Systematic Exploration of the Interactions between Pyrite and Coal from the View of Density Functional Theory
Abstract
:1. Introduction
2. Calculation Methods and Model
2.1. The Frontier Orbital Calculation
2.2. DFT Calculation
3. Results and Discussion
3.1. Frontier Orbital Analysis
3.2. DFT Calculation
3.2.1. Adsorption Configurations and Adsorption Energy
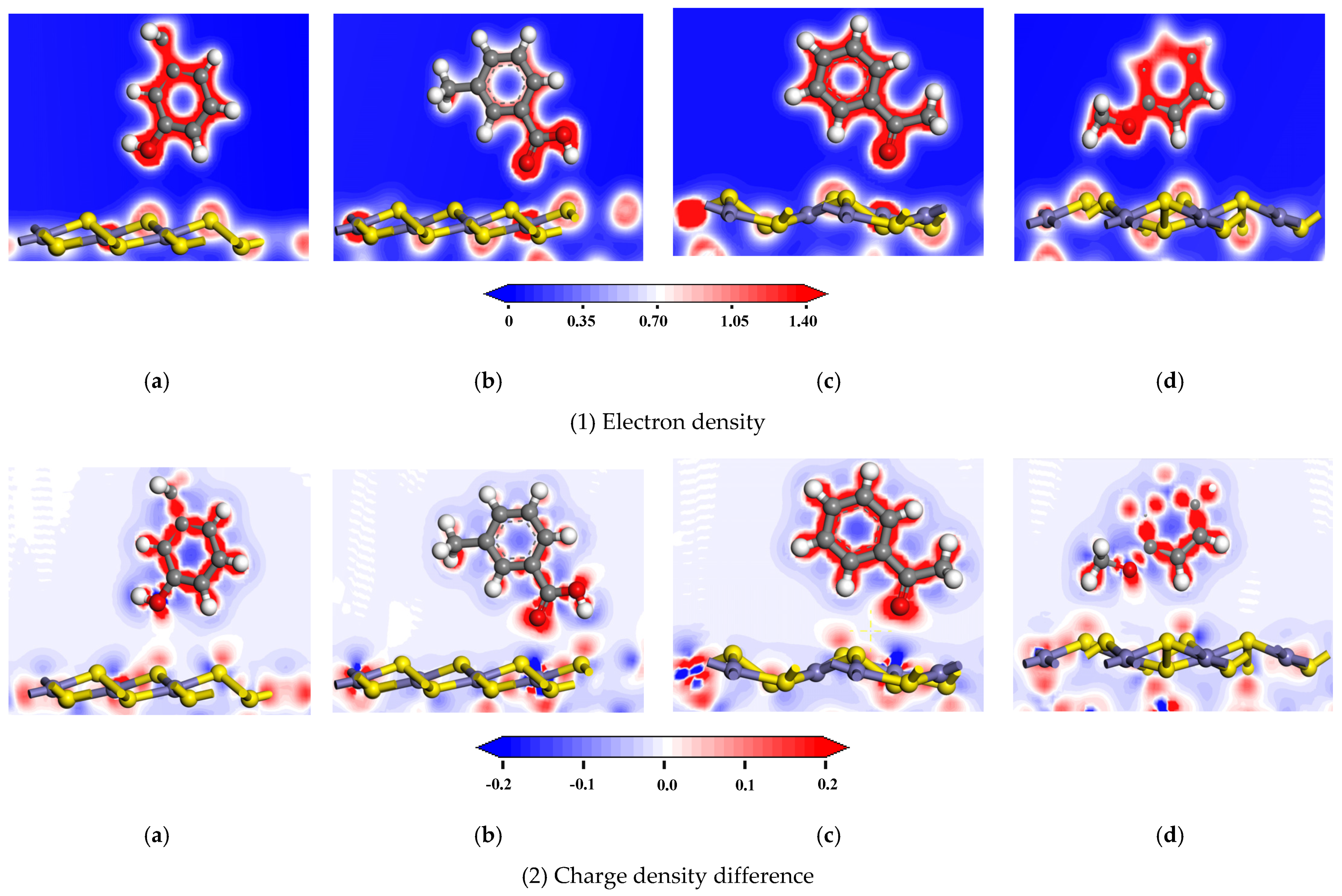
3.2.2. Analysis of Bonding
3.2.3. Charge Transfer
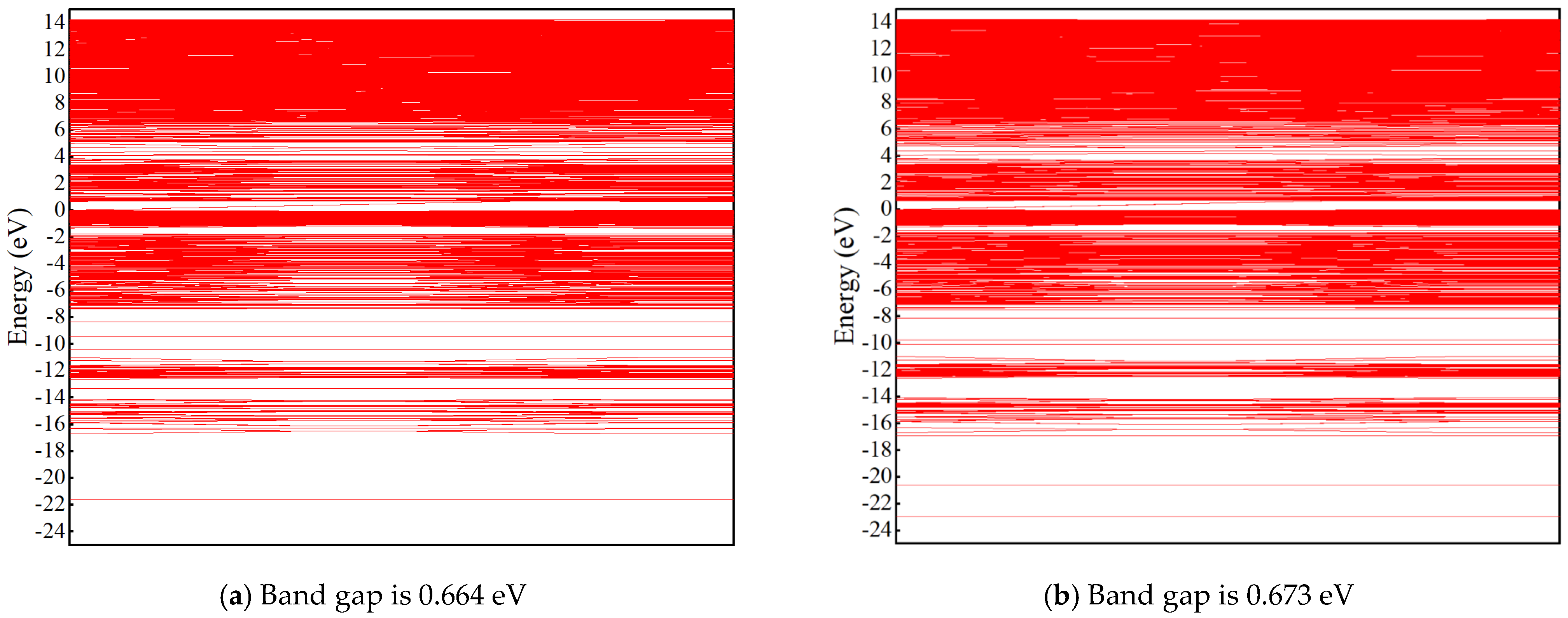
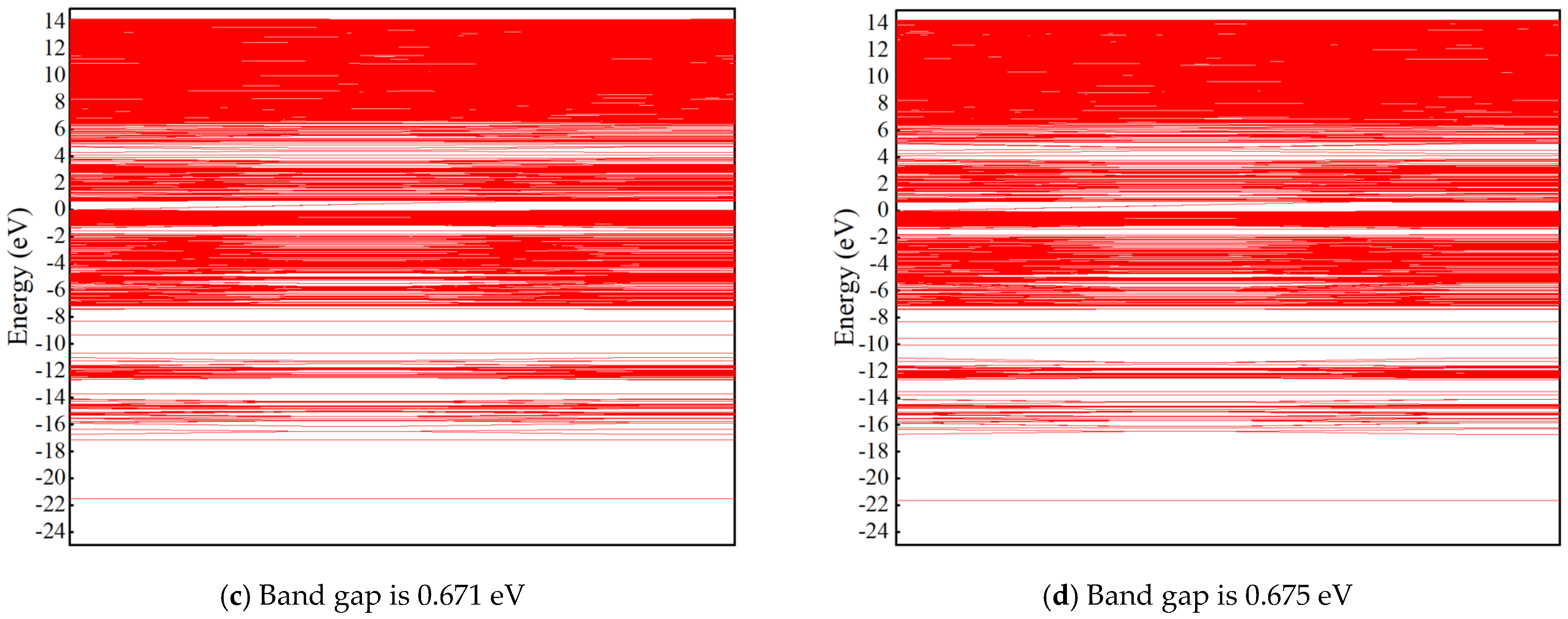
3.2.4. Density of States (DOS) and Band Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.R. Consideration of clean coal strategy in China. J. Heilongjiang Univ. Sci. Technol. 2004, 14, 261–264. [Google Scholar]
- Lu, M.X.; Liu, W.L. Sulfur Distribution of High Sulfur Coal and Feasibility Study of Coal Desulfurization Before Combustion. Coal Sci. Technol. 1999, 27, 42–45. [Google Scholar]
- Cao, S.M.; Cao, Y.J.; Ma, Z.L.; Liao, Y.F. The flotation separation of fine pyrite locked in coking coal. J. China Univ. Min. Technol. 2019, 48, 1366–1374. [Google Scholar]
- Zhu, H.; Li, H.L.; Ou, Z.S.; Wang, D.Z. Effect of Pyrite Surface Oxidation on the Desulurization in Coal Flotation. Clean Coal Technol. 2000, 6, 5–8. [Google Scholar]
- Yoon, R.H.; Lagno, M.; Luttrel, G.H.; Mirlvzarski, J.A. On the hydrophobicity of coal pyrite. In Processing and Utilization of High-Sulfur Coals IV; Dugan, P.R., Quiley, D.R., Attia, Y.A., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1991; pp. 241–253. [Google Scholar]
- Niu, X.P. Correlation of Surface Oxidation of Galena, Chalcopyrite and Pyrite with Their Floatability. Ph.D. Dissertation, Chinese Academy of Sciences, Institute of Process Engineering, Beijing, China, 2019. [Google Scholar]
- Xi, P.; Shi, C.X.; Yan, P.K. DFT study on the influence of sulfur on the hydrophobicity of pyrite surfaces in the process of oxidation. Appl. Surf. Sci. 2018, 466, 964–969. [Google Scholar] [CrossRef]
- Çetinkaya, Z. Investigation of removal of ash and sulfur from Kangal (Sivas/Turkey) lignite with the application of combined methods. Int. J. Coal Prep. Util. 2024, 2391908, 1–18. [Google Scholar] [CrossRef]
- Channa, F.H.; Khoso, S.A.; Soomro, S.A.; Uqaili, M.A. Studies on sulfur liberation characteristics of Pakistani high sulfur low-rank coal using Hardgrove grinding. Int. J. Coal Prep. Util. 2024, 2341953, 1–20. [Google Scholar] [CrossRef]
- Xi, P.; Liu, W.L.; Han, Y.H. Study on the mechanism of coal pyrite crystal lattice defects and floatability. J. China Coal. Soc. 2016, 41, 997–1003. [Google Scholar]
- Xi, P.; Liu, W.L.; Yang, Z.Y. Quantum chemistry investigation on influence of carbon atom adsorption in carbon material to the coal pyrite hydrophobicity. J. China Coal. Soc. 2017, 42, 1290–1296. [Google Scholar]
- Xi, P.; Ma, R.X.; Liu, W.L. Research on the Effect of Carbon Defects on the Hydrophilicity of Coal Pyrite Surface from the Insight of Quantum Chemistry. Molecules 2019, 24, 2285. [Google Scholar] [CrossRef] [PubMed]
- Xi, P.; Wang, D.H.; Liu, W.L. DFT Study into the Influence of Carbon Material on the Hydrophobicity of a Coal Pyrite Surface. Molecules 2019, 24, 3534. [Google Scholar] [CrossRef] [PubMed]
- Kawatra, S.K.; Eisele, T.C. Pyrite recovery mechanisms in coal flotation. Int. J. Miner. Process. 1997, 50, 187–201. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Eisele, T.C.; Johnson, H. Recovery of liberated pyrite in coal flotation: Entrainment or hydrophobicity. In Processing and Utilization of High-Sulfur Coals IV; Dugan, P.R., Quiley, D.R., Attia, Y.A., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1991; pp. 255–277. [Google Scholar]
- Mkhonto, P.P.; Zhang, X.R.; Lu, L. Adsorption mechanisms and effects of thiocarbamate collectors in the separation of chalcopyrite from pyrite minerals: DFT and experimental studies. Miner. Eng. 2022, 176, 107318. [Google Scholar] [CrossRef]
- Mkhonto, P.P.; Zhang, X.R.; McFadzean, B. Incorporating pH into DFT-D+U and microflotation recovery studies on heterocyclic collector-pyrite interactions. Sep. Purif. Technol. 2024, 337, 126430. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, J.H.; Li, Y.Q. Adsorption behaviors of dibutyl dithiophosphate and sodium-diisobutyl dithiophosphinate (3418A) on chalcopyrite: A combined experimental and theoretical study. Appl. Surf. Sci. 2023, 636, 157810. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, J.H.; Li, Y.Q. First-principles study on the co-adsorption of water and oxygen molecules on chalcopyrite (112)-M surface. Int. J. Min. Sci. Technol. 2023, 33, 1055–1063. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Chen, J.H.; Li, Y.Q. Application of a new self-consistent-charge density-functional tight-binding(SCC-DFTB) parameter set for simulating the adsorption of flotation reagents on the surface of typical lead minerals. Miner. Eng. 2024, 209, 108631. [Google Scholar] [CrossRef]
- Feng, X.L.; Jian, S.; Chen, H.M.; Chen, J.H. Synergistic adsorption of ethyl xanthate and butyl xanthate on pyrite surfaces: A DFT study. Quantum Chem. 2024, 124, e27448. [Google Scholar] [CrossRef]
- Chen, P.; Sun, W.; Yue, T. Dynamics simulation of tributyltetradecylphosphonium chloride on kaolinite (001) plane. J. China Univ. Min. Technol. 2014, 43, 294–299. [Google Scholar]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrated and the CASTEP code. J. Phys. Condens. Matter. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B Condens. Matter Mater. Phys. 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Zhao, C. Influence of external electronic field on the electronic structure and optical properties of pyrite. RSC Adv. 2017, 7, 56676–56681. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B Solid State 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pack, J.D.; Monkhorst, H.J. “Special points for Brillouin-zone integrations”—A reply. Phys. Rev. B Solid State 1977, 16, 1748–1749. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.L.; Zhou, J. Interactions between kaolinite Al-OH surface and sodium hexametaphosphate. Appl. Surf. Sci. 2016, 387, 759–765. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.L.; Chen, J.H. DFT simulation of the adsorption of sodium silicate species on kaolinite surfaces. Appl. Surf. Sci. 2016, 370, 403–409. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, W.; Zhang, H. DFT study of the adsorption of 3-chloro-2-hydroxypropyl trimethylammonium chloride on montmorillonite surfaces in solution. Appl. Surf. Sci. 2018, 436, 58–65. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO-MO molecular wave functions. IV. bonding and antibonding in LCAO and Valence-bond theories. J. Chem. Phys. 1955, 23, 2343–2346. [Google Scholar] [CrossRef]
- Šolc, R.; Gerzabek, M.H.; Lischka, H.; Tunega, D. Wettability of kaolinite (001) surfaces—Molecular dynamic study. Geoderma 2011, 169, 47–54. [Google Scholar] [CrossRef]
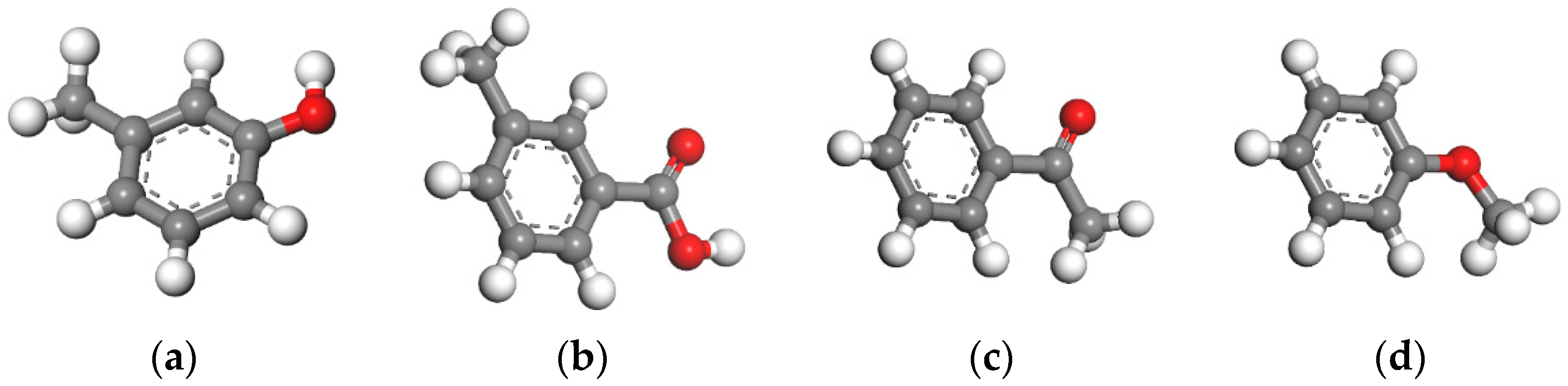
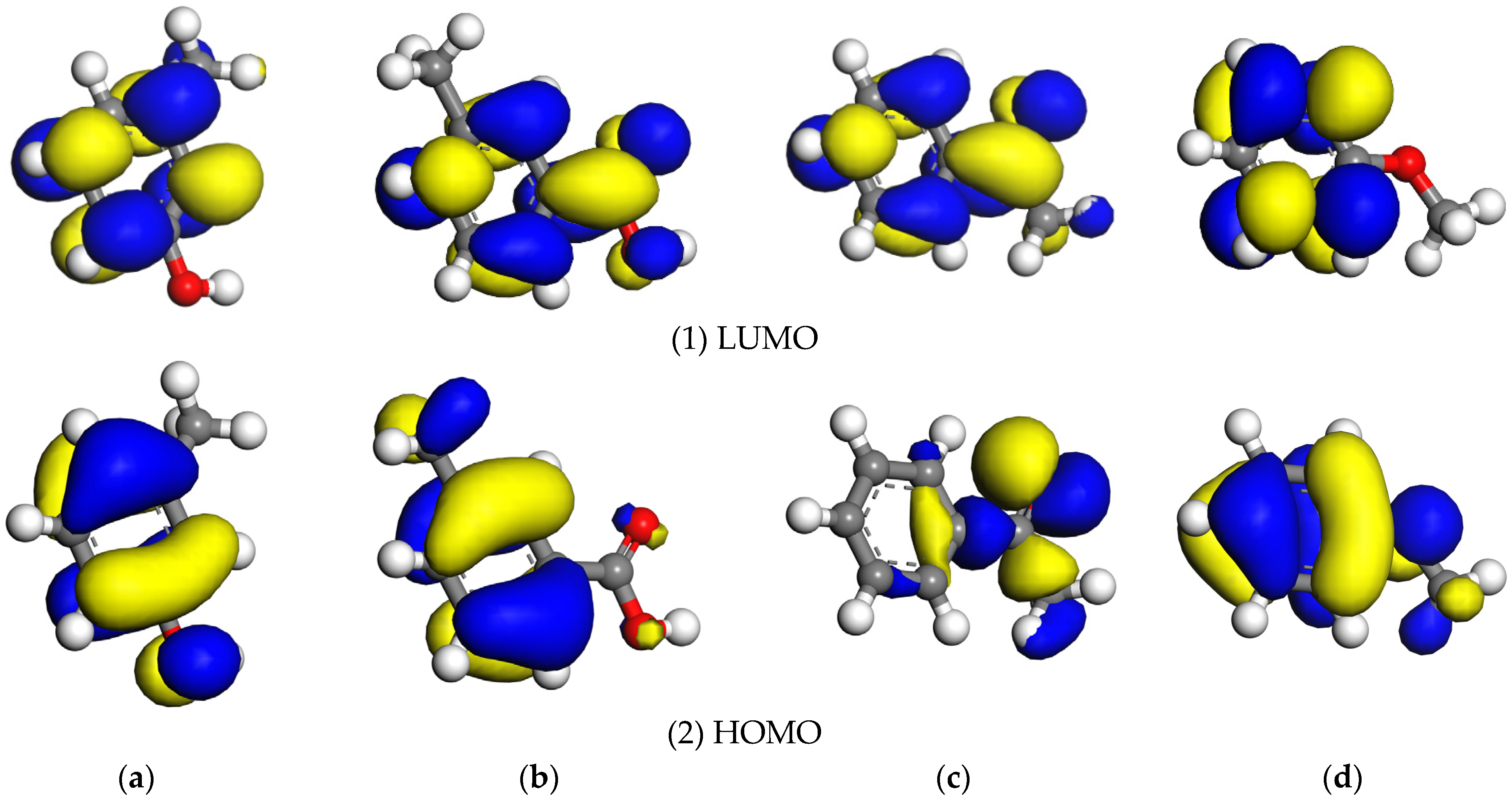


| Model | Frontier Orbital Energy/eV | Frontier Orbital Energy Difference/eV | |||
|---|---|---|---|---|---|
| HOMO | LUMO | ΔE1 | ΔE2 | ||
| Coal structural units | Ph-OH | −5.692 | −2.656 | 3.023 | 1.232 |
| Ph-COOH | −5.371 | −1.030 | 4.649 | 0.911 | |
| Ph-C=O | −5.390 | −1.027 | 4.652 | 0.930 | |
| Ph-O- | −6.361 | −2.388 | 3.291 | 1.901 | |
| Pyrite | −5.679 | −4.460 | |||
| Coal Structural Units | Eads/kJ·mol−1 | Adsorption Configuration of Coal Structural Units |
|---|---|---|
| Ph-OH/FeS2 | 24.90 | F |
| 24.82 | P | |
| 2.84 | LPS | |
| 1.85 | HPS | |
| Ph-COOH/FeS2 | −23.57 | F |
| 14.46 | P | |
| −62.19 | LPS | |
| −1.99 | HPS | |
| Ph-CO-CH3/FeS2 | −25.39 | F |
| 7.40 | P | |
| −10.77 | LPS | |
| 7.92 | HPS | |
| Ph-O-CH3/FeS2 | 42.49 | F |
| 42.55 | P | |
| 25.82 | LPS | |
| 1.9 | HPS |
| Adsorption Model | Interaction | Population | Length/Å |
|---|---|---|---|
| Ph-OH/FeS2 | H-S | 0.00 | 2.990 |
| Ph-COOH/FeS2 | H-S | 0.05 | 2.475 |
| Fe-O | 0.13 | 2.797 | |
| Ph-CO-CH3/FeS2 | Fe-O | 0.13 | 2.820 |
| H-S | 0.00 | 2.895 | |
| Ph-O-CH3/FeS2 | H-S | −0.01 | 2.597 |
| H-S | 0.00 | 2.595 |
| Adsorption Model | Atomic Label | Adsorption Status | s | p | d | Total | Charge/e |
|---|---|---|---|---|---|---|---|
| Ph-OH/FeS2 | H | B | 0.46 | 0.00 | 0.00 | 0.46 | 0.54 |
| A | 0.48 | 0.00 | 0.00 | 0.48 | 0.52 | ||
| S | B | 1.86 | 4.26 | 0.00 | 6.12 | −0.11 | |
| A | 1.86 | 4.26 | 0.00 | 6.12 | −0.11 | ||
| Ph-COOH/FeS2 | H | B | 0.44 | 0.00 | 0.00 | 0.44 | 0.55 |
| A | 0.52 | 0.00 | 0.00 | 0.52 | 0.48 | ||
| S | B | 1.84 | 4.26 | 0.00 | 6.12 | −0.12 | |
| A | 1.82 | 4.19 | 0.00 | 6.01 | −0.01 | ||
| Fe | B | 0.35 | 0.44 | 7.14 | 7.93 | 0.07 | |
| A | 0.34 | 0.44 | 7.12 | 7.90 | 0.10 | ||
| O | B | 1.82 | 4.74 | 0.00 | 6.56 | −0.57 | |
| A | 1.82 | 4.72 | 0.00 | 6.54 | −0.54 | ||
| Ph-CO-CH3/FeS2 | H | B | 0.66 | 0.00 | 0.00 | 0.66 | 0.33 |
| A | 0.72 | 0.00 | 0.00 | 0.72 | 0.27 | ||
| S | B | 1.86 | 4.26 | 0.00 | 6.12 | −0.11 | |
| A | 1.86 | 4.24 | 0.00 | 6.08 | −0.09 | ||
| Fe | B | 0.35 | 0.44 | 7.14 | 7.93 | 0.07 | |
| A | 0.34 | 0.44 | 7.12 | 7.90 | 0.10 | ||
| O | B | 1.82 | 4.70 | 0.00 | 6.52 | −0.52 | |
| A | 1.82 | 4.68 | 0.00 | 6.50 | −0.49 | ||
| Ph-O-CH3/FeS2 | H | B | 0.68 | 0.00 | 0.00 | 0.68 | 0.31 |
| A | 0.78 | 0.00 | 0.00 | 0.78 | 0.23 | ||
| S | B | 1.86 | 4.26 | 0.00 | 6.12 | −0.11 | |
| A | 1.86 | 4.26 | 0.00 | 6.12 | −0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, P.; Sun, F.; Tang, X.; Fan, X.; Lu, A.; Lu, K.; Zhuo, Q. Systematic Exploration of the Interactions between Pyrite and Coal from the View of Density Functional Theory. Processes 2024, 12, 2125. https://doi.org/10.3390/pr12102125
Xi P, Sun F, Tang X, Fan X, Lu A, Lu K, Zhuo Q. Systematic Exploration of the Interactions between Pyrite and Coal from the View of Density Functional Theory. Processes. 2024; 12(10):2125. https://doi.org/10.3390/pr12102125
Chicago/Turabian StyleXi, Peng, Fengling Sun, Xiaoyu Tang, Xiaoping Fan, An Lu, Kaifei Lu, and Qiming Zhuo. 2024. "Systematic Exploration of the Interactions between Pyrite and Coal from the View of Density Functional Theory" Processes 12, no. 10: 2125. https://doi.org/10.3390/pr12102125







