Enhancement of Natural Dye-Sensitized Solar Cell Efficiency Through TiO2 Hombikat UV100 and TiO2 P25 Photoanode Optimization
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Elaboration of TiO2 Photoanodes
2.3. Preparation of Electrolyte Solution (3I−/I3−)
2.4. DSSC-N Device Setup
3. Results and Discussion
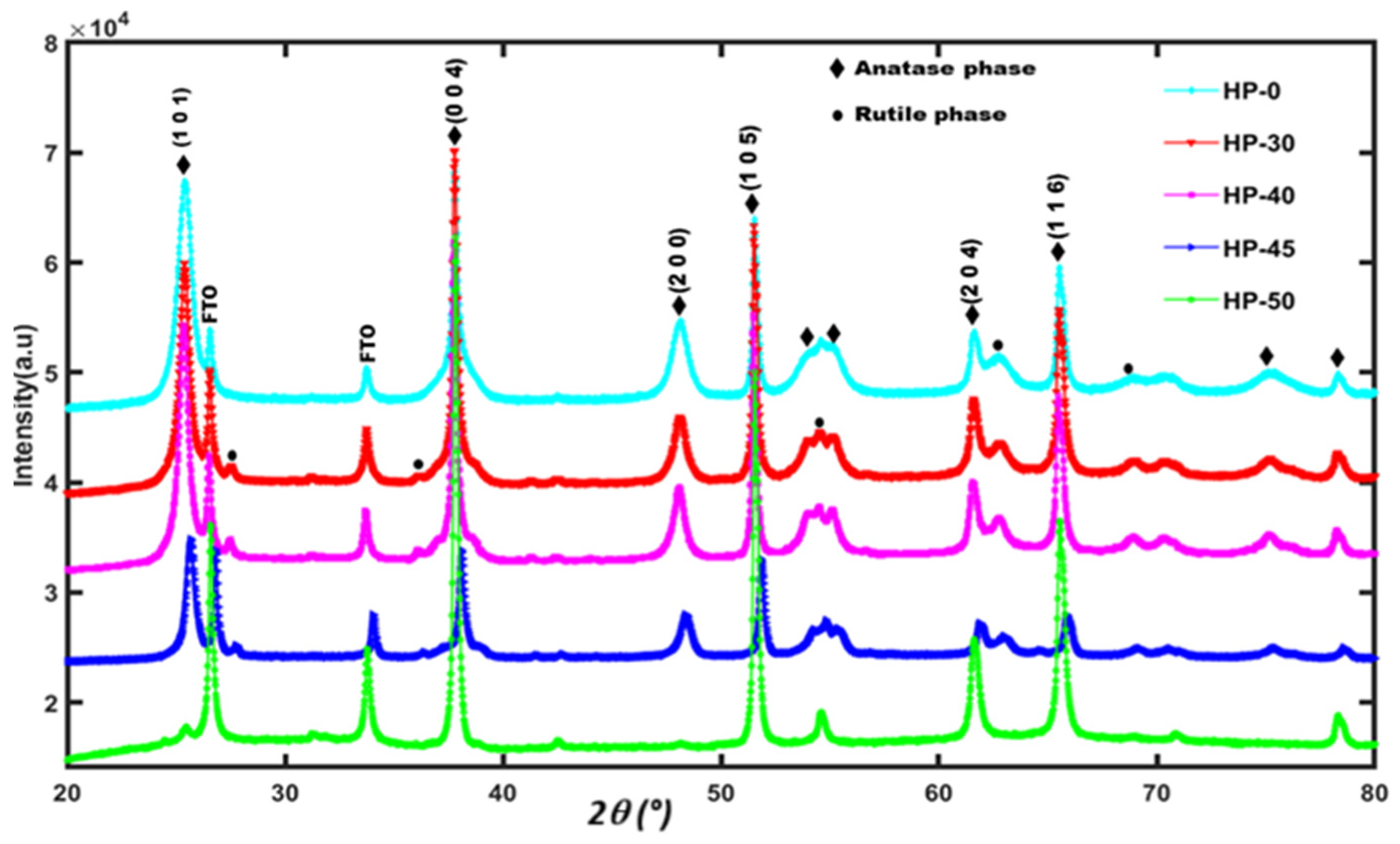
3.1. XRD Pattern Analysis
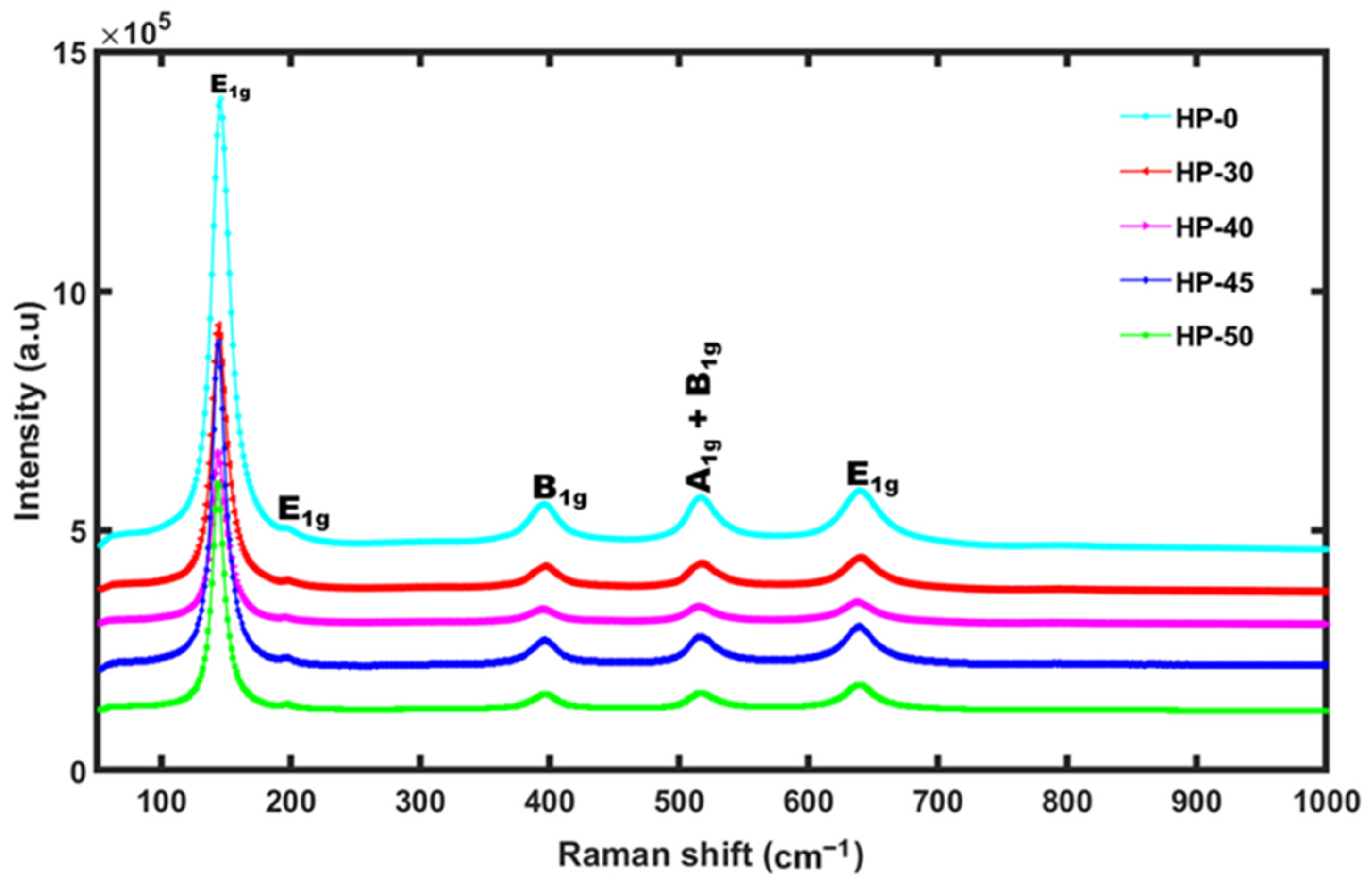
3.2. Raman Spectra Study
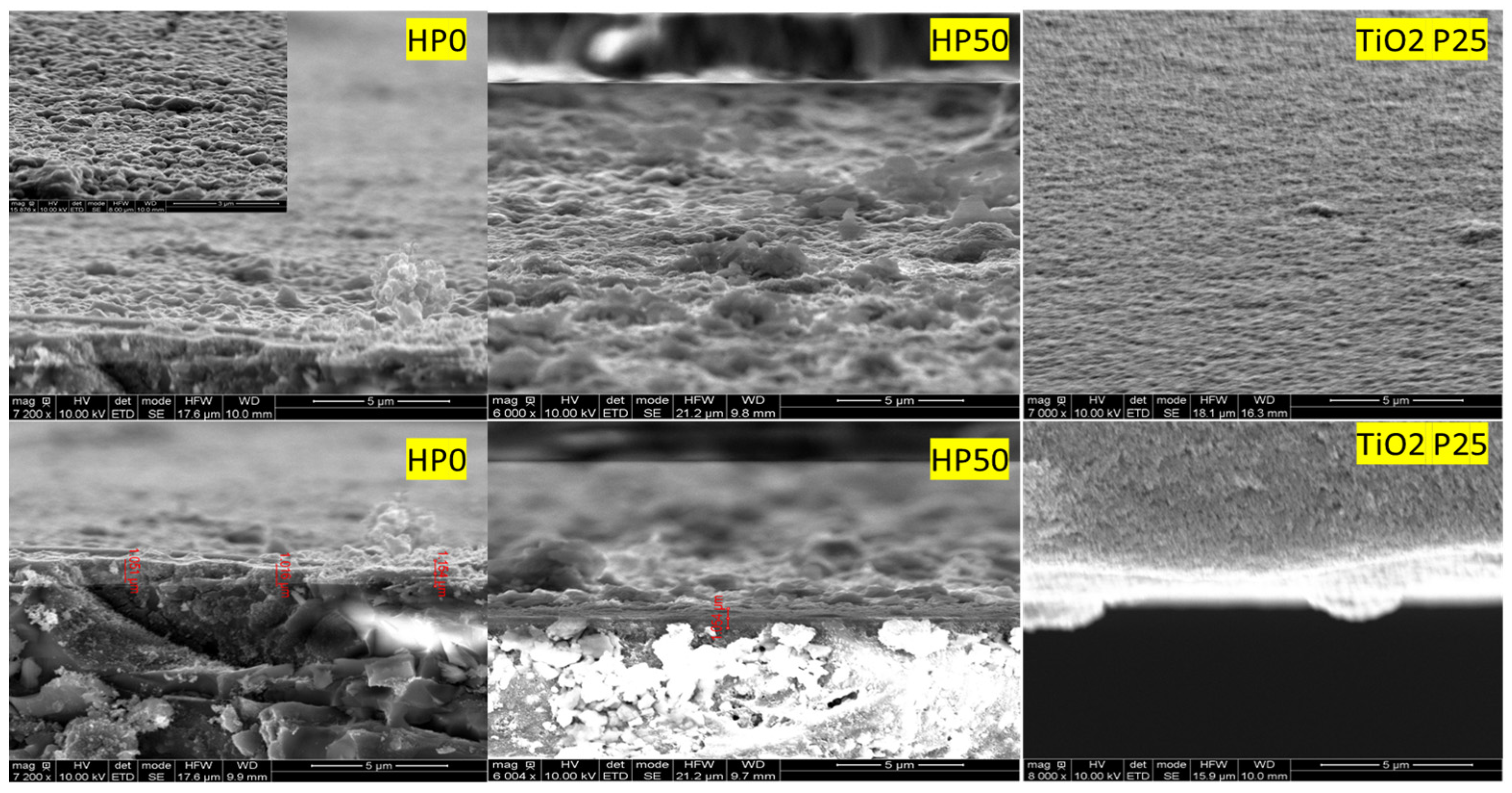
3.3. SEM Study
4. Optical Properties
4.1. Reflectance and Transmittance
4.2. Absorbance Spectra and Tauc’s Diagram Analysis
5. Evaluation of the DSSC-Ns’ Efficiency and J-V Curve Analysis
- -
- VOC represents the open-circuit voltage;
- -
- JSC denotes the short-circuit current density;
- -
- represents the incident light power;
- -
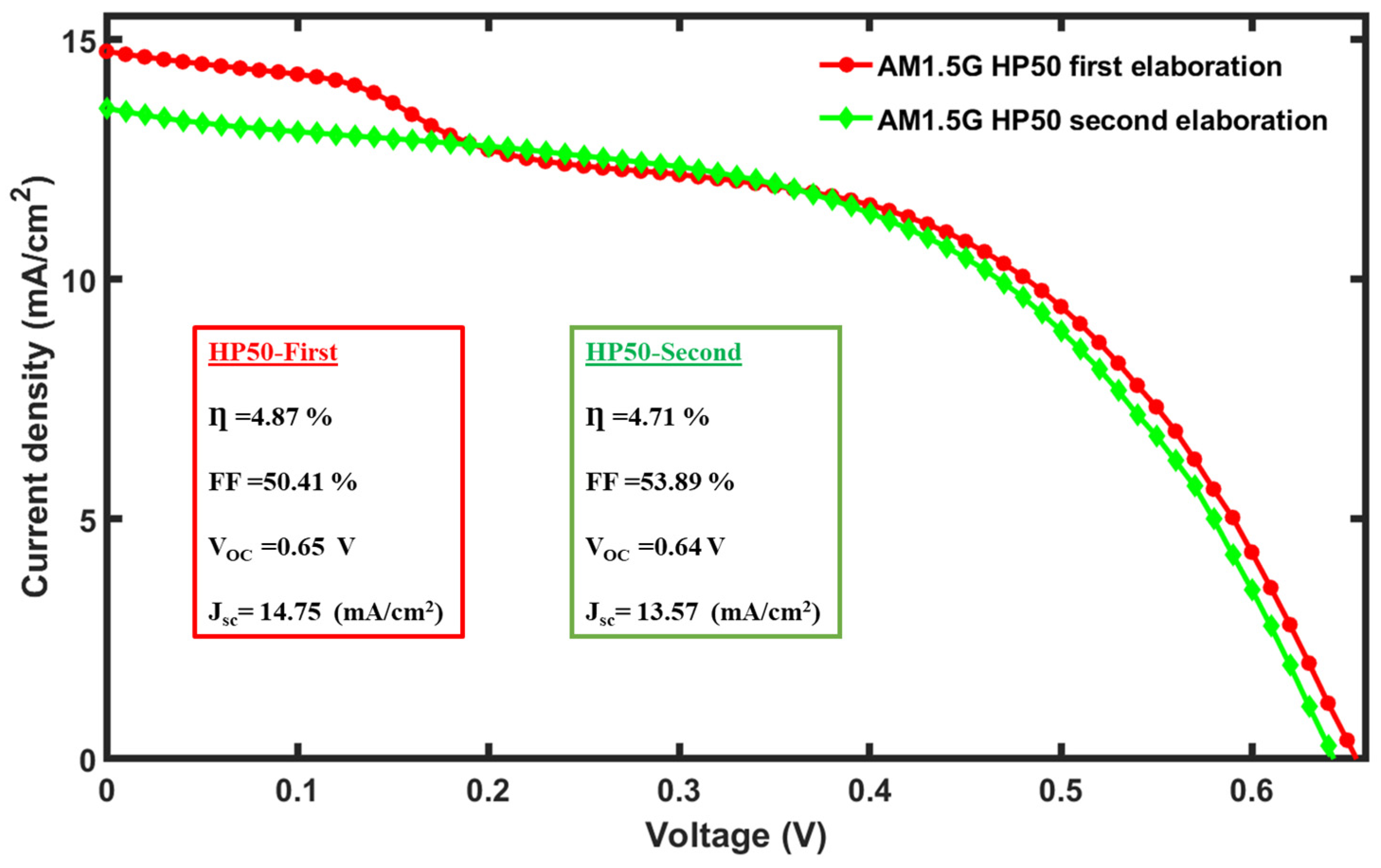
6. Discussion and Reproducibility of Results
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A Perspective on Mesoporous TiO2 Materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Tasić, N.; Marinković Stanojević, Z.; Branković, Z.; Lačnjevac, U.; Ribić, V.; Žunić, M.; Novaković, T.; Podlogar, M.; Branković, G. Mesoporous Films Prepared from Synthesized TiO2 Nanoparticles and Their Application in Dye-Sensitized Solar Cells (DSSCs). Electrochim. Acta 2016, 210, 606–614. [Google Scholar] [CrossRef]
- Jesus, M.A.M.L.; Ferreira, A.M.; Lima, L.F.S.; Batista, G.F.; Mambrini, R.V.; Mohallem, N.D.S. Micro-Mesoporous TiO2/SiO2 Nanocomposites: Sol-Gel Synthesis, Characterization, and Enhanced Photodegradation of Quinoline. Ceram. Int. 2021, 47, 23844–23850. [Google Scholar] [CrossRef]
- Tsotetsi, D.; Dhlamini, M.; Mbule, P. Sol-Gel Derived Mesoporous TiO2: Effects of Non-Ionic Co-Polymers on the Pore Size, Morphology, Specific Surface Area and Optical Properties Analysis. Results Mater. 2022, 14, 100266. [Google Scholar] [CrossRef]
- Koozegar Kaleji, B.; Gorgani, M. Comparison of Sol-Gel and Hydrothermal Synthesis Methods on the Structural, Optical and Photocatalytic Properties of Nb/Ag Codoped TiO2 Mesoporous Nanoparticles. Int. J. Environ. Anal. Chem. 2022, 102, 3357–3372. [Google Scholar] [CrossRef]
- Noorasid, N.S.; Arith, F.; Aliyaselvam, O.V.; Salehuddin, F.; Mustafa, A.N.; Chelvanathan, P.; Azam, M.A.; Amin, N. Low-Temperature Sol-Gel Synthesized TiO2 with Different Titanium Tetraisopropoxide (TTIP) Molarity for Flexible Emerging Solar Cell. J. Sol-Gel Sci. Technol. 2024, 109, 826. [Google Scholar] [CrossRef]
- Yeoh, M.-E.; Chan, K.-Y.; Wong, H.-Y.; Low, P.-L.; How Thien, G.S.; Ng, Z.-N.; Ananda Murthy, H.C.; Balachandran, R. Hydrothermal Duration Effect on the Self-Assembled TiO2 Photo-Anode for DSSC Application. Opt. Mater. 2023, 141, 113907. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Zaferani, M.; Kierulf, A.; Abbaspourrad, A. Shape-Controlled Fabrication of TiO2 Hollow Shells toward Photocatalytic Application. Appl. Catal. B Environ. 2018, 227, 519–529. [Google Scholar] [CrossRef]
- Marycleopha, M.; Yaou Balarabe, B.; Adjama, I.; Moussa, H.; Anandaram, H.; Abdoul Razak, M.W. Anhydrous Sol-Gel Synthesis of TiO2 Nanoparticles: Evaluating Their Impact on Protein Interactions in Biological Systems. J. Trace Elem. Miner. 2024, 7, 100114. [Google Scholar] [CrossRef]
- Nakamoto, T.; Higuchi, K.; Taguchi, K. Effectiveness of Light Scattering Layer Using Mesoporous SiO2-TiO2 Core-Shell on Dye-Sensitized Solar Cells. IEEJ Trans. Electr. Electron. Eng. 2024, 19, 157–159. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Liao, H.-J.; Chen, Y.-T.; Wei, C.-Y.; Chang, C.-C.; Chen, Y.-C.; Chang, Y.-H.; Lin, J.-C.; Lee, Y.-C.; Wen, C.-Y.; et al. Extended Visible to Near-Infrared Harvesting of Earth-Abundant FeS2–TiO2 Heterostructures for Highly Active Photocatalytic Hydrogen Evolution. Green Chem. 2018, 20, 1640–1647. [Google Scholar] [CrossRef]
- Ye, Y.-J.; Wu, T.-C. Preparation of Photoanode Composite Layers with Different Concentrations of Silver Nanowires Combined with TiO2 for Dye-Sensitized Solar Cells. In Proceedings of the 2021 IEEE 3rd Eurasia Conference on IOT, Communication and Engineering (ECICE), Yunlin, Taiwan, 29–31 October 2021; pp. 534–537. [Google Scholar]
- Ge, Z.; Yin, A.; Fang, M.; Zhu, W.; Li, C. dSiO2@TiO2-CsPbBr3 Hierarchical Microspheres for Dye-Sensitized Solar Cells to Enhance Photovoltaic Conversion Efficiency. J. Mater. Sci. Mater. Electron. 2024, 35, 1183. [Google Scholar] [CrossRef]
- Ho, Y.-R.; Lin, C.-H.; Huang, J.-J. Effect of TiO2 Compact Layer and ITO Texturing on DSSC Efficiency Improvement by Chemical Deposition and Etching Process. J. Mater. Sci. Mater. Electron. 2021, 32, 2618–2626. [Google Scholar] [CrossRef]
- Subramanian, A.; Murugapoopathi, S.; Amesho, K.T.T. Enhancing the Performance of Nanocrystalline TiO2 Dye-Sensitized Solar Cells with Phenothiazine-Doped Blended Solid Polymer Electrolyte. Electrocatalysis 2024, 15, 226–238. [Google Scholar] [CrossRef]
- Duarte, A.I.E.; Cruz, A.L.L.; Marquina, A.V.; Martínez, J.A.A.; Juárez, A.G.; Islas, C.Z. One-Step Method to Simultaneously Grow TiO2 Compact and Porous Layers for DSSC Photoelectrodes. Appl. Nanosci. 2024, 14, 819. [Google Scholar] [CrossRef]
- Siah, W.R.; Lintang, H.O.; Shamsuddin, M.; Yuliati, L. High Photocatalytic Activity of Mixed Anatase-Rutile Phases on Commercial TiO2 Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012005. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, T.; Liu, P.; Oeser, M. Microstructures and Optical Performances of Nitrogen-Vanadium Co-Doped TiO2 with Enhanced Purification Efficiency to Vehicle Exhaust. Environ. Res. 2021, 193, 110560. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, L.; Lin, S. First-Principles Study on Transition Metal-Doped Anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Ahmed, M.B.; Zhou, J.L. Photocatalytic Degradation of Pharmaceutical Residues from Water and Sewage Effluent Using Different TiO2 Nanomaterials. Nanomaterials 2024, 14, 135. [Google Scholar] [CrossRef]
- Chnirheb, A.; Harir, M.; Kanawati, B.; Azzouzi, M.E.; Gebefügi, I.; Schmitt-Kopplin, P. Efficiency and Degradation Products Elucidation of the Photodegradation of Mefenpyrdiethyl in Water Interface Using TiO2 P-25 and Hombikat UV100. J. Environ. Sci. 2012, 24, 1686–1693. [Google Scholar] [CrossRef]
- Zumeta, I. Comparative Study of Nanocrystalline TiO2 Photoelectrodes Based on Characteristics of Nanopowder Used. Sol. Energy Mater. Sol. Cells 2003, 76, 15–24. [Google Scholar] [CrossRef]
- Kraidy, A.F.; Yapi, A.S.; El Marssi, M.; Penton Madrigal, A.; Gagou, Y. Structural Refinement and Optoelectrical Properties of Nd2Ru2O7 and Gd2Ru2O7 Pyrochlore Oxides for Photovoltaic Applications. Materials 2024, 17, 2571. [Google Scholar] [CrossRef] [PubMed]
- Mejica, G.F.C.; Unpaprom, Y.; Ramaraj, R. Fabrication and Performance Evaluation of Dye-Sensitized Solar Cell Integrated with Natural Dye from Strobilanthes Cusia under Different Counter-Electrode Materials. Appl. Nanosci. 2023, 13, 1073–1083. [Google Scholar] [CrossRef]
- Gu, P.; Yang, D.; Zhu, X.; Sun, H.; Wangyang, P.; Li, J.; Tian, H. Influence of Electrolyte Proportion on the Performance of Dye-Sensitized Solar Cells. AIP Adv. 2017, 7, 105219. [Google Scholar] [CrossRef]
- Sreethawong, T.; Yamada, Y.; Kobayashi, T.; Yoshikawa, S. Catalysis of Nanocrystalline Mesoporous TiO2 on Cyclohexene Epoxidation with H2O2: Effects of Mesoporosity and Metal Oxide Additives. J. Mol. Catal. Chem. 2005, 241, 23–32. [Google Scholar] [CrossRef]
- Uddin, M.T.; Mukhlish, B.; Zobayer, M.; Mondal, J.; Shafiul, H. Hydrothermal Synthesis of Mesoporous TiO2 Nanoparticles for Enhanced Photocatalytic Degradation of Organic Dye. Indian J. Chem. Technol. 2023, 30, 320–330. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the “Debye-Scherrer Equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Kumar, C.P.; Gopal, N.O.; Wang, T.C.; Wong, M.-S.; Ke, S.C. EPR Investigation of TiO2 Nanoparticles with Temperature-Dependent Properties. J. Phys. Chem. B 2006, 110, 5223–5229. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman Spectra of Titanium Dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman Spectrum of Anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chan, C.K.; Porter, J.F.; Guo, W. Micro-Raman Spectroscopic Characterization of Nanosized TiO2 Powders Prepared by Vapor Hydrolysis. J. Mater. Res. 1998, 13, 2602–2609. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Ali, N.; Jabeen, M.; Khesro, A.; Ahmed, R.; Alrobei, H.; Kalam, A.; Al-Sehemi, A.G. Combinatorial Synthesis of Tin Antimony Sulfide Thin Films for Solar Cell Application. Int. J. Energy Res. 2021, 45, 21527–21533. [Google Scholar] [CrossRef]
- Kraidy, A.F.; El Radaf, I.M.; Zeinert, A.; Lahmar, A.; Peláiz-Barranco, A.; Gagou, Y. Optoelectrical Properties of the Ternary Chalcogenide SnSb2S5 as a New Absorber Layer for Photovoltaic Application. J. Phys. Appl. Phys. 2024, 57, 205102. [Google Scholar] [CrossRef]
- Xu, K. Silicon Electro-Optic Micro-Modulator Fabricated in Standard CMOS Technology as Components for All Silicon Monolithic Integrated Optoelectronic Systems*. J. Micromechanics Microeng. 2021, 31, 054001. [Google Scholar] [CrossRef]
- Belghachi, A. Theoretical Calculation of the Efficiency Limit for Solar Cells. In Solar Cells—New Approaches and Reviews; Kosyachenko, L.A., Ed.; InTech: London, UK, 2015; ISBN 978-953-51-2184-8. [Google Scholar]
- Kumar, T.S.; Shalini, S.; Roy, T.A.; Prasanna, S.; Balasundaraprabhu, R.; Sundaram, S. Solvent Selection for Anthrocyanin Dye Extraction from Kigelia Africana and Hibiscus Sabdariffa for Dye Sensitized Solar Cells. J. Photochem. Photobiol. 2024, 20, 100233. [Google Scholar] [CrossRef]
- El-Ahmar, M.H.; El-Sayed, A.-H.M.; Hemeida, A.M. Mathematical Modeling of Photovoltaic Module and Evalute the Effect of Varoius Paramenters on Its Performance. In Proceedings of the 2016 Eighteenth International Middle East Power Systems Conference (MEPCON), Cairo, Egypt, 27–29 December 2016; pp. 741–746. [Google Scholar]
- Taretto, K.; Soldera, M.; Troviano, M. Accurate explicit equations for the fill factor of real solar cells—Applications to thin-film solar cells. Prog. Photovolt. Res. Appl. 2013, 21, 1489–1498. [Google Scholar] [CrossRef]
- Tayeb, A.M.; Solyman, A.A.A.; Hassan, M.; Abu el-Ella, T.M. Modeling and Simulation of Dye-Sensitized Solar Cell: Model Verification for Different Semiconductors and Dyes. Alex. Eng. J. 2022, 61, 9249–9260. [Google Scholar] [CrossRef]
- Vaillant, L.; Vigil, E.; Forcade, F.; Thami, T.; Adnani, H.; Yacou, C.; Ayral, A.; Saint-Grégoire, P. On Fundamental Mechanisms in Dye Sensitized Solar Cells through the Behaviour of Different Mesoporous Titanium Dioxide Films. Eur. Phys. J. Appl. Phys. 2015, 72, 20404. [Google Scholar] [CrossRef][Green Version]



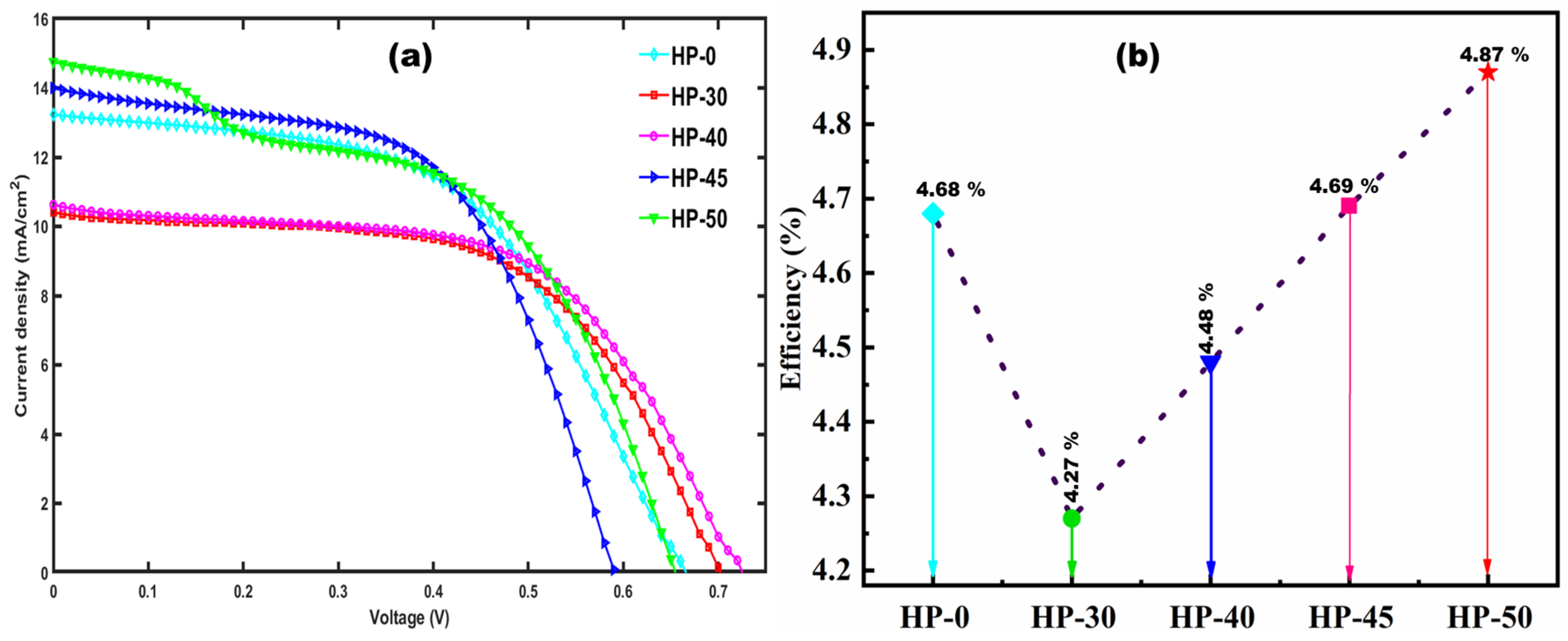
| Photoanode | Jsc (mA/cm2) | Voc (V) | FF (%) | η (%) |
|---|---|---|---|---|
| HP-0 | 13.22 | 0.66 | 53.65 | 4.68 |
| HP-30 | 10.40 | 0.70 | 58.54 | 4.27 |
| HP-40 | 10.62 | 0.73 | 58.09 | 4.48 |
| HP-45 | 13.99 | 0.59 | 56.74 | 4.69 |
| HP-50 | 14.75 | 0.65 | 50.41 | 4.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraidy, A.F.; Yapi, A.S.; Saint-Gregoire, P.; Vaillant-Roca, L.; Eke, S.; Mouangue, R.; Jamali, A.; Gagou, Y. Enhancement of Natural Dye-Sensitized Solar Cell Efficiency Through TiO2 Hombikat UV100 and TiO2 P25 Photoanode Optimization. Processes 2024, 12, 2481. https://doi.org/10.3390/pr12112481
Kraidy AF, Yapi AS, Saint-Gregoire P, Vaillant-Roca L, Eke S, Mouangue R, Jamali A, Gagou Y. Enhancement of Natural Dye-Sensitized Solar Cell Efficiency Through TiO2 Hombikat UV100 and TiO2 P25 Photoanode Optimization. Processes. 2024; 12(11):2481. https://doi.org/10.3390/pr12112481
Chicago/Turabian StyleKraidy, Assohoun Fulgence, Abé Simon Yapi, Pierre Saint-Gregoire, Lídice Vaillant-Roca, Samuel Eke, Ruben Mouangue, Arash Jamali, and Yaovi Gagou. 2024. "Enhancement of Natural Dye-Sensitized Solar Cell Efficiency Through TiO2 Hombikat UV100 and TiO2 P25 Photoanode Optimization" Processes 12, no. 11: 2481. https://doi.org/10.3390/pr12112481
APA StyleKraidy, A. F., Yapi, A. S., Saint-Gregoire, P., Vaillant-Roca, L., Eke, S., Mouangue, R., Jamali, A., & Gagou, Y. (2024). Enhancement of Natural Dye-Sensitized Solar Cell Efficiency Through TiO2 Hombikat UV100 and TiO2 P25 Photoanode Optimization. Processes, 12(11), 2481. https://doi.org/10.3390/pr12112481









