | 0.0235 |
| Neohesperidin | 0.0297 | 0.7984 | <0.0001 | 0.3242 | 0.0434 | 0.7298 | 0.5838 |
| Naringenin | 0.3483 | 0.0651 | <0.0001 | <0.0001 | 0.0078 | 0.1065 | 0.1957 |
| Apigenin | 0.0010 | 0.0913 | <0.0001 | 0.78 | 0.7784 | 0.0331 | 0.1322 |
| Diosmetin | 0.0394 | 0.2556 | 0.0046 | 0.3214 | 0.3621 | 0.9092 | 0.5612 |
| Phenolic Compound | Antioxidant Capacity (DPPH) | ||
|---|---|---|---|
| C. aurantium | C. sinensis | C. limon | |
| TPC | 0.5564 | 0.1051 | 0.2280 |
| Gallic acid | 0.0000 | −0.2372 | −0.3056 |
| Protocatechuic acid | 0.0000 | −0.0315 | −0.0460 |
| Catechin | 0.3395 | 0.5492 | −0.1918 |
| Chlorogenic acid | −0.0394 | 0.1211 | 0.0000 |
| Rutin | 0.4420 | 0.5211 | −0.3178 |
| Q + L | 0.7706 | 0.5747 | 0.6023 |
| Kaempferol | 0.7110 | 0.4312 | 0.3271 |
| Vanillin | 0.4496 | 0.8072 | 0.8487 |
| Hesperidin | 0.1803 | −0.2467 | 0.0456 |
| Neohesperidin | 0.7486 | 0.7129 | 0.6004 |
| Naringenin | 0.3353 | 0.6091 | 0.6722 |
| Apigenin | −0.3562 | 0.3604 | −0.4075 |
| Diosmetin | 0.2755 | −0.2951 | 0.7863 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Sucre, M.O.; Avilés-Betanzos, K.A.; López-Martínez, A.; Rodríguez-Buenfil, I.M. Evaluation of Polyphenol Profile from Citrus Peel Obtained by Natural Deep Eutectic Solvent/Ultrasound Extraction. Processes 2024, 12, 2072. https://doi.org/10.3390/pr12102072
Ramírez-Sucre MO, Avilés-Betanzos KA, López-Martínez A, Rodríguez-Buenfil IM. Evaluation of Polyphenol Profile from Citrus Peel Obtained by Natural Deep Eutectic Solvent/Ultrasound Extraction. Processes. 2024; 12(10):2072. https://doi.org/10.3390/pr12102072
Chicago/Turabian StyleRamírez-Sucre, Manuel Octavio, Kevin Alejandro Avilés-Betanzos, Anahí López-Martínez, and Ingrid Mayanin Rodríguez-Buenfil. 2024. "Evaluation of Polyphenol Profile from Citrus Peel Obtained by Natural Deep Eutectic Solvent/Ultrasound Extraction" Processes 12, no. 10: 2072. https://doi.org/10.3390/pr12102072
APA StyleRamírez-Sucre, M. O., Avilés-Betanzos, K. A., López-Martínez, A., & Rodríguez-Buenfil, I. M. (2024). Evaluation of Polyphenol Profile from Citrus Peel Obtained by Natural Deep Eutectic Solvent/Ultrasound Extraction. Processes, 12(10), 2072. https://doi.org/10.3390/pr12102072









