Abstract
The valorization of industrial waste in the production of new products is a growing trend, with food waste showing significant promise as a raw material for various industries. Thus, this research aimed to investigate the production of Geotrichum candidum lipase using industrial waste, such as mozzarella cheese whey (MCW) and corn steep liquor (CSL), and to analyze how effectively it catalyzes the esterification of oleic acid with methanol. Lipase production was carried out in medium containing MCW and CSL, with fermentation conditions optimized using a fractional factorial experimental design and central composite experimental design. The highest activities (16.71 U/mL in 24 h and 17.80 U/mL in 48 h) were found in conditions of 13.6% (%w/v) CSL and 37.1% (%w/v) MCW, with corn oil fixed at 1% (%w/v) and pH fixed at 6,00. Esterification conditions were evaluated at atmospheric pressure, both in a solvent-free environment and using hexane, as well as under high pressures with supercritical carbon dioxide (SC-CO2). The produced lipase demonstrated high catalytic activity for the esterification reaction of oleic acid with methanol under SC-CO2 with an increase of 39.41% in the yield. The yields found confirm the feasibility of producing lipase from industrial waste, thus presenting it as a sustainable and efficient alternative for industrial processes, and show that there is no need to use toxic organic solvents in esterification reactions.
1. Introduction
The search for the utilization of industrial waste to manufacture other economically valuable products is one of today’s major trends. Food industry residues have considerable promise for being employed as raw materials in other various industrial areas [1,2,3]. Among the waste generated by the food industry is whey from cheese production or whey (lactoserum). Typically, its composition consists of around 93% water, 5% lactose, 0.5% lipids, 0.7% soluble proteins, 0.2% lactic acid, and small amounts of other minerals. Whey contains about 55% of the total solids of milk and is commonly used as a raw resource for manufacturing yogurt, ricotta, and other dairy products [4,5].
It is estimated that for the fabrication of 1 kg of cheese, 9 kg of whey is produced, and the reuse of whey is important because, among the food industry sectors, the dairy product sector is considered to be the most polluting, with about 50% of the whey produced not being reused [4,6,7]. Industrially produced whey, if not treated, can contribute a very high pollutant load requiring about 120,000 mg·L⁻1 of biochemical oxygen demand (BOD5) to be degraded. Considering that whey cannot be stored for very long periods, there is a need to find alternative ways to reuse this waste [4,7].
It is well known that whey is rich in nutrients, especially proteins, lactose, vitamins, and minerals, and has various industrial applications. For human consumption, whey protein is widely used in protein supplements for athletes, diets, and enriched food products [4,8]. Additionally, whey can be processed into powder and used in the manufacture of yogurts, dairy beverages, confectionery, and baked goods. Regarding animal feed, whey is frequently used in the diets of pigs, cattle, and poultry, serving as a valuable source of proteins and nutrients [4]. In the biotechnological and chemical industries, whey serves as a raw material for the production of organic acids, such as lactic acid, and also for the manufacture of enzymes [4,7]. Furthermore, whey is utilized in fermentation for the production of ethanol, biogas, and other chemical compounds [4,6,9,10].
Another important waste in the food industry is corn steep liquor (CSL), a secondary product obtained through the wet milling of corn used to produce corn flour. This byproduct is considered to be low-cost and available on a large scale. Additionally, it provides a significant amount of proteins, amino acids, minerals, vitamins, reducing sugars, organic acids, enzymes, and other nutrients, such as nitrogen. The percentage of nitrogen in CSL can vary, but it typically ranges from 0.05% to 0.1%. CSL is also a waste that can pose environmental problems if disposed of improperly, as the BOD for its complete degradation is around 11,000 mg·L⁻1 [8,11,12].
CSL is also reused in the industry in various ways. For animal feed, it is often incorporated as a nutritious ingredient in feed for pigs, cattle, and poultry due to its content of nutrients and proteins. In the biofuel industry, CSL is employed as a fermentation medium for ethanol production [11,12]. Additionally, this liquor is used to manufacture enzymes, such as amylase, which is essential for converting starch into sugars during fermentation processes. Another important application of CSL is in the production of organic acids, such as lactic acid [8].
CSL, MCW, and corn oil (used in this study) can be employed as growth inducers for microorganisms, providing essential sources of carbon, nitrogen, hydrogen, and lipids. However, it is important that these nutrients are at adequate levels for microorganisms to thrive. Insufficient concentrations can lead to slow growth and reduced production efficiency. On the other hand, excess nutrients can cause growth inhibition, resulting in toxicity or competition among different substrates, which can negatively impact fermentation yield [13]. Additionally, the amount of oil in the fermentation medium plays an important role. An adequate amount of oil can stimulate cell growth and metabolite production. However, high concentrations can be toxic, affecting cell membrane permeability and compromising the viability of microorganisms [5].
Another determining factor is the pH of the culture medium. Each microorganism has an optimal pH range for its growth and metabolic activity. When the pH deviates from this range, growth inhibition can occur, adversely affecting enzymatic activity and reducing the production of desired products. Therefore, ensuring the proper supply of nutrients, combined with maintaining the pH close to the optimal growth range, is essential for optimizing the development and activity of microorganisms [5,13].
The reuse of industrial waste as raw materials for other production chains represents a sustainable approach, allowing for a balance between production and environmental quality. Thus, both MCW and CSL are rich in nutrients that can be valuable not only for the previously mentioned applications but also for lipase production [5,14].
Lipase is an enzyme of the hydrolase class that catalyzes the hydrolysis of long-chain esters and alcohols and free fatty acids, and it can be used as a catalyst in esterification reactions. It can be obtained from plant or animal sources or produced by various microorganisms such as Aspergillus, Mucor, Culvaria, Fusarium, and Geotrichum candidum [5,15,16].
Geotrichum candidum is a fungus that can reproduce under various conditions of pH, temperature, pressure, and different sources of carbon, nitrogen, and lipids. The lipase produced by this microorganism is known as a fatty acid-specific lipase because it acts on the cis-9 double bond of long-chain fatty acid molecules, which prevents secondary reactions in the reaction medium during the hydrolysis of vegetable or soybean oils [17,18,19,20]. Geotrichum candidum produces enzymes that are useful in the food industry for the fermentation of cheeses and other dairy products, contributing to the texture, flavor, and aroma of cheeses and other foods. Its ability to ferment carbohydrates is also valuable in the production of probiotics. Being a microorganism naturally present in various foods, Geotrichum candidum is generally considered safe for human consumption and has a history of use in food production [5].
Esterification reactions are extensively utilized in the food and cosmetics industries to obtain bioflavors. Among the bioflavors produced in the industry are rose, pineapple, banana, and others. Most reactions use chemical catalysts such as acids or bases, usually H2SO4 or NaOH, to initiate the esterification reaction and organic solvents as the reaction medium. However, the use of biological catalysts, such as enzymes, and a less toxic reaction medium is becoming increasingly common. Additionally, non-polluting or environmentally friendly methods are always a more desirable choice [19,21,22,23,24].
Lipase as a catalyst enables the use of a reaction medium at lower temperatures and a pH closer to neutrality. Additionally, complex separation steps after the esterification stage are not necessary, as lipase replaces conventional chemical catalysts [20,25,26]. However, most enzymatic processes require significant amounts of water in the reaction medium, which can lead to environmental problems if disposed of improperly, as the waste may contain a high organic load that needs to be degraded.
There is also the possibility of using organic co-solvents for the esterification reaction, such as hexane. A medium with hexane may yield better results compared to those in solvent-free conditions. However, hexane, in addition to being detrimental to the environment and toxic to humans, can reduce the reaction yield when used in large quantities due to its toxicity to microorganisms themselves [25,27].
Supercritical fluids offer an alternative to organic solvents in esterification reactions. Since lipase exhibits good stability in pressurized fluids, it can be used as a catalyst in esterification reactions in reaction medium under pressures higher than atmospheric pressure, thus reducing the amount of water used in the process and the waste generated after the reaction. Supercritical carbon dioxide (SC-CO2) is an example of such a medium. Processes that utilize SC-CO2 are sustainable, environmentally friendly, cost-efficient, and provide the potential to produce a product with tailored properties. Additionally, SC-CO2 is considered a green solvent because all the CO2 can be recovered after the esterification process [5,28,29,30,31,32,33,34].
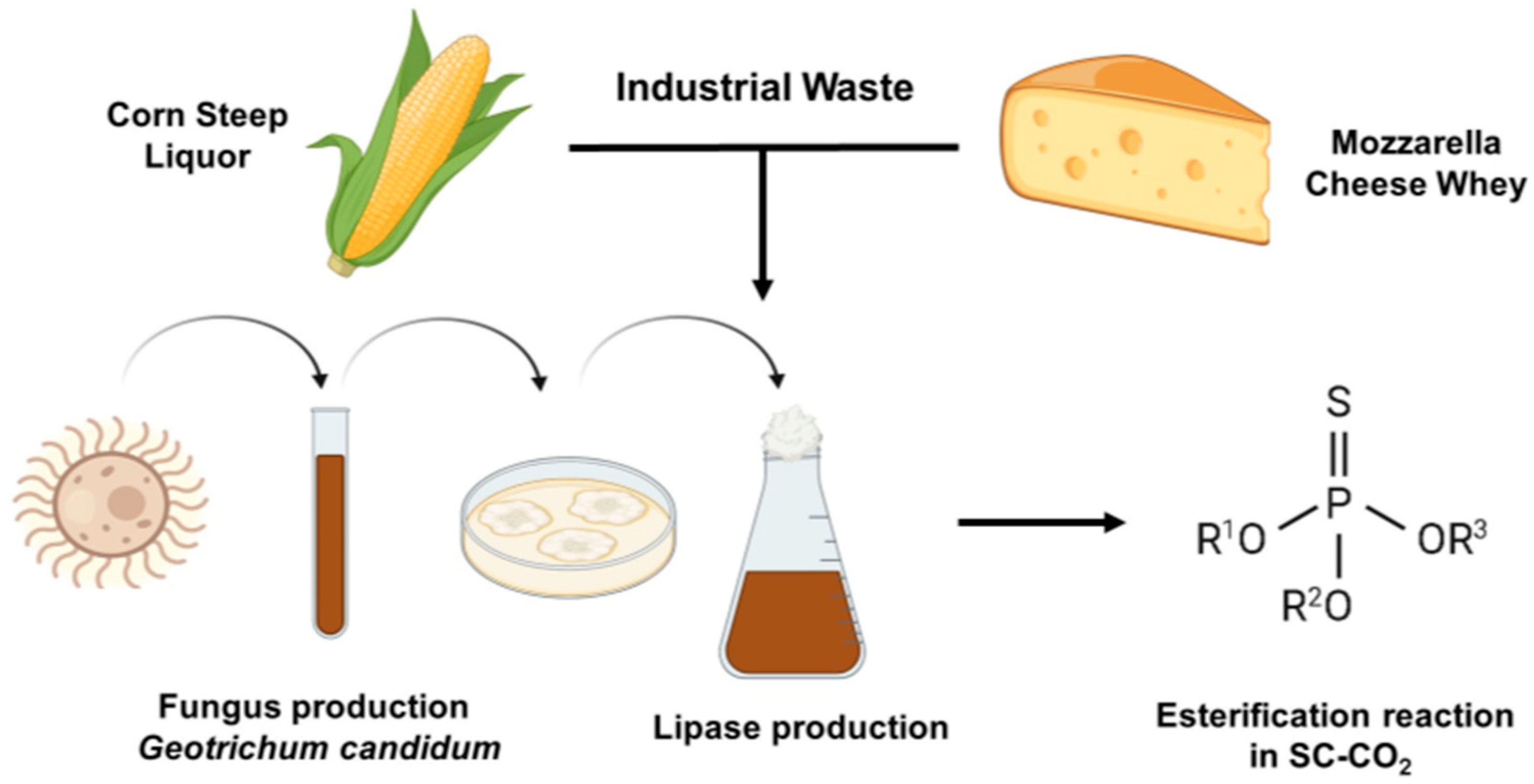
In addition, it is important to note that most of the literature describing Geotrichum candidum lipase in esterification reactions uses commercial lipases, that is, those produced industrially [14,19,26,35,36]. Thus, studies relating to Geotrichum candidum lipase produced in the laboratory with MCW, CSL, and SC-CO2 are scarce. Figure 1 illustrates the processes involved in this work.
Figure 1.
Diagram of processes involved in lipase production from Geotrichum candidum and esterification reaction in SC-CO2 medium.
This study aimed to assess the efficiency of the esterification reaction of oleic acid with methanol in supercritical carbon dioxide (SC-CO2) using Geotrichum candidum lipase, produced from mozzarella cheese whey (MCW) and corn steep liquor (CSL), both of which are byproducts of the food industry with low economic value. Their feasibility for lipase production justified the choice of these substrates. The results support the biotechnological use of this enzyme and demonstrate the potential of these byproducts of the food industry as raw materials for lipase production while also advancing the understanding of pressurized fluids and esterification reactions.
2. Materials and Methods
2.1. The Production of the Microorganism Geotrichum Candidum
The microorganism employed was Geotrichum candidum (Y-552), sourced from NRRL (USA). It was cultured on Yeast Malt (YM) Agar, which contained (% w/v) 1.0% dextrose (Inlab, São Paulo, SP, Brazil), 0.5% peptone (Kasvi, Pinhais, PR, Brazil), 0.3% malt extract (Himedia Laboratories PVT LTD, Mumbai, Maharashtra, India), 0.3% yeast extract (Acumedia, Lansing, MI, USA), and 3.0% bacteriological agar (Acumedia, Lansing, MI, USA), and was stored at 4 °C.
The reagents used in the pre-fermentation and fermentation were Ammonium Sulfate ((NH4)2SO4) (Sigma-Aldrich, Jurubatuba, SP, Brazil), Sodium Hydroxide (NaOH) (Sigma-Aldrich, Jurubatuba, SP, Brazil), Arabic Gum (Sigma-Aldrich, Jurubatuba, SP, Brazil) and Ethanol 99.5% (Sigma-Aldrich, Jurubatuba, SP, Brazil). All reagents are of analytical grade. Commercial corn oil (Liza, Mairinque, SP, Brazil) was also employed.
Pre-fermentation, fermentation in larger volumes, and the analysis of enzymatic activity and productivity were based on work by Maldonado et al. [13] and Maldonado et al. [15], adapted in this work.
For pre-fermentation, a 1 cm2 circular section of inoculum was excised and placed into a 250 mL Erlenmeyer flask. This flask contained a mixture of 5% peptone, 1% corn oil, 0.25% ammonium sulfate ((NH4)2SO4), and water, resulting in a total solution volume of 100 mL. The fermentation was performed in a shaker (Tecnal, TE-424, Piracicaba, SP, Brazil) at 30 °C for 48 h.
Following pre-fermentation, 10% (v/v) aliquots were transferred to a fermentation medium that included MCW and CSL. MCW production was supplied by the dairy school of the Faculty of Animal Science and Food Engineering campus of the University of São Paulo (FZEA-USP). To preserve its initial properties and ensure consistency, MCW was stored in bottles and frozen, maintaining the same batch throughout this study.
CSL (53% (w/w) dry weight, 43% (w/w) total protein, and 15.5% (w/w) total minerals) was generously supplied by Ingredion, located in Mogi Guaçu, Brazil-SP, and was stored in plastic containers and frozen. Additionally, corn oil, which was bought from a local market, was used and stored in its original packaging.
Both MCW and CSL were removed from the freezer one day before use for acclimatization and were previously homogenized to obtain a representative sample of the compounds. pH adjustment, when required, was performed using 0.5 M NaOH.
The phase of evaluating enzymatic activity was divided into two stages. The initial stage employed a 24−1 fractional factorial design to evaluate how CSL concentration, MCW, corn oil, and pH affect the lipase’s enzymatic activity. The choice of the fractional model was motivated by its ability to identify trends in the results with fewer experiments compared to other response surface design models. This leads to a reduction in the consumption of reagents and raw materials, thereby minimizing waste. Table 1 presents the levels used in the design and the results of enzymatic activity and productivity.

Table 1.
Fractional Factorial Design for four variables, activity and productivity of Geotrichum candidum lipase obtained with mozzarella cheese whey (MCW) and corn steep liquor (CSL).
After identifying the main effects using statistical methods (Table 2), a new 22 factorial design and Rotational central composite design (RCCD) with CSL and MCW variables was performed to identify the optimized production conditions for lipase (Table 3) [17].

Table 2.
Main effects of Geotrichum candidum lipase obtained with mozzarella cheese whey (MCW) and corn steep liquor (CSL).

Table 3.
Rotational central composite design (RCCD) for two variables, activity, and productivity of Geotrichum candidum lipase employing mozzarella cheese whey (MCW) and corn steep liquor (CSL).
The fermentation was conducted in a shaker (Tecnal TE—424, Piracicaba, SP, Brazil) at 30 °C for 96 h for all tests. The temperature and fermentation time were analyzed. During the fermentation process, 1 mL aliquots were taken every 24 h for enzymatic activity analysis (U). The aliquots were transferred to a 125 mL Erlenmeyer flask with 5 mL of 1 M phosphate buffer and 2 mL of gum Arabic solution constituted of 75% gum solution (7% w/v in water) and 25% corn oil) and maintained in a 30 °C shaking water bath for 30 min.
Subsequently, 1 mL aliquots of this solution were transferred to a 125 mL Erlenmeyer flask containing 10 mL of a 1:1 (v/v) water:alcohol solution to halt enzymatic activity. The solution was then titrated with 0.05 M NaOH and phenolphthalein. The volume of base used was correlated with the enzymatic activity over each period (24 h). After identifying the optimal lipase production conditions, fermentation was conducted on a larger scale for the experiments of this study.
The raw broth obtained from these fermentations (total of 6 L) was centrifuged using a centrifuge (Eppendorf Merck, KGaA, Darmstadt, Germany) to isolate Geotrichum candidum (precipitate) from the lipase which remained in the supernatant.
Enzyme precipitation was carried out with ethanol (99% v/v) added to the raw broth at a constant rate of 15 mL/min in a system with magnetic stirring and temperature control at 0 °C. Ethanol was added in a 2:1 (ethanol/raw broth) ratio using a burette until the final ethanol concentration reached 70% v/v saturation, and the temperature was maintained as low as possible to prevent lipase denaturation.
After a 30 min decantation period, centrifugation was performed at 5000 rpm for 3 min to separate the precipitated enzyme from the residual broth. The enzyme was then resuspended in a small volume of 0.1 mol/L phosphate buffer, pH 7.0, frozen, and then transferred to a freeze dryer (Terroni—LC 1500, São Carlos, SP, Brazil) [36].
2.2. Esterification Reactions
The esterification reaction was conducted in two distinct ways: in a non-pressurized environment and in a pressurized reactor with SC-CO2, both following the methodology of Ramos et al. [5]. In the non-pressurized environment, the reaction was performed in both a solvent-free and hexane medium as a co-solvent. The reagents used in the esterification reaction were methanol (Dinâmica, Jaraguá do Sul, SC, Brazil), oleic acid (Dutral, Campinas, SP, Brazil), and hexane (98.5%) (Exodus Científica, Sumaré, SP, Brazil).
A full factorial design for 3 independent variables was used for conducting reactions in a solvent-free and hexane medium. The molar ratio of acid/alcohol varied at 1:1, 1:2, and 1:3; lipase concentration in the medium varied at 2.5, 5.0, and 7.5 U/g; and temperature varied at 30, 40, and 50 °C. For the esterification reaction in a reactor with SC-CO2 as the solvent, in addition to the already-listed parameters, pressure was also analyzed, varying at 10, 20, and 30 MPa.
After the esterification reactions, the mass of ester formed (Equation (1)) and the esterification reaction yield (Equation (2)) were calculated for the process under atmospheric pressure and in SC-CO2.
where mester—ester mass formed; mtheoretical—theoretical ester mass; Mester—molar weight of ester; MNaOH—molar weight of NaOH; and Y—yield of ester
2.2.1. Esterification Reaction in Solvent-Free and Hexane Medium
For the reaction in a solvent-free medium, 1 mole of oleic acid and a corresponding amount of alcohol (1, 2, or 3 moles) were added to 125 mL Erlenmeyer flasks. Lipase was subsequently introduced into the reaction mixture. Table 4 displays the experimental design employed for the esterification reaction in both the solvent-free medium and hexane as a co-solvent medium.

Table 4.
Yield of esterification reactions in solvent-free and hexane medium utilizing Geotrichum candidum lipase obtained with mozzarella cheese whey (MCW) and corn steep liquor (CSL).
The reaction was carried out with stirring at 200 rpm in a shaker (Tecnal, TE-424, Piracicaba, SP, Brazil) for 1 h at a constant temperature. Following this, 1 mL aliquots were taken and mixed with 10 mL of ethanol (1:1 v/v) solution in a 125 mL Erlenmeyer flask to halt the reaction. The residual oleic acid in the reaction medium was then quantified by titration with 0.1 M NaOH.
The effect of adding hexane to the reaction medium was investigated by incorporating hexane at a 10% v/v ratio relative to alcohol. After 1 h of stirring, 1 mL aliquots were withdrawn and added to Erlenmeyer flasks containing an alcohol (1:1 v/v) solution. The response obtained in both cases was the residual amount of oleic acid in the reaction medium, and the reaction yield was calculated stoichiometrically.
2.2.2. Esterification Reaction Using SC-CO2 as Solvent
Table 5 presents the experimental design for the esterification reaction in SC-CO2. A total of 1 mole of oleic acid was combined with the specific molar ratio of methanol to be tested (1, 2, or 3 moles) in a 125 mL Erlenmeyer flask. This blend, along with lipase, was then placed in the supercritical extraction and reaction unit SFE-500 (Thar SCF Waters, Milford, CT, USA).

Table 5.
The yield of the esterification reaction in SC-CO2 using Geotrichum candidum lipase as the catalyst.
The reaction was performed at a constant temperature and pressure (Table 5) with stirring at 200 rpm for 1 h. The system was gradually de-pressurized (1 MPa/min) at the end of the reaction, and a 1 mL aliquot was transferred to an Erlenmeyer flask containing a 1:1 (v/v) alcohol mixture to halt the reaction. The mixture was then titrated with 0.1 M NaOH to determine the residual oleic acid, and the yield was calculated stoichiometrically.
All statistical analyses, estimates, and models obtained, as well as tables and contour curve figures, were carried out and obtained using the software Statistica 13.5® (version 13.5, TIBCO Software Inc., Tulsa, OK, USA).
3. Results and Discussion
3.1. Geotrichum candidum Lipase Production
Table 1 presents the findings from the preliminary studies on enzyme activity, focusing on the effects of CSL, MCW, corn oil, and pH. Enzyme activities were evaluated over 96 h with intervals every 24 h. This interval was chosen because some authors reported that enzymatic activity could be high after 96 h of fermentation [36,37].
It was found that, after 24 h of fermentation, enzyme activity ranged from 9.86 to 21.52 U/mL·h (test 6, Table 1), with a maximum productivity of 0.90 U/mL also at 24 h of fermentation. Table 2 shows the main effects of the independent variables for the production of lipase using MCW for 24, 48, and 72 h of fermentation. Neither of the variables presented significant effects after 72 h of fermentation, so the discussions were focused on the 24 and 48 h fermentation times.
CSL exhibited a positive and significant effect after 24 h (with 89% confidence) and 48 h (with 98% confidence), indicating that increasing the CSL concentration enhanced lipase production. Consequently, the CSL concentration was increased from 5 to 15% for the full factorial design.
Similar results have been reported in the literature. Tommaso et al. [14] studied the production of Candida rugosa lipase in submerged fermentation using whey, malt extract, yeast extract, Tween 80, olive oil, and brewery waste, compared to the use of whey alone. The authors reported a 180% increase in lipolytic activity when using supplements in the reaction medium compared to using whey alone.
Ramos et al. [5] studied lipase production using fresh whey and CSL. The authors reported that the lipase produced showed good activity (19.87 U/mL) when 6% CSL and 40% fresh Minas cheese whey were used.
Maldonado et al. [38] and Maldonado, Macedo, and Rodrigues [39] investigated the influence of CSL on the enzymatic activity of Geotrichum candidum and Geotrichum sp lipase. They found that the highest activity was achieved with 15% (w/v) CSL for Geotrichum sp. lipase and 8% CSL (w/v) for Geotrichum candidum lipase.
MCW exhibited a negative and significant effect at both 24 and 48 h and also at 72 h (considering 89% confidence), leading to the decision to significantly reduce the concentration of MCW (despite the initial proposal to use the maximum possible). Thus, the full factorial design range was set between 20% and 40% (w/v). Compared with the literature, the production of lipase by Candida rugosa was also higher with lower concentrations of MCW [14].
Corn oil did not show significant effects and was fixed at 1.0% (central point condition), and pH also did not show significant effects and was fixed at 6.0. This pH value was selected based on previous work from our research group, where Geotrichum candidum lipase was more stable over time [5,36].
The data obtained from the full factorial design (Table 3) show that the maximum lipolytic activity achieved (17.80 U/mL) (test 4, Table 3) was lower than that in the fractional factorial design (31.65 U/mL) (test 6, Table 1). Such a result is not unexpected, as Geotrichum candidum is an imperfect fungus, where the standardization of the results is challenging, and relatively high variations (20 to 30% in lipase production), even under optimized conditions, have been observed in previous studies [39].
Variations in the results may also occur due to the variability in the composition of the byproducts used, which are also difficult to standardize. However, the average productivity, considering the 12 assays conducted, was 0.47 U/mL·h after 24 h of fermentation, which is similar to a previous study using CSL that achieved 0.44 U/mL·h under optimal conditions [39].
The analysis of variance (ANOVA) showed a better fit for the first-order model, with R2 = 0.79. Thus, Equation (3) represents lipase production.
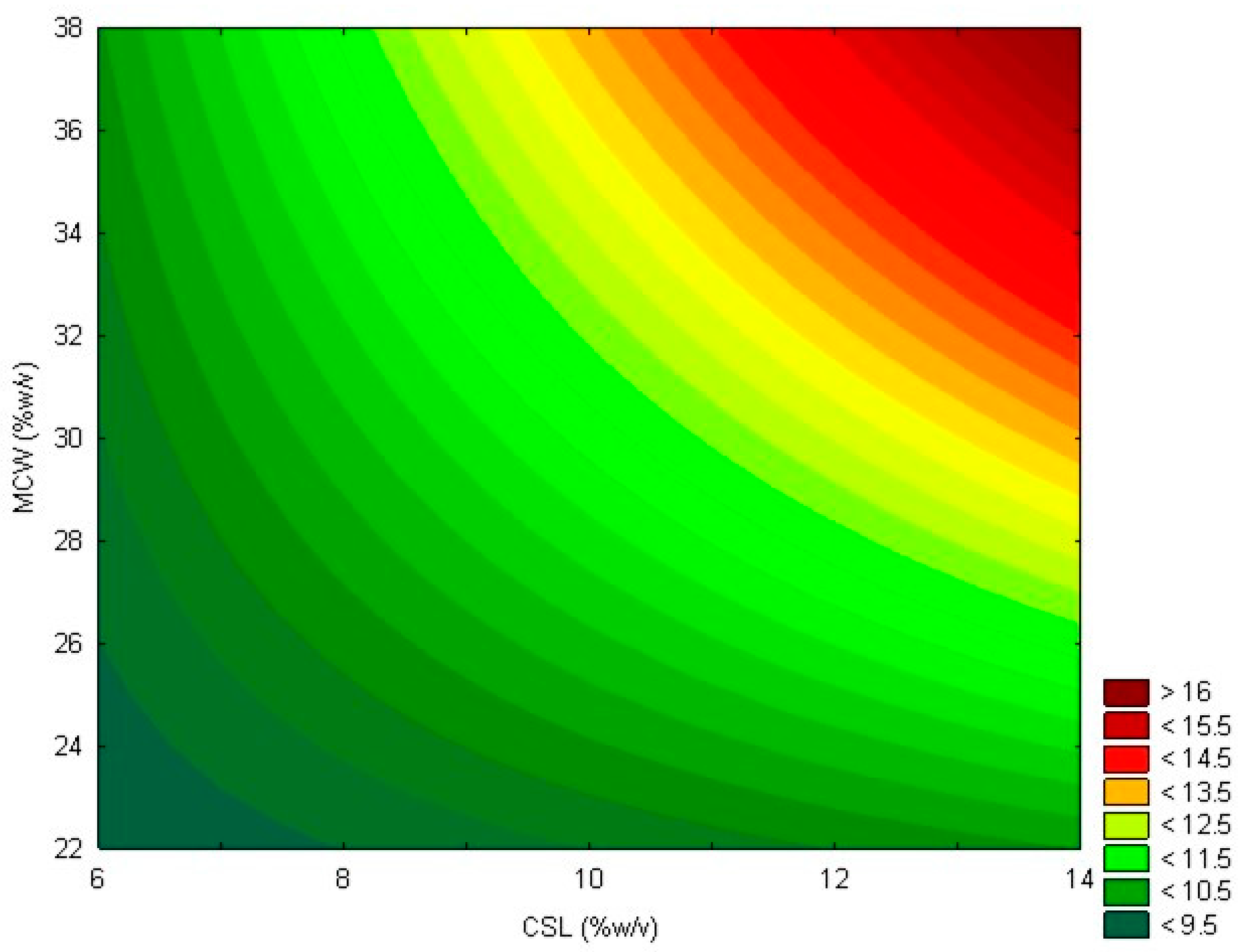
The model represented by Equation (3) can be illustrated by the contour plot shown in Figure 2.
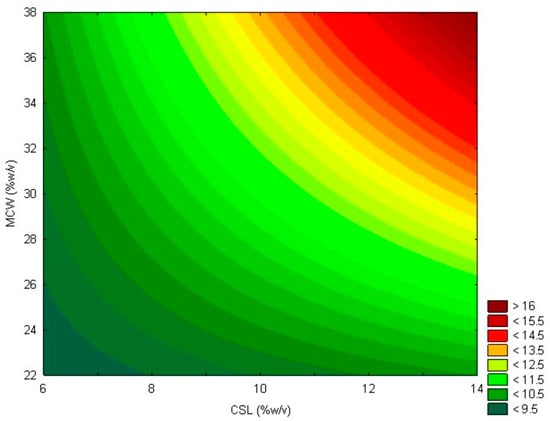
Figure 2.
Contour plot showing lipase activity (U/mL) as a function of mozzarella cheese whey (MCW) and corn steep liquor (CSL) for the first-order model.
Within the studied range, increasing the concentrations of CSL and MCW led to an increase in lipolytic activity. However, the axial points obtained show that a more substantial increase in the concentrations of both substrates did not lead to higher levels of lipolytic activity. Therefore, within the studied range, a condition of 13.6% CSL and 37.1% MCW is deemed optimal for the production of Geotrichum candidum lipase, achieving a maximum lipolytic activity of 17.80 U/mL after 48 h of fermentation and the highest productivity of 0.70 U/mL·h after 24 h of fermentation. Studies that used only CSL in the production of Geotrichum candidum lipase reported a productivity that was 1.6 times lower than that obtained in this research [39,40].
3.2. Esterification Reactions at Atmospheric Pressure
Table 4 shows the experimental design and yields of the esterification reactions conducted in a solvent-free medium and with hexane medium, using lipase obtained with MCW and CSL. The yield increased when the molar ratio of acid/alcohol was higher (1:3) in the solvent-free medium. Notably, the highest yields were observed in assays 4 and 6, with yields of 10.51% and 10.33%, respectively.
Hexane as a co-solvent in the reaction medium enhanced an increase in yield in assays where the molar ratio of acid/alcohol was higher. The highest yield occurred in assay 2, which, under the conditions of a 1:3 molar ratio of acid/alcohol, 2.5 U/g of lyophilized lipase concentration, and 30 °C, achieved a yield of 10.10%. The authors highlight that the use of hexane as a co-solvent can increase the yield of the esterification reaction. However, the increase shown was very small, which does not justify using hexane in these reactions due to its high toxicity [25,41,42].
Ramos et al. [5] also utilized lipase from Geotrichum candidum, which was produced using Minas Frescal cheese whey, for esterification reactions in solvent-free medium and with hexane as a co-solvent. The authors found that increasing the molar fraction had a positive impact on the yield.
The results of the analysis of the estimated effects for the esterification reaction in a solvent-free medium and with hexane medium are shown in Table 6. It was observed that the molar ratio was the only significant effect in the analysis for both reactions and that temperature was statistically significant for the esterification reaction with hexane as a co-solvent.

Table 6.
The estimated effects of variables in the esterification reaction in solvent-free medium and with hexane using Geotrichum candidum Lipase as a catalyst.
It was observed that the temperature had a negative impact, suggesting that the yield of the reaction decreased as the temperature increased. The reduction in the esterification reaction yield was likely due to the denaturation of the lipase at higher temperatures. It is important to consider the temperature variable in enzymatic reactions to identify the ideal condition for each enzyme, as this variable is specific to different types of enzymes [5,43].
The model is predictive and statistically significant at 90% confidence (R2 = 0.97) for the esterification reaction in solvent-free medium and at 95% confidence (R2 = 0.90) for the reaction in hexane medium, according to the analysis of variance. Such values are justified by the imperfection of the fungus used to produce lipase in the present study, as previously mentioned. Ramos et al. [5] similarly performed esterification and obtained R2 ~ 0.90. These values corroborate those obtained in this work. Equations (4) and (5) show the yield in the esterification reaction at atmospheric pressure for the solvent-free medium and with hexane, respectively.
where Y—yield; MR—molar ratio (M/M); L—lipase (U/g freeze-dried lipase); T—temperature (°C).
3.3. Esterification Reaction with SC-CO2 as Solvent
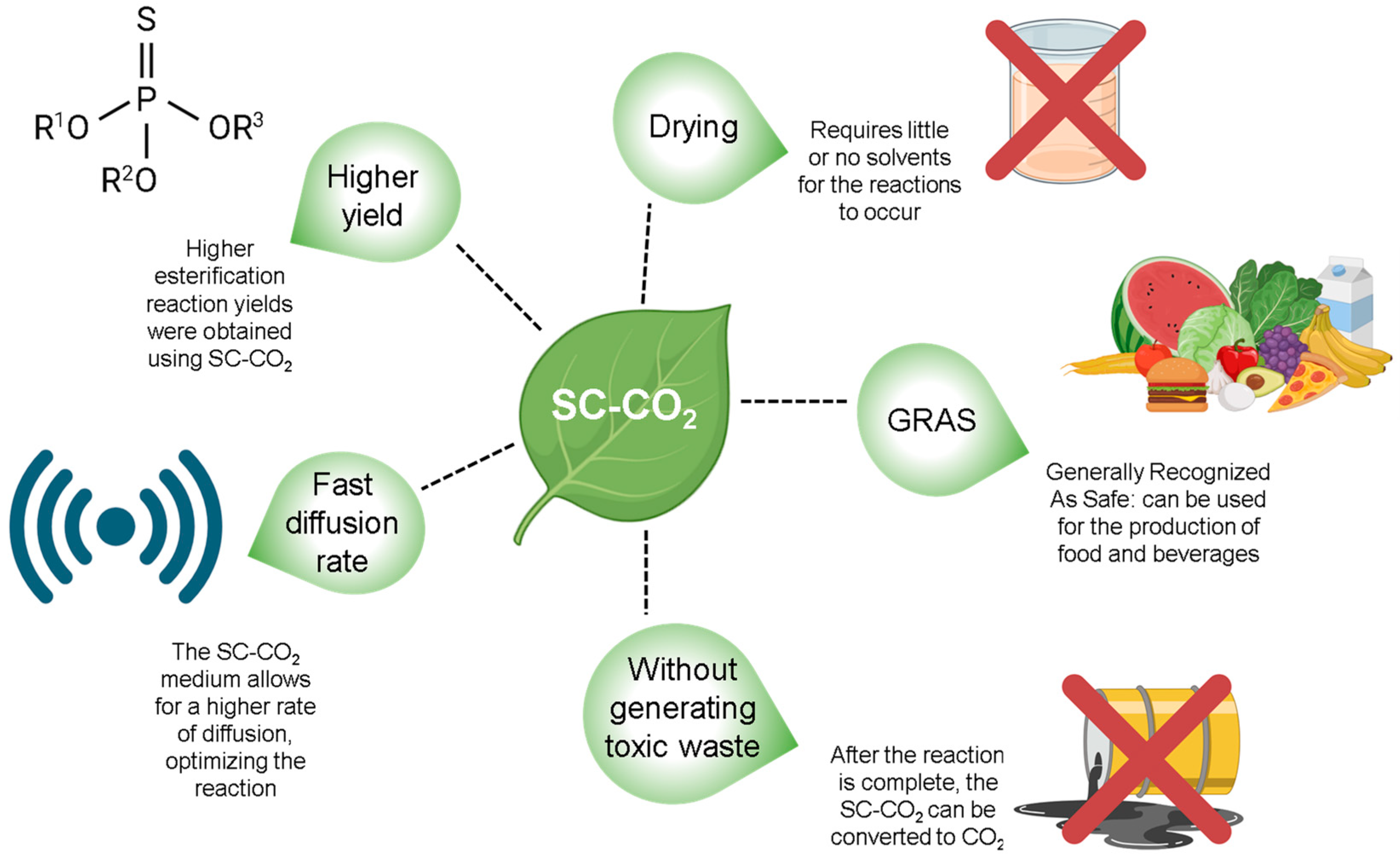
The use of a pressurized environment for the esterification reaction was based on the fact that at higher pressures, the diffusivity of the medium increases, which theoretically promotes an increase in the yield of reactions, extractions, and mass transfer processes without the use of toxic organic solvents, since SC-CO2 is a GRAS (Generally Recognized As Safe) solvent [44,45,46]. Figure 3 highlights the advantages of using SC-CO2 as a solvent.
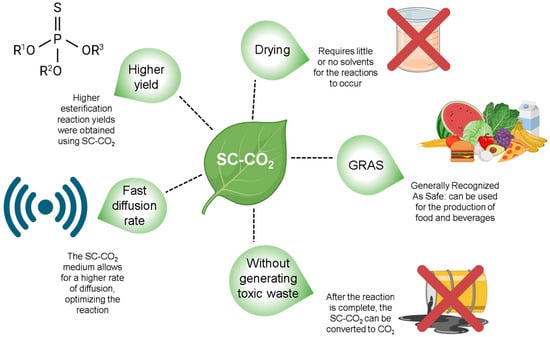
Figure 3.
The advantages of using SC-CO2 as a solvent in esterification reactions.
The impact of the molar ratio, lipase amount, pressure, and temperature on the esterification reaction using SC-CO2 as a solvent was also evaluated for Geotrichum candidum lipase produced from MCW and CSL. Table 5 shows the yield of this reaction.
Under conditions where the molar ratio of acid to alcohol was 1:3, the highest yields were observed. The maximum yield (39.41%) occurred under conditions where the molar ratio was 1:3, the lipase concentration was 2.5 U/g of lyophilized lipase, the pressure was 10 MPa, and the temperature was 50 °C.
From the experiments, it is generally observed that SC-CO2 as a solvent had a significant effect on the yield of the esterification reaction of oleic acid with methanol. For comparison, lipase showed yields of 10.51% in a solvent-free medium, 10.10% in a medium with hexane as a co-solvent, and 39.41% in a medium with SC-CO2. These results demonstrate that SC-CO2 positively influences yield.
It is also noted that for the pressures of 10, 20, and 30 MPa used in the trials, there was no significant effect. However, the change from ambient pressure (0.101 MPa) to pressurized environments resulted in an increased yield, as shown in the previous paragraph.
The highest yield can be attributed to the reduction in the viscosity and density of SC-CO2, which increases the diffusion coefficient of the substrate within the reaction medium. Furthermore, elevated pressures improve the distribution of the substrate and the lipase active sites, thereby enhancing the conversion in the esterification reaction [47,48]
Rosa et al. [22] studied the esterification reaction of vinyl acetate with geraniol in the synthesis of geranyl acetate using the organic solvent hexane and Candida rugosa lipase as the catalyst. The variables analyzed included temperature, lipase mass concentration, and the molar ratio of vinyl acetate to geraniol. The authors found an increase in the process conversion when the temperature was raised to 45 °C compared to 30 °C. They also demonstrated that the formation of geranyl acetate increased as the reaction time was extended.
In another study, Escandell et al. [47] investigated butyl acetate synthesis using Candida antarctica lipase with two different solvents: the organic solvent hexane and SC-CO2. The authors reported an increase in butyl acetate production under SC-CO2, reaching 76.2% at a pressure of 12 MPa, indicating that supercritical conditions are favorable for butyl acetate production.
Dos Santos et al. [48] explored the synthesis of eugenyl acetate in SC-CO2 as the solvent and two types of commercial enzymes: Lipozyme 435 and Novozym 435. They studied the esterification reaction between acetic anhydride and eugenol under varying conditions, including enzyme mass concentration (1–10% m/m), substrate molar ratios (1:1 and 1:5), different pressures (10–30 MPa), and temperatures (40–60 °C). Their study showed that higher temperatures and lower pressures increased reaction yields, with the optimal conditions being identified as 50 °C and 10 MPa.
Table 7 shows the effects for the esterification reaction using lipase obtained from MCW and CSL in a SC-CO2 medium.

Table 7.
Estimated effects for esterification reaction in SC-CO2 medium using lipase obtained from MCW and CSL.
The molar ratio of acid to alcohol was the only statistically significant effect and demonstrated a positive effect, as an increase in the molar ratio also resulted in an increase in the yield. Equation (6) shows the yield of the esterification reaction using SC-CO2 as a solvent.
where Y—yield; MR—molar ratio (M/M).
In general, the molar ratio was the only variable, across all the studied esterification reactions, that had a significant effect. This is because a higher amount of alcohol, which serves as the limiting substrate, promotes the reaction towards ester formation, thereby increasing yields.
This finding aligns with those of Anschau et al. [49], who achieved a 94% conversion in the synthesis of isoamyl butyrate using an acid molar ratio of 1:3 with Lipozyme TL IM as the catalyst. Similarly, Macedo, Pastore, and Rodrigues [50] observed effective results in their study, where they used Rhizopus sp. lipase for the synthesis of isoamyl butyrate and achieved a 75% conversion with an acid molar ratio of 1:1.5.
Generally, it can be observed that the use of a pressurized environment is beneficial in the esterification reaction, increasing its yield. In a study conducted by Melgosa et al. [51], the influence of the substrate molar ratio, pressure, and temperature on the yield of the fish oil ethanolysis reaction catalyzed by Lipozyme RM IM in SC-CO2 was evaluated. The authors reported that when the pressure was increased from 10 to 30 MPa, there was a 195% increase in the reaction rate.
In another study, the effect of pressure, temperature, and enzyme and substrate concentration on the synthesis of eugenol acetate in SC-CO2 employing Lipozyme 435 and Novozym 435 as catalysts was assessed. It was found that as temperature and pressure increased, there was an increase in the reaction conversion rate [51]. Similarly, our results also confirm that the SC-CO2 reaction medium can enhance the yield of ester production using lipase as a catalyst.
3.4. Potential and Sustainability in Lipase Production from Industrial Waste: Alignment with Circular Economy and Green Chemistry
The production of lipase from MCW and CSL, which are industrial wastes, represents a valuable opportunity for various industries, including food, biodiesel, cosmetics, pharmaceuticals, and bioremediation [20,52]. In the food industry, for example, lipases enhance the quality and flavor of dairy products. In biodiesel production, they act as catalysts in the conversion of oils into biofuels. In the cosmetics sector, they are used in formulations that promote skin hydration. Additionally, in environmental applications, lipases assist in the biodegradation of lipid pollutants, contributing to waste management.
The incorporation of SC-CO2 as a medium in the process also reduces the use of toxic solvents and reagents, favoring methods that are less harmful to the environment. Energy efficiency is optimized, as the process utilizes residual materials, decreasing the carbon footprint associated with enzyme production [52].
To further increase the efficiency and sustainability of lipase production on an industrial scale, various strategies can be implemented. Optimizing fermentation conditions, such as pH and temperature, can enhance the yield and activity of enzymes. Thus, integrating a process that uses SC-CO2 as a solvent with existing processing operations can further reduce energy and resource consumption, representing an important step toward a more sustainable and efficient future [4,6,9,10].
4. Conclusions
The use of mozzarella cheese whey and corn steep liquor was effective for the production of Geotrichum candidum lipase, achieving an activity of 16.71 U/mL after 24 h of fermentation (with a productivity of 0.70 U/mL·h) and 17.80 U/mL after 48 h of fermentation (with a productivity of 0.37 U/mL·h). In both solvent-free and hexane environments under atmospheric pressure, lipase yielded poor results in the esterification reaction, showing yields of 10.51% and 10.10%, respectively. However, when SC-CO2 was used as the reaction medium, lipase showed a significantly higher yield of 39.41%. These results highlight that supercritical carbon dioxide not only improves the esterification reaction but also represents a promising, non-toxic, and environmentally friendly alternative for producing Geotrichum candidum lipase.
Author Contributions
Conceptualization, E.S.K.; Validation, P.R.R.; Formal Analysis, P.R.R.; Investigation, P.R.R.; Resources, A.L.d.O. and E.S.K.; Data Curation, P.R.R.; Writing—Original Draft Preparation, P.R.R.; Writing—Review & Editing, P.R.R., E.S.K., A.L.d.O., G.V.C.R.; Supervision, E.S.K.; Project Administration, E.S.K.; Funding Acquisition, E.S.K.; Visualization, P.R.R., E.S.K., G.V.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Process no 2018/18024-7, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—Processes no 304573/2019-1 and no 306317/2016-8) for its financial support. P.R.Ramos thanks the Brazilian agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship (Process no 001).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, M.A.; Silva, T.A.L.; Salgueiro, A.A.; Campos-Takaki, G.M.; Tambourgi, E.B. Reuse of Whey Cheese for Lipase Production by Candida Lipolytica. Chem. Eng. Trans. 2015, 43, 331–336. [Google Scholar] [CrossRef]
- Churton, H.; McCabe, B.K. Advancing a Food Loss and Waste Bioproduct Industry: A Critical Review of Policy Approaches for Application in an Australian Context. Heliyon 2024, 10, e32735. [Google Scholar] [CrossRef] [PubMed]
- Subramani, D.; Kumaraguruparaswami, M.; Senthilkumar, K.; Arunachalam, S.; Naveenkumar, M. Recent Advances in Valorization of Wastes from Food Industries. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2024; Chapter 8; pp. 135–155. [Google Scholar] [CrossRef]
- Deshmukh, N.; Rao, P.S.; Sharma, H.; Kumar, M. Waste to Nutrition: The Evolution of Whey, a Byproduct to Galactooligosaccharides Production. Food Chem. Adv. 2024, 4, 100642. [Google Scholar] [CrossRef]
- Ramos, P.R.; Kamimura, E.S.; Pires, N.A.M.; Maldonado, R.R.; Oliveira, A.L. de Esterification Reaction in SC-CO2 Catalyzed by Lipase Produced with Corn Steep Liquor and Minas Frescal Cheese Whey. Bioresour. Technol. Rep. 2021, 14, 100670. [Google Scholar] [CrossRef]
- Lavelli, V.; Beccalli, M.P. Cheese Whey Recycling in the Perspective of the Circular Economy: Modeling Processes and the Supply Chain to Design the Involvement of the Small and Medium Enterprises. Trends Food Sci. Technol. 2022, 126, 86–98. [Google Scholar] [CrossRef]
- Besediuk, V.; Yatskov, M.; Korchyk, N.; Kucherova, A.; Maletskyi, Z. Whey—From Waste to a Valuable Resource. J. Agric. Food Res. 2024, 18, 101280. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, J.; Ma, Y.; Cai, L.; Zheng, L.; Gong, W.; Liu, Q. Corn Steep Liquor: Green Biological Resources for Bioindustry. Appl. Biochem. Biotechnol. 2022, 194, 3280–3295. [Google Scholar] [CrossRef]
- Rodrigues, M.H.P.; Gräff, C.A.; Tupuna-Yerovi, D.S.; Schmitz, C.; Camargo de Lima, J.; Timmers, L.F.S.M.; Lehn, D.N.; Volken de Souza, C.F. The Bioactive Potential of Cheese Whey Peptides from Different Animal Origins (Bovine, Goat, Sheep, Buffalo, and Camel): A Systematic Review and Meta-Analysis. Food Res. Int. 2024, 196, 115053. [Google Scholar] [CrossRef]
- Caltzontzin-Rabell, V.; Feregrino-Pérez, A.A.; Gutiérrez-Antonio, C. Bio-Upcycling of Cheese Whey: Transforming Waste into Raw Materials for Biofuels and Animal Feed. Heliyon 2024, 10, e32700. [Google Scholar] [CrossRef]
- Suresh, G.; Ragunathan, R.; Johney, J. Assessing the Impact of Corn Steep Liquor as an Inducer on Enhancing Laccase Production and Laccase Gene (Lac1) Transcription in Pleurotus Pulmonarius during Solid-State Fermentation. Bioresour. Technol. Rep. 2024, 27, 101905. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; de Paula, D.R.; Medeiros, A.B.P.; de Carvalho, J.C.; Molina, D.; Soccol, C.R. Hydrogen Production by Dark Fermentation Using a New Low-Cost Culture Medium Composed of Corn Steep Liquor and Cassava Processing Water: Process Optimization and Scale-Up. Bioresour. Technol. 2021, 320, 124370. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.R.; Aguiar-Oliveira, E.; Fogaça, F.M.; Ramos, G.G.; Macedo, G.A.; Rodrigues, M.I. Evaluation of Partial Purification and Immobilization of Lipase from Geotrichum Candidum. Biocatal. Agric. Biotechnol. 2015, 4, 321–326. [Google Scholar] [CrossRef]
- Tommaso, G.; de Moraes, B.S.; Macedo, G.C.; Silva, G.S.; Kamimura, E.S. Production of Lipase from Candida Rugosa Using Cheese Whey through Experimental Design and Surface Response Methodology. Food Bioprocess Technol. 2011, 4, 1473–1481. [Google Scholar] [CrossRef]
- Maldonado, R.R.; Aguiar-Oliveira, E.; Pozza, E.L.; Costa, F.A.A.; Maugeri Filho, F.; Rodrigues, M.I. Production of Lipase from Geotrichum Candidum Using Corn Steep Liquor in Different Bioreactors. AOCS 2014, 91, 1999–2009. [Google Scholar] [CrossRef]
- Abro, A.A.; Qureshi, A.S.; Khushk, I.; Jatt, A.N.; Ali, C.H.; Karimi, K.; Rajper, S.B.; Khan, M.S. Enhanced Lipase Production from Ionic Liquid Tolerant Klebsiella Aerogenes Using Mustard Oilcake for Efficient Biodiesel Production. Renew. Energy 2024, 232, 121143. [Google Scholar] [CrossRef]
- Resende, R.; Eduardo, M.; Pozza, L.; Aguiar, E.; Aparecida, F.; Costa, A.; Maugeri, F.; Rodrigues, M.I. Characterization of Crude and Partially Purified Lipase from Geotrichum Candidum Obtained with Different Nitrogen Sources. J. Am. Oil Chem. Soc. 2016, 93, 1355–1364. [Google Scholar] [CrossRef]
- Botha, A.; Botes, A. Geotrichum. Encycl. Food Microbiol. 2014, 2, 88–93. [Google Scholar] [CrossRef]
- Eliskases-Lechner, F.; Guéguen, M.; Panoff, J.M. Geotrichum Candidum. Encycl. Dairy Sci. 2022, 14, 561–568. [Google Scholar] [CrossRef]
- Çağatay, Ş.; Aksu, Z. Use of Different Kinds of Wastes for Lipase Production: Inductive Effect of Waste Cooking Oil on Activity. J. Biosci. Bioeng. 2021, 132, 234–240. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, L.; Shi, W.; Long, J. Investigation of Enzymatic Activity, Stability and Structure Changes of Pectinase Treated in Supercritical Carbon Dioxide. J. Clean. Prod. 2016, 125, 331–340. [Google Scholar] [CrossRef]
- Rosa, B.H.; Silva, G.S.; Conceição, G.J.A.; Carvalho, R.A.; Aguiar-Oliveira, E.; Maldonado, R.R.; Kamimura, E.S. Application of Partially Concentrated Candida Rugosa Lipase in the Enzymatic Synthesis of Geranyl Acetate in Organic Solvent. Biocatal. Agric. Biotechnol. 2017, 12, 90–95. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Teleken, J.G.; de Cinque Almeida, V.; da Silva, C. Biodiesel from Waste Frying Oils: Methods of Production and Purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sarkar, D.; Bhattacharya, T.; Das, T.; Sengupta, A.; Preetam, S.; Ulaganathan, V.; Rustagi, S. Reaction Kinetics Involved in Esterification between the Fatty Acids in Castor Oil and Furfuryl Alcohol. Ind. Crops Prod. 2024, 213, 118393. [Google Scholar] [CrossRef]
- Dos Santos, P.; Rezende, C.A.; Martínez, J. Activity of Immobilized Lipase from Candida Antarctica (Lipozyme 435) and Its Performance on the Esterification of Oleic Acid in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2016, 107, 170–178. [Google Scholar] [CrossRef]
- Santos, L.F.S.; Silva, M.R.L.; Ferreira, E.E.A.; Gama, R.S.; Carvalho, A.K.F.; Barboza, J.C.S.; Luiz, J.H.H.; Mendes, A.A.; Hirata, D.B. Decyl Oleate Production by Enzymatic Esterification Using Geotrichum Candidum Lipase Immobilized on a Support Prepared from Rice Husk. Biocatal. Agric. Biotechnol. 2021, 36, 102142. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.C.; Zhang, G. Enzymatic Transesterification for Biodiesel Production from Used Cooking Oil, a Review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Saito, M. History of Supercritical Fluid Chromatography: Instrumental Development. J. Biosci. Bioeng. 2013, 115, 590–599. [Google Scholar] [CrossRef]
- Liu, S.; Yang, F.; Zhang, C.; Ji, H.; Hong, P.; Deng, C. The Journal of Supercritical Fluids Optimization of Process Parameters for Supercritical Carbon Dioxide Extraction of Passiflora Seed Oil by Response Surface Methodology. J. Supercrit. Fluids 2009, 48, 9–14. [Google Scholar] [CrossRef]
- Wen, D.; Jiang, H.; Zhang, K. Supercritical Fluids Technology for Clean Biofuel Production. Prog. Nat. Sci. 2009, 19, 273–284. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical Fluid Extraction of Bioactive Compounds. TrAC—Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Melfi, D.T.; dos Santos, K.C.; Ramos, L.P.; Corazza, M.L. Supercritical CO2 as Solvent for Fatty Acids Esterification with Ethanol Catalyzed by Amberlyst-15. J. Supercrit. Fluids 2020, 158, 104736. [Google Scholar] [CrossRef]
- Kanda, H.; Shimakata, M.; Wang, T.; Zhu, L.; Wahyudiono; Goto, M. Supercritical Methanol-Induced Esterification of Microalgal Lipids Employing Biomineralized Cell Walls as the Catalyst. Fuel 2022, 330, 125707. [Google Scholar] [CrossRef]
- Da Silva Junior, V.A.; Shigueyuki Kanda, L.R.; Zandoná-Filho, A.; Corazza, M.L.; Sutile De Lima, C. Effect of Supercritical Carbon Dioxide over the Esterification of Levulinic Acid with Ethanol Using Montmorillonite K10 as Catalyst. J. CO2 Util. 2020, 39, 101158. [Google Scholar] [CrossRef]
- Matsuda, T.; Marukado, R.; Mukouyama, M.; Harada, T.; Nakamura, K. Asymmetric Reduction of Ketones by Geotrichum Candidum: Immobilization and Application to Reactions Using Supercritical Carbon Dioxide. Tetrahedron Asymmetry 2008, 19, 2272–2275. [Google Scholar] [CrossRef]
- Maldonado, R.R.; Aguiar-oliveira, E.; Pozza, E.L.; Mazutti, M.A.; Maugeri, F.; Rodrigues, M.I. Application of Yeast Hydrolysate in Extracellular Lipase Production by Geotrichum Candidum in Shaken Flasks, Stirred Tank, and Airlift Reactors. Can. J. Chem. Eng. 2015, 93, 1524–1530. [Google Scholar] [CrossRef]
- Potumarthi, R.; Subhakar, C.; Vanajakshi, J.; Jetty, A. Effect of Aeration and Agitation Regimes on Lipase Production by Newly Isolated Rhodotorula Mucilaginosa—MTCC 8737 in Stirred Tank Reactor Using Molasses as Sole Production Medium. Appl. Biochem. Biotechnol. 2008, 151, 700–710. [Google Scholar] [CrossRef]
- Maldonado, R.R.; Burkert, J.F.M.; Mazutti, M.A.; Maugeri, F.; Rodrigues, M.I. Evaluation of Lipase Production by Geotrichum Candidum in Shaken Flasks and Bench-Scale Stirred Bioreactor Using Different Impellers. Biocatal. Agric. Biotechnol. 2012, 1, 147–151. [Google Scholar] [CrossRef]
- Maldonado, R.R.; Macedo, G.A.; Rodrigues, M.I. Lipase Production Using Microorganisms from Different Agro-Industrial By- Products. Int. J. Appl. Sci. Technol. 2014, 4, 108–115. [Google Scholar]
- Maldonado, R.R.; Aguiar-oliveira, E.; Durrant, L.R.; Mazutti, M.; Maugeri Filho, F.; Rodrigues, M.I. Elucidation of the Effects of Inoculum Size and Age on Lipase Production by Geotrichum Candidum. Biotecnol. Apl. 2014, 31, 216–221. [Google Scholar]
- Aljawish, A.; Heuson, E.; Bigan, M.; Froidevaux, R. Lipase Catalyzed Esterification of Formic Acid in Solvent and Solvent-Free Systems. Biocatal. Agric. Biotechnol. 2019, 20, 101221. [Google Scholar] [CrossRef]
- Shin, S.K.; Sim, J.E.; Kishimura, H.; Chun, B.S. Characteristics of Menhaden Oil Ethanolysis by Immobilized Lipase in Supercritical Carbon Dioxide. J. Ind. Eng. Chem. 2012, 18, 546–550. [Google Scholar] [CrossRef]
- Zulkeflee, S.A.; Sata, S.A.; Rohman, F.S.; Aziz, N. Modelling of Immobilized Candida Rugosa Lipase Catalysed Esterification Process in Batch Reactor Equipped with Temperature and Water Activity Control System. Biochem. Eng. J. 2020, 161, 107669. [Google Scholar] [CrossRef]
- Ramos, P.R.; Vicentini-Polette, C.M.; Mazalli, M.R.; de Lima Caneppele, F.; de Oliveira, A.L. Pressurized Liquid Extraction of Soybean Oil Using Intermittent Process with Ethanol and Hexane as Solvent: Extraction Yield and Physicochemical Parameters Comparison. J. Food Process Eng. 2024, 47, e14562. [Google Scholar] [CrossRef]
- Rodrigues, L.d.C.; Bodini, R.B.; Caneppele, F.L.; Dacanal, G.C.; Crevelin, E.J.; de Moraes, L.A.B.; de Oliveira, A.L. Pressurized Liquid Extraction (PLE) in an Intermittent Process as an Alternative for Obtaining Passion Fruit (Passiflora edulis) Leaf Hydroalcoholic Extract (Tincture). Processes 2023, 11, 2308. [Google Scholar] [CrossRef]
- Ramos, P.R.; Sponchiado, J.; Echenique, J.V.F.; Dacanal, G.C.; Oliveira, A.L. de Kinetics of Vegetable Oils (Rice Bran, Sunflower Seed, and Soybean) Extracted by Pressurized Liquid Extraction in Intermittent Process. Processes 2024, 12, 1107. [Google Scholar] [CrossRef]
- Escandell, J.; Wurm, D.J.; Belleville, M.P.; Sanchez, J.; Harasek, M.; Paolucci-Jeanjean, D. Enzymatic Synthesis of Butyl Acetate in a Packed Bed Reactor under Liquid and Supercritical Conditions. Catal. Today 2015, 255, 3–9. [Google Scholar] [CrossRef]
- dos Santos, P.; Zabot, G.L.; Meireles, M.A.A.; Mazutti, M.A.; Martínez, J. Synthesis of Eugenyl Acetate by Enzymatic Reactions in Supercritical Carbon Dioxide. Biochem. Eng. J. 2016, 114, 1–9. [Google Scholar] [CrossRef]
- Anschau, A.; Aragão, V.C.; Porciuncula, B.D.A.; Kalil, S.J.; Burkert, C.A.V.; Burkert, J.F.M. Enzymatic Synthesis Optimization of Isoamyl Butyrate. J. Braz. Chem. Soc. 2011, 22, 2148–2156. [Google Scholar] [CrossRef]
- Macedo, G.A.; Pastore, G.M.; Rodrigues, M.I. Optimising the Synthesis of Isoamyl Butyrate Using Rhizopus Sp. Lipase with a Central Composite Rotatable Design. Process Biochem. 2004, 39, 687–693. [Google Scholar] [CrossRef]
- Melgosa, R.; Teresa Sanz, M.; Solaesa, Á.G.; De Paz, E.; Beltrán, S.; Lamas, D.L. Supercritical Carbon Dioxide as Solvent in the Lipase-Catalyzed Ethanolysis of Fish Oil: Kinetic Study. J. CO2 Util. 2017, 17, 170–179. [Google Scholar] [CrossRef]
- Singh, B.; Jana, A.K. Agri-Residues and Agro-Industrial Waste Substrates Bioconversion by Fungal Cultures to Biocatalyst Lipase for Green Chemistry: A Review. J. Environ. Manag. 2023, 348, 119219. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).