Challenges and Advances in Tertiary Waste Water Treatment for Municipal Treatment Plants
Abstract
1. Introduction
2. The Issue of the Tertiary Stage of Waste Water Treatment Plants as a Decisive Factor in the Removal of Polluting Toxic Substances
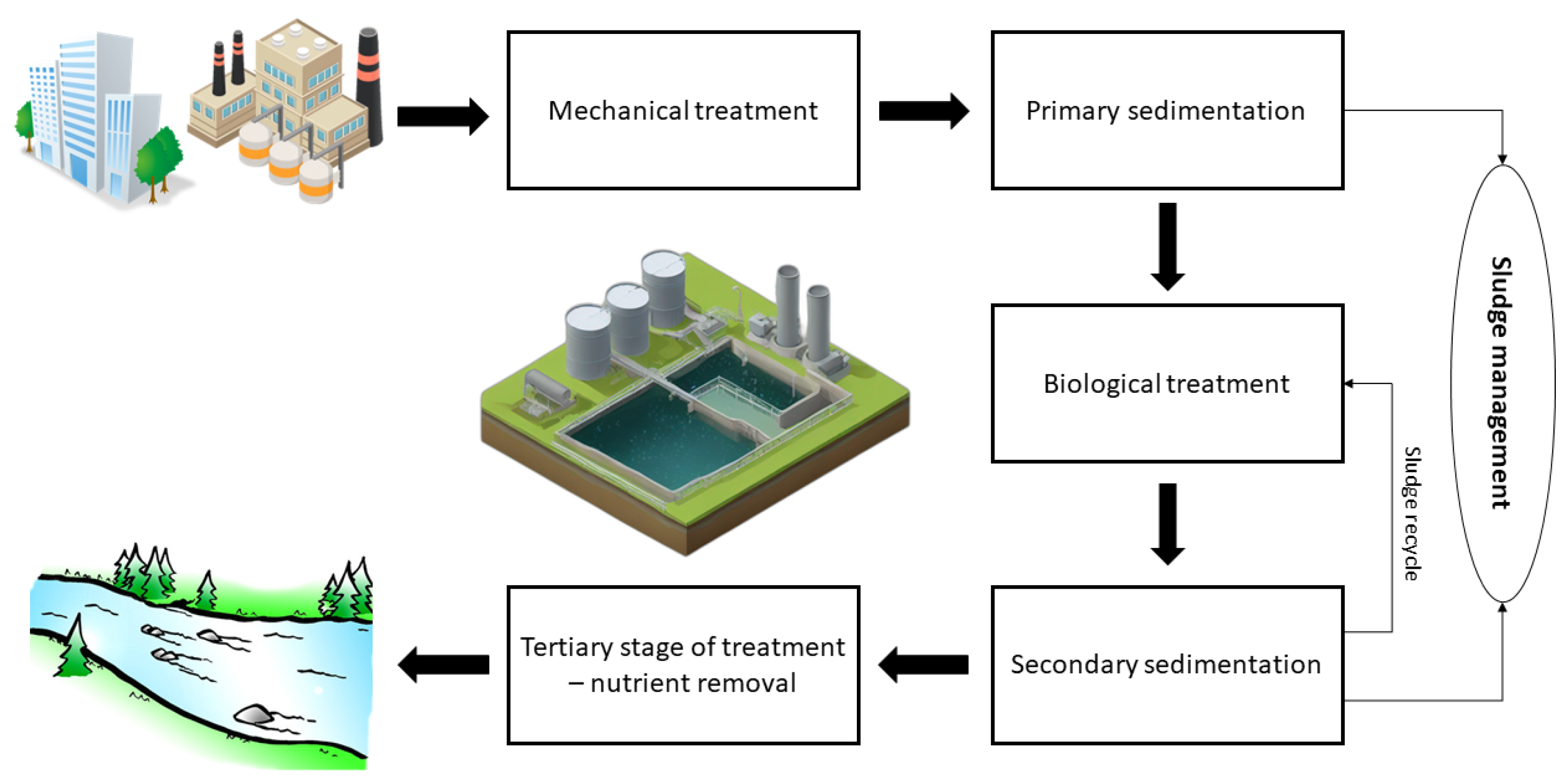
The Typical Organization of Individual Processes in WWTPs
3. Micropollutants in the Waste Water and Effluents Discharged from Most Current WWTPs
4. Waste Water Treatment Plants as Point Sources of Environmental Contamination, Especially by Newly Monitored Pollutants
5. Removal of Microplastics from the Waste Water Entering Treatment Plants
5.1. The Detection of Microplastics Entering Treatment Plants
5.2. Possibilities for the Removal of Microplastics from Waste Water Treated in Small Waste Water Treatment Plants
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valdes Ramos, A.; Aguilera Gonzalez, E.N.; Tobón Echeverri, G.; Samaniego Moreno, L.; Díaz Jiménez, L.; Carlos Hernández, S. Potential Uses of Treated Municipal Wastewater in a Semiarid Region of Mexico. Sustainability 2019, 11, 2217. [Google Scholar] [CrossRef]
- Zema, D.A.; Carrà, B.G.; Sorgonà, A.; Zumbo, A.; Lucas-Borja, M.E.; Miralles, I.; Ortega, R.; Soria, R.; Zimbone, S.M.; Calabrò, P.S. Sustainable Use of Treated Municipal Wastewater after Chlorination: Short-Term Effects on Crops and Soils. Sustainability 2023, 15, 11801. [Google Scholar] [CrossRef]
- EPA United States Environmental Protection Agency. Potable Water Reuse and Drinking Water. Available online: https://www.epa.gov/ground-water-and-drinking-water/potable-water-reuse-and-drinking-water (accessed on 29 August 2024).
- Binns, C. The Cleanest Drinking Water Is Recycled. Available online: https://engineering.stanford.edu/news/cleanest-drinking-water-recycled (accessed on 29 August 2024).
- 2017 UN World Water Development Report, Wastewater: The Untapped Resource. Available online: https://www.unep.org/resources/publication/2017-un-world-water-development-report-wastewater-untapped-resource (accessed on 29 August 2024).
- Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 29 August 2024).
- Śalas, E.B. Proportion of Domestic Wastewater Flow Safely Treated Worldwide in 2022, by Region. Available online: https://www.statista.com/statistics/746428/wastewater-treatment-global-share-by-region/ (accessed on 29 August 2024).
- Wang, N.; Sun, X.; Zhao, Q.; Wang, P. Treatment of Polymer-Flooding Wastewater by a Modified Coal Fly Ash-Catalysed Fenton-like Process with Microwave Pre-Enhancement: System Parameters, Kinetics, and Proposed Mechanism. Chem. Eng. J. 2021, 406, 126734. [Google Scholar] [CrossRef]
- Yumin, W.; Lei, W.; Yanhong, F. Cost Function for Treating Wastewater in Rural Regions. Desalination Water Treat. 2016, 57, 17241–17246. [Google Scholar] [CrossRef]
- Chatterjee, P.; Ghangrekar, M.M.; Rao, S. Low Efficiency of Sewage Treatment Plants due to Unskilled Operations in India. Environ. Chem. Lett. 2016, 14, 407–416. [Google Scholar] [CrossRef]
- Boguniewicz-Zabłocka, J.; Capodaglio, A.G. Sustainable Wastewater Treatment Solutions for Rural Communities’: Public (Centralized) or Individual (On-Site)—Case Study. Econ. Environ. Stud. 2017, 17, 1103–1119. [Google Scholar] [CrossRef]
- Marcantonio, C.D.; Chiavola, A.; Dossi, S.; Cecchini, G.; Leoni, S.; Frugis, A.; Spizzirri, M.; Boni, M.R. Occurrence, Seasonal Variations and Removal of Organic Micropollutants in 76 Wastewater Treatment Plants. Process Saf. Environ. Prot. 2020, 141, 61–72. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Speth, T. PFAS TREATMENT in Drinking Water and Wastewater; PFAS Science Webinars for EPA Region 1 and State & Tribal Partners; US EPA Office of Research and Development: Washington, DC, USA, 2020.
- The Four Stages of Wastewater Treatment Plants. Available online: https://www.idrica.com/blog/stages-of-wastewater-treatment-plants/ (accessed on 29 August 2024).
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016; Volume 15. [Google Scholar]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Muruganandam, L.; Kumar, M.P.S.; Jena, A.; Gulla, S.; Godhwani, B. Treatment of Waste Water by Coagulation and Flocculation Using Biomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 32006. [Google Scholar] [CrossRef]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Mao, D.; Yu, S.; Rysz, M.; Luo, Y.; Yang, F.; Li, F.; Hou, J.; Mu, Q.; Alvarez, P.J.J. Prevalence and Proliferation of Antibiotic Resistance Genes in Two Municipal Wastewater Treatment Plants. Water Res. 2015, 85, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M. Effects of Advanced Treatment Systems on the Removal of Antibiotic Resistance Genes in Wastewater Treatment Plants from Hangzhou, China. Environ. Sci. Technol. 2013, 47, 8157–8163. [Google Scholar] [CrossRef] [PubMed]
- Alavian Petroody, S.S.; Hashemi, S.H.; van Gestel, C.A.M. Transport and Accumulation of Microplastics through Wastewater Treatment Sludge Processes. Chemosphere 2021, 278, 130471. [Google Scholar] [CrossRef]
- Bella, G.D.; Corsino, S.F.; De Marines, F.; Lopresti, F.; La Carrubba, V.; Torregrossa, M.; Viviani, G. Occurrence of Microplastics in Waste Sludge of Wastewater Treatment Plants: Comparison between Membrane Bioreactor (MBR) and Conventional Activated Sludge (CAS) Technologies. Membranes 2022, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Prasetya, K.D.; Hanun, J.N.; Bui, H.M.; Rajendran, S.; Kataria, N.; Khoo, K.S.; Wang, Y.-F.; You, S.-J.; Jiang, J.-J. Microplastic Contamination in Sewage Sludge: Abundance, Characteristics, and Impacts on the Environment and Human Health. Environ. Technol. Innov. 2023, 31, 103176. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Aatik, A.E.; Aliste, M.; Navarro, G.; Fenoll, J.; Navarro, S. Removal of Contaminants of Emerging Concern from a Wastewater Effluent by Solar-Driven Heterogeneous Photocatalysis: A Case Study of Pharmaceuticals. Water Air Soil. Pollut. 2023, 234, 55. [Google Scholar] [CrossRef]
- de Vidales, M.; Prieto, R.; Galán-Lucarelli, G.; Sánchez, E.; Martinez, F.F. Removal of Contaminants of Emerging Concern by Photocatalysis with a Highly Ordered TiO2 Nanotubular Array Catalyst. Catal. Today 2023, 413–415, 113995. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Bampos, G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes 2022, 10, 2304. [Google Scholar] [CrossRef]
- Zambrano, J.; Irusta-Mata, R.; Jiménez, J.J.; Bolado, S.; García-Encina, P.A. Photocatalytic Removal of Emerging Contaminants in Water and Wastewater Treatments: A Review. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 543–572. [Google Scholar] [CrossRef]
- Borges, M.E.; de Paz Carmona, H.; Gutiérrez, M.; Esparza, P. Photocatalytic Removal of Water Emerging Pollutants in an Optimized Packed Bed Photoreactor Using Solar Light. Catalysts 2023, 13, 1023. [Google Scholar] [CrossRef]
- Neumann, S.; Fatula, P. Principles of Ion Exchange in Wastewater Treatment. Asian Water. Techno Focus 2009, 19, 14–19. [Google Scholar]
- Goutham, R.; Rohit, P.; Vigneshwar, S.S.; Swetha, A.; Arun, J.; Gopinath, K.P.; Pugazhendhi, A. Ionic Liquids in Wastewater Treatment: A Review on Pollutant Removal and Degradation, Recovery of Ionic Liquids, Economics and Future Perspectives. J. Mol. Liq. 2022, 349, 118150. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Shehata, N.; Egirani, D.; Olabi, A.G.; Inayat, A.; Abdelkareem, M.A.; Chae, K.-J.; Sayed, E.T. Membrane-Based Water and Wastewater Treatment Technologies: Issues, Current Trends, Challenges, and Role in Achieving Sustainable Development Goals, and Circular Economy. Chemosphere 2023, 320, 137993. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, M. Advanced Membrane Technologies for Wastewater Treatment and Recycling. Membranes 2023, 13, 558. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Yousefi, M.; Mohammadi, A.A.; Changani, F. Recent Trends in Disposal and Treatment Technologies of Emerging-Pollutants—A Critical Review. Trends Anal. Chem. 2020, 122, 115744. [Google Scholar] [CrossRef]
- Khan, M.M.; Siddiqi, S.A.; Farooque, A.A.; Iqbal, Q.; Shahid, S.A.; Akram, M.T.; Rahman, S.; Al-Busaidi, W.; Khan, I. Towards Sustainable Application of Wastewater in Agriculture: A Review on Reusability and Risk Assessment. Agronomy 2022, 12, 1397. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging Pollutants in the Environment: A Challenge for Water Resource Management. Int. Soil. Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Krishnakumar, S.; Singh, D.S.H.; Godson, P.S.; Thanga, S.G. Emerging Pollutants: Impact on Environment, Management, and Challenges. Environ. Sci. Pollut. Res. 2022, 29, 72309–72311. [Google Scholar] [CrossRef]
- Bayabil, H.K.; Teshome, F.T.; Li, Y.C. Emerging Contaminants in Soil and Water. Front. Environ. Sci. 2022, 10, 873499. [Google Scholar] [CrossRef]
- Europian Commision. Endocrine Disruptors Overview. Available online: https://health.ec.europa.eu/endocrine-disruptors/overview_en (accessed on 29 August 2024).
- Tortajada, C. Contributions of Recycled Wastewater to Clean Water and Sanitation Sustainable Development Goals. NPJ Clean. Water 2020, 3, 22. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A Review on Effective Removal of Emerging Contaminants from Aquatic Systems: Current Trends and Scope for Further Research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Halleux, V. Urban Wastewater Treatment; European Parliamentary Research Service: Brussels, Belgium, 2024.
- Ben, W.; Zhu, B.; Yuan, X.; Zhang, Y.; Yang, M.; Qiang, Z. Occurrence, Removal and Risk of Organic Micropollutants in Wastewater Treatment Plants across China: Comparison of Wastewater Treatment Processes. Water Res. 2018, 130, 38–46. [Google Scholar] [CrossRef]
- Nam, S.-W.; Jo, B.-I.; Yoon, Y.; Zoh, K.-D. Occurrence and Removal of Selected Micropollutants in a Water Treatment Plant. Chemosphere 2014, 95, 156–165. [Google Scholar] [CrossRef]
- Thompson, K.A.; Mortazavian, S.; Gonzalez, D.J.; Bott, C.; Hooper, J.; Schaefer, C.E.; Dickenson, E.R.V. Poly- and Perfluoroalkyl Substances in Municipal Wastewater Treatment Plants in the United States: Seasonal Patterns and Meta-Analysis of Long-Term Trends and Average Concentrations. ACS EST Water 2022, 2, 690–700. [Google Scholar] [CrossRef]
- Lenka, S.P.; Kah, M.; Padhye, L.P. A Review of the Occurrence, Transformation, and Removal of Poly- and Perfluoroalkyl Substances (PFAS) in Wastewater Treatment Plants. Water Res. 2021, 199, 117187. [Google Scholar] [CrossRef]
- McGrath, A. 66 Things Wastewater Treatment Plant Owners Need to Know about PFAS. Available online: https://www.stantec.com/en/ideas/6-things-wastewater-treatment-plant-owners-need-to-know-about-pfas (accessed on 24 August 2022).
- Tavasoli, E.; Luek, J.L.; Malley, J.P.; Mouser, P.J. Distribution and Fate of Per- and Polyfluoroalkyl Substances (PFAS) in Wastewater Treatment Facilities. Environ. Sci. Process Impacts 2021, 23, 903–913. [Google Scholar] [CrossRef]
- O’Connor, J.; Bolan, N.S.; Kumar, M.; Nitai, A.S.; Ahmed, M.B.; Bolan, S.S.; Vithanage, M.; Rinklebe, J.; Mukhopadhyay, R.; Srivastava, P.; et al. Distribution, Transformation and Remediation of Poly- and per-Fluoroalkyl Substances (PFAS) in Wastewater Sources. Process Saf. Environ. Prot. 2022, 164, 91–108. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and Polyfluoroalkyl Substances in Water and Wastewater: A Critical Review of Their Global Occurrence and Distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Hammoudani, Y.E.; Dimane, F.; Haboubi, K.; Benaissa, C.; Benaabidate, L.; Bourjila, A.; Achoukhi, I.; Boudammoussi, M.E.; Faiz, H.; Touzani, A.; et al. Micropollutants in Wastewater Treatment Plants: A Bibliometric—Bibliographic Study. Desalination Water Treat. 2024, 317, 100190. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E.; Galamon, M.; Bernat, P. Kinetics of Biological Removal of the Selected Micropollutants and Their Effect on Activated Sludge Biomass. Water Air Soil. Pollut. 2018, 229, 356. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ray, N.M.; Wan, J.; Khan, A.; Chakraborty, T.; Ray, M.B. Micropollutants in Wastewater: Fate and Removal Processes. In Physico-Chemical Wastewater Treatment and Resource Recovery; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Belete, B.; Desye, B.; Ambelu, A.; Yenew, C. Micropollutant Removal Efficiency of Advanced Wastewater Treatment Plants: A Systematic Review. Environ. Health Insights 2023, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Lundqvist, J.; Orn, S.; Ahrens, L. Assessing the Cumulative Pressure of Micropollutants in Swedish Wastewater Effluents and Recipient Water Systems Using Integrated Toxicological and Chemical Methods. SLU. Available online: https://www.diva-portal.org/smash/get/diva2:1430097/FULLTEXT01.pdf (accessed on 30 August 2024).
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging Pollutants in Wastewater: A Review of the Literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A Review on Emerging Contaminants in Wastewaters and the Environment: Current Knowledge, Understudied Areas and Recommendations for Future Monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Puchovsky, M. Výskyt Metabolitů Nelegálních Drog v Odpdních Vodách; Vysoká Škola Báňská—Technická Univerzita Ostrava: Ostrava, Czech Republic, 2019. [Google Scholar]
- Huang, Y.; Dsikowitzky, L.; Yang, F.; Schwarzbauer, J. Emerging Contaminants in Municipal Wastewaters and Their Relevance for the Surface Water Contamination in the Tropical Coastal City Haikou, China. Estuar. Coast. Shelf Sci. 2020, 235, 106611. [Google Scholar] [CrossRef]
- Madhogaria, B.; Banerjee, S.; Kundu, A.; Dhak, P. Efficacy of New Generation Biosorbents for the Sustainable Treatment of Antibiotic Residues and Antibiotic Resistance Genes from Polluted Waste Effluent. Infect. Med. 2024, 3, 100092. [Google Scholar] [CrossRef]
- Zhao, X.; Su, H.; Xu, W.; Hu, X.; Xu, Y.; Wen, G.; Cao, Y. Removal of Antibiotic Resistance Genes and Inactivation of Antibiotic-Resistant Bacteria by Oxidative Treatments. Sci. Total Environ. 2021, 778, 146348. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X. Removal of Antibiotic Resistance Genes (ARGs) in Various Wastewater Treatment Processes: An Overview. Crit. Rev. Environ. Sci. Technol. 2022, 52, 571–630. [Google Scholar] [CrossRef]
- Chen, P.; Yu, X.; Zhang, J.; Wang, Y. New and Traditional Methods for Antibiotic Resistance Genes Removal: Constructed Wetland Technology and Photocatalysis Technology. Front. Microbiol. 2023, 13, 1110793. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment: Five Decades of Experience. Environ. Sci. Technol. 2011, 45, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ahmad, S.; Liu, L.; Wang, L.; Tang, J. A Review of Antibiotics and Antibiotic Resistance Genes (ARGs) Adsorption by Biochar and Modified Biochar in Water. Sci. Total Environ. 2023, 858, 159815. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Jain, R.; Ahammad, S.Z.; Ali, S.W. Removal of Antibiotic Resistance Genes from Wastewater Using Diethylaminoethyl Cellulose as a Promising Adsorbent. J. Water Process Eng. 2023, 55, 104109. [Google Scholar] [CrossRef]
- El-Monaem, E.M.A.; Eltaweil, A.S.; Elshishini, H.M.; Hosny, M.; Alsoaud, M.M.A.; Attia, N.F.; El-Subruiti, G.M.; Omer, A.M. Sustainable Adsorptive Removal of Antibiotic Residues by Chitosan Composites: An Insight into Current Developments and Future Recommendations. Arab. J. Chem. 2022, 15, 103743. [Google Scholar] [CrossRef]
- Tobey, E. New Approach Methodologies in Ecotoxicology Bibliography; USDA National Agricultural Library: Beltsville, MD, USA, 2023.
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Bokhari, A.; Karimian, M.; Zahra, M.M.A.; Sillanpää, M.; Panchal, H.; Alrubaie, A.J.; Rezakhani, Y. A Comprehensive Review of Various Approaches for Treatment of Tertiary Wastewater with Emerging Contaminants: What Do We Know? Environ. Monit. Assess. 2022, 194, 884. [Google Scholar] [CrossRef]
- Bracamontes-Ruelas, A.R.; Ordaz-Díaz, L.A.; Bailón-Salas, A.M.; Ríos-Saucedo, J.C.; Reyes-Vidal, Y.; Reynoso-Cuevas, L. Emerging Pollutants in Wastewater, Advanced Oxidation Processes as an Alternative Treatment and Perspectives. Processes 2022, 10, 1041. [Google Scholar] [CrossRef]
- Ramrakhiani, L.; Ghosh, S.; Majumdar, S. Emerging Contaminants in Water and Wastewater: Remediation Perspectives and Innovations in Treatment Technologies. In Impact of COVID-19 on Emerging Contaminants; Springer: Singapore, 2022; pp. 253–284. [Google Scholar] [CrossRef]
- Shehu, Z.; Kalu, K.M.; Lamayi, D.W.; Akinterinwa, A.; Irimiya, A.; Emmanuel, M.; Kenneth, R.; Nyakairu, G.W.A. A Review of Global Occurrence of Emerging Pollutants in Wastewater: Present Status, Source/Pathway, Extraction and Detection Techniques. Asian J. Curr. Res. 2023, 8, 24–61. [Google Scholar] [CrossRef]
- Haddaoui, I.; Mateo-Sagasta, J. A Review on Occurrence of Emerging Pollutants in Waters of the MENA Region. Environ. Sci. Pollut. Res. 2021, 28, 68090–68110. [Google Scholar] [CrossRef]
- Vidal-Dorsch, D.E.; Bay, S.M.; Maruya, K.A.; Snyder, S.A.; Trenholm, R.A.; Vanderford, B.J. Contaminants of Emerging Concern in Municipal Wastewater Effluents and Marine Receiving Water. Environ. Toxicol. Chem. 2011, 31, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores, F.G.; Isac-García, J.; Dobado, J.A. Emerging Pollutants: Origin, Structure and Properties; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2017. [Google Scholar] [CrossRef]
- Peivasteh-roudsari, L.; Barzegar-bafrouei, R.; Sharifi, K.A.; Azimisalim, S.; Karami, M.; Abedinzadeh, S.; Asadinezhad, S.; Tajdar-oranj, B.; Mahdavi, V.; Alizadeh, A.M.; et al. Origin, Dietary Exposure, and Toxicity of Endocrine-Disrupting Food Chemical Contaminants: A Comprehensive Review. Heliyon 2023, 9, e18140. [Google Scholar] [CrossRef] [PubMed]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-Disruptive Chemicals as Contaminants of Emerging Concern in Wastewater and Surface Water: A Review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef] [PubMed]
- Ismanto, A.; Hadibarata, T.; Kristanti, R.A.; Maslukah, L.; Safinatunnajah, N.; Kusumastuti, W. Endocrine Disrupting Chemicals (EDCs) in Environmental Matrices: Occurrence, Fate, Health Impact, Physio-Chemical and Bioremediation Technology. Environ. Pollut. 2022, 302, 119061. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; de Souza, A.O.; Bernardes, M.F.F.; Pazin, M.; Tasso, M.J.; Pereira, P.H.; Dorta, D.J. A Perspective on the Potential Risks of Emerging Contaminants to Human and Environmental Health. Environ. Sci. Pollut. Res. 2015, 22, 13800–13823. [Google Scholar] [CrossRef]
- Encarnação, T.; Pais, A.A.C.C.; Campos, M.G.; Burrows, H.D. Endocrine Disrupting Chemicals: Impact on Human Health, Wildlife and the Environment. Sci. Prog. 2019, 102, 3–42. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, P.; Wu, X.; Shi, H.; Huang, H.; Wang, H.; Gao, S. Insight into Chain Scission and Release Profiles from Photodegradation of Polycarbonate Microplastics. Water Res. 2021, 195, 116980. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in Wastewater Treatment Plants: Detection, Occurrence and Removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic Contamination in an Urban Area: A Case Study in Greater Paris. Environ. Chem. 2015, 12, 592. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Li, M.-Y.; Qi, Z.-Z.; Fu, M.; Sun, T.-F.; Elsheikha, H.M.; Cong, W. Waterborne Protozoan Outbreaks: An Update on the Global, Regional, and National Prevalence from 2017 to 2020 and Sources of Contamination. Sci. Total Environ. 2022, 806, 150562. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Islam, N.; Tasannum, N.; Mehjabin, A.; Momtahin, A.; Chowdhury, A.A.; Almomani, F.; Mofijur, M. Microplastic Removal and Management Strategies for Wastewater Treatment Plants. Chemosphere 2024, 347, 140648. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Arenas, L.; Ramseier Gentile, S.; Zimmermann, S.; Stoll, S. Nanoplastics Adsorption and Removal Efficiency by Granular Activated Carbon Used in Drinking Water Treatment Process. Sci. Total Environ. 2021, 791, 148175. [Google Scholar] [CrossRef]
- Valiyaveettil Salimkumar, A.; Kurisingal Cleetus, M.C.; Ehigie, J.O.; Onogbosele, C.O.; Nisha, P.; Kumar, B.S.; Prabhakaran, M.P.; Rejish Kumar, V.J. Adsorption Behavior and Interaction of Micro-Nanoplastics in Soils and Aquatic Environment. In Management of Micro and Nano-Plastics in Soil and Biosolids; Springer: Cham, Switzerland, 2024; pp. 283–311. [Google Scholar] [CrossRef]
- Spacilova, M.; Dytrych, P.; Lexa, M.; Wimmerova, L.; Masin, P.; Kvacek, R.; Solcova, O. An Innovative Sorption Technology for Removing Microplastics from Wastewater. Water 2023, 15, 892. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, Y.; Zhou, G.; Zhang, Y.; Yue, J.; Wang, X.; Yang, Z.; Chen, J.; Wang, Q.; Zhang, J. Mechanisms of Polystyrene Nanoplastics Adsorption onto Activated Carbon Modified by ZnCl2. Sci. Total Environ. 2023, 876, 162763. [Google Scholar] [CrossRef]
- Sutherland, B.R.; Dhaliwal, M.S.; Thai, D.; Li, Y.; Gingras, M.; Konhauser, K. Suspended Clay and Surfactants Enhance Buoyant Microplastic Settling. Commun. Earth Environ. 2023, 4, 393. [Google Scholar] [CrossRef]
- Ahn, J.; Moon, J.; Pae, J.; Kim, H.-K. Microplastics as Lightweight Aggregates for Ultra-High Performance Concrete: Mechanical Properties and Autoignition at Elevated Temperatures. Compos. Struct. 2023, 321, 117333. [Google Scholar] [CrossRef]
- Abomohra, A.; Hanelt, D. Recent Advances in Micro-/Nanoplastic (MNPs) Removal by Microalgae and Possible Integrated Routes of Energy Recovery. Microorganisms 2022, 10, 2400. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Mo, A.; Jiang, J.; Liang, Y.; Cao, X.; He, D. Removal of Microplastics in Water: Technology Progress and Green Strategies. Green. Anal. Chem. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Tang, W.; Li, H.; Fei, L.; Wei, B.; Zhou, T.; Zhang, H. The Removal of Microplastics from Water by Coagulation: A Comprehensive Review. Sci. Total Environ. 2022, 851, 158224. [Google Scholar] [CrossRef]
- Girish, N.; Parashar, N.; Hait, S. Coagulative Removal of Microplastics from Aqueous Matrices: Recent Progresses and Future Perspectives. Sci. Total Environ. 2023, 899, 165723. [Google Scholar] [CrossRef] [PubMed]
- Takács, D.; Szabó, T.; Jamnik, A.; Tomšič, M.; Szilágyi, I. Colloidal Interactions of Microplastic Particles with Anionic Clays in Electrolyte Solutions. Langmuir 2023, 39, 12835–12844. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Gao, S.-H.; Ge, C.; Gao, Q.; Huang, S.; Kang, Y.; Luo, G.; Zhang, Z.; Fan, L.; Zhu, Y.; et al. Removing Microplastics from Aquatic Environments: A Critical Review. Environ. Sci. Ecotechnol. 2023, 13, 100222. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.S.; Tahir, I.; Ali, A.; Ayub, I.; Nasir, A.; Abbas, N.; Sajjad, U.; Hamid, K. Innovative Technologies for Removal of Micro Plastic: A Review of Recent Advances. Heliyon 2024, 10, e25883. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Ahmad, M.; Hossain, M.F.; Nawab, A.; Ahmad, I.; Ahmad, K.; Panyametheekul, S. Microplastic Removal by Coagulation: A Review of Optimizing the Reaction Conditions and Mechanisms. Water Emerg. Contam. Nanoplastics 2023, 2, 22. [Google Scholar] [CrossRef]
- Acarer, S. A Review of Microplastic Removal from Water and Wastewater by Membrane Technologies. Water Sci. Technol. 2023, 88, 199–219. [Google Scholar] [CrossRef]
- Ali, I.; Ding, T.; Peng, C.; Naz, I.; Sun, H.; Li, J.; Liu, J. Micro- and Nanoplastics in Wastewater Treatment Plants: Occurrence, Removal, Fate, Impacts and Remediation Technologies—A Critical Review. Chem. Eng. J. 2021, 423, 130205. [Google Scholar] [CrossRef]

| Group of Pollutants | Micropollutants |
|---|---|
| Pharmaceuticals | Acetaminophen, Diclofenac, Ketoprofen, Mefenamic acid, Naproxen, Salicylic acid, Carbamazepine, Bezafibrate, Clofibric acid, Gemfibrozil |
| Antibiotics | Erythromycin, Sulfamethoxazole, Trimethoprim, Azithromycin, Amoxicillin, Sulfamethoxazole, Lincomycin, Metronidazole, Flumequine—all in hundreds of ng/L |
| β-blockers | Atenolol, Metoprolol |
| Nervous stimulants | Caffeine, Salbutamol, Diazepam, Primidone, Carbamazepine—in hundreds of ng/L, with carbamazepine generally considered difficult to degrade |
| Fragrances, personal care products | Tonalide, Galaxolide, Diethyltoluamide, 4-benzophenone, 1,4-dioxane, methyl-propyl parabens |
| Steroid hormones | 17α-Ethynylestradiol, Estrone, Estradiol, Estriol |
| Surfactants | Nonylphenol, Octylphenol |
| Plasticizers and fire retardants | Dimethyl adipate and Tris (1-chloro-2-propyl) phosphate |
| Phosphates and surfactants | Tweens (Polysorbates) and sodium lauryl sulfate, Bis-(2-ethylhexyl) phthalate, Tris (2-chloroisopropyl) phosphate |
| Industrial chemicals | Plasticizers (Bisphenol A), Fire retardant, Pesticides (Atrazine, Diuron, Diazinon, Clotrimazole, Tebuconazole, Chlorpyrifos, Malathion, Simazine) |
| Per- and polyfluorinated compounds | Perfluorooctanoic acid and perfluorooctanesulfonate (PFAS). This pollutant, often called “eternal”, is currently the most popular and possibly the most closely monitored waste water contaminant, usually in concentrations of ng/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solcova, O.; Dlaskova, M.; Kastanek, F. Challenges and Advances in Tertiary Waste Water Treatment for Municipal Treatment Plants. Processes 2024, 12, 2084. https://doi.org/10.3390/pr12102084
Solcova O, Dlaskova M, Kastanek F. Challenges and Advances in Tertiary Waste Water Treatment for Municipal Treatment Plants. Processes. 2024; 12(10):2084. https://doi.org/10.3390/pr12102084
Chicago/Turabian StyleSolcova, Olga, Martina Dlaskova, and Frantisek Kastanek. 2024. "Challenges and Advances in Tertiary Waste Water Treatment for Municipal Treatment Plants" Processes 12, no. 10: 2084. https://doi.org/10.3390/pr12102084
APA StyleSolcova, O., Dlaskova, M., & Kastanek, F. (2024). Challenges and Advances in Tertiary Waste Water Treatment for Municipal Treatment Plants. Processes, 12(10), 2084. https://doi.org/10.3390/pr12102084








