Fe2NiSe4 Nanowires Array for Highly Efficient Electrochemical H2S Splitting and Simultaneous Energy-Saving H2 Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Fe2NiSe4/FeNi3
2.3. Characterization
2.4. Electrochemical Measurement
3. Results and Discussion
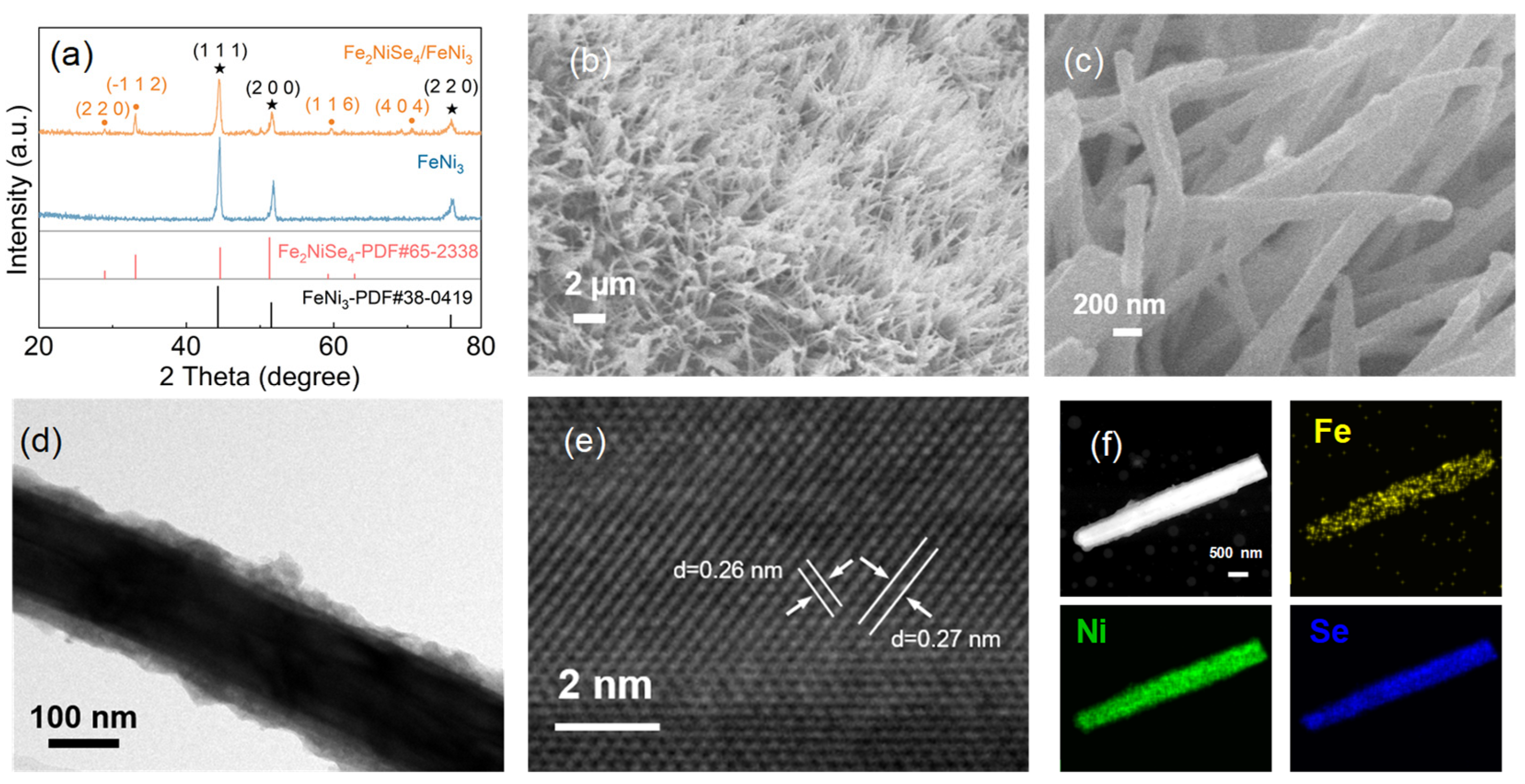
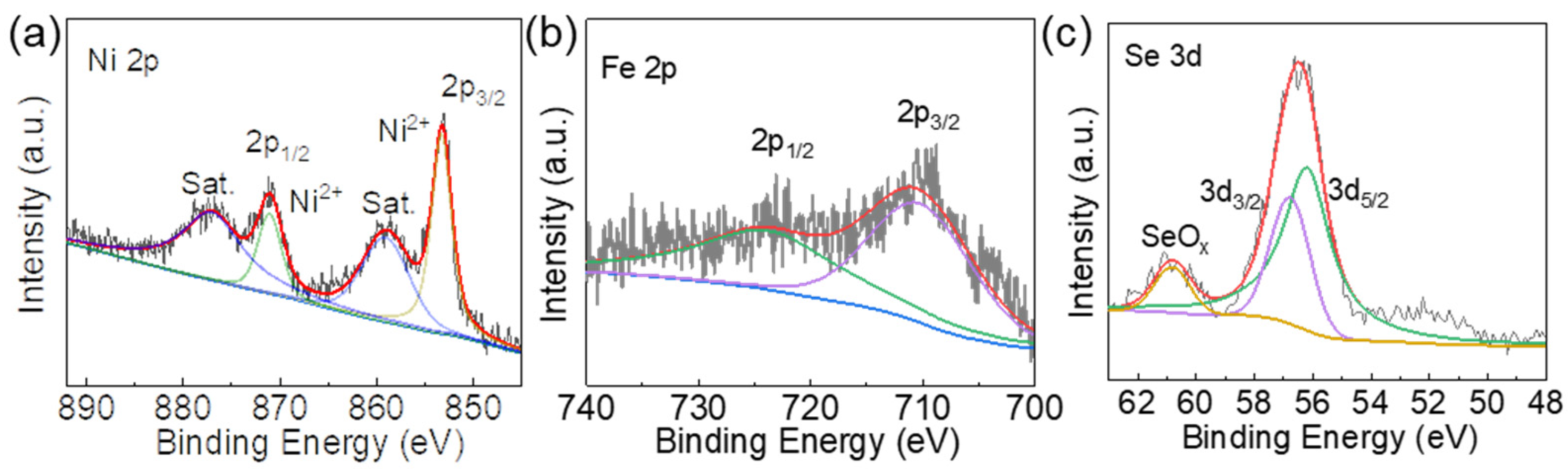
3.1. Characterization of Fe2NiSe4/FeNi3
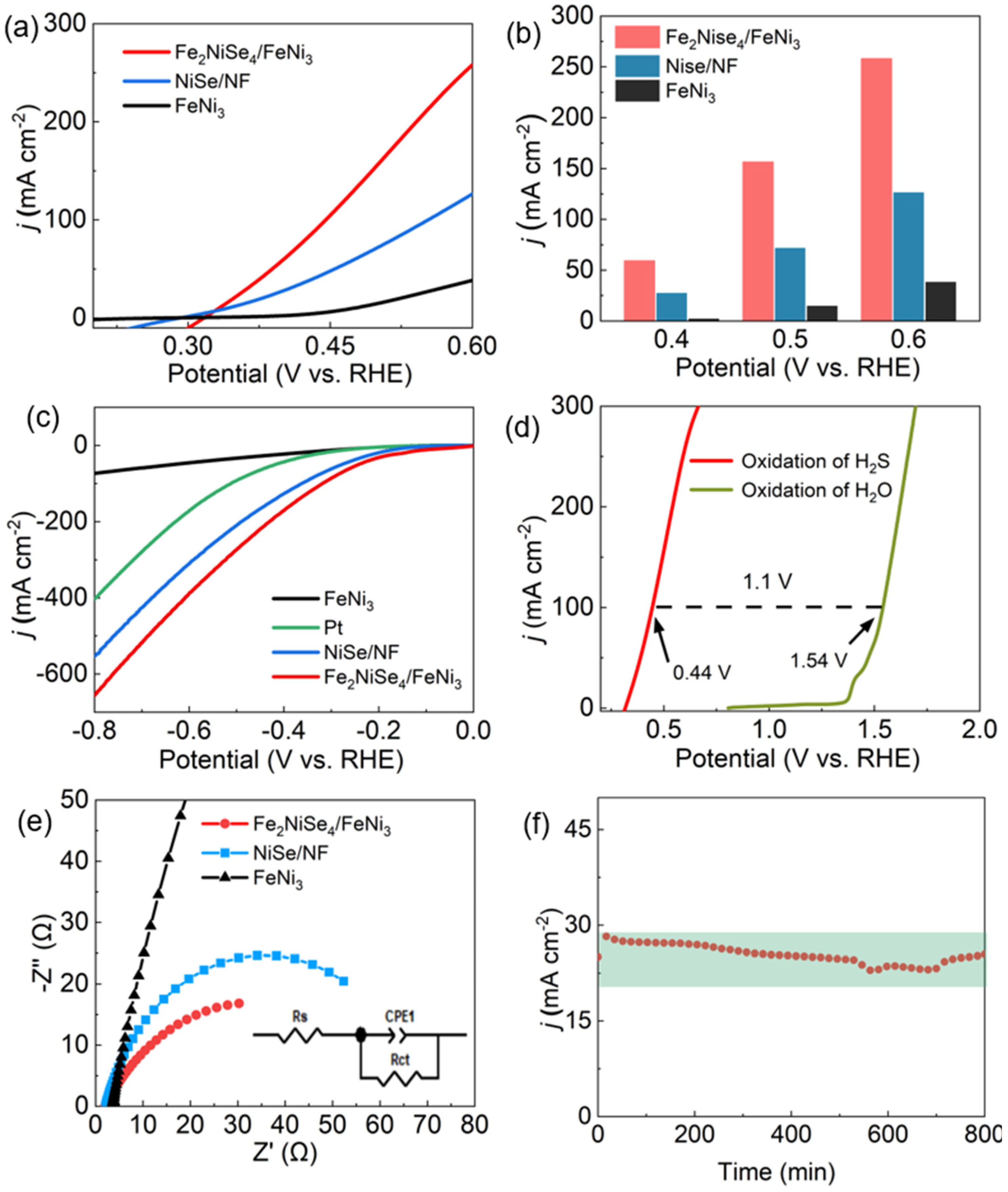
3.2. Electrocatalytic Performance
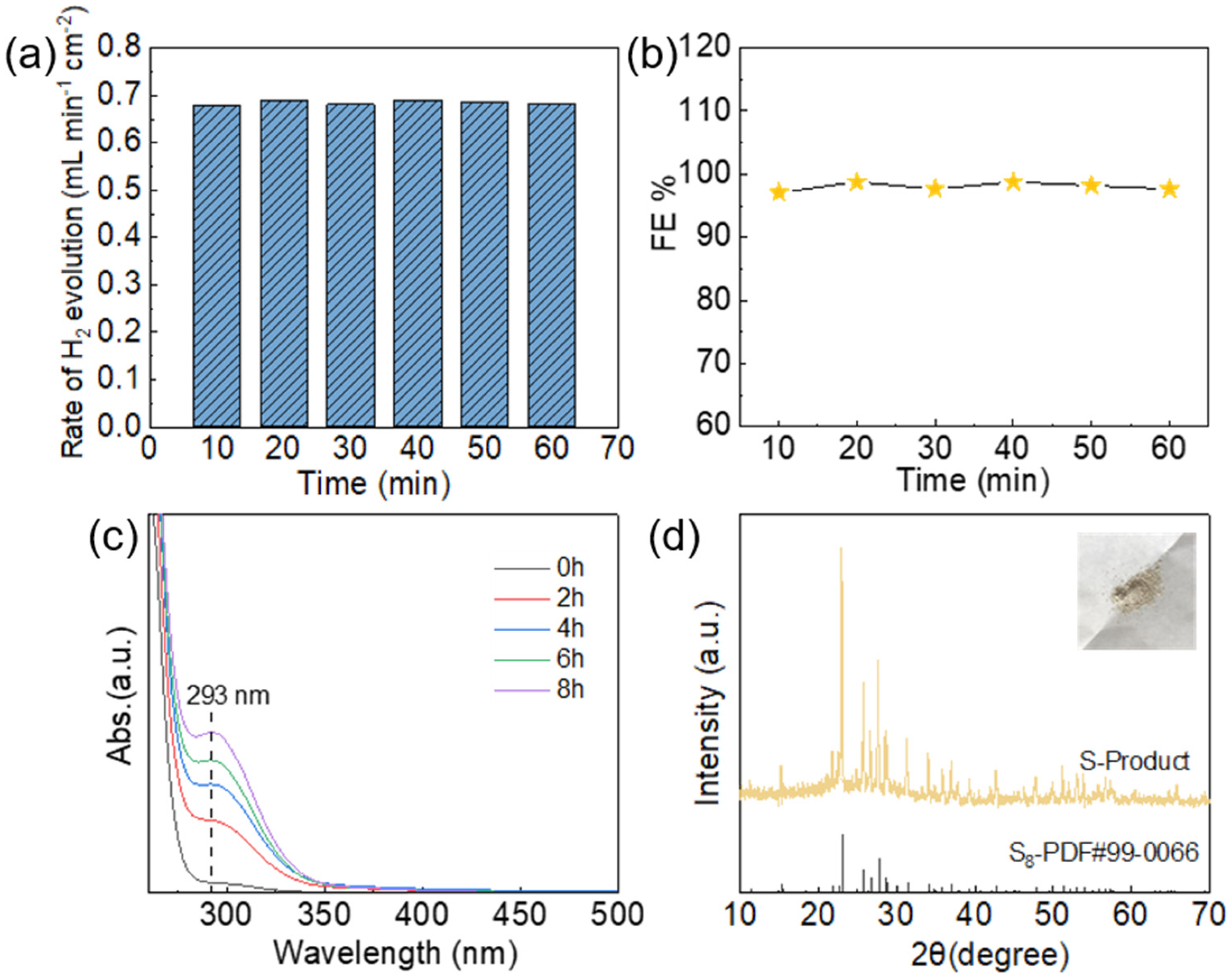
3.3. Products Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, C.; Yang, Z.; He, D.; Wei, Y.; Li, J.; Jia, A.; Chen, J.; Zhao, Q.; Li, Y.; Li, J. Theory, Technology and Prospects of Conventional and Unconventional Natural Gas. Pet. Explor. Dev. 2018, 45, 604–618. [Google Scholar] [CrossRef]
- Dan, M.; Yu, S.; Li, Y.; Wei, S.; Xiang, J.; Zhou, Y. Hydrogen Sulfide Conversion: How to Capture Hydrogen and Sulfur by Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100339. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Zhao, Z.; Qiu, J. Review of H2S Selective Oxidation over Carbon-Based Materials at Low Temperature: From Pollutant to Energy Storage Materials. New Carbon. Mater. 2022, 37, 675–694. [Google Scholar] [CrossRef]
- Liu, S.; Gu, X.; Yang, S.; Qian, Y. Can Green Hydrogen and Waste Heat Utilization Improve Energy Conservation and Emission Reduction of Coal-Based Cogeneration Processes? J. Clean. Prod. 2023, 389, 136045. [Google Scholar] [CrossRef]
- Segawa, Y.; Endo, N.; Shimoda, E.; Yamane, T.; Maeda, T. Pilot-Scale Hydrogen Energy Utilization System Demonstration: A Case Study of a Commercial Building with Supply and Utilization of off-Site Green Hydrogen. Int. J. Hydrogen Energy 2023, 50, S0360319923032731. [Google Scholar] [CrossRef]
- Hwang, J.; Maharjan, K.; Cho, H. A Review of Hydrogen Utilization in Power Generation and Transportation Sectors: Achievements and Future Challenges. Int. J. Hydrogen Energy 2023, 48, 28629–28648. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, L.; He, Y.; Wang, Y.; Hao, Q.; Zhu, Y. Carbon Dioxide Utilization Based on Exergoenvironmental Sustainability Assessment: A Case Study of CO2 Hydrogenation to Methanol. Energy 2023, 273, 127219. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef] [PubMed]
- Abdelghafar, F.; Xu, X.; Jiang, S.; Shao, Z. Designing Single-Atom Catalysts toward Improved Alkaline Hydrogen Evolution Reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Lu, W.; Wang, Z.; Liu, D.; Hao, S.; Du, G.; Abdullah, M.A.; Sun, X. Energy-saving electrolytic hydrogen generation: Ni2P nanoarray as a high-performance non-noble-metal electrocatalyst. Angew. Chem. Int. Ed. 2017, 56, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, C. Recent Progress in Electrochemical Hydrogen Sulfide Splitting: Strategies for Enabling Sulfur-Tolerant Anodic Reactions. Chem. Eng. J. 2023, 469, 143861. [Google Scholar] [CrossRef]
- Kumar, M.; Nagaiah, T.C. Efficient Production of Hydrogen from H 2 S via Electrolysis Using a CoFeS2 Catalyst. J. Mater. Chem. A 2022, 10, 7048–7057. [Google Scholar] [CrossRef]
- Kumar, M.; Nagaiah, T.C. Pure Hydrogen and Sulfur Production from H2S by an Electrochemical Approach Using a NiCu–MoS2 Catalyst. J. Mater. Chem. A 2022, 10, 13031–13041. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, C.; Wang, J.; Qian, Y.; Wang, B.; Zhang, Q.; Tang, A.; Yang, H. Bifunctional Co3S4 Nanowires for Robust Sulfion Oxidation and Hydrogen Generation with Low Power Consumption. Adv. Funct. Mater. 2023, 33, 2212183. [Google Scholar] [CrossRef]
- Pei, Y.; Cheng, J.; Zhong, H.; Pi, Z.; Zhao, Y.; Jin, F. Sulfide-Oxidation-Assisted Electrochemical Water Splitting for H2 Production on a Bifunctional Cu2S/Nickel Foam Catalyst. Green Chem. 2021, 23, 6975–6983. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Q.; Shen, Z.; Jin, X.; Zhang, Y.; Shi, M.; Zhou, J.; Liu, J.; Lu, Z.; Zhou, Y. Sulfophobic and Vacancy Design Enables Self-Cleaning Electrodes for Efficient Desulfurization and Concurrent Hydrogen Evolution with Low Energy Consumption. Adv. Funct. Mater. 2021, 31, 2101922. [Google Scholar] [CrossRef]
- Li, Y.; Duan, Y.; Zhang, K.; Yu, W. Efficient Anodic Chemical Conversion to Boost Hydrogen Evolution with Low Energy Consumption over Cobalt-Doped Nickel Sulfide Electrocatalyst. Chem. Eng. J. 2022, 433, 134472. [Google Scholar] [CrossRef]
- Yu, H.; Wang, W.; Mao, Q.; Deng, K.; Xu, Y.; Wang, Z.; Li, X.; Wang, H.; Wang, L. Electrocatalytic Sulfion Recycling Assisted Energy-Saving Hydrogen Production Using CuCo-Based Nanosheet Arrays. J. Mater. Chem. A 2023, 11, 2218–2224. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, L.; Zhang, Y.; Liu, L.; Sa, B.; Lin, J.; Wang, L.; Peng, D.-L.; Xie, Q. Dual-Functional Lithiophilic/Sulfiphilic Binary-Metal Selenide Quantum Dots Toward High-Performance Li–S Full Batteries. Nano-Micro Lett. 2023, 15, 67. [Google Scholar] [CrossRef]
- Naaz, F.; Ahmad, T. Ag-Doped WO 3 Nanoplates as Heterogenous Multifunctional Catalyst for Glycerol Acetylation, Electrocatalytic and Enhanced Photocatalytic Hydrogen Production. Langmuir 2023, 39, 9300–9314. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Tang, C.; Yu, S.; Li, L.; Li, J.; Zhou, Y. Efficient Electrocatalytic Desulfuration and Synchronous Hydrogen Evolution from H2S via Anti-Sulfuretted NiSe Nanowire Array Catalyst. Appl. Catal. B Environ. 2023, 324, 122255. [Google Scholar] [CrossRef]
- Yang, J.; Lei, C.; Wang, H.; Yang, B.; Li, Z.; Qiu, M.; Zhuang, X.; Yuan, C.; Lei, L.; Hou, Y. High-Index Faceted Binary-Metal Selenide Nanosheet Arrays as Efficient 3D Electrodes for Alkaline Hydrogen Evolution. Nanoscale 2019, 11, 17571–17578. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Z.; Tan, K.; He, H.; Yin, S. Novel Hierarchical FeWO4–FeNi3 Heterostructure Nanosheets for Highly Active Water Electrolysis at Large Current Density. Mater. Today Phys. 2023, 35, 101138. [Google Scholar] [CrossRef]
- Lambert-Andron, B.; Berodias, G.; Babot, D. Structures magnetiques des composes MFe2Se4 (M= Ti, V, Cr, Fe, Co, Ni). J. Phys. Chem. Solids 1972, 33, 87–94. [Google Scholar] [CrossRef]
- Xiao, K.; Zhou, L.; Shao, M.; Wei, M. Fabrication of (Ni,Co)0.85 Se Nanosheet Arrays Derived from Layered Double Hydroxides toward Largely Enhanced Overall Water Splitting. J. Mater. Chem. A 2018, 6, 7585–7591. [Google Scholar] [CrossRef]
- Liang, J.; Li, S.; Li, F.; Zhang, L.; Jiang, Y.; Ma, H.; Cheng, K.; Qing, L. Defect Engineering Induces Mo-Regulated Co9Se8/FeNiSe Heterostructures with Selenium Vacancy for Enhanced Electrocatalytic Overall Water Splitting in Alkaline. J. Colloid. Interface Sci. 2024, 655, 296–306. [Google Scholar] [CrossRef]
- Xu, R.; Wu, R.; Shi, Y.; Zhang, J.; Zhang, B. Ni3Se2 Nanoforest/Ni Foam as a Hydrophilic, Metallic, and Self-Supported Bifunctional Electrocatalyst for Both H2 and O2 Generations. Nano Energy 2016, 24, 103–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, T.; Cen, N.; Fan, R.; Li, L.; Du, Y.; Tang, C.; Guo, H.; Li, Y.; Liu, Z. Fe2NiSe4 Nanowires Array for Highly Efficient Electrochemical H2S Splitting and Simultaneous Energy-Saving H2 Production. Processes 2024, 12, 2111. https://doi.org/10.3390/pr12102111
Ding T, Cen N, Fan R, Li L, Du Y, Tang C, Guo H, Li Y, Liu Z. Fe2NiSe4 Nanowires Array for Highly Efficient Electrochemical H2S Splitting and Simultaneous Energy-Saving H2 Production. Processes. 2024; 12(10):2111. https://doi.org/10.3390/pr12102111
Chicago/Turabian StyleDing, Tong, Nanheng Cen, Rui Fan, Long Li, Yonghong Du, Chun Tang, Heng Guo, Yiping Li, and Zongshe Liu. 2024. "Fe2NiSe4 Nanowires Array for Highly Efficient Electrochemical H2S Splitting and Simultaneous Energy-Saving H2 Production" Processes 12, no. 10: 2111. https://doi.org/10.3390/pr12102111




