Enhanced Hydrolysis of Carbonyl Sulfide in Coking Oven Gas Utilizing an Efficient Ca-Ba-γ-Al2O3 Catalyst
Abstract
:1. Introduction
2. Experimental
2.1. Material Synthesis
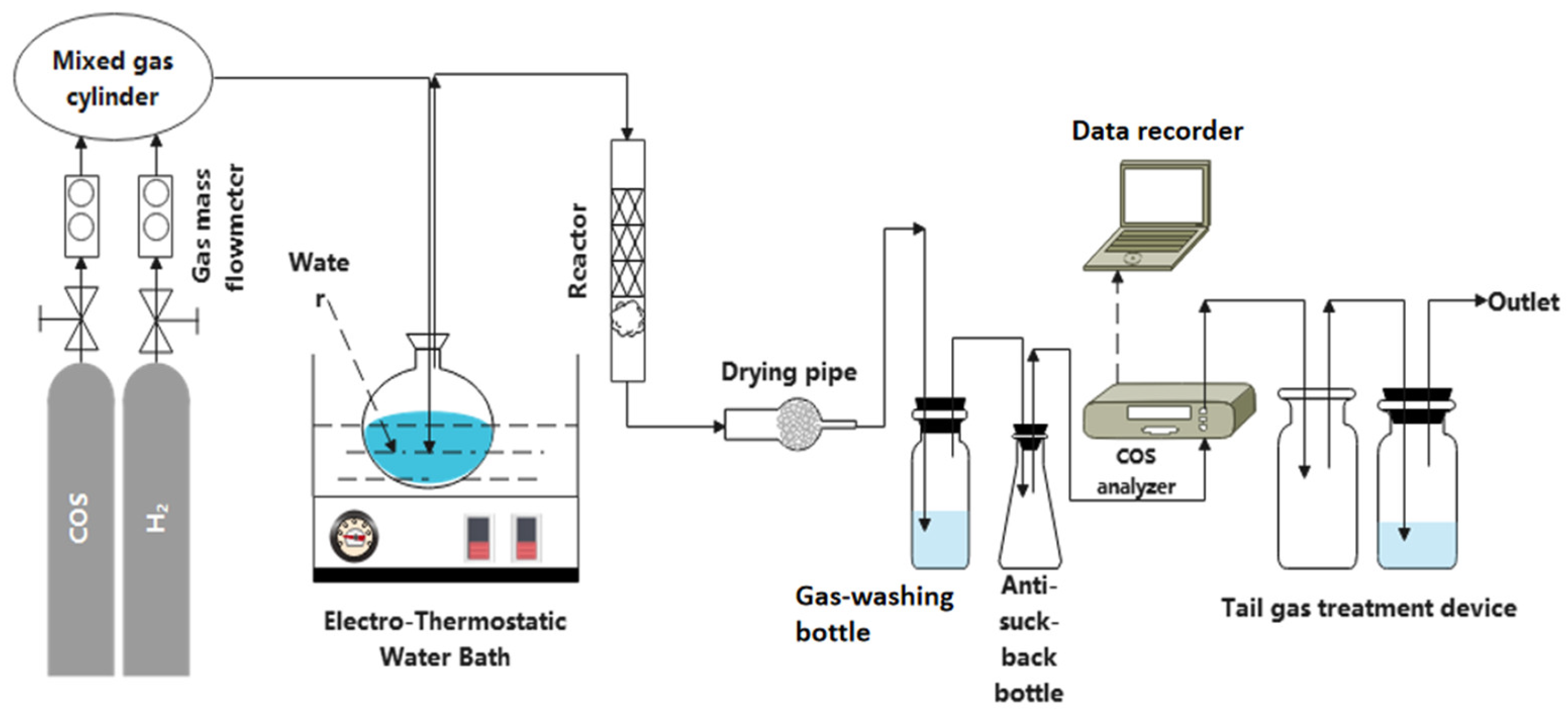
2.2. Experimental Instrument
2.3. Experimental Process
2.4. Evaluation Criteria
3. Results and Discussion
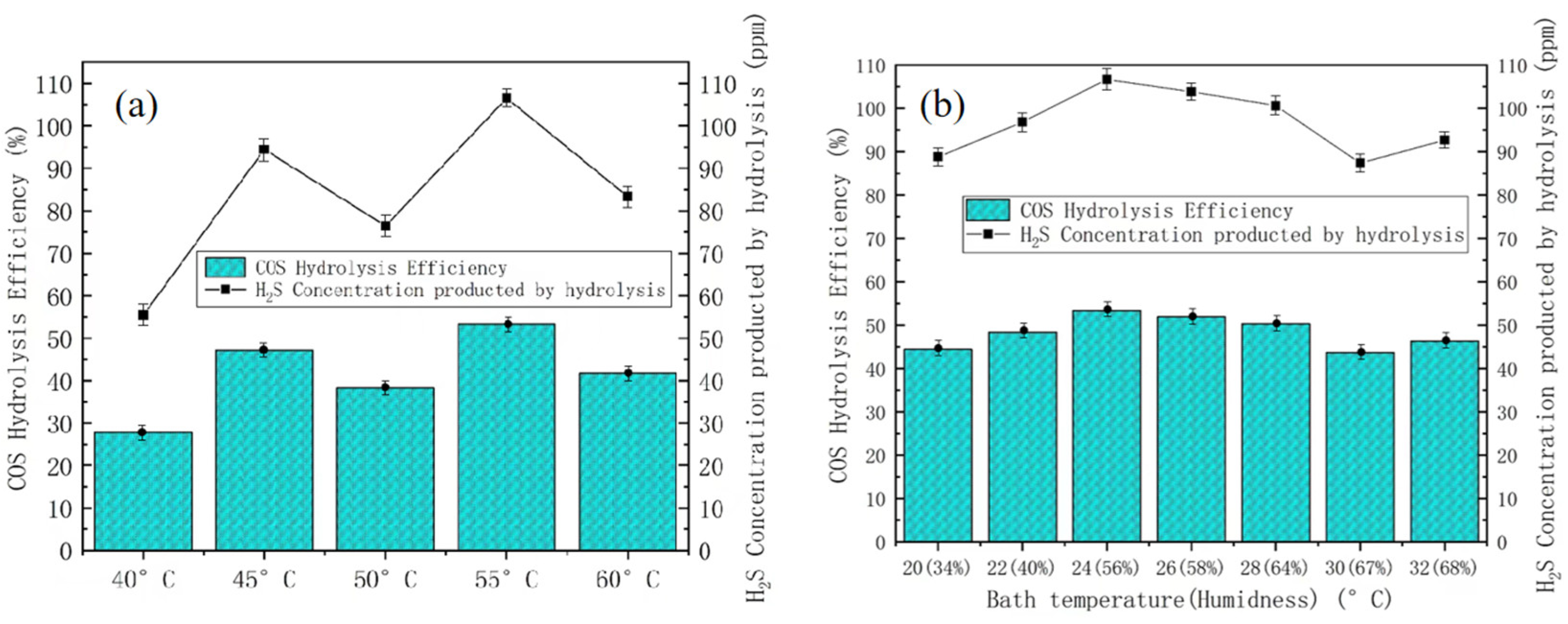
3.1. Catalyst Support Experiment
3.2. Active Component on the COS Hydrolysis Efficiency
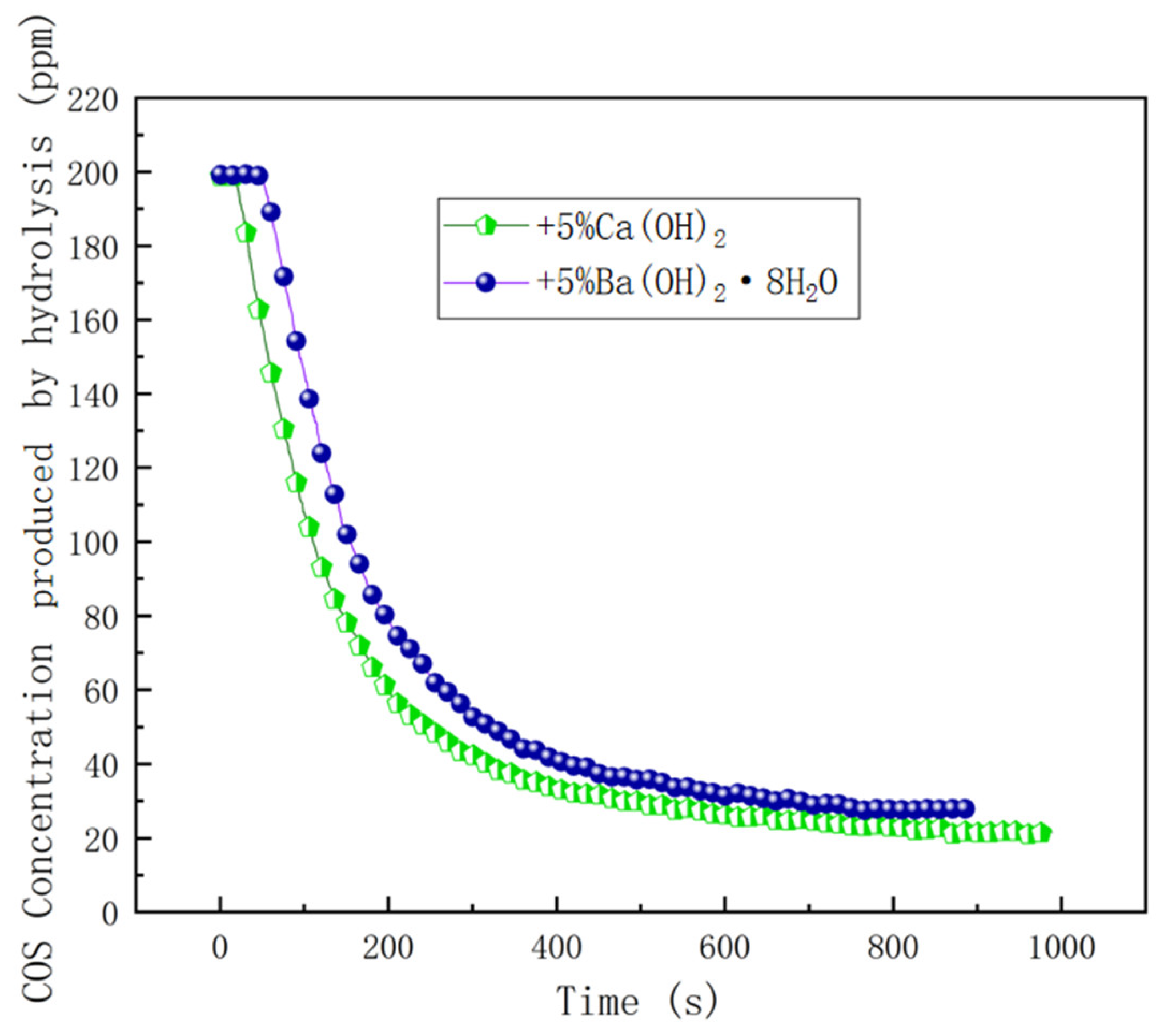
3.3. Preparation of Ca-Ba-γ-Al2O3 Catalyst
3.4. Characterization Results and Discussion
3.5. Analysis of COS Hydrolysis Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, Z.; Bai, J.; Li, W. Comprehensive utilization of coke oven gas and CO2 emission reduction potential analysis. Clean Coal Technol. 2016, 22, 90–95. [Google Scholar] [CrossRef]
- World Steel in Figures 2022. World Steel Association. 2022. Available online: https://worldsteel.org/data/world-steel-in-figures-2022 (accessed on 7 June 2022).
- Cao, R.; Ning, P.; Wang, X.; Wang, L.; Ma, Y.; Xie, Y.; Zhang, H.; Qu, J. Low-temperature hydrolysis of carbonyl sulfide in blast furnace gas using Al2O3-based catalysts with high oxidation resistance. Fuel 2022, 310, 122295. [Google Scholar] [CrossRef]
- Shen, L.; Wang, G.; Zheng, X.; Cao, Y.; Guo, Y.; Lin, K.; Jiang, L. Tuning the growth of Cu-MOFs for efficient catalytic hydrolysis of carbonyl sulfide. Chin. J. Catal. 2017, 38, 1373–1381. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Liu, N.; Yu, Z.; Li, Y.; Qiu, J. Synthesis of metallic Ni-Co/graphene catalysts with enhanced hydrodesulfurization activity via a low-temperature plasma approach. Catal. Today 2015, 256, 203–208. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, X.; Zhang, F.; Xie, Q.; Zhao, S.; Hao, Z. Boosting carbonyl sulfide catalytic hydrolysis performance over N-doped Mg-Al oxide derived from MgAl-layered double hydroxide. J. Hazard. Mater. 2021, 407, 124546. [Google Scholar] [CrossRef] [PubMed]
- He, E.; Huang, G.; Fan, H.; Yang, C.; Wang, H.; Tian, Z.; Wang, L.; Zhao, Y. Macroporous alumina- and titania-based catalyst for carbonyl sulfide hydrolysis at ambient temperature. Fuel 2019, 246, 277–284. [Google Scholar] [CrossRef]
- Kong, X.; Ding, J.; Xie, L.; Qin, J.; Wang, J. Lanthanum–bismuth mixed oxide catalyst with improved activity for carbonyl sulfide hydrolysis. J. Environ. Chem. Eng. 2023, 11, 109830. [Google Scholar] [CrossRef]
- Zeng, Z.; Dlugogorski, B.Z.; Oluwoye, I.; Altarawneh, M. Combustion chemistry of COS and occurrence of intersystem crossing. Fuel 2021, 283, 119257. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Zhang, X.; Demkov, A.A.; Li, H.; Hu, X.; Wei, Y.; Kulik, J. Atomic and electronic structure of theSi/SrTiO3interface. Phys. Rev. B 2003, 68, 125323. [Google Scholar] [CrossRef]
- Díaz-Terán, J.; Nevskaia, D.; Fierro, J.; López-Peinado, A.; Jerez, A. Study of chemical activation process of a lignocellulosic material with KOH by XPS and XRD. Microporous Mesoporous Mater. 2003, 60, 173–181. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Paereli, S. Sorption of Hydrogen Chloride on Solid Sorbents; NTNU: Trondheim, Norway, 2015. [Google Scholar]
- Kim, J.; Do, J.Y.; Nahm, K.; Park, N.-K.; Chi, J.; Hong, J.-P.; Kang, M. Capturing ability for COS gas by a strong bridge bonding of a pair of potassium anchored on carbonate of activated carbon at low temperatures. Sep. Purif. Technol. 2019, 211, 421–429. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, J.; Ning, P.; Ren, X.; Li, Z.; Yin, Z.; Chen, W.; Liu, W. Adsorption/desorption of low concentration of carbonyl sulfide by impregnated activated carbon under micro-oxygen conditions. J. Hazard. Mater. 2012, 229-230, 128–136. [Google Scholar] [CrossRef]
- Tian, X. Study on Catalytic Hydrolysis and Absorption Characteristics of Coke Oven Gas Desulfurization Process; North China Electric Power University: Beijing, China, 2021. [Google Scholar] [CrossRef]
- Amararene, F.; Bouallou, C. Kinetics of Carbonyl Sulfide (COS) Absorption with Aqueous Solutions of Diethanolamine and Methyldiethanolamine. Ind. Eng. Chem. Res. 2004, 43, 6136–6141. [Google Scholar] [CrossRef]
- Bacsik, Z.; Hedin, N. Adsorption of Carbonyl Sulfide on Propylamine Tethered to Porous Silica. Langmuir 2018, 34, 7708–7713. [Google Scholar] [CrossRef]
- Seo, M.W.; Yun, Y.M.; Cho, W.C.; Ra, H.W.; Yoon, S.J.; Lee, J.G.; Kim, Y.K.; Kim, J.H.; Lee, S.H.; Eom, W.H.; et al. Methanol absorption characteristics for the removal of H2S (hydrogen sulfide), COS (carbonyl sulfide) and CO2 (carbon dioxide) in a pilot-scale biomass-to-liquid process. Energy 2014, 66, 56–62. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, B.; Sun, H.; Liu, J.; Liu, L. Rational Formulation Design and Commercial Application of a New Hybrid Solvent for Selectively Removing H2S and Organosulfurs from Sour Natural Gas. Energy Fuels 2016, 30, 12–19. [Google Scholar] [CrossRef]
- Lammers, J.; Haringa, J.; Littel, R. Effect of polyhydroxyalcohols on COS absorption in aqueous methyldiethanolamine. Chem. Eng. J. Biochem. Eng. J. 1995, 60, 123–129. [Google Scholar] [CrossRef]
- Wang, K. Removal of COS in Natural Gas. Chem. Eng. Oil Gas 2007, 36, 28–36. [Google Scholar] [CrossRef]
- Huang, H.; Young, N.; Williams, B.P.; Taylor, S.H.; Hutchings, G. High temperature COS hydrolysis catalysed by γ-Al2O3. Catal. Lett. 2006, 110, 243–246. [Google Scholar] [CrossRef]
- Wang, G.; Liu, D.; Bing, L. Preparation and desulfurization mechanism of LaCoO3/γ-Al2O3 and La2O3-Co2O3-Al2O3 catalysts. In Proceedings of the 2011 AASRI Conference on Artificial Intelligence and Industry Application (AASRI-AIIA 2011), Male, Maldives, 23–24 May 2011; Volume 4, pp. 312–315. [Google Scholar]
- Xiong, J.; Gong, C.; Hu, Y.; Zhu, W.; Chen, D.; Lu, J.; Ai, T.; Liu, J.; Luo, Y. Boosting catalytic hydrolysis of carbonyl sulfide over K+ modified CeO2 nanospheres at low-temperature. Appl. Surf. Sci. 2024, 663, 160143. [Google Scholar] [CrossRef]
- Nimthuphariyha, K.; Usmani, A.; Grisdanurak, N.; Kanchanatip, E.; Yan, M.; Suthirakun, S.; Tulaphol, S. Hydrolysis of carbonyl sulfide over modified Al2O3 by platinum and barium in a packed-bed reactor. Chem. Eng. Commun. 2021, 208, 539–548. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xue, L.; Yu, Y.; He, H. Oxygen Poisoning Mechanism of Catalytic Hydrolysis of OCS over Al2O3 at Room Temperature. Acta Phys.-Chim. Sin. 2007, 23, 997–1002. [Google Scholar] [CrossRef]
- Wang, G.; Huang, X.; Ye, J.; Wang, J.; Kong, Y. Preparation of T (504) type COS hydrolysis catalyst at room temperature. Hubei Chem. Ind. 1995, 1, 24–28. [Google Scholar]
- Renda, S.; Barba, D.; Palma, V. Recent Solutions for Efficient Carbonyl Sulfide Hydrolysis: A Review. Ind. Eng. Chem. Res. 2022, 61, 5685–5697. [Google Scholar] [CrossRef]
- Cao, Q.; Lin, Y.; Li, Y.; Tian, J.; Liu, H.; Zhu, T.; Wang, J. Hydrolysis of Carbonyl Sulfide in Blast Furnace Gas Using Alkali Metal-Modified γ-Al2O3 Catalysts with High Sulfur Resistance. ACS Omega 2023, 8, 35608–35618. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Mu, Y. Heterogeneous reactivity of carbonyl sulfide on α-Al2O3 and γ-Al2O3. Atmos. Environ. 2008, 42, 960–969. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, H.; Tang, X. Research status and progress of carbonyl sulfur and CS2 hydrolysis catalysts and desulfurization mechanism. New Chem. Mater. 2020, 48, 266–270. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, M.; Yang, Z.; Duan, X.; Jiang, G.; Li, G.; Zhang, F.; Hao, Z. Oxygen vacancy-engineered titanium-based perovskite for boosting H2O activation and lower-temperature hydrolysis of organic sulfur. Proc. Natl. Acad. Sci. USA 2023, 120, e2217148120. [Google Scholar] [CrossRef]
- Song, X.; Ning, P.; Wang, C.; Li, K.; Tang, L.; Sun, X.; Ruan, H. Research on the low temperature catalytic hydrolysis of COS and CS2 over walnut shell biochar modified by Fe–Cu mixed metal oxides and basic functional groups. Chem. Eng. J. 2017, 314, 418–433. [Google Scholar] [CrossRef]
- Poolwong, J.; Del Gobbo, S.; D’Elia, V. Transesterification of dimethyl carbonate with glycerol by perovskite-based mixed metal oxide nanoparticles for the atom-efficient production of glycerol carbonate. J. Ind. Eng. Chem. 2021, 104, 43–60. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Guan, K.; Liu, Y.; Li, Y.; Sun, F.; Wang, X.; Zhang, C.; Feng, S.; Zhang, T. Influence of oxygen vacancies on the performance of SnO2 gas sensing by near-ambient pressure XPS studies. Sens. Actuators B Chem. 2023, 393, 134252. [Google Scholar] [CrossRef]





| Name | Model Number | Manufacturer |
|---|---|---|
| Desktop ultrasonic cleaning machine | PS-30T | Guangdong Foheng Instrument Co., LTD, Foshan, China |
| Heat collection type constant-temperature heating magnetic stirrer | DF-101S | Zhengzhou Keda Mechanical instrument Equipment Co., LTD, Zhengzhou, China |
| Carbonyl sulfide detector | APEQ-DCOS-2N | Shenzhen Ampal Technology Co., LTD, Shenzhen, China |
| Hydrogen sulfide test analyzer | CGM10-70 | Shenzhen Angwei Electronics Co., LTD, Shenzhen, China |
| Hygrograph | AS108D | Guangzhou Aosong Electronics Co., LTD, Guangzhou, China |
| Box-type resistance furnace | XH4L-16 | Zhengzhou Xinhan Instrument Equipment Co., LTD, Zhengzhou, China |
| Electric oven | 101-1A | Beijing Zhongxing Weiye Century Instrument Co., LTD, Beijing, China |
| Planetary ball mill | KE-0.4L | Qidong Honghong instrument equipment factory, Qidong, China |
| Mass flow controller | D07 | Beijing seven star Huachuang flowmeter Co., LTD, Beijing, China |
| Rotor flowmeter | LZB-2 | Changzhou double ring thermal instrument Co., LTD, Changzhou, China |
| Electronic analytical balance | FA1604A | Shanghai Jingtian Electronic Instrument Co., LTD, Shanghai, China |
| Method | Manufacturer | Model Number | Test Parameter |
|---|---|---|---|
| BET | Beijing Jingwei Gao Bo Science and Technology Co., LTD, Beijing, China | JW-BK122W | 1 atmosphere, 77K, drying for 8 h, 473.15K |
| XRD | Bruker, Ettlingen, Germany, DE | Bruker D8 | Voltage 40 KV, current 40MA, test range 5–90 degrees, Pace 0.02; the target material is Cu target. |
| Sample | Single Point BET Specific Surface Area (m2g−1) | Pore Volume (cm3g−1) | Aperture (nm) |
|---|---|---|---|
| Pure γ-Al2O3 | 57.494 | 0.162 | 12.765 |
| Ca-Ba-γ-Al2O3 | 50.646 | 0.161 | 14.416 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Wang, L.; Fu, D.; Zhang, P. Enhanced Hydrolysis of Carbonyl Sulfide in Coking Oven Gas Utilizing an Efficient Ca-Ba-γ-Al2O3 Catalyst. Processes 2024, 12, 2150. https://doi.org/10.3390/pr12102150
Li K, Wang L, Fu D, Zhang P. Enhanced Hydrolysis of Carbonyl Sulfide in Coking Oven Gas Utilizing an Efficient Ca-Ba-γ-Al2O3 Catalyst. Processes. 2024; 12(10):2150. https://doi.org/10.3390/pr12102150
Chicago/Turabian StyleLi, Kangrui, Lemeng Wang, Dong Fu, and Pan Zhang. 2024. "Enhanced Hydrolysis of Carbonyl Sulfide in Coking Oven Gas Utilizing an Efficient Ca-Ba-γ-Al2O3 Catalyst" Processes 12, no. 10: 2150. https://doi.org/10.3390/pr12102150







