Abstract
Biotechnology is increasingly being used as a tool to replace traditional production methods due to concerns about the increasing damage caused by global warming. Bacteria, yeasts, fungi, and microalgae are microorganisms able to transform residues into value-added bioproducts. They produce microbial biomass that can result in the production of several products, such as biofuels, microbial proteins, fatty acids, bioactive polysaccharides, carotenoids, industrial enzymes, polyhydroxyalkanoates, and biofertilizers, among others. To improve microbial biomass and lipid yield, modern genetic engineering techniques can be applied as a way of optimizing processes and conditions. This review aims to explore the latest trends and technological advances in microbial biomass and lipid production, including optimization strategies for cultivation conditions and the use of genetic engineering to enhance yields and efficiency. It also discusses the challenges and future prospects for scaling up production for industrial applications. The potential of microbial lipids to contribute to a sustainable bioeconomy, as well as their applications in renewable energy and food industries, underscores their importance in a world increasingly seeking alternatives to fossil fuel dependence and unsustainable agricultural practices.
1. Introduction
Lipids play essential roles in several biological and industrial functions, being crucial for both the health of organisms and for commercial applications. Biologically, lipids are components of cell membranes, ensuring the structural integrity of cells and facilitating the transportation of substances. In addition, they act as a source of energy for organisms and can be precursors of essential hormones [1]. They are utilized industrially in the production of biodiesel, as ingredients in nutritional supplements, and as components in the formulation of creams, lotions, and pharmaceuticals [2]. Traditionally, lipids are found in meat fats, dairy products, fish oils, vegetable oils, nuts, and seeds [3]. In the last few years, microbial lipids have emerged as a sustainable and innovative alternative to traditional lipids. Produced from microorganisms such as yeast and fungi, these lipids offer several advantages, including faster and more efficient production, a lower environmental impact, and the ability to be cultivated on a variety of substrates, such as agricultural waste.
The production of microbial biomass and lipids has increasingly attracted industrial interest due to its potential application in various sectors. Among the markets interested in these technologies, we can highlight the biofuels industry and food and chemical products. According to Grand View Research, the global biodiesel market was valued at USD 32.09 billion and is expected to grow at a compound annual growth rate (CAGR) of 10% from 2022 to 2030 [4]. In the biofuels industry, leveraging oleaginous microorganisms—including bacteria, yeasts, fungi, and microalgae—enables the production of lipids that can be transformed into biodiesel, reducing dependence on conventional vegetable oils [5].
In addition, the application of microbial lipids in the food industry has gained prominence, particularly in the production of omega-3 fatty acids and other functional lipids used in supplements and enriched foods [6]. Polyunsaturated fatty acids (PUFAs) are fatty acids with more than one double bond in their chemical structure. They are categorized as n-3 (ω-3) and n-6 (ω-6), depending on the location of the first double bond at the methyl end of the fatty acid chain. PUFAs are recognized as active components that promote health. Among the health benefits, the development and function of the brain, nerves, and eyes, cardioprotective effects, anti-inflammatory and anticancer properties, vitamin absorption, cholesterol regulation, and potential beneficial effects on the immune system stand out [7]. Another product that is now produced in a microbial way is cocoa butter, whose price has increased to over USD 8000 per ton since the 1980s [8].
Regarding the protein sector for human and animal food, single-cell proteins (SCPs) have also gained prominence. SCPs are dry cells that can be used to produce microbial meat, supplements, and protein-rich ingredients for animal and human feed [9]. These proteins can be produced from agricultural waste or by-products from the food industry [10], mainly with the help of fungal strains. Yeast biomass is rich in essential amino acids, making it an excellent option for animal feed, especially in poultry and swine diets [5]. Microalgae such as Spirulina and Chlorella are widely used in animal nutrition due to their high nutritional content, which includes proteins, vitamins, and essential fatty acids. These microalgae are especially popular in feed for fish and other aquatic organisms [11]. Additionally, Spirulina and Chlorella are gaining recognition in human nutrition due to their remarkable health benefits. They are rich in high-quality proteins, providing all essential amino acids, which makes them an excellent alternative for plant-based diets. Furthermore, both microalgae are packed with vitamins such as B1, B2, B3, and B12, as well as minerals like iron, magnesium, and calcium [12]. Their antioxidant and anti-inflammatory properties help reduce oxidative stress and may lower the risk of chronic diseases [13]. The SCP global market size was valued at around USD 13.1 billion in 2022 and is estimated to reach USD 75.9 billion by 2032, which is a reflection of population growth in food demand [14].
Technological innovations, such as gene editing by CRISPR/Cas9, have made it possible to modify microorganisms to increase lipid production and improve the efficiency of fermentation processes [15]. In addition, advances in bioprocessing, including the optimization of fermenter operating modes, have shown a potential to increase the production of microbial biomass [16]. The use of substrates from agricultural waste and industrial by-products not only improves process efficiency but also promotes sustainability, contributing to the circular economy [17]. Nevertheless, significant challenges remain to be addressed. The high production and operational costs, particularly concerning lipid recovery, pose substantial barriers to the economic feasibility of these technologies [18]. In addition, market acceptance of products intended for supplementation and human nutrition that are derived from genetically modified organisms also presents a challenge, requiring efforts to educate consumers and ensure the safety of these products [19].
In this review, we will explore the advances in biomass and lipid production by microbes, presenting the processes and the bioproducts generated from biomass and lipids and the challenges surrounding this area of research. Furthermore, we will discuss the potential of microbial lipids in various applications, including biofuels, food supplements, and pharmaceuticals, highlighting the importance of optimizing production processes to enhance yields and efficiency. Additionally, we will also conduct a comprehensive search of patents related to these processes and products, examining the innovations that have emerged in this field and analyzing trends and prospects in this evolving field. Ultimately, this review aims to provide a comprehensive overview of microbial biomass and lipid production, emphasizing their significance in promoting sustainability and contributing to a circular economy.
2. Microbial Biomass Production
2.1. Bacterial Biomass
The composition of bacterial biomass is variable and depends on several factors [20]. Generally, proteins make up 50–83% of the dry weight, and this high protein content makes bacterial biomass a promising alternative to traditional protein sources. Bacterial biomass contains all the essential amino acids necessary for human and animal nutrition. The amino acid profile is similar to that of fishmeal, making it a valuable ingredient in animal feed. Taran and Asadi, in 2014 [21], produced bacterial biomass using Haloarcula sp. IRU1 and achieved 76.4% single-cell protein relative to the cell dry weight under optimal conditions. However, bacteria have a high nucleic acid content, typically ranging from 15% to 16%. This high content can be harmful to humans and may lead to health issues. Furthermore, bacterial biomass contains carbohydrates, lipids, vitamins, and minerals [22].
Bacterial biomass production for lipid generation has garnered significant attention due to its potential in biofuels and sustainable energy sources. Certain bacteria, such as Rhodococcus opacus, Acinetobacter calcoaceticus, and Acinetobacter spp., have the ability to accumulate intracellular lipids, primarily in the form of triacylglycerols (TAGs), which can be efficiently converted into biodiesel [23]. Compared to traditional oil crops, lipid-producing bacteria offer faster growth rates, higher yields per biomass unit, and the ability to thrive on a variety of carbon sources, including waste products from industrial processes [24]. This versatility in substrate utilization not only enhances the economic feasibility of bacterial lipid production but also contributes to waste valorization and circular economy practices.
Oleaginous microbes are defined as those that store over 20% of their biomass in the form of lipids, generally TAGs, which can reach 70% of the total biomass. Though the utilization of bacteria for biofuel production is still limited compared to other sources, it is important to recognize the underlying potential of the group to this end [25]. R. opacus, A. calcoaceticus, and Acinetobacter spp. are able to accumulate 87% of their dry biomass in oil content. Other than those, species such as Streptomyces sp., Nocardia sp., Mycobacterium sp., Dietzia sp., Gordionia sp., Serratia sp., and Bacillus sp. have also been classified as oleaginous due to TAGs accumulation [24,26]. Their biomass composition varies according to growth conditions. For instance, R. opacus can generate high lipid levels (over 80%) when utilizing simple sugars and aromatic compounds, but its lipid production decreases when grown on more complex lignin-based substrates [27].
In bacteria, TAG accumulation is a result of cell stress. Its biochemical route can be described in two distinct steps: (1) a growing phase in a carbon-rich environment, in which nutrients are mainly destined for biomass synthesis; (2) an oleaginous phase, initiated when the availability of an essential nutrient, commonly nitrogen, is limited. This causes biomass growth to be interrupted. Though the assimilation of carbon is continued, its excess is directed towards the synthesis and accumulation of storage lipids, such as TAGs [28]. Hence, the choice of substrate significantly influences lipid accumulation, which is decisive for establishing economic viability.
Bacteria can utilize various carbon sources, including glucose, sucrose, and organic acids. Glucose is easily metabolized by various different species of bacteria and normally renders adequate amounts of lipid accumulation [29,30]. However, its relatively high cost might be an impediment to process scale-up. Alternatively, low-cost substrates, such as industrial and agricultural wastes, offer an economically viable route and increase the sustainability of the industrial platform. The challenge lies in identifying, among these residues, high-quality carbon sources that allow for high-cell-density batches of bio-oil-producing bacteria.
Several studies report lipid production by R. opacus using biomass hydrolysates as a substrate, particularly sugarcane bagasse. The research conducted by Mahmood and Singh [31] demonstrated that the strain R. opacus DSM 43205 is capable of accumulating lipids up to 64.47% of its cell dry weight (CDW) when cultured in a medium containing sugarcane bagasse hydrolysate. The lipid productivity achieved was 0.015 g/L/h. These values are significantly higher than those reported in other studies using different substrates and microorganisms. On the other hand, Mondala et al. [32] utilized sugarcane bagasse hydrolysate as a carbon source to stimulate oil production by activating sludge microbial cultures. Pre-cultivation of microorganisms in a high xylose concentrated medium was necessary so that they could be acclimated for assimilating xylose, the main component of the hydrolysate. Furthermore, pre-cultivation allowed for a high cellular concentration, which mitigated potential growth inhibition caused by bagasse hydrolysis byproducts. The study observed lipid accumulation ranging from 40% to 47% of the CDW under a high C:N ratio following feeding with bagasse hydrolysate. The authors further suggest that a sequential fed-batch strategy using hydrolysate with and without nutrient supplementation may be ideal for achieving high cell density and lipid accumulation while minimizing dilution.
Though a decent strategy, the use of lignocellulosic sources demands an extra step of raw material pre-treatment, which allows the bacteria to have access to fermentable sugars. Ongoing research aims to establish efficient and affordable pre-treatment methods to break down lignin materials. Various methods, such as organosolv pre-treatment, oxygen oxidation, laccase supplementation, and alkaline or alkaline–peroxide pre-treatment, demonstrate varying degrees of effectiveness. The optimal method depends on factors such as the type of biomass, the microbial strain used, and the economic feasibility of the process [27]. The literature highlights that the pre-treatment of lignocellulosic biomass, particularly lignin, is crucial for enhancing microbial lipid production.
Moreover, different batch processes can significantly influence lipid accumulation by bacteria, primarily through the controlled management of nutrient availability and growth conditions. Fed-batch fermentation emerges as particularly effective due to its ability to extend the growth phase and optimize nutrient limitations, leading to higher lipid yields compared to traditional batch processes. In a fed-batch process, nutrients are added gradually over time, allowing for extended bacterial growth and controlled nutrient limitation [33]. By carefully regulating the supply of nitrogen and carbon sources, fed-batch cultivation can enhance lipid accumulation, as bacteria can switch from biomass growth to lipid storage more efficiently.
For instance, Thanapimmetha et al. demonstrated the advantage of employing a fed-batch over a batch strategy for the cultivation of R. opacus PD630 and lipid accumulation in a modified salt-medium containing glycerol as the carbon source [34]. The specific feeding profile used in the study involved supplementing the culture with ammonium acetate on the first day, followed by acetic acid on the second and third days, and then alternating between the two feedings until the end of the cultivation. This approach was designed to prevent nitrogen limitation, enhance glycerol consumption, and consequently promote both growth and metabolite production. One of the key benefits of the batch-feeding strategy was the ability to modulate pH levels without the need for external control. Feeding with ammonium acetate initially raised the pH, while subsequent feeding with acetic acid neutralized this effect, thereby creating a self-regulating pH cycle within an optimal range for the growth of R. opacus. This illustrates how a carefully designed feeding profile can enhance metabolite production and optimize conditions such as the pH, demonstrating the potential of fed-batch cultivation to significantly improve lipid yields in bacterial systems like R. opacus.
On the other hand, in a continuous batch process, nutrients are continuously supplied, and cells are constantly harvested, maintaining the bacteria in a dynamic state. This method allows for the constant monitoring and optimization of growth conditions, improving the overall yield of lipids over time [35]. This process can be tailored to maximize lipid accumulation by modifying environmental conditions like carbon source availability, pH, oxygen levels, and temperature.
Gupta et al. explored three different bioreactor operation modes: fed-batch, continuous, and continuous cell recycling, aiming to determine the most effective method for maximizing lipid production and chemical oxygen demand (COD) removal from dairy wastewater using R. opacus [36]. In terms of lipid accumulation, the continuous culture with cell recycling yielded the best results, reaching a lipid concentration of 3.4 g/L, with a COD removal efficiency of 97%, while fed-batch and the continuous culture reached lipid concentrations of 1.1 g/L and 1.8 g/L, respectively. The high lipid production is attributed to the increased biomass in the bioreactor caused by cell recycling [37]. This approach highlights the potential for integrating bioprocesses to improve sustainability in industrial applications.
In summary, optimizing microbial biomass production conditions is critical for maximizing lipid accumulation in bacterial systems like R. opacus and Acinetobacter spp. These oleaginous bacteria exhibit significant potential for sustainable biofuel production due to their ability to utilize diverse carbon sources, including waste products, and efficiently convert them into lipids. Among the various bioprocess strategies, fed-batch and continuous cell recycling modes have proven particularly effective. Additionally, the use of low-cost substrates such as sugarcane bagasse hydrolysate demonstrates the potential for integrating circular economy practices into microbial lipid production. By carefully managing nutrient availability and growth conditions, these processes offer scalable solutions for bio-oil production, contributing to advancements in sustainable energy technologies.
2.2. Fungi Biomass
The composition of fungal biomass exhibits significant variability and is heavily influenced by the cultivation medium, environmental conditions, and the age of the fungal strain. Typically, polysaccharides dominate the composition, representing up to 80% of the dry weight of fungal biomass [38]. Among the polysaccharides found in fungal biomass, glucans, chitosan, and polyuronides are particularly noteworthy [39]. Generally, proteins constitute 3% to 20% of the dry weight of the biomass and play a fundamental role in adhesion and providing hydrophobic properties. Kam et al. [40] demonstrate that certain species of fungi, such as Aspergillus niger, can produce SCP-rich biomass, with protein content reaching 52%. Lipids represent around 1% to 10% of the fungal biomass dry weight. They serve as energy storage material and structural components of the cell membrane [41]. Fungi can accumulate lipids in the form of triglycerides, free fatty acids, polar lipids, sterols, hydrocarbons, and pigments.
Fungal species known for their ability to accumulate lipids are increasingly being studied for applications in biodiesel production and other biotechnological processes. Among the species used to produce lipids, Mucor circinelloide is recognized for its significant lipid production, especially when cultivated under aerobic conditions and high glucose concentrations [42]. Aspergillus terreus is identified as one of the most potent lipid producers, accumulating up to 38.33% lipids of its dry biomass weight under optimized culture conditions. Its fatty acid profile is rich in oleic and stearic acids, making it a promising candidate for biodiesel feedstock [43].
Similar to A. terreus, A. niger has been shown to effectively accumulate lipids, with studies indicating significant lipid production under specific nutrient conditions [44]. It is often screened alongside other Aspergillus species for its potential in biodiesel applications. Aspergillus flavus also demonstrates considerable lipid accumulation capabilities and is often included in studies assessing the potential of various fungi for sustainable biodiesel production [45]. Trichoderma viride is known for its ability to synthesize lipids and can achieve lipid accumulation of about 32% of its dry biomass weight when grown on nitrogen-deficient media, highlighting its potential in biotechnological applications [46]. Trichoderma harzianum species has also been studied for lipid production, achieving around 17% lipid accumulation under specific growth conditions, although it generally produces less biomass compared to T. viride [47].
Fungal lipid accumulation generally occurs in two stages: cell proliferation and lipid accumulation [48]. During the cell proliferation process, fungi actively grow and multiply [49]. In this stage, fungal cells rapidly consume available nutrients in the growth medium, primarily carbon sources. The metabolic processes are predominantly focused on cell division and biomass increase. As the process progresses, the conditions shift towards lipid accumulation. This stage typically occurs when nitrogen sources are depleted while carbon sources remain abundant. In these circumstances, the fungi halt substantial cell proliferation and shift their metabolic emphasis toward the production of lipids [50]. Fungi produce lipids by converting surplus carbohydrates via innovative fatty acid biosynthesis pathways. The transition to lipid synthesis is facilitated by the availability of acetyl-CoA, which is derived from carbohydrate metabolism [51]. The accumulated lipids are stored as TAGs, which serve as energy reserves for the fungi. This accumulation can be influenced by various factors, including temperature, pH, and the presence of metal ions that can enhance or inhibit lipid production [52].
Some factors must be considered when trying to produce fungal biomass and/or fungal lipids. To accumulate lipids, the carbon-to-nitrogen (C/N) ratio is critical. Higher ratios promote lipid accumulation, while lower ratios support biomass growth. To achieve optimal lipid production, the C/N ratio must be greater than 20 but may decline at ratios exceeding 70 [48]. Furthermore, macronutrients such as phosphorus and potassium and micronutrients like zinc and magnesium are essential for optimal growth and lipid synthesis [50]. Fungi have specific temperature ranges that optimize their growth rates. For example, some species exhibit increased proliferation at temperatures around 25 °C but may transition to a different growth form (e.g., yeast to mycelium) at higher temperatures (37 °C) to adapt to environmental changes [53]. The acidity or alkalinity of the growth medium can influence enzyme activity and nutrient availability, thereby affecting cell division and overall growth rates [54]. Adequate oxygen levels are crucial for aerobic fungi as they rely on oxidative metabolism for energy production. Inadequate levels of oxygen can significantly hinder growth rates and disrupt metabolic pathways [55].
The fungal production of lipids can be carried out using two main methods. Submerged fermentation (SF) involves the growth of microorganisms in a nutrient-enriched liquid medium, where agitation is used to ensure medium homogenization. Solid-state fermentation (SSF), on the other hand, occurs through the growth of microorganisms on the surface of a solid substrate, usually agro-industrial residues. Compared to SSF, SF provides a more controlled environment. This allows for more precise monitoring of critical parameters, such as temperature, pH, dissolved oxygen, and nutrient levels, enhancing the overall fermentation process [56]. This process control is crucial to optimizing microbial growth and the production of biomass and lipids. The environment provided by the bioreactors used in SF significantly reduces the risk of contamination by undesirable microorganisms [57]. Moreover, this technology is already widely used and well-established in the bioprocess industry. This technological maturity translates into more efficient and reliable processes for large-scale lipid production.
Another crucial factor is the efficiency of mass transfer. SF processes provide efficient agitation of the fermentation medium, ensuring a more uniform distribution of nutrients. Aeration can also be controlled by adjusting the amount of oxygen required for microbial growth and the production of the desired product. SF generally results in higher biomass yields due to better nutrient solubility and accessibility [58]. For instance, studies show that certain fungi produce more biomass in SF compared to SSF due to optimal growth conditions [59].
Although SF offers valuable advantages for the production of fungal biomass and lipids, SSF remains the most recommended mode of operation. Lipid production by SSF has several advantages over SF, particularly regarding economic and sustainability aspects [60]. SSF allows the use of agro-industrial residues as a fermentation substrate, which are significantly cheaper than the pure substrates used in SF. Examples of such residues include sugarcane bagasse and soybean hulls [61]. Furthermore, solid-state processes demand considerably less water, leading to decreased energy consumption for both mixing and aeration. SSF does not require sophisticated bioreactors, simplifying infrastructure and reducing the implementation costs of the production plant [62]. Regarding the suitability of filamentous fungi for the fermentation environment [63], SSF allows the simulation of the natural environment of these fungi, which generally show greater efficiency in lipid production compared to yeasts [64]. The bioprocess is also consolidated, as enzyme production, biomass hydrolysis, and fermentation occur in a single system.
2.3. Yeast Biomass
The main yeast genera used in lipid production are Yarrowia, Candida, Rhodotorula, Rhodosporidium, Cryptococcus, Trichosporon, and Lipomyces [65]. Of all the known yeast species, less than 30 can accumulate more than 20% of their biomass as intracellular lipids, which qualifies them as oleaginous yeasts [66]. The most studied species for lipid production are Yarrowia lipolytica, Rhodotorula glutinis, Rhodosporidium toruloides, Cryptococcus curvatus, and Lipomyces starkeyi. However, high productivity has also been obtained with other species, such as Trichosporon fermentans [67].
The dry biomass of oleaginous yeasts is generally composed of 40–60% proteins, which are rich in essential amino acids and are an important source of protein for animal feed and human food. A total of 20–30% are lipids that can be used in the production of biodiesel or as functional ingredients in foods. A total of 10–20% are carbohydrates, and these carbohydrates may include glycogen, mannan, and other polysaccharides [68]. Cui et al. [69] produced SCP from Y. lipolytica, reaching a biomass production of 20.1 g/L with 47.5% crude protein. The strain had the introduction of the exo-inulinase gene from Kluyveromyces marxianus, which allowed the yeast to grow on media containing inulin. Gao et al. [70] produced biomass containing 56.42% of crude protein using soy molasses as a substrate. The biomass and SCP produced can be used in animal and human feed, as well as in supplement composition.
The production of biomass and lipids in yeast is significantly impacted by a variety of factors that shape cellular metabolism and enhance the efficiency of synthesizing valuable compounds. When there is excess carbon in the medium, whether from sources such as glucose, sucrose, glycerol, or fatty acids, lipid accumulation may be induced, especially under conditions of the limitation of other nutrients [71]. Nitrogen limitation, for example, is one of the most important factors for lipid accumulation since yeast diverts excess carbon from protein production and cell growth (biomass) to lipid synthesis. This results in the increased production of TAGs, the main storage lipids in cells [72].
TAGs are the most abundant type of lipid found in oleaginous yeasts, typically representing 80% to 90% of the total lipid content [73]. Their structure consists of a glycerol molecule linked to three fatty acids. In addition, the high C/N ratio favors lipid accumulation [74]. Oleaginous yeast has a high activity of lipogenic pathways. Enzymes such as glycerol-3-phosphate acyltransferase (GPAT) and diacylglycerol acyltransferase (DGAT) play critical roles in the incorporation of fatty acids into TAGs [75].
Oxygen availability affects lipid metabolism, especially for the synthesis of unsaturated fatty acids, which require oxygen. Under aerobic conditions, yeasts accumulate lipids, mainly TAGs, since oxygen plays a fundamental role in the oxidation of carbohydrates in the Krebs cycle [76]. Studies show that yeast types such as Y. lipolytica present a higher production of lipids under aerobic conditions due to the increased activity of ATP-citratolyase, which converts citrate into acetyl-CoA, the precursor molecule for the synthesis of fatty acids [75]. Under anaerobic conditions, the production of lipids by oleaginous yeasts is significantly reduced. This occurs because oxygen is an essential cofactor in several enzymes involved in the synthesis of fatty acids. Without oxygen, yeasts redirect their metabolism to alcoholic fermentation or other fermentative pathways, prioritizing the production of ethanol and other fermentative products instead of lipids. The absence of oxygen also results in a lack of sufficient ATP for lipid synthesis since the production of ATP via fermentation is much less efficient than through aerobic respiration [77,78]. In certain scenarios, low oxygen levels (microaeration) may be beneficial for lipid production. Microaeration can provide sufficient oxygen to keep oxygen-dependent enzymes active without stimulating oxidative metabolism to the point where all available carbon is consumed for cell growth rather than lipid synthesis [79,80].
Phosphorus is essential for cell growth and the production of phospholipids, which are essential components of cell membranes [81]. Oxygen limitation can affect both growth and lipid production. Environmental conditions, such as the temperature and pH of the medium, also affect the efficiency of yeast metabolism [82]. Optimized temperatures (generally between 28 °C and 32 °C) favor the biosynthesis of biomass and lipids. Changes in pH can influence fatty acid metabolism and cell permeability [83]. Yeasts, such as Y. lipolytica and Rhodotorula, require oxygen for lipid synthesis, especially for the production of unsaturated fatty acids [84]. Adequate aeration favors biomass accumulation, but oxygen limitation can, in some cases, stimulate lipid accumulation, depending on the yeast species [85].
Bioreactor operating modes (batch, fed-batch, and continuous) differ significantly in their control dynamics and productivity, directly affecting the production of yeast-derived biomass and lipids. Fed-batch fermentation allows for the controlled addition of nutrients over time, which helps to maintain optimal growth conditions for yeast. This approach prevents nutrient depletion and supports sustained yeast activity, leading to higher biomass and lipid production [86]. By gradually feeding substrates, fed-batch systems can minimize the inhibitory effects of high substrate concentrations that may occur in batch processes. This helps to improve overall fermentation performance and product yields [87].
High initial concentrations of the carbon source, although potentially desirable for high lipid yields, can inhibit cell growth in the early phase of batch cultivation. This effect can be pronounced in yeasts that are sensitive to high substrate concentrations. This inhibition can lead to a prolonged lag phase and reduce total biomass and lipid production. Additionally, insufficient nitrogen levels in the culture medium, which are critical for stimulating lipid accumulation, may hinder cell growth throughout batch cultivation. Since nitrogen is an essential nutrient for cell growth, its early depletion in the culture medium can impair biomass production, consequently limiting the final lipid yield, even if the intention is to direct metabolism toward lipid production [88].
Batch fermentation is straightforward to set up and operate, requiring less complex equipment and fewer control systems compared to continuous or fed-batch processes. This simplicity can lead to lower initial capital costs and easier maintenance. Since all ingredients are added at once and the fermentation runs to completion before harvesting, there is a reduced risk of contamination that can occur with continuous systems where media is constantly added. Fed-batch is effective in maximizing biomass production and then promoting lipid accumulation in oleaginous yeasts, especially under nitrogen-limited conditions [89].
Studies have shown that fed-batch fermentation can lead to higher lipid concentrations compared to batch fermentation due to better substrate utilization and reduced inhibitory effects [90]. Fed-batch systems require careful monitoring and control of nutrient additions, which can complicate the operation and increase the likelihood of errors. This complexity may necessitate skilled personnel to manage the process effectively, leading to higher operational costs. Prolonged exposure of yeast to high cell concentrations and potential inhibitors can occur in fed-batch systems. This accumulation can negatively affect yeast performance and reduce overall fermentation efficiency [91].
In continuous mode, the environment is constantly maintained, allowing cells to adapt and thrive, which can lead to higher biomass concentrations and lipid yields over time [90]. This mode allows for sustained production without the downtime associated with batch processes. Continuous feeding of nutrients ensures that yeast cells are always in an optimal growth phase, leading to higher overall productivity [92]. This method offers the advantage of a constant C/N ratio and constant production yields at a steady state [88]. Continuous operation facilitates better nutrient management, as fresh substrates can be supplied while waste products are removed regularly. This reduces the risk of nutrient depletion and toxic accumulation, which can hinder growth. This can lead to higher lipid content in the biomass, making it suitable for biodiesel production and other applications [93].
However, continuous systems require sophisticated control mechanisms to maintain optimal conditions, such as nutrient concentrations and pH levels. This complexity can lead to higher operational costs and the need for skilled personnel to manage the process effectively. In addition, the continuous introduction of fresh media and removal of culture broth increases the risk of contamination by undesirable microorganisms. This can compromise product quality and yield, necessitating stringent sterilization protocols. Lipid production is lower compared to other operating modes, as the continuous system favors cell growth rather than the accumulation of lipid reserves [94].
2.4. Microalgae Biomass
Microalgae biomass composition varies significantly across species and growth conditions. Protein content ranges from 18 to 56%, lipids from 7 to 48%, and carbohydrates from 15 to 46% of dry weight [95]. Microalgae are rich in essential amino acids and polyunsaturated fatty acids, including EPA and DHA [96]. Oliveira et al. produced an SCP-rich biomass from Spirulina maxima, reaching 71% of protein content [97]. Microalgae also contain valuable pigments like carotenoids and chlorophyll [37]. Of course, there are significant phylogenetic differences that reflect on the macromolecular composition (protein, lipid, carbohydrate) of the microalgae [95]).
Feller et al. compared the biochemical composition of five different microalgae. It was found that the green microalgae Chlorella vulgaris and Scenedesmus spp. exhibited notably high protein contents, with values of 56.1% and 49.0%, respectively. Additionally, these species also stood out for their lipid contents, achieving the highest levels among the microalgae studied, with C. vulgaris at 12.5% and Scenedesmus spp. at 12.1%. In terms of fatty acid composition, Spirulina platensis and Phaeodactylum tricornutum were rich in saturated fatty acids, particularly palmitic acid, while Porphyridium cruentum was characterized by high monounsaturated fatty acid content. Conversely, Scenedesmus spp. and C. vulgaris had elevated levels of polyunsaturated fatty acids, which highlights their diverse macromolecular profiles [98].
Different algae species exhibit varying growth rates and biomass accumulation capacities. Selecting an appropriate species with a high growth rate and strong biomass accumulation potential is crucial. For instance, C. vulgaris is known for its high biomass productivity and ability to accumulate lipids, especially under nitrogen deprivation conditions [98]. Furthermore, combining nitrogen deficiency with carbon enrichment in heterotrophic cultures can be an effective strategy to increase biomass and lipid accumulation. While traditional autotrophic cultivation relies on sunlight and CO2 for algae growth, heterotrophic cultivation uses organic carbon sources in the absence of light. This method can significantly boost biomass production by achieving higher cell densities and faster growth rates in a shorter time. Heterotrophic cultivation also allows greater flexibility as it can be performed in conventional fermenters, reducing the need for expensive photobioreactors, lowering production costs, and removing the dependence on sunlight, enabling continuous production regardless of weather or seasonal change [99].
In contrast to lipid-producing bacteria, microalgae are much more explored in that sense. This is due to the versatility of microalgal lipids, which opens up multiple routes for sustainable applications across various industries. They may be converted into fatty acid methyl esters (FAME) for use in biofuels; used in dietary supplements and functional foods, providing an alternative to fish oils; utilized in the pharmaceutical industry for drug development or as ingredients in cosmetic products due to their skin-nourishing properties; and used as raw material for producing bioplastics and other biochemicals [100]. Microalgae lipid is, hence, a focal point in efforts to promote renewable resources and reduce environmental impact.
Lipid production in microalgae is favored under stress conditions, in which TAG is accumulated as an energy storage compound [101]. One of the most powerful triggers for lipid accumulation in microalgae is nutrient limitation, especially of nitrogen and phosphorus. This scarcity compels the organisms to adapt their metabolism to conserve energy and resources, mirroring the responses observed in bacteria [102]. Moreover, extreme temperatures can also act as stressors. For instance, temperatures above the optimal growth range may lead to cell damage and increased lipid production as a survival strategy, while low temperatures may enhance unsaturated fatty acid production [103]. Additionally, protective mechanisms that result in lipid accumulation can be triggered by excessive lighting, salinity stress, pH variations, and chemical exposure [104]. Understanding how microalgae respond to these stresses allows for the optimization of cultivation strategies that maximize lipid accumulation while avoiding compromising the growth and viability of the algae.
The study of Morowvat and Ghasemi investigates the heterotrophic cultivation of the microalga C. vulgaris using glucose as a carbon source in shake flasks and stirred-tank bioreactors as an alternative to traditional autotrophic cultivation, with the aim of improving biomass and lipid production for biodiesel. It was found that cell growth was significantly higher in the heterotrophic culture, particularly with increased glucose concentration in the medium. The biomass productivity was 3.68 times greater than in autotrophic conditions, with total lipid content reaching 44% of dry cell weight after 21 days. In bioreactors, heterotrophic cultivation further improved results, achieving a biomass concentration of 4.95 g/L and a total lipid content of 2.18 g/L after just 5 days. These findings demonstrate the potential of heterotrophic cultivation to enhance biomass and lipid production. However, the authors recognize that the use of glucose as a carbon source may not be economically feasible and therefore suggest the employment of alternative and cheaper carbon sources [99].
In that context, heterotrophic cultivations may be leveraged to utilize waste materials as carbon sources, offering a more sustainable pathway for producing microalgal lipids. In a more synergistic approach, heterotrophic and autotrophic cultivations can be combined in what is called mixotrophic cultivation. By leveraging the benefits of both light-driven and organic carbon utilization, mixotrophy enhances growth rates and overall productivity [105]. This makes it an attractive option for large-scale microalgal biomass production, especially in applications aimed at biofuel generation and other biotechnological processes.
Hang et al. explored the benefits of mixotrophic cultivation of Chlamydomonas reinhardtii using treated wastewater (nitrogen and phosphorous source) supplemented with either acetate, fructose, glucose, molasses, sucrose, or corn steep liquor (CSL) (carbon source) as low-cost culture media to enhance biomass and lipid production for biodiesel. The addition of organic carbon sources significantly increased biomass and lipid production compared to purely phototrophic conditions, with the exception of glucose and sucrose. Molasses provided the highest growth rate, followed by acetate and CSL, while acetate resulted in the highest lipid content, followed by molasses and CSL. The findings suggest that mixotrophic cultivation not only provides a sustainable and economical alternative to traditional methods but also leverages organic waste as a nutrient source, positioning C. reinhardtii as a viable candidate for biodiesel production [106].
Similarly, Jasimone et al. investigated the dual objectives of lipid production and wastewater treatment using microalgae cultivated in urban wastewater. The study examines how varying light intensities and nutrient concentrations affect the biomass growth rate of microalgae, their efficiency in removing nutrients like nitrogen and phosphorus, their ability to self-flocculate for sedimentation, and their lipid accumulation. This approach addresses two critical issues: reducing pollution from wastewater and providing a sustainable source of biomass for energy production. Results showed that higher light intensity enhanced biomass growth, while lower light levels improved self-flocculation efficiency. Increased nutrient concentrations improved biomass production but decreased flocculation efficiency, with optimal lipid accumulation occurring at low initial nutrient levels. Overall, the findings highlight the significant impact of light and nutrient management on achieving effective wastewater treatment alongside maximizing lipid yields in microalgae cultivation [107].
In Table 1, the main characteristics of the three types of cultivation discussed are highlighted.

Table 1.
Cultivation of microalgae by trophic mode employed.
Regarding autotrophic cultivation, light intensity and duration are essential as they drive photosynthesis. The optimal light and dark photoperiods for maximizing microalgae biomass growth can vary depending on the species of microalgae and the specific cultivation conditions. However, general trends and recommendations can be outlined based on research findings. A light/dark cycle of 18 h light to 6 h dark (18:6) is often cited as optimal for maximizing biomass production in several microalgae species, including C. vulgaris and Desmodesmus subspicatus) [108,109]. This cycle allows for metabolic processes that promote lipid synthesis. In the absence of light, microalgae harness their stored carbohydrates to produce lipids, especially when nutrients are scarce. This metabolic transformation is crucial for optimizing lipid accumulation within the cells.
The effectiveness of different wavelengths in combination with photoperiods in biomass growth has also been explored. For instance, using mixed wavelengths (e.g., blue and green light) for specific durations can enhance growth performance, indicating that not just duration but also the quality of light matters [110]. Moreover, it has been shown that different microalgal species exhibit varying responses to light intensity.
Ho et al. investigated the optimization of lipid production in a newly isolated marine microalga, Chlamydomonas sp. JSC4, focusing on light intensity and nitrogen depletion as key factors. Through experiments with different light intensities, the authors found that the specific growth rate of Chlamydomonas sp. JSC4 increased with light intensity up to a saturation point. Beyond this intensity, growth was inhibited, suggesting a delicate balance between light supply and photoinhibition. However, in order to promote lipid accumulation, optimized light intensity had to be combined with nitrogen depletion. The optimal combination of high light intensity (300 μmol m−2 s−1) for robust growth and nitrogen depletion for lipid accumulation resulted in a maximum lipid productivity of 328 mg L−1 d−1 and a lipid content of 34.1%. Further studies performed indicated that sufficient light supply promotes the formation of essential precursors for TAG synthesis, leading to increased lipid accumulation [111].
Another important factor that drives photosynthesis is the presence of CO2. Higher concentrations of CO2 generally enhance microalgae growth and biomass production by providing more inorganic carbon for photosynthesis. Numerous studies have shown that CO2 supplementation can significantly boost both biomass and lipid productivity, with optimal concentrations usually falling between 2% and 6% [112,113,114]. For instance, Omar Faruque et al. found the highest lipid content (31.6%) at 6% CO2 in Scenedesmus dimorphus, while Chen et al. found 6% CO2 to be optimal for Nannochloropsis salina [115,116].
Nevertheless, excessive CO2 levels can negatively impact microalgae growth, leading to growth inhibition or even cell death. This is often due to factors such as medium acidification, which lowers pH and disrupts optimal growth conditions, enzymatic activity alterations that affect essential metabolic pathways like photosynthesis, and nutrient imbalances due to over-prioritization of carbon fixation [117]. To optimize CO2 uptake, strategies such as optimizing CO2 concentrations and improved aeration methods are key to maximizing biomass and lipid yields in microalgae cultivation systems enriched with CO2. For instance, by employing sequential bioreactors or special aerators that generate smaller bubbles, the dissolution and utilization of CO2 can significantly increase [118].
From a sustainable perspective, CO2 supply can be combined with flue gas treatment, an industrial waste stream. Flue gas CO2 has shown promise as a sustainable carbon source for microalgal cultivation, offering both environmental and economic benefits, as studies have demonstrated that various microalgae species can effectively utilize flue gas CO2 for growth and biomass production [119,120]. The capture and utilization of CO2 from flue gases emitted by thermal power plants and other industries present a promising opportunity to reduce greenhouse gas emissions while providing a sustainable carbon source for microalgae cultivation. However, the high concentrations of CO2 and other potentially toxic compounds in flue gases can inhibit the growth of some microalgae species.
Paylou et al. conducted a study focused on identifying and evaluating CO2-tolerant microalgae species for use in 3G biorefineries by analyzing biomass growth and biomolecule accumulation, including lipids. The authors carried out cultivation experiments using 13 microalgae species from Greek collections in flasks and bioreactors to assess their tolerance to 10% CO2, representing the upper limit found in flue gas mixtures. The strain Stichococcus sp. Wild-TUC was identified as a promising candidate for 3G biorefineries due to its high CO2 tolerance, rapid growth, and high carbohydrate and protein productivity. However, the authors noted that the biomass yields were lower than those reported in the literature for lower CO2 concentrations, highlighting the importance of optimizing cultivation conditions to maximize biomass production under high CO2 levels [121].
In such a context, it becomes clear that understanding the intricate balance of light intensity, CO2 concentration, and nutrient management is crucial for maximizing microalgal growth and lipid accumulation. As research continues to identify effective species and optimize cultivation techniques, microalgae are poised to play a vital role in promoting renewable resources and reducing the environmental impact of industrial processes, making them a key player in the transition toward a more sustainable future. Table 2 below presents a comparative analysis of the production of each type of microorganism.

Table 2.
Production of biomass and lipids and lipid profiles of key oil-producing microbial species.
The analysis presented in Table 2 demonstrates that oleaginous fungi and yeasts have a superior performance in the production of biomass and lipids compared to bacteria and microalgae. This superiority can be attributed to the intrinsic capacity of these microorganisms to metabolize a wide range of substrates, resulting in high yields of both biomass and lipids. The main fatty acids synthesized by these microorganisms include palmitic acid (C16:0), oleic acid (C18:1), and linoleic acid (C18:2). These fatty acids are widely valued in several commercial applications. Palmitic acid is frequently used in the food, cosmetic, and pharmaceutical industries due to its emulsifying and stabilizing properties. Oleic acid, in turn, is a fundamental component of olive oil, known for its benefits to cardiovascular health, in addition to being used in cosmetics and cleaning products. Linoleic acid, classified as an essential fatty acid, is crucial for human nutrition and also has applications in the formulation of food and cosmetic products [136].
In addition, some microorganisms have the ability to synthesize less common fatty acids, such as α-linoleic acid (C18:3), γ-linoleic acid (C18:3), hexadecadienoic acid (C16:2), hexadecatrienoic acid (C16:3) and myristic acid (C14:0). α-linoleic acid, for example, is an essential fatty acid recognized for its anti-inflammatory properties, arousing considerable interest in nutrition and disease prevention [90]. γ-linoleic acid is often used in dietary supplements and can help manage conditions such as arthritis [137]. Hexadecadienoic and hexadecatrienoic acids have potential applications in the biofuel industry, given their chemical structure that favors more efficient conversion into biodiesel [138]. Myristic acid, in turn, is used in the cosmetics industry and is present in several personal care products [139].
The lipid production by fungi and yeasts not only showcases remarkable yield advantages but also highlights their substantial commercial and industrial potential. The diversification of applications of the fatty acids produced emphasizes the importance of continuing research and optimization in fermentation processes involving these microorganisms.
3. Microbial Lipids Production
3.1. Impact of Microbial Lipids
The production of microbial lipids offers several advantages over traditional sources such as vegetable oils and animal fats, particularly in terms of production efficiency and environmental impact. Table 3 summarizes these comparisons, highlighting key factors such as production efficiency, environmental impact, raw material use, and cost-effectiveness. In terms of efficiency, microbial lipids have shorter production cycles, ranging from days to weeks, whereas traditional sources depend on longer, seasonal cycles, making microbial lipid production more stable and less susceptible to climatic variations.
Moreover, microbial lipid production requires significantly fewer natural resources, such as land and water, than traditional sources. Microorganisms like algae can thrive in saline or wastewater environments, reducing the demand for freshwater resources in water-scarce regions. This capability is particularly beneficial in areas where freshwater use needs to be minimized [140]. Another important advantage is that microorganisms can be cultivated on non-arable land, avoiding competition with food crops, which allows for higher-density cultivation without the need for fertile soil [141].
A distinctive feature of microbial lipids is their ability to utilize non-edible by-products, such as agricultural waste or glycerol, as feedstocks. This reduces competition with food production and contributes to a circular bioeconomy [142]. Additionally, microbial lipid production has the potential to lower the carbon footprint associated with fuel production by using CO2 from industrial emissions or atmospheric sources. Microorganisms such as algae and bacteria can convert carbon into lipids through photosynthesis or fermentation processes, sequestering carbon and helping to mitigate greenhouse gas emissions [143]. When integrated with existing industrial processes, microbial lipid production can result in a net reduction in emissions, contributing to a more positive environmental impact.
Furthermore, microbial lipid production can effectively promote waste valorization by transforming organic waste materials, such as food processing and agricultural residues, into valuable lipids. This not only reduces landfill usage but also contributes to efficient waste management and lowers the environmental impact associated with waste disposal [140].
While microbial lipids offer numerous benefits, they still face challenges related to cost-effectiveness and scalability. Traditional lipid production benefits from established infrastructure and economies of scale, making it more financially advantageous at present. However, with advancements in fermentation technologies and the reduction in costs associated with bioreactors and feedstock optimization, microbial lipids are expected to become increasingly competitive. Although scalability remains a challenge, ongoing technological advancements hold the potential for microbial lipid production to become a viable and sustainable alternative to traditional lipid sources in the future.

Table 3.
Comparison between microbial lipids and traditional lipid sources.
Table 3.
Comparison between microbial lipids and traditional lipid sources.
| Aspect | Traditional Lipids | Microbial Lipids | Ref. |
|---|---|---|---|
| Production Efficiency | Seasonal and climate-dependent. | Independent of seasons or climate | [144] |
| Long production cycles (e.g., months to years). | Short production cycles (e.g., days to weeks). | ||
| Environmental impact | High land and water usage for crops or livestock. | Minimal land use; can utilize non-arable areas. | [145,146] |
| Associated with deforestation and biodiversity loss. | Lower ecological footprint; utilizes waste substrates. | ||
| Raw material | Edible feedstocks (e.g., soy, palm, animals). | Can use waste or non-edible feedstocks (e.g., glycerol, lignocellulose). | [144] |
| Cost-effectiveness | Relatively lower cost for established production methods. | Currently higher production costs due to scalability and process optimization challenges. | [147] |
| Vulnerable to market fluctuations (e.g., crop yields, feed costs). | Potential for cost reduction with technological advances. | [148] | |
| Scalability | Well-established and large-scale industrial infrastructure. | Emerging technology; scalability still a challenge. | [100] |
| Limitations | Environmental degradation and limited resource availability. | Requires further development to achieve economic parity. | [148,149] |
| Ethical concerns with animal fat production. | Public acceptance of microbial-derived products. | [6] |
3.2. Integrated Metabolic Pathway
The production of microbial lipids involves a series of interconnected metabolic pathways, where primary metabolites, such as glucose or acetate, are converted into energy reserves and structural lipids. A central point in this process is the synthesis of acetyl-CoA, which serves as a precursor for various lipid biosynthesis routes. The production of acetyl-CoA can occur through different pathways depending on the microorganism type and environmental conditions (Figure 1A and Figure 2A,H). During glucose metabolism, pyruvate decarboxylation is essential: glucose is initially converted into pyruvate, which is subsequently transformed into acetyl-CoA. In eukaryotic organisms, such as yeasts, fungi, and microalgae, this process takes place in the mitochondria; however, the acetyl-CoA required for lipid biosynthesis is exported to the cytosol through specific transporters. In bacteria, the conversion occurs in the cytoplasm, facilitating the direct utilization of acetyl-CoA for lipid synthesis [150].
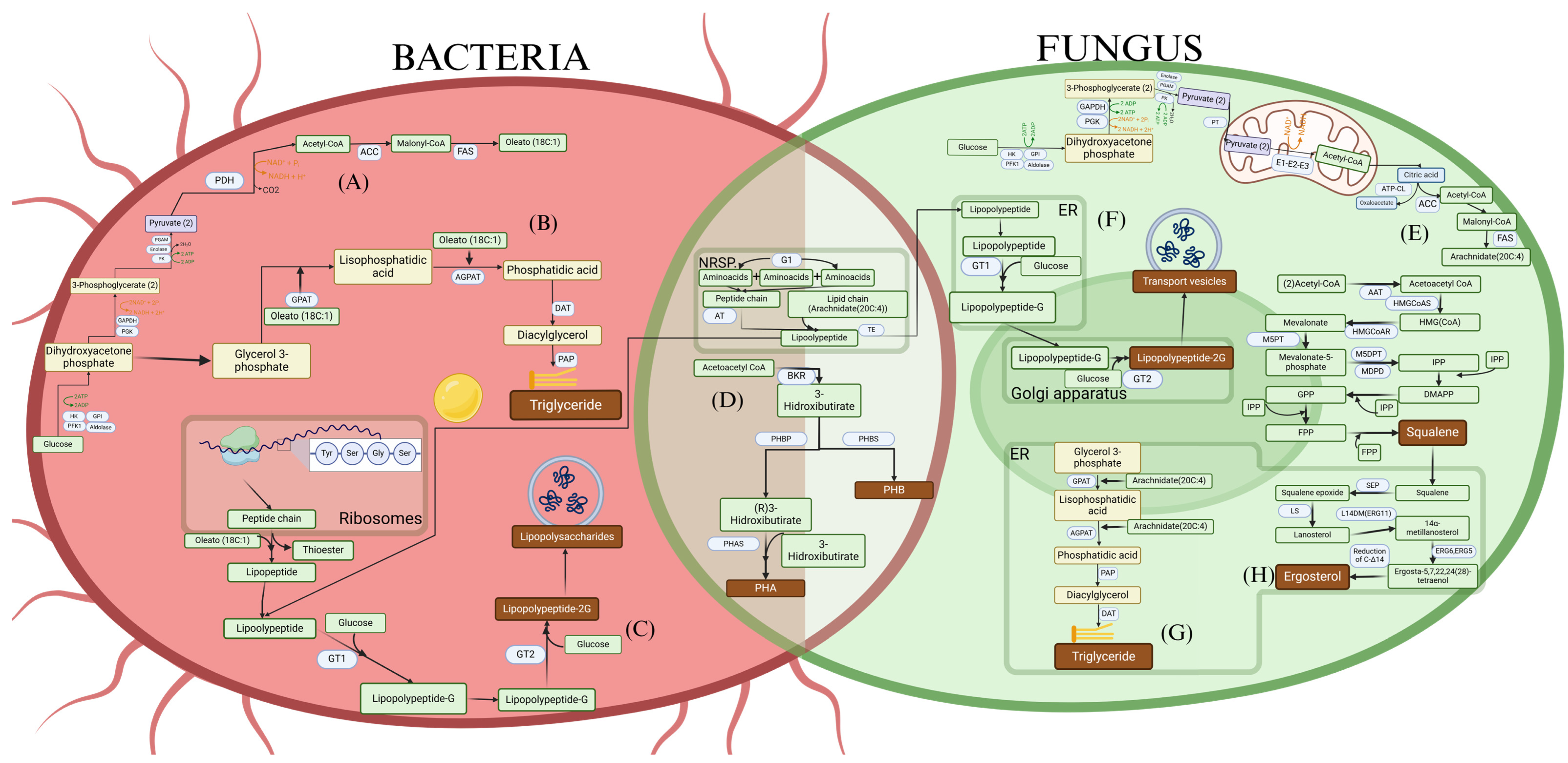
Figure 1.
Metabolic pathways for (A) Production of acetyl-CoA and fatty acids from glucose in bacteria. (B) Production of triglycerides in bacteria. (C) Production of lipopeptides in bacteria from ribosomes and NRPS (non-ribosomal peptide synthetases). (D) Production of polyhydroxyalkanoates (PHA) and polyhydroxybutyrates (PHB) from acetyl-CoA in bacteria and fungi. (E) Production of acetyl-CoA and fatty acids from glucose in fungi. (F) Production of lipopeptides in fungi from NRPS. (G) Production of triglycerides in fungi. (H) Production of squalene and ergosterol from acetyl-CoA in fungi.
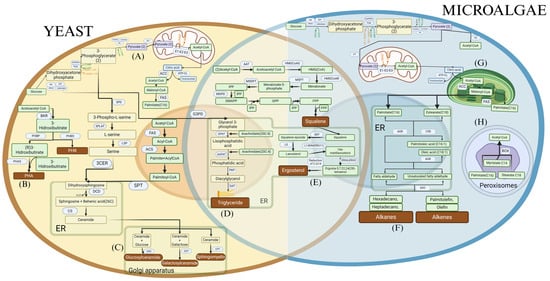
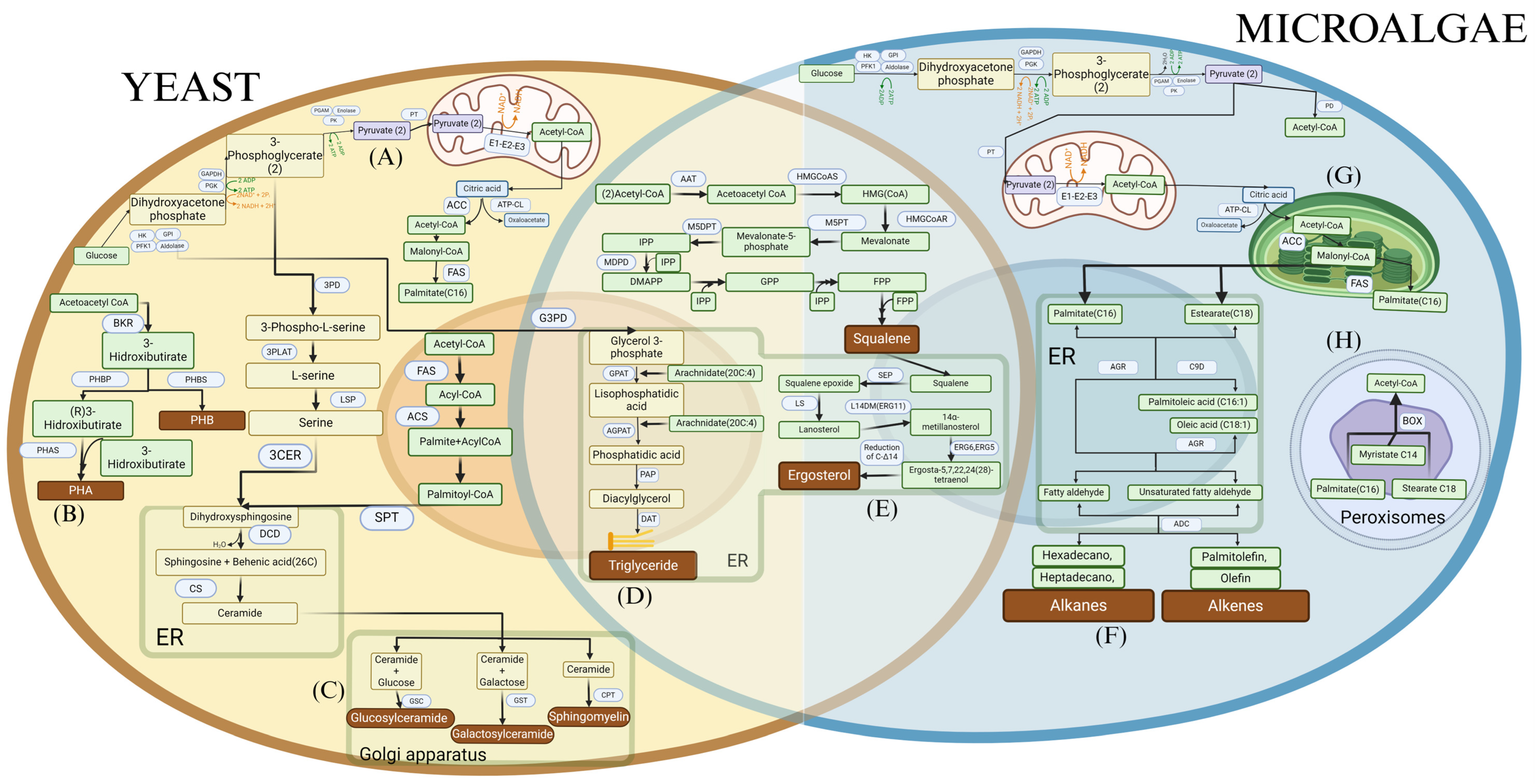
Figure 2.
Metabolic pathways for (A) Production of acetyl-CoA and fatty acids from glucose in yeasts. (B) Production of polyhydroxyalkanoates (PHA) and polyhydroxybutyrates (PHB) from acetyl-CoA. (C) Production of sphingolipids in yeasts. (D) Production of triglycerides in yeasts and microalgae. (E) Production of squalene and ergosterol in yeasts and microalgae. (F) Production of hydrocarbons in yeasts. (G) Production of acetyl-CoA and fatty acids in microalgae. (H) Production of acetyl-CoA in microalgae through peroxisomal degradation.
Fatty acid synthesis is another key process for lipid production, where acetyl-CoA is incorporated into elongation cycles catalyzed by the fatty acid synthase (FAS) complex (Figure 1E and Figure 2G). Each cycle adds two carbon units, generating saturated and unsaturated fatty acids that can be incorporated into triglycerides, phospholipids, and other lipid compounds. Conditions favoring this route include the availability of carbon-rich sources and nitrogen limitation, as nitrogen restriction activates anabolic pathways and promotes the accumulation of storage lipids. Oleaginous microorganisms, such as Y. lipolytica and R. toruloides, exhibit a high capacity to store lipids under such conditions, reaching up to 70% of their dry weight in lipids [151].
In addition to fatty acids, other metabolic products, such as squalene, ergosterol, and polyhydroxyalkanoates (PHAs), are synthesized from acetyl-CoA-derived precursors. Squalene and ergosterol, for instance, are products of the mevalonate pathway and are involved in the production of sterols and isoprenoid compounds, essential for cell membrane structure (Figure 1H and Figure 2E) [152,153]. Maximizing the production of these compounds requires an optimal supply of acetate or glucose, along with the fine-tuning of pH and temperature. PHAs, which serve as carbon reserves in bacteria, accumulate primarily under conditions of carbon excess and nutrient limitation (such as nitrogen or phosphorus), enabling the synthesis of these biopolymers with industrial applications in bioplastics production [154] (Figure 1D and Figure 2B).
The accumulation of triglycerides primarily occurs through the esterification of fatty acids with glycerol-3-phosphate, forming triacylglycerols that are stored in lipid bodies [155]. The efficiency of this process depends not only on enzymatic activity but also on the cell’s energy balance. Through fermentative processes, such as fed-batch, it is possible to maximize both biomass production and lipid accumulation, particularly under nitrogen-limited conditions and high carbon concentrations. The use of agro-industrial residues, such as residual glycerol, is being explored as a sustainable strategy to enhance economic feasibility and reduce production costs. Due to their ability to store triglycerides, yeasts are considered prime candidates for biofuel production [156]. Many fungi are used to produce omega-3 fatty acids and other high-value oils thanks to their ability to store unsaturated fatty acids (Figure 1G) [157]. Microalgae, on the other hand, are known for their ability to store triglycerides under adverse conditions, specifically when nutrients like nitrogen are scarce, allowing them to survive unfavorable conditions (Figure 2D). In bacteria, unlike eukaryotic cells, triglycerides are not as abundant (Figure 1B) [158]. Only oleaginous bacteria can accumulate oils in their cellular structures, where they can synthesize and store triglycerides as carbon and energy reserves, such as Rhodococcus and Acinetobacter [159].
3.3. Conditions and Optimization Processes
Optimal parameters for microbial lipid production can significantly enhance the yield and efficiency of the process. Inoculum concentration directly affects microbial growth rate. A very low inoculum can result in a long lag phase, delaying the onset of biomass and lipid production. On the other hand, an excessively high inoculum can lead to rapid nutrient depletion, limiting growth and lipid production [125,160]. The ideal inoculum concentration for Rhodotorula kratochvilovae was determined to be 10% (v/v), which resulted in a maximum dry biomass of 14.28 g/L and a lipid yield of 5.32 g/L. Lower inoculum concentrations led to longer lag phases, while concentrations above 10% resulted in a decrease in lipid yield [160].
Chemical factors, such as a high C/N ratio (typically above 100) [161], are essential to stimulate lipid production, as nitrogen limitation combined with excess carbon triggers the accumulation of TAGs as energy reserves. R. toruloides was able to accumulate up to 60% intracellular lipids when cultivated on xylose with a C/N ratio of 120 [162]. In addition, the choice of carbon source can significantly impact lipid production. Different carbon sources influence microbial metabolic pathways and lipid synthesis rates. Simple sugars, like glucose and sucrose, are more easily metabolized than complex carbohydrates, such as starch. The carbon source can significantly affect lipid production, with glycerol yielding higher lipid levels than sugars like xylose and glucose. Glycerol, for example, can produce up to 69% lipid yield from consumed carbon, making it one of the most effective substrates for lipid synthesis [74]. Sucrose has proven to be a superior carbon source compared to glucose and xylose for lipid production in R. toruloides [163].
The nitrogen source plays a key role in microbial lipid production by affecting growth dynamics and lipid accumulation. Inorganic nitrogen sources, such as sodium nitrate (NaNO3), are more effective in enhancing lipid production than organic sources like urea or ammonium sulfate. For example, Crypthecodinium cohnii produced 26.9% lipid content with NaNO3 [164]. The optimal carbon-to-nitrogen (C/N) ratio is crucial, with ratios between 30 and 120 being effective for R. toruloides, leading to increased lipid accumulation at higher ratios. Nitrogen limitation, such as high C/N ratios, can induce metabolic pathways that enhance lipid production, as seen in Y. lipolytica. Common nitrogen sources like ammonium sulfate and ammonium nitrate support high lipid yields, while urea has emerged as a sustainable alternative with similar results. Sodium nitrate, ammonium nitrate, ammonium sulfate, and urea are all considered optimal for boosting microbial lipid production [165].
The pH of the cultivation medium is a critical factor for the optimal performance of lipid-producing strains. pH variations can trigger undesirable responses and reduce process yields. It is important to note that the optimal pH range may vary depending on the strain, but it is generally between four and six. For Saitozyma podzolica DSM 27192, a pH of four was ideal for lipid production, while a pH of six resulted in a 50% reduction in lipid yield [161].
Physical and environmental factors also impact lipid production. Cultivation temperature, like pH, can affect strain performance and may even inactivate it. Assessing the microorganism’s tolerance range and its optimal growth temperature can lead to higher yields. Aeration, another key factor, is essential for the growth and metabolism of many oleaginous microorganisms. Optimizing the airflow rate in an airlift bioreactor enhanced lipid production by Candida tropicalis ASY2. Proper agitation ensures the homogeneity of the culture medium, nutrient availability, and oxygen transfer, influencing both growth and lipid production [166].
Process optimization can benefit from various tools that assist in experimental design and result prediction. Response surface methodology (RSM) is a powerful statistical technique used to simultaneously optimize multiple parameters. In the study by Thangavelu et al., RSM was employed to determine the optimal conditions for starch content, yeast extract, and airflow rate for lipid production in a bioreactor. This approach also enabled the optimization of the biomass-to-methanol ratio, catalyst concentration, and reaction time to maximize FAME yield in the transesterification process [166]. Similarly, mathematical modeling can be used to predict lipid yield and optimize cultivation conditions. In a study with Aspergillus wentii Ras101, the LINGO optimization program was applied to predict maximum lipid production by considering process variables [167]. Genetic modification of oleaginous microorganisms is another optimization strategy, capable of enhancing lipid production and altering fatty acid composition to improve biodiesel properties [142].
4. Lipids Extraction Process
Most lipids produced by oleaginous microorganisms consist of short- and long-chain fatty acids, ranging from 6 to 36 carbon atoms, and can be classified as saturated (SUFA), monounsaturated (MUFA), or PUFA, depending on the number of double bonds in the carbon chain [25,168,169]. Yeasts, microalgae, filamentous fungi, and bacteria can produce oils rich in SUFA and MUFA, which have strong applicability in biofuel production. Others, such as microalgae and thraustochytrids, are excellent sources of PUFA, with applications in nutraceutical production [25].
The extraction of microbial lipids is a vital process that enables the accessibility of intracellular lipids, paving the way for their recovery for various applications. Nonetheless, achieving lipid recovery on an industrial scale poses significant challenges. This difficulty arises from the fact that lipids are not stored in uniform compartments and possess varying polarities; some are polar, such as phospholipids, while others are nonpolar, like triacylglycerols [25].
Lipids can be synthesized either intracellularly or extracellularly, which affects the type of extraction method and the complexity of the process. The extraction of extracellular lipids can be easily performed using physical processes, such as centrifugation or filtration, while intracellular lipids require a pre-treatment step (Figure 3) [170].
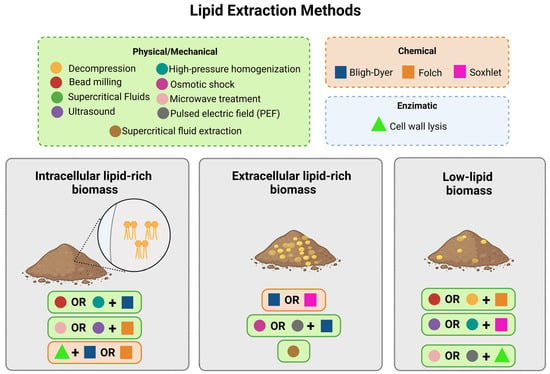
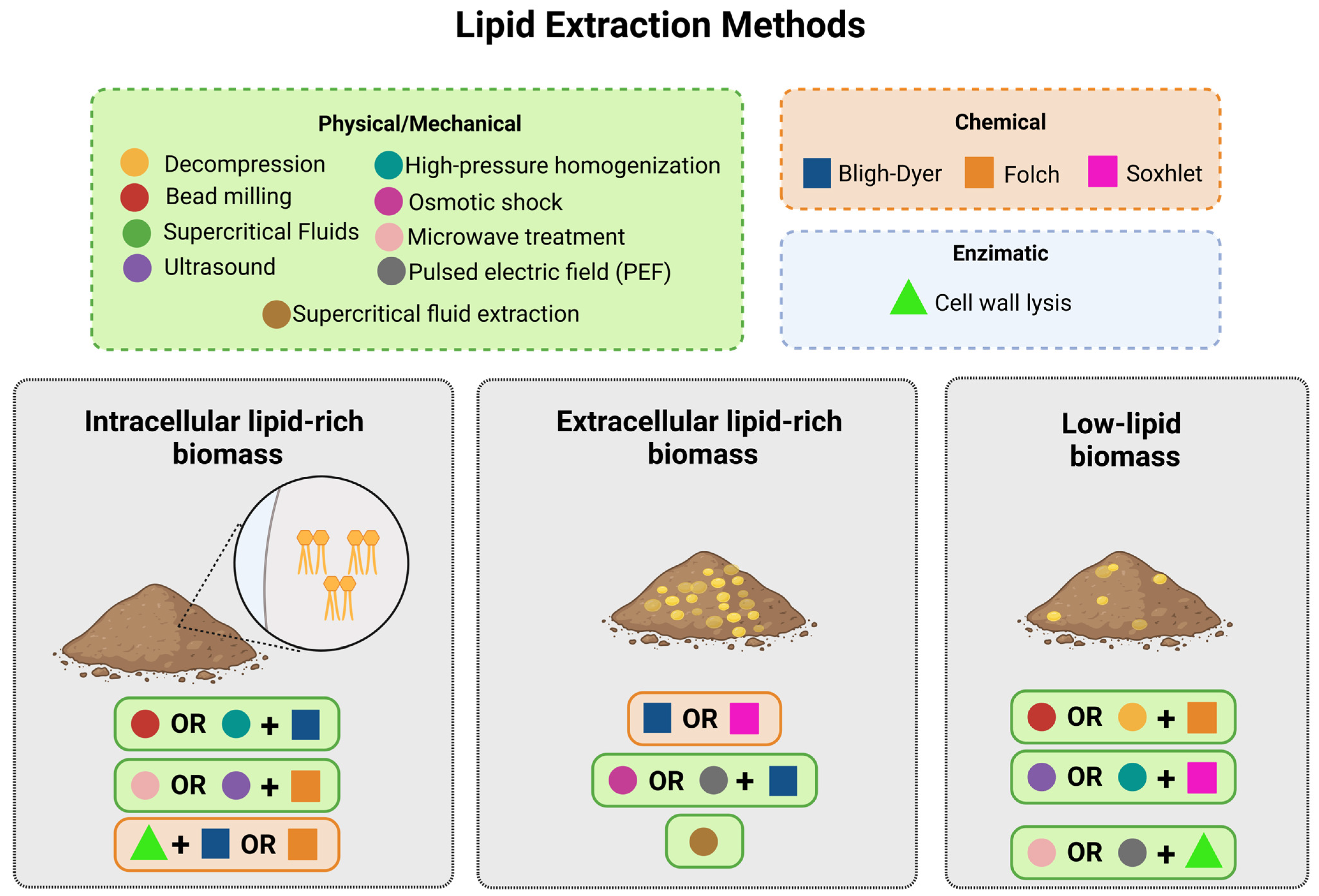
Figure 3.
The figure presents combinations of lipid extraction techniques for different types of biomasses, adjusted according to the location of the lipids. For intracellular lipid-rich biomass, it is essential to disrupt the cells by combining mechanical methods, such as bead milling or high-pressure homogenization, with or without chemical methods like Bligh–Dyer or Folch, or by applying enzymatic lysis followed by chemical extraction. In extracellular lipid-rich biomass, chemical techniques like Soxhlet or Bligh–Dyer are sufficient but can be enhanced with physical processes such as osmotic shock or supercritical fluids. For low-lipid biomass, more intensive combinations are required: mechanical methods (ultrasound or microwave treatment) assist in cell disruption, followed by chemical techniques such as Soxhlet to ensure maximum recovery of dispersed lipids.
One effective pre-treatment method for biomass to optimize mass transfer and enhance the efficiency of downstream processing of intracellular lipids involves disrupting or weakening the microbial cell wall [26]. This disruption can be achieved by two routes: using wet or dry biomass. The wet biomass route uses organic solvent extraction and is more advantageous than the dry biomass route due to reduced costs associated with lower energy demands. However, depending on the material, this disruption may be affected by lipid accessibility, mass transfer, and emulsion formation. The dry biomass route relies on physical damage to the microbial cell wall in the presence of a co-solvent [23,170].
Cell disruption represents one of the main obstacles to achieving the economic feasibility of microbial lipid production. Therefore, the choice of the optimal cell disruption and lipid extraction method depends on the structure of the oleaginous microorganism [25,171]. There are various methods available for lipid recovery at a laboratory scale, including chemical, physical, and enzymatic methods [171].
4.1. Chemical Process of Lipid Extraction
Organic solvent extraction is one of the most widely used chemical methods for extracting lipids from microbial sources, as it promotes cell disruption without requiring additional pre-treatment due to the affinity of lipids for organic solvents [172]. The efficiency of the solvent extraction process depends on the proper selection of the solvent, the solubility of lipids in the chosen solvent, and the ability to separate complex lipids from other macromolecules [173].
The Bligh–Dyer and Folch methods are the most commonly used techniques for microbial lipid extraction, utilizing methanol and chloroform as solvents, which have a high affinity for and solubility in polar lipids, resulting in high lipid recovery yields [172]. The Folch method is specifically designed for small sample sizes, whereas the Bligh–Dyer method is commonly utilized for extracting lipids from samples that are high in moisture and low in fat. Both techniques make use of a methanol-chloroform mixture for effective extraction [173]. In these methods, the dry biomass of the microorganism is homogenized or mixed with a chloroform and methanol solution in a 2:1:0.8 ratio and then filtered through a tube. The addition of a potassium chloride solution to this mixture promotes the distribution of lipids into two phases: an aqueous phase (top) and an organic phase (bottom), with chloroform containing the lipids due to the salting-out effect [25,174]. Phase separation can be carried out using a decantation funnel or centrifugation, and lipids are recovered by evaporating the chloroform. However, disadvantages include the high toxicity and health risks associated with the solvents, as well as the multiple steps involved, which complicate their use at both laboratory and industrial scales [173].
Martinez-Burgos et al. used soybean hulls rich in lignocellulosic residues as a carbon source for biolipid production by Lipomyces starkeyi LPB 53. These authors obtained 42.5% lipids from a maximum lyophilized cell biomass concentration of 26.5 g/L using a chloroform and methanol solution. The extracted oil was composed of oleic, palmitic, palmitoleic, linoleic, and stearic fatty acids [175].
Another widely utilized technique is the Soxhlet method, which uses hexane as its organic solvent. This method is applied to solid samples, which are subjected to several washing cycles with the solvent at high temperatures. It is often combined with mechanical pressing or expeller extraction methods, allowing for greater lipid extraction efficiency from the residual cake. However, large quantities of solvents are required, and lipids are recovered by distillation, achieving yields of up to 95%. Disadvantages include the long extraction time (~6 h), the potential for thermal degradation of unsaturated fatty acids, and the need for large volumes of solvent [176].
Due to the growing demand for extraction methods that utilize green technologies and solvents with minimal environmental impact, bio-solvents have emerged. Breil et al. demonstrated that a combination of ethyl acetate and ethanol in a 1.794:0.784 (w/w) ratio, using 0.08 g of wet sample, was more efficient for lipid extraction from the yeast Y. lipolytica IFP29. This was followed by the addition of water (containing 0.8% KCl) and ethyl acetate in a 1:2 (v/v) ratio to separate the aqueous and organic phases. These results suggest that the classic extraction system is as efficient as the alternative system in terms of lipid yield from microbial tissues, regardless of their apparent hydrophilicity [174].
4.2. Physical and Mechanical Process of Lipid Extraction
Physical and mechanical techniques for disrupting the cell wall in oleaginous microorganisms offer energy-efficient solutions but face several drawbacks, including limitations to small-scale applications and reduced overall efficiency. Among the methods employed are decompression, bead milling, high-pressure homogenization, osmotic shock, microwave treatment, ultrasound, pulsed electric fields, supercritical CO2 extraction, and drying [6].
Cell disruption by decompression involves mixing microbial cells with a pressurized supercritical gas, followed by a sudden release of pressure, which causes cell expansion and rupture due to changes in pressure. Various gases can be used, such as nitrogen or carbon dioxide. Carbon dioxide is highly efficient under optimized conditions of pressure (40 atm), temperature (40 °C), and time (3 h) in wet yeast cells. In contrast, nitrogen, although inert and pH-neutral, exhibits low efficiency in wet yeast cells due to its low solubility in water [6,177].
Among mechanical methods, bead milling is simple and highly efficient, making it suitable for yeasts, bacteria, algae, and filamentous fungi. This method disrupts microbial cells through the abrasive action of small glass or stainless-steel beads, releasing lipids. The ideal bead diameter is 0.5 mm for most microorganisms; however, for bacteria, smaller beads (0.1 mm) improve cell disruption efficiency. One significant advantage of this method is the straightforward removal of the beads through gravitational force, followed by lipid recovery via solvent extraction [178].
High-pressure homogenization employs pressures between 150 and 200 MPa to effectively disrupt cell structures. One of the primary benefits of this technique is its scalability and versatility for a wide range of microorganisms. However, it is important to note that filamentous fungi can pose a challenge, as their mycelial structure may lead to valve blockages. This method can increase lipid extraction yield by up to four times compared to solvent extraction. Furthermore, its combination with bead milling enhances lipid extraction while reducing energy consumption [178].
Osmotic shock exposes microbial cells to a high osmotic pressure medium using high concentrations of solutes, such as salts or sugars. Cell lysis occurs as water migrates into the cell, increasing internal pressure. However, microorganisms with rigid cell walls resist lysis and undergo only weakening. Additionally, the high cost of additives limits the application of this method on a small scale [179].
Microwave treatment has a high potential for cell disruption as electromagnetic waves (300 MHz to 300 GHz) interact with water molecules in cellular compartments, causing water to heat, expand, and increase internal pressure, leading to cell rupture. This method is advantageous due to its speed, safety, low cost, and industrial scalability without the need for pre-drying. However, the heat generated may degrade the quality of the extracted lipids, particularly unsaturated fatty acids [6,178].
Ultrasound is a method that accelerates the extraction process by interacting with the solvent and matrix through the periodic collapse of cavitation bubbles. This method involves cavitation effects that destroy cells and enhance lipid mass transfer. It is compatible with various materials, such as plants, microalgae, animals, and fungi, improving extraction efficiency and lipid quality [180].
The combination of extraction methods with bio-solvents, such as ethanol and ethyl acetate, can improve extraction yield, lipid purity, and composition, including fatty acid content in algae. However, the Folch method was more efficient for the extraction of polar lipids (glycolipids and phospholipids) and pigments (chlorophylls and carotenoids). Conversely, bio-solvents reduced SFAs and PUFAs [181].
In another study, the combination of hexane/isopropanol and ultrasound-assisted extraction for lipids from microalgae Nannochloropsis resulted in similar lipid, free fatty acid, and pigment extraction efficiencies compared to the control protocol using chloroform/methanol (1:1 v/v). The success of ultrasound-assisted extraction is largely attributed to the ultrasound-induced temperature increase. However, the scalability of this method remains challenging for industrial implementation [182].
Pulsed electric field (PEF) technology is an innovative, non-thermal extraction method that enhances industrial oil extraction through environmentally friendly processes. By improving mass transfer at lower temperatures and shorter extraction times, PEF significantly reduces energy and solvent usage. This technology works by applying brief, high-voltage electrical pulses that disrupt microbial cell membranes, increasing their permeability. As a result, PEF facilitates the extraction of intracellular compounds, including lipids, while also allowing for higher incorporation of health-promoting phytochemicals, such as antioxidants, thus enhancing oxidative stability [183,184].
PEF technology has shown great potential in increasing lipid recovery from microalgae Auxenochlorella protothecoides, utilizing anaerobic digestion compared to other physical methods like bead milling and freeze-drying. In small laboratory-scale experiments (using microalgae volumes around 5 mL), the PEF method achieved a lipid yield 16 times higher than bead-milled and freeze-dried methods, reaching 98.1% lipid yield compared to only 6.1% with the physical methods. However, when scaling up to larger volumes (around 300 mL of concentrated microalgae), the lipid extraction efficiency of PEF slightly decreases, with yields ranging from 59.5% to 78.7%. Despite this reduction, the PEF process remains highly efficient and environmentally advantageous, as it does not require biomass drying and uses minimal energy (approximately 1.5 MJ/kg of cell dry weight) [185].
Supercritical fluid extraction is a technique that uses a fluid over its critical point, and at this condition, the fluid diffuses in a similar way to gas and demonstrates solvent capacity close to that of organic liquids [186,187]). Carbon dioxide is an ideal supercritical fluid because of its environmentally benign, non-toxic, non-flammable, non-polluting, recoverable characteristics and its ability to solubilize lipophilic substances [188].
This method is considered an alternative method for lipid extraction from different materials, which allows using environmentally friendly processes and low consumption of potentially toxic solvents [186,188,189]. The advantages of this extraction method when compared to traditional extraction methods (hexane, petroleum ether, chloroform/ethanol) for lipids recovering from algae are higher selectivity, shorter extraction time, and the non-use of toxic organic solvents [190]. Additionally, this extraction process can be performed at low temperatures, enabling a gentle extraction of thermosensitive compounds and protection against oxidation [6].
One significant drawback of lipid extraction methods for microorganisms, such as yeast, is the need for sample pre-treatment. This step is crucial because traditional processes are often inadequate for effectively disrupting the cells. The challenge in extracting lipids from intact cells primarily stems from the rigidity of the yeast cell wall, which hinders lipid recovery through mere contact with supercritical CO2. Additionally, the high costs associated with the necessary equipment further complicate the extraction process [6,187,191].
A study compared five different cell wall disruption techniques with supercritical fluid extraction, combined with ultrasound pre-treatment (SFE + US), for lipid extraction from oleaginous yeast Candida sp. LEB-M3 cultivated in crude glycerol. The findings revealed significant differences in lipid recovery efficiency across the methods. Thermal hydrolysis in an autoclave at 121 °C for 15 min using 2 M HCl yielded the highest lipid recovery (155%), followed by shear abrasion with glass beads in a vortex for 120 min (125.32%) and acid hydrolysis with 5 M HCl (107.13%). On the other hand, methods with lower yields included supercritical CO2 extraction combined with prior cell disruption via ultrasound (20%), thermal hydrolysis in an autoclave with water (34.21%), ultrasound-assisted extraction after 45 min (32.75%), high-pressure homogenization and maceration with diatomaceous earth (47.56%), and freeze-thaw methods using an ultrafreezer (−80 °C) and liquid nitrogen, resulting in 39.57% and 36.90%, respectively. These results highlight the variability in lipid extraction efficiency depending on the method used, with thermal hydrolysis and shear abrasion outperforming other techniques in terms of lipid recovery [187].
4.3. Enzymatic Process of Lipid Extraction
The use of enzymes in lipid extraction promotes lysing of the rigid cell walls of oleaginous microorganisms, such as algae, while releasing intracellular components under mild conditions. This approach offers several advantages, including high biological specificity, energy efficiency, and minimal damage to intercellular compounds. However, this approach is more used for the treatment of substrates or by-products used for oleaginous microorganisms [175,192].
For instance, a study on enzymatic cell disruption followed by the application of an imposed electric potential for enhanced lipid extraction from wet algal biomass utilizing a photosynthetic microbial fuel cell showed a remarkable increase in lipid yield. Specifically, fungal enzymes effectively disrupted algal cells, resulting in the recovery of up to 79.0% of lipids after applying a potential of +3.0 V versus a standard hydrogen electrode, maintaining a pH of six and using a 3.5% (v/v) of fungi-extracted crude enzymes mixture over 16 h. This method exemplifies a successful alternative to traditional solvent-based extraction processes, highlighting the potential of enzymatic approaches in achieving high yields while minimizing environmental impact [193].
The comparison of lipid extraction methods reveals that chemical processes, such as solvent extraction, provide high efficiency but come with significant environmental and health risks due to the use of toxic solvents and high operational costs. Mechanical methods like bead milling and high-pressure homogenization offer a more sustainable alternative with the potential for scalability, although they often require considerable energy input.
In contrast, physical methods such as PEF and ultrasonication represent a shift towards greener technologies, with PEF demonstrating particularly high lipid yields and low energy consumption, making it a promising option for large-scale, environmentally friendly lipid extraction. The scalability of these innovative techniques is critical for industrial applications, as they not only enhance extraction efficiency but also reduce reliance on hazardous solvents and improve overall process sustainability. These advancements in physical and mechanical extraction methods underscore the importance of integrating sustainable practices in bioproduct production while minimizing environmental impact and aligning with the growing demand for sustainable production in the industry.
5. Bioproducts from Microbial Lipids
Microbial lipids have emerged as promising sources for sustainable bioproducts, meeting the growing demand for alternatives to petrochemical-derived materials and animal-based resources. These compounds exhibit high versatility, with applications spanning energy, food, cosmetics, and biodegradable materials [194]. Their production potential is significantly augmented by the capacity to utilize agro-industrial residues as substrates, resulting in high synthesis efficiency under controlled conditions. This aspect further cements their importance within the bioeconomy framework. Microbial lipids can produce a diverse array of bioproducts, encompassing biosurfactants, carotenoids, dietary supplements, biofuels, and various pharmaceutical and cosmetic products.
Biosurfactants are low molecular weight surface-active compounds with high industrial interest due to their chemical properties and stability in various environmental conditions. They are produced by different species of bacteria, yeasts, fungi, and archaea. They have the ability to act as foam producers, emulsifiers, and solubilizing agents, allowing the solubilization, dispersion, and desorption of priority environmental pollutants, such as petroleum derivatives, polycyclic aromatic hydrocarbons (PAHs), and heavy metals [195,196].
Biosurfactants have hydrophobic and hydrophilic parts, which together promote the formation of interfaces between fluids with different polarities. These compounds are generally produced by species of Pseudomonas, Bacillus, Candida, Rhodococcus, and Corynebacterium [197]. The specific structure of the lipids used in the production of biosurfactants varies according to the producing microorganism and the carbon source used in the fermentation process. For example, rhamnolipids, which are produced mainly by strains of Pseudomonas aeruginosa, are formed by rhamnose mono- and disaccharides linked to a β-hydroxy fatty acid molecule [198]. Sophorolipids, produced by yeasts of the genus Starmerella, have a cyclic or acyclic structure, with variations in the sophorose sugar and the length of the fatty acid [199].
Pi et al. treated offshore marine oil samples with different doses of biosurfactants, achieving a 73.94% removal efficiency with 15.00% crude oil, using a Pseudomonas consortium [200]. This result highlights how biosurfactants contribute to sustainability by providing biodegradable and eco-friendly alternatives to synthetic surfactants, driving the growth of the global biosurfactant market—valued at USD 3.13 billion in 2023—as industries increasingly adopt greener solutions [201].
Carotenoids are a diverse group of fat-soluble pigments that exhibit vibrant colors, ranging from yellow to orange to red. They are isoprenoid lipids formed by the linkage of 5-carbon isoprene molecules. Bacteria, yeast, filamentous fungi, and microalgae can synthesize carotenoids. The microalgae Dunaliella salina, for example, can accumulate more than 8% of its dry weight in β-carotene [202], while Staphylococcus gallinarum, a bacterium in the intestinal microflora of the silkworm Bombyx mori, produces staphyloxanthin, a carotenoid with antimicrobial, antioxidant, and anticancer properties [203]. Carotenoids have strong antioxidant activity, allowing the neutralization of free radicals and protecting cells against oxidative damage. In addition, they have anti-inflammatory activity [204].
Industrially, carotenoids are widely used as natural colorants in processed foods, beverages, dairy products, and bakery products. Astaxanthin is widely used to improve the pigmentation of meat and eggs. β-carotene, lutein, and zeaxanthin are often included in dietary supplements due to their health benefits. In the cosmeceutical industry, carotenoids are incorporated into products to protect the skin against damage caused by free radicals [205].
The advantage of producing carotenoids by microbial means instead of chemical synthesis and/or plant extraction is the possibility of using agro-industrial waste as substrates, making the process cheaper and more sustainable, contributing to the circular economy, in addition to allowing the optimization of production processes as a way to maximize yields [204]. This approach aligns with market growth trends, as the global carotenoids market size was valued at USD 1.48 billion in 2023 and is projected to grow at a CAGR of 3.5% from 2024 to 2030, driven by increasing demand for natural and sustainable solutions in various industries [206].
According to the International Grains Council (IGC) and reported by Germany’s Union for the Promotion of Oil and Protein Plants, by the end of 2024, the global production of biodiesel is estimated to hit 76.3 million tons [207]. However, most of the biodiesel produced still comes from vegetable oils. Lipids from microorganisms are a promising source for the production of biofuels, including biodiesel and biokerosene. Transesterification of microbial lipids can produce high-quality biodiesel, a sustainable alternative to fossil diesel, using the same principle applied to conventional vegetable oils [208].
Furthermore, biokerosene can be produced from various microorganisms, such as lipids produced by R. glutinis, which, through processes such as hydrogenation or pyrolysis, transform fatty acids into hydrocarbons that resemble conventional fuels. The use of lignocellulosic biomass as a raw material for biokerosene production reduces dependence on fossil fuels and contributes to the reduction in CO2 emissions, one of the main gases responsible for the greenhouse effect. In addition, biokerosene, after hydrotreatment, is a “drop-in” fuel, which means it is compatible with existing aviation infrastructure without the need for significant modifications to aircraft or fueling systems [209].
Microbial lipids can be converted into various bioproducts suitable for dietary supplements, leveraging their rich nutritional profiles. The PUFAs are polyunsaturated fatty acids and may belong to the omega-3 family or omega-6 family. Both omega-3 and omega-6 fatty acids are essential for human health, but mammals cannot synthesize them, so they must be obtained through diet. The omega-3 family includes a-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), while the omega-6 family includes γ-glinolenic acid (GLA), linoleic acid (LA), arachidonic acid (ARA), and conjugated linolenic acid (CLA) [210]. Omega-3 fatty acids are known for their health benefits, such as protecting against cardiovascular diseases and promoting proper neural and visual function [211]. On the other hand, omega-6 fatty acids play roles in infant formulas and in the treatment of conditions like atopic eczema, rheumatoid arthritis, multiple sclerosis, and premenstrual syndrome [212]. Additionally, compounds like ARA are direct precursors of various eicosanoids, which regulate lipoprotein metabolism, blood fluidity, leukocyte function, and platelet activation [210].
Known as eco-friendly or bioplastics, PHAs and PHBs are the more promising candidates to substitute traditional petrochemical plastics. They are biodegradable, biocompatible and versatile [213]. Although PHA and PHB are not lipids, their synthesis is related to lipid metabolism in certain microorganisms. The production of these polymers can occur from lipid sources or carbon-rich compounds, such as fatty acids and vegetable oils. Because they are biodegradable, they are used in the manufacture of bioplastics for packaging, medical devices, and disposable products [214]. There are several types of PHA, depending on the monomers that make up their structure, such as PHB, PHV (polyhydroxyvalerate) and PHBV (copolymer of 3-hydroxybutyrate and 3-hydroxyvalerate). The incorporation of different monomers can modify the mechanical properties of the polymer, making it more flexible or more rigid depending on the application [215]. PHAs and PHBs are promising for integrating a circular economy model since they can be biotically recycled (through microorganisms) and reintegrated into new production cycles.
6. Challenges for Industrial Production Scaling Up
The scale-up of microbial biomass and lipid production for industrial applications faces several intrinsic challenges in production and extraction processes. Each stage presents unique hurdles that can impact efficiency, yield, and overall feasibility. In the production stage, substrate choice plays a critical role in determining the cost-effectiveness and sustainability of the process [216]. Commonly utilized substrates include glucose and other simple sugars, which are readily metabolized by microorganisms for biomass and lipid production [217]. However, reliance on pure substrates is economically burdensome and raises concerns about competition with food production. To address this, the optimization of microbial strains through genetic engineering can improve strain performance, such as enhancing lipid production or increasing the efficiency of waste substrate utilization, reducing the dependency on food-grade substrates [218].
As a solution, waste materials such as agricultural residues or by-products from food processing provide a cost-effective and environmentally sustainable alternative, as they often contain organic compounds suitable as carbon sources for microbial growth. Nevertheless, the inherent heterogeneity of industrial waste streams results in variability in nutrient composition, complicating process optimization and scalability [148]. To address these challenges, strategies such as rigorous characterization of raw materials, pre-treatment to remove inhibitors and standardize composition, blending residues to balance nutrient profiles, and real-time monitoring for dynamic nutrient supplementation are essential [219]. Additionally, the use of flexible production systems and resilient microbial strains can mitigate inconsistencies, enhance process reliability, and enable efficient scale-up while minimizing costs and environmental impact.
In addition to the carbon source, fermentation parameters such as pH, temperature, and aeration must be carefully optimized for each microorganism to maximize both growth and lipid synthesis [220]. Deviations from these optimal conditions can lead to suboptimal yields, rendering the process economically unfeasible. The optimization of these parameters should begin at the laboratory scale, where a series of tests can be conducted to identify the ideal conditions. This approach ensures that, upon scaling up to industrial levels, significant adjustments to the process are not required, thereby minimizing resource waste and ensuring a smoother transition to larger-scale production [221]. The use of automation and real-time process monitoring can also help optimize fermentation conditions and ensure consistency at larger scales, reducing manual errors and improving overall efficiency [219].
In the extraction step, the efficiency of lipid recovery is influenced by factors such as the type of solvent used and the ability to minimize the co-extraction of non-lipid materials, such as proteins and carbohydrates [222]. Using a combination of solvents can significantly enhance extraction efficiency. For instance, solvent mixtures like chloroform/methanol/water have been shown to achieve higher lipid yields compared to single solvents [223]. Additionally, adopting a sequential extraction approach, where fresh solvent is applied after an initial extraction, can capture residual lipids that remain in the biomass [224]. Studies have demonstrated that residual lipids often persist after the first extraction, suggesting that sequential extractions maximize recovery. However, the rigid cell walls of microalgae and certain yeast strains pose a challenge to solvent penetration, necessitating energy-intensive pre-treatment methods for effective cell disruption. Moreover, many highly effective solvents, such as chloroform, present environmental and health risks, adding to production costs. To address these issues, modern extraction techniques like supercritical CO2 extraction offer advantages in both yield and processing time, reducing the need for toxic solvents [225]. Combining physical, chemical, and solvent extraction methods can further improve overall efficiency [226]. Pre-treatment techniques, such as enzymatic hydrolysis or mechanical disruption, can enhance solvent penetration, leading to better lipid yields while minimizing energy consumption. Additionally, exploring low-cost, sustainable alternatives to traditional solvents, along with the recovery and reuse of solvents, can significantly reduce operational costs and support environmentally friendly practices [226]. Finally, evaluating the economic feasibility of scaling up the process, including cost-benefit analyses and market competition with traditional lipid sources, is crucial to ensure the long-term viability of microbial lipid production at industrial scales [227].
Together, optimizing production and extraction processes, alongside innovative pre-treatment and extraction techniques, can significantly improve the feasibility of scaling up microbial biomass and lipid production, ensuring a more cost-effective and sustainable industrial application.
7. Patent: History and Innovation
The biotechnology product market is witnessing remarkable advancements in biomass and lipid production by microorganisms. This ongoing growth is propelled by critical factors such as sustainability, cost-effective production, and the high standards that biotechnology strives to meet. With this context in mind, this article will delve into an analysis of patents filed in the past five years (2020–2024) using the Derwent Innovations Index platform.
To obtain search results, it was necessary to develop a search algorithm for the patent database. Given the extensive range of International Patent Classification (IPC) codes pertinent to this search, certain codes were excluded, and the focus was narrowed to keywords for topic and code searches from the Derwent platform itself. The classification code employed was “D16”, which encompasses the fermented products industry, fermentation equipment, the manufacture of fermented products, the production of microorganisms, the production of pharmaceutical products, microbiology, the production of vaccines and antibodies, cell and tissue culture, and genetic engineering. The final algorithm was thus formulated: (microb*)) OR (bacteria*)) OR (fung*)) OR (yeast)) OR (oleaginous)) OR (microorganism*)) AND (lipid*)) OR (oil*)) OR (fatty acid)) AND (biomass)) AND (D16)).
The patents were classified according to their respective applications. The identified patents pertained to a range of applications, including the enhancement of microbiological biomass and bio-oil production, the generation of biofuels (biodiesel), the development of functional foods and animal feed, the advancement of cosmetics and pharmaceuticals, and the extraction and purification of bio-oils.
The patents were classified according to their respective applications. The patents identified pertained to a range of applications, including the enhancement of microbiological biomass and bio-oil production, the generation of biofuels (biodiesel), the development of functional foods and animal feed, the creation of cosmetics and pharmaceuticals, and the extraction and purification of bio-oils. In the majority of the documents, the most prevalent strains were microalgae and oleaginous yeasts, such as Schizochytrium sp., Mortierella sp., Chlorella sp., and Y. lipolytica.
The application of this algorithm resulted in the registration of 4079 patents. By refining the search parameters and filtering out patents filed in the last five years, the number of identified patents was reduced to 1.117. Following a detailed examination of the search results, patents related to the production of biomass, bio-oil, and derived products were identified as the most pertinent. Consequently, 190 patents were deemed to meet the requisite criteria.
7.1. Current Prospects for Innovation
7.1.1. Biomass and Lipids
In the 21st century, particularly over the past five years (2020–2024), the average annual number of patents filed in the field discussed in this article has consistently been around 38. Noteworthy innovations include methods that enhance the production and extraction of biomass and microbial bio-oils, which have significant potential for creating bioproducts in various sectors, such as nutraceuticals, dietary supplements, animal feed, pharmaceuticals, cosmetics, and biofuels.
It is important to highlight a key observation. Among the 190 patents identified and analyzed in the Derwent Innovations Index, an overwhelming majority—99 patents—focus on enhancing the production of biomass, microbial lipids, or a combination of both. Specifically, 13 patents address biomass production exclusively, while 50 are dedicated to improving bio-oil production, and 34 tackle both biomass and lipid production.
In terms of bioproducts developed, the areas with the highest number of patent applications relate to the production of nutraceutical foods, food supplements, animal feed, and biofuels. Of the total, 42 patents related to food for humans and animals, with 24 patents focused on the development of food for both humans and animals, 8 related to food for humans, and 5 to food for animals. The production of biofuels accounted for 41 patents. The area with the lowest number of patents was bio-oils used in the pharmaceutical and cosmetic industries, with only nine patents.
To make the discussion more illustrative and informative, some examples of patents relating to each of the above types of applications are provided. Patent US2022017929-A1, filed by Evonik Operations GmbH in 2022, describes a process that can increase biomass production and the content of polyunsaturated fatty acids in its composition. The bioprocess is divided into two stages. The first stage is the growth phase, in which 50 g/L to 220 g/L of Schizochytrium biomass is produced using both alcoholic and non-alcoholic carbon sources, as well as organic and inorganic nitrogen sources. The second stage is oil production, which can be enhanced by the controlled and continuous addition of 5–100 mg (preferably 10–50 mg) of sulfate per 1 kg of fermentation medium [228].
Patent IN202021039478-A1, filed by Reliance Industries Limited in 2022, presents a novel method for inducing better lipid production by fungi of the species Aspergillus, Mucor, Chrysosporium, Fusarium, Scedosporium, Scytalidium, Populariopsis, Cladosporium, Scopulariopsis, and Penicillium. Instead of following the conventional approach of using solvents or substrates, this methodology utilizes infrared rays, preferably at 1100 nm for 15 min. This exposure results in irradiated fungi, leading to an increase in neutral lipid yield of 2–80% and an increase in total lipid yield of 1–12% [229].
Another notable example is patent CN113512567-A1, filed by Anhui Normal University in 2021, which aims to create a photo-heterotrophic microalgae production system to enhance bio-oil production. The invention utilizes the Rhodobacter capsulatus strain, which is cultivated at a temperature of 22–26 °C and a light intensity of 6000–10,000 lux for 6 days. The autotrophic environment was optimized with inorganic substrates. The final result was an increase of more than 65% in biomass yield and 30% in fatty acid yield compared to the microalgae-only system [230].
7.1.2. Bio-Oil Used in the Animal and Human Food Industry
In the realm of bioproducts, the area that has received the most innovation and investment in terms of patent applications is nutraceutical food for humans and animal feed production. Regarding food production for both humans and animals, we can start with patent BR102020026212-A2, filed at the Federal University of Paraná in 2022. This patent describes a bioprocess for producing acid-rich biomass, specifically DHA, from agro-industrial waste, which can be used to produce animal feed. Residues such as sugarcane molasses, millet, and yeast cream are used as the growth medium, along with artificial seawater. The microorganism utilized is Schizochytrium limacium SR21. The result of the bioprocess is a biomass production of 39.29–40 g/L and lipid production of 16.1–14.98 g/L. The final composition of the produced biomass includes 30.5–35.43% DHA, 85–87.5% dry matter, 30.1–31.4% protein, 38.13–40% lipids, 8.17–10.1% carbohydrates, and 9.8–10.2% ash [231].
Another patent targets a broader market niche. Filed in 2024 under number WO2024050590-A1 by Nourish Ingredients Pty Ltd. (Mitchell, QLD, Australia), its purpose is to utilize the lipids produced to formulate food, beverages, or feed. The biomass and lipids of Mortierella sp. are generated using a synthetic culture medium containing glucose, ribose, yeast extract, glutamic acid, and sea salt. The biomass is converted into powder, although the drying method is not specified. Finally, this powder is added to the food, beverage, or feed by heating it at a temperature of 130 °C for 1 h. This process is expected to produce a product with an aroma and taste similar to that of meat [232].
7.1.3. Bio-Oil Used in the Biofuel Industry
With the advent of the climate crisis and the growing need for sustainable, affordable, and energetic fuels, bio-oils have emerged as a promising feedstock for biofuel production. Patent IN202041037060-A1, filed by a small group of inventors in 2020, covers a process for producing biodiesel from the bio-oil contained in microalgae biomass. The methodology involves isolating non-specific oleaginous microalgae species from agro-industrial wastewater, cultivating the microalgae using optimized heterotrophic growth media, extracting the lipids, and finally converting the extracted lipids into biodiesel using magnetic nanoparticles immobilized with lipase [233].
A recent example is patent WO2023180925-A1, filed by Versalis S.P.A. (San Donato Milanese, Italy) in 2023, which aims to create a bioprocess using oleaginous yeasts capable of producing lipids useful for biofuel production in diesel engines. While many yeast species have been documented as effective, the preferred species for this technique is Rhodosporidium azoricum. The bioprocess utilizes an optimized heterotrophic production medium under ideal physicochemical conditions and is conducted in two stages. The expected lipid yield is approximately 15.68 g/L to 64.2 g/L in the first stage and 12.02 g/L to 39.8 g/L in the second stage [234].
7.1.4. Bio-Oil Used in Pharmaceutical and Cosmetic Industries
Although this area has seen few patent registrations in the last five years, it is still worth analyzing the trends that bio-oils are addressing in the market for therapeutic and cosmetic products. Patent BR102019025709-A2, filed by the Federal University of Paraná in 2021, aims to create a cosmetic product for the prevention and treatment of hair loss due to nutritional deficiencies. This invention utilizes lipid-accumulating microbial cultures (Chlorella, Neochloris, Chaetoceros, Rhodospiridium, Lipomyces, Rhodotorula, Yarrowia, Trichosporon, Cunninghamella, Mortierella, Rhodococcus, Streptomyces, Gordonia, Nocardia, and Schizochytrium) in optimized synthetic media. The biomass produced is then recovered and dried to be added to the other components of the cosmetic formulation through an emulsifying tube. The final product contains a cosmetic composition with 1.5–4% by weight of microbial lipids derived from the dried and enriched biomass [235].
Another example with broader claimed applications is patent WO2022104063-A2, filed by C16 Biosciences, Inc. (New York, NY, USA) in 2022. This patent aims to develop a bio-oil produced by oleaginous yeast for various cosmetic products. The preferred yeast is Rhodotorula toruloides, which is cultivated in an optimized production medium. The oil extracted from the biomass undergoes a process that removes toxins and yields a microbial oil safe for human consumption. This process employs techniques such as fractionation, interesterification, transesterification, hydrogenation, steam hydrolysis, distillation, saponification, or combinations thereof. The final product is a bio-oil available in solid, liquid, cream, lotion, spray, gel, or foam form, which can be used to create products such as cleansing oil, lip balm, lotion, cream, makeup, deodorant, hair color, liquid soap, shampoo, conditioner, liquid cleanser, or bar soap [236].
7.1.5. Bio-Oil Extraction Methods
In addition to the production methods and bioproducts that have been patented, there has been significant interest in innovative methods for extracting bio-oils that are less polluting, more cost-effective, and more efficient. Patent WO2023220060-A1, filed by C16 Biosciences, Inc. (New York, NY, USA) in 2023, presents a method for the extraction and isolation of yeast (Rhodosporidium) bio-oils that aims to preserve the integrity of the oils while minimizing contamination risks. This method involves treating yeast cells with β-1,3-glucomannase to produce an enzymatically lysed sample. Subsequently, this sample undergoes lipid phase isolation utilizing solvents such as heptane, hexane, ethyl acetate, ethanol, chloroform, and/or methanol. The anticipated fatty acid profile at the conclusion of the process is expected to contain more than 40% saturated fatty acids, over 40% monounsaturated fatty acids, and less than 20% polyunsaturated fatty acids [237].
The Brazilian patent BR102019003055-A2, filed by the Federal University of Paraíba in 2020, develops a method for the extraction of not only lipids but also the majority of bioactive compounds produced by microalgae, using biomass derived from the species Scenedesmus quadricauda. The extraction process involves the recovery of saponifiable compounds, including fatty acids and unsaponifiable compounds, through a saponification reaction in an alcoholic solution. The saponifiable compounds, which are solid, are separated from the unsaponifiable compounds, which remain in the liquid phase by filtration. The liquid phase containing the unsaponifiable compounds is then treated with a mixture of polar and apolar solvents, specifically hexane and water, resulting in the separation of the apolar and polar liquid fractions. The apolar phase, generated by the partitioning of the mother water, is purified through successive washes with distilled water until a neutral pH is reached. Once a neutral pH is achieved, the apolar solvent is evaporated, yielding bioactive compounds from microalgae that are rich in carotenoids [238].
8. Global Trends in the Microbial Biomass and Lipids and Its Derivates
The industrial production of microbial oils for the food industry has already been initiated by several companies, including DuPont, DSM (formerly Martek Biosciences Inc., Kaiseraugst, Switzerland), Cargill Alking Bioengineering (Wuhan, China) Co. Ltd. (CABIO), Nestlé S.A., and Nutricia. Various commercial products are based on microbial oils, including javanicus oil, which is rich in linolenic fatty acid and serves as a substitute for evening primrose oil; Life’s OMEGA, which is rich in EPA and DHA; DHASCO-B, high in DHA; ARASCO, rich in ARA; ingredients for infant formula. Notably, microbial oils, especially those containing ARA and DHA, have been recognized as Generally Recognized as Safe (GRAS) by the U.S. Food and Drug Administration (FDA) for both adult and infant consumption since 2002 [239].
In the biofuel industry, biofuel produced from microbial biomass, particularly algae, is classified as third-generation biofuel, and its production has been well established [172]. The industrial-scale production of biodiesel using microbial oil from Rhodotorula toruloides DEBB 5533 has also achieved success. Economic viability analyses indicate that microbial biodiesel is cost-competitive at USD 0.76/L compared to biodiesel derived from vegetable oils, which is priced at USD 0.81/L. Additionally, the yield of biodiesel from microbial oil is 6.3 times higher than that of standard biodiesel, highlighting the significant potential of microbial lipids in sustainable energy production [240].
Microbial lipids, derived from renewable sources, play a crucial role in significantly lowering greenhouse gas emissions, thereby contributing to the decarbonization of the energy sector. In contrast to traditional biofuel feedstocks that require extensive cultivable land and significant water resources, microbial lipid production harnesses a wide variety of cost-effective substrates, including agro-industrial waste. This approach not only conserves natural resources but also mitigates competition with food crops, making it a more sustainable option for biofuel production [171].
Moreover, the process of microbial lipid production enhances the principles of a circular economy by transforming waste streams into valuable precursors for biodiesel. Integrating microbial lipid production into existing industrial frameworks not only promotes waste valorization but also facilitates the closure of carbon loops, thereby minimizing environmental impacts. The utilization of waste-derived substrates directly supports global sustainability initiatives, particularly those outlined in the United Nations Sustainable Development Goals (SDGs), specifically SDG 7 (Affordable and Clean Energy) and SDG 13 (Climate Action) [241].
Current global trends in microbial lipid production are focused on improving process efficiency through innovations in genetic engineering, high-efficiency bioreactors, and advanced extraction technologies, such as PEF and enzymatic cell disruption [6,184,193].
These advancements aim to reduce energy consumption and decrease the reliance on hazardous solvents, thereby increasing the environmental and economic feasibility of microbial lipid extraction [186]. For instance, PEF has demonstrated the capacity to significantly enhance lipid yields while maintaining lower energy input, positioning it as a sustainable alternative for large-scale production [185]. Moreover, genetic engineering efforts have successfully optimized microbial strains for higher lipid productivity, further refining the process for industrial applications [172].
Innovative systems such as photosynthetic microbial fuel cells (PMMFCs) have also demonstrated promising potential in bioenergy recovery and wastewater treatment, offering dual benefits in sustainability and cost-efficiency [242]. These systems rely on optimizing parameters like light intensity and light/dark cycles to enhance the growth of microalgae (C. vulgaris) and increase lipid production (145 mg per gram of dry biomass).
Microbial lipids are increasingly recognized not only for their role in biofuel production but also for their vast potential in high-value sectors such as pharmaceuticals, nutraceuticals, cosmetics, and food. The combination of their sustainable production, scalability, and broad utility makes them a critical component of future bioproducts, aligning with global trends toward reducing dependency on fossil fuels and promoting the circular economy.
9. Conclusions
The production of microbial biomass and lipids constitutes a crucial step forward in the quest for sustainable biotechnological approaches that inform both the current trends of environmental degradation and the need for alternative energy sources. By utilizing the metabolic capabilities of bacteria, fungi, yeasts, microalgae, etc., the above processes have a revolutionizing potential in diverse sectors, including biofuels, pharmaceuticals, functional foods, and so on. The last decades, however, witnessed tremendous developments in the scale and efficiency of microbial production systems, with precision genetic engineering, metabolic pathway targeting, and agro-industrial waste utilization being notable examples.
However, there are still some problems that must be overcome, in particular, the elevated price for downstream processing, particularly in lipid extraction, and the lack of public and government support for genetically modified organisms. Overcoming these hurdles will entail a combination of disciplines, including the optimization of processes to incorporate new approaches for synthetic biology and bioprocess engineering. In addition, the promotion of awareness campaigns among the public and improvement of legislation will be necessary in order to facilitate acceptance in the market.
The role of microbial lipids in the industrial sectors is expected to be crucial in the shift towards a bioproduct economy. With further developments and upscaling of these methods, microbial biomass and lipid technologies are expected to become the mainstay of sustainable development, aiding significant global aspirations in energy, food, and environmental challenges.
Author Contributions
Conceptualization, G.d.S.C. and W.J.M.-B.; methodology, G.d.S.C. and W.J.M.-B.; investigation, G.d.S.C., G.A.d.R., Y.P.P., F.R.V., J.L.S. and S.d.M.C.; writing—original draft preparation, G.d.S.C., G.A.d.R., Y.P.P., F.R.V., J.L.S. and S.d.M.C.; writing—review and editing, W.J.M.-B. and C.R.; supervision, C.R.S.; project administration, C.R.S.; funding acquisition, C.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordination for the Improvement of Higher Education Personnel (CAPES-Brazil).
Data Availability Statement
Dara sharing is not applicable.
Acknowledgments
We acknowledge the support of the Department of Bioprocess Engineering and Biotechnology of the Federal University of Paraná (Brazil); the Department of Food Engineering of the University of Córdoba (Colombia) and the Department of Food Technology of the Federal Institute of Maranhão (Brazil).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lenaz, G. The Role of Lipids in the Structure and Function of Membranes. In Subcellular Biochemistry; Roodyn, D.B., Ed.; Springer: New York, NY, USA, 1979; Volume 6, pp. 233–343. ISBN 978-1-4615-7945-8. [Google Scholar]
- Li-Beisson, Y.; Nakamura, Y.; Hardwood, J. Lipids: From Chemical Structures, Biosynthesis, and Analyses to Industrial Applications. In Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1, pp. 1–18. ISBN 978-3-319-25977-2. [Google Scholar]
- Serventi, L.; Yang, K.; Liu, C.; Tanyitiku, M.; Mohajer, M. Lipids. In Sustainable Food Innovation; Serventi, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2023; Volume 1, pp. 59–71. [Google Scholar]
- Grand View Research. Biodiesel Market Size, Share & Trends Analysis Report by Feedstock (Vegetable Oils, Animal Fats), by Application (Fuel, Power Generation), by Region (Europe, APAC), And Segment Forecasts, 2022–2030. 2024. Available online: https://www.grandviewresearch.com/press-release/global-biodiesel-market#:~:text=Biodiesel%20Market%20Worth%20%2473.05%20Billion%20By%202030%20%7C%20CAGR%3A%2010.0%25&text=The%20global%20biodiesel%20market%20size,by%20Grand%20View%20Research%2C%20Inc (accessed on 29 September 2024).
- Brandenburg, J.; Blomqvist, J.; Shapaval, V.; Kohler, A.; Sampels, S.; Sandgren, M.; Passoth, V. Oleaginous Yeasts Respond Differently to Carbon Sources Present in Lignocellulose Hydrolysate. Biotechnol. Biofuels 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production Strategies and Applications of Microbial Single Cell Oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Yang, W.; Mohamed, H.; Zhang, Y.; Song, Y. Microbes: A Hidden Treasure of Polyunsaturated Fatty Acids. Front. Nutr. 2022, 9, 827837. [Google Scholar] [CrossRef] [PubMed]
- Ghazani, S.M.; Marangoni, A.G. Microbial Lipids for Foods. Trends Food Sci. Technol. 2022, 119, 593–607. [Google Scholar] [CrossRef]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal Proteins: Production Strategies and Nutritional and Functional Properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef]
- Abramova, I.M.; Soloviev, A.O.; Turshatov, M.V.; Krivchenko, V.A.; Kononenko, V.V. Protein Feedstuff Production Based on Microbial Biomass. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Krasnoyarsk, Russia, 18–20 June 2020; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 548. [Google Scholar] [CrossRef]
- Keita, V.M.; Lee, Y.Q.; Lakshmanan, M.; Ow, D.S.W.; Staniland, P.; Staniland, J.; Savill, I.; Tee, K.L.; Wong, T.S.; Lee, D.Y. Evaluating Oleaginous Yeasts for Enhanced Microbial Lipid Production Using Sweetwater as a Sustainable Feedstock. Microb. Cell Fact. 2024, 23, 63. [Google Scholar] [CrossRef]
- Merchant, R.E.; Andre, C.A. A Review of Recent Clinical Trials of the Nutritional Supplement Chlorella pyrenoidosa in the Treatment of Fibromyalgia, Hypertension, and Ulcerative Colitis. Altern. Ther. 2001, 7, 79. [Google Scholar]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in Clinical Practice: Evidence-Based Human Applications. Evid.-Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- Global Market Insights Single Cell Protein Market—By Source (Bacteria, Yeast, Algae {Chlorella, Spirulina, Haematococcus}, Fungi), by Application (Food And Beverages {Meat Analogs, Bakery, Dairy Alternatives, Cereals & Snacks, Beverages} Animal Feed, Dietary Supplements), & Forecast, 2023–2032. Available online: https://www.gminsights.com/industry-analysis/single-cell-protein-market (accessed on 29 September 2024).
- Lakhawat, S.S.; Malik, N.; Kumar, V.; Kumar, S.; Sharma, P.K. Implications of CRISPR-Cas9 in Developing Next Generation Biofuel: A Mini-Review. Curr. Protein Pept. Sci. 2022, 23, 574–584. [Google Scholar] [CrossRef]
- Li, Z.; Waghmare, P.R.; Dijkhuizen, L.; Meng, X.; Liu, W. Research Advances on the Consolidated Bioprocessing of Lignocellulosic Biomass. Eng. Microbiol. 2024, 4, 100139. [Google Scholar] [CrossRef]
- Rafiq, S.; Bhat, M.I.; Sofi, S.A.; Muzzafar, K.; Majid, D.; Dar, B.N.; Makroo, H.A. Bioconversion of Agri-Food Waste and by-Products into Microbial Lipids: Mechanism, Cultivation Strategies and Potential in Food Applications. Trends Food Sci. Technol. 2023, 139, 104118. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced Microalgal Lipid Production for Biofuel Using Different Strategies Including Genetic Modification of Microalgae: A Review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Buiatti, M.; Christou, P.; Pastore, G. The Application of GMOs in Agriculture and in Food Production for a Better Nutrition: Two Different Scientific Points of View. Genes Nutr. 2013, 8, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Gangola, S.; Joshi, S.; Bhandari, G.; Bhatt, P.; Kumar, S.; Pandey, S.C. Omics Approaches to Pesticide Biodegradation for Sustainable Environment. In Advanced Microbial Techniques in Agriculture, Environment, and Health Management; Academic Press: Cambridge, MA, USA, 2023; pp. 191–203. [Google Scholar] [CrossRef]
- Taran, M.; Asadi, N. A Novel Approach for Environmentally Friendly Production of Single Cell Protein from Petrochemical Wastewater Using a Halophilic Microorganism in Different Conditions. Pet. Sci. Technol. 2014, 32, 625–630. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial Protein: Future Sustainable Food Supply Route with Low Environmental Footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef]
- Dong, T.; Knoshaug, E.P.; Pienkos, P.T.; Laurens, L.M.L. Lipid Recovery from Wet Oleaginous Microbial Biomass for Biofuel Production: A Critical Review. Appl. Energy 2016, 177, 879–895. [Google Scholar] [CrossRef]
- Koreti, D.; Kosre, A.; Jadhav, S.K.; Chandrawanshi, N.K. A Comprehensive Review on Oleaginous Bacteria: An Alternative Source for Biodiesel Production. Bioresour. Bioprocess. 2022, 9, 47. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef]
- Shields-Menard, S.A.; Amirsadeghi, M.; French, W.T.; Boopathy, R. A Review on Microbial Lipids as a Potential Biofuel. Bioresour. Technol. 2018, 259, 451–460. [Google Scholar] [CrossRef]
- Mahan, K.M.; Le, R.K.; Yuan, J.; Ragauskas, A.J. A Review on The Bioconversion of Lignin to Microbial Lipid with Oleaginous Rhodococcus opacus. J. Biotechnol. Biomater. 2017, 7. [Google Scholar] [CrossRef]
- Dourou, M.; Aggeli, D.; Papanikolaou, S.; Aggelis, G. Critical Steps in Carbon Metabolism Affecting Lipid Accumulation and Their Regulation in Oleaginous Microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 2509–2523. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M.A.L.R.M.; de Carvalho, C.C.C.R. Effect of Carbon Sources on Lipid Accumulation in Rhodococcus Cells. Biochem. Eng. J. 2015, 94, 100–105. [Google Scholar] [CrossRef]
- Janßen, H.J.; Ibrahim, M.H.A.; Bröker, D.; Steinbüchel, A. Optimization of Macroelement Concentrations, PH and Osmolarity for Triacylglycerol Accumulation in Rhodococcus Opacus Strain PD630. AMB Express 2013, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Z.; Singh, L. Rhodococcus opacus High-Cell-Density Batch Cultivation with a Bagasse Hydrolysate for Possible Triacylglycerol Synthesis. Biomed. Biotechnol. Res. J. 2023, 7, 209. [Google Scholar] [CrossRef]
- Mondala, A.; Hernandez, R.; French, T.; Green, M.; McFarland, L.; Ingram, L. Enhanced Microbial Oil Production by Activated Sludge Microorganisms from Sugarcane Bagasse Hydrolyzate. Renew. Energy 2015, 78, 114–118. [Google Scholar] [CrossRef]
- Behera, B.; Unpaprom, Y.; Ramaraj, R.; Maniam, G.P.; Govindan, N.; Paramasivan, B. Integrated Biomolecular and Bioprocess Engineering Strategies for Enhancing the Lipid Yield from Microalgae. Renew. Sustain. Energy Rev. 2021, 148, 111270. [Google Scholar] [CrossRef]
- Thanapimmetha, A.; Suwaleerat, T.; Saisriyoot, M.; Chisti, Y.; Srinophakun, P. Production of Carotenoids and Lipids by Rhodococcus opacus PD630 in Batch and Fed-Batch Culture. Bioprocess. Biosyst. Eng. 2017, 40, 133–143. [Google Scholar] [CrossRef]
- Penia Kresnowati, M.T.A.; Chen, X.D. Continuous Operation. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 527–535. [Google Scholar]
- Gupta, N.; Manikandan, N.A.; Pakshirajan, K. Real-time lipid production and dairy wastewater treatment using Rhodococcus opacus in a bioreactor under fed-batch, continuous and continuous cell recycling modes for potential biodiesel application. Biofuels 2018, 9, 239–245. [Google Scholar] [CrossRef]
- Menegol, T.; Diprat, A.B.; Rodrigues, E.; Rech, R. Effect of Temperature and Nitrogen Concentration on Biomass Composition of Heterochlorella Luteoviridis. Food Sci. Technol. 2017, 37, 28–37. [Google Scholar] [CrossRef]
- Tigini, V.; Prigione, V.; Donelli, I.; Freddi, G.; Varese, G.C. Influence of Culture Medium on Fungal Biomass Composition and Biosorption Effectiveness. Curr. Microbiol. 2012, 64, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Mondala, A. Solid-State Direct Fungal Bioconversion of Biomass to Fuels and Chemicals: The Next Frontier in Sustainable. Austin Chem. Eng. 2014, 1, 1007. [Google Scholar]
- Kam, S.; Kenari, A.A.; Younesi, H. Production of Single Cell Protein in Stickwater by Lactobacillus acidophilus and Aspergillus niger. J. Aquat. Food Product. Technol. 2012, 21, 403–417. [Google Scholar] [CrossRef]
- Isaza-Pérez, F.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Potential of Residual Fungal Biomass: A Review. Environ. Sci. Pollut. Res. 2020, 27, 13019–13031. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Mohamed, H.; Fazili, A.B.A.; Yang, W.; Song, Y. Investigating the Effect of Alcohol Dehydrogenase Gene Knockout on Lipid Accumulation in Mucor Circinelloides WJ11. J. Fungi 2022, 8, 917. [Google Scholar] [CrossRef]
- Youssef, G.A.; Elrefaey, A.M.; El-Aassar, S. Evaluating Oleaginous Fungi for Sustainable Biodiesel Production: Screening, Identification and Optimization of Lipid Production. Egypt. J. Bot. 2021, 61, 693–708. [Google Scholar] [CrossRef]
- Youssef, G.; Elrefaey, A.; El-Assar, S. Potential Assessment of Oleaginous Fungi for Sustainable Biodiesel Production: Screening, Identication and Lipid Production Optimization. Egypt. J. Bot. 2021. [Google Scholar] [CrossRef]
- Li, D.D.; Wang, Y.; Kim, E.L.; Hong, J.; Jung, J.H. A New Fungal Triterpene from the Fungus Aspergillus flavus Stimulates Glucose Uptake without Fat Accumulation. Mar. Drugs 2022, 20, 203. [Google Scholar] [CrossRef]
- Aidemark, M.; Tjellström, H.; Sandelius, A.S.; Stålbrand, H.; Andreasson, E.; Rasmusson, A.G.; Widell, S. Trichoderma Viride Cellulase Induces Resistance to the Antibiotic Pore-Forming Peptide Alamethicin Associated with Changes in the Plasma Membrane Lipid Composition of Tobacco BY-2 Cells. BMC Plant Biol. 2010, 10, 274. [Google Scholar] [CrossRef]
- Serrano-Carreon, L.; Hathout, Y.; Bensoussan, M.; Belin, J.-M. Lipid Accumulation in Trichoderma Species. FEMS Microbiol. Lett. 1992, 93, 181–187. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, B.; Huang, B.C.; Wang, F.B.; Zhang, Y.Q.; Zhao, S.G.; Li, M.; Wang, H.Y.; Yu, X.J.; Liu, X.Y.; et al. Production, Biosynthesis, and Commercial Applications of Fatty Acids From Oleaginous Fungi. Front. Nutr. 2022, 9, 873657. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, S.I.; Ezeokoli, O.T.; Roopnarain, A.; Ndaba, B.; Sekoai, P.T.; Habimana, O.; Pohl, C.H. The Potential of Single-Cell Oils Derived From Filamentous Fungi as Alternative Feedstock Sources for Biodiesel Production. Front. Microbiol. 2021, 12, 637381. [Google Scholar] [CrossRef] [PubMed]
- Dzurendova, S.; Zimmermann, B.; Tafintseva, V.; Kohler, A.; Horn, S.J.; Shapaval, V. Metal and Phosphate Ions Show Remarkable Influence on the Biomass Production and Lipid Accumulation in Oleaginous Mucor Circinelloides. J. Fungi 2020, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- van Aarle, I.M.; Olsson, P.A. Fungal Lipid Accumulation and Development of Mycelial Structures by Two Arbuscular Mycorrhizal Fungi. Appl. Environ. Microbiol. 2003, 69, 6762–6767. [Google Scholar] [CrossRef] [PubMed]
- Nosheen, S.; Naz, T.; Yang, J.; Hussain, S.A.; Fazili, A.B.A.; Nazir, Y.; Li, S.; Mohamed, H.; Yang, W.; Mustafa, K.; et al. Role of Snf-β in Lipid Accumulation in the High Lipid-producing Fungus Mucor Circinelloides WJ11. Microb. Cell Fact. 2021, 20. [Google Scholar] [CrossRef]
- Patriarca, E.J.; Kobayashi, G.S.; Maresca, B. Mitochondrial Activity and Heat-Shock Response during Morphogenesis in the Pathogenic Fungus Histoplasma capsulatum. Biochem. Cell Biol. 1992, 70, 207. [Google Scholar] [CrossRef]
- Gao, D.; Zeng, J.; Yu, X.; Dong, T.; Chen, S. Improved Lipid Accumulation by Morphology Engineering of Oleaginous Fungus Mortierella Isabellina. Biotechnol. Bioeng. 2014, 111, 1758–1766. [Google Scholar] [CrossRef]
- Vasconcelos, B.; Teixeira, J.C.; Dragone, G.; Teixeira, J.A. Oleaginous Yeasts for Sustainable Lipid Production-from Biodiesel to Surf Boards, a Wide Range of “Green” Applications. Appl. Microbiol. Biotechnol. 2019, 103, 3651–3667. [Google Scholar] [CrossRef]
- Meeuwse, P.; Sanders, J.P.M.; Tramper, J.; Rinzema, A. Lipids from Yeasts and Fungi: Tomorrow’s Source of Biodiesel? Biofuels Bioprod. Biorefin. 2013, 7, 512–524. [Google Scholar] [CrossRef]
- Ali, H.; Zulkali, M.D. Design Aspects of Bioreactors for Solid-State Fermentation: A Review. Chem. Biochem. Eng. 2011, 25, 255–266. [Google Scholar] [CrossRef]
- Subramaniam, R.; Thirumal, V.; Chistoserdov, A.; Bajpai, R.; Bader, J.; Popovic, M. High-Density Cultivation in the Production of Microbial Products. Chem. Biochem. Eng. Q. 2018, 32, 451–464. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Fernández, F.J.; Montiel-González, A.; Sánchez, C.; Díaz-Godínez, G. Growth and Laccase Production by Pleurotus ostreatus in Submerged and Solid-State Fermentation. Appl. Microbiol. Biotechnol. 2008, 81, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Cerda, A.; Artola, A.; Barrena, R.; Font, X.; Gea, T.; Sánchez, A. Innovative Production of Bioproducts From Organic Waste Through Solid-State Fermentation. Front. Sustain. Food Syst. 2019, 3, 63. [Google Scholar] [CrossRef]
- Khot, M.B. Solid-State Fermentation: An Alternative Approach to Produce Fungal Lipids as Biodiesel Feedstock. In Status and Future Challenges for Non-Conventional Energy Sources; Joshi, J., Sen, R., Sharma, A., Salam, P.A., Eds.; Springer: Singapore, 2022; Volume 2, pp. 123–137. ISBN 978-981-16-4509-9. [Google Scholar]
- de Mello, A.F.M.; de Souza Vandenberghe, L.P.; Herrmann, L.W.; Letti, L.A.J.; Burgos, W.J.M.; Scapini, T.; Manzoki, M.C.; de Oliveira, P.Z.; Soccol, C.R. Strategies and Engineering Aspects on the Scale-up of Bioreactors for Different Bioprocesses. Syst. Microbiol. Biomanuf. 2024, 4, 365–385. [Google Scholar] [CrossRef]
- Li, Y.; Peng, X.; Chen, H. Comparative Characterization of Proteins Secreted by Neurospora sitophila in Solid-State and Submerged Fermentation. J. Biosci. Bioeng. 2013, 116, 493–498. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R. Fungi in Fermentation and Biotransformation Systems. In Biology of Microfungi; De-Wei, L., Ed.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 525–541. ISBN 978-3-319-29137-6. [Google Scholar]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily Yeasts as Oleaginous Cell Factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Sargeant, L.A.; Chuck, C.J.; Donnelly, J.; Bannister, C.D.; Scott, R.J. Optimizing the Lipid Profile, to Produce Either a Palm Oil or Biodiesel Substitute, by Manipulation of the Culture Conditions for Rhodotorula glutinis. Biofuels 2014, 5, 33–43. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Zong, M.H.; Wu, H. Efficient Lipid Production with Trichosporonfermentans and Its Use for Biodiesel Preparation. Bioresour. Technol. 2008, 99, 7881–7885. [Google Scholar] [CrossRef]
- Caporusso, A.; Capece, A.; De Bari, I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Ferment 2021, 7, 50. [Google Scholar] [CrossRef]
- Cui, W.; Wang, Q.; Zhang, F.; Zhang, S.C.; Chi, Z.M.; Madzak, C. Direct Conversion of Inulin into Single Cell Protein by the Engineered Yarrowia lipolytica Carrying Inulinase Gene. Process Biochem. 2011, 46, 1442–1448. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Liu, Y. Production of Single Cell Protein from Soy Molasses Using Candida tropicalis. Ann. Microbiol. 2012, 62, 1165–1172. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Maiti, M.K. Lipid Production by Oleaginous Yeasts. Adv. Appl. Microbiol. 2021, 116, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Chopra, J.; Sen, R. Process Optimization Involving Critical Evaluation of Oxygen Transfer, Oxygen Uptake and Nitrogen Limitation for Enhanced Biomass and Lipid Production by Oleaginous Yeast for Biofuel Application. Bioprocess. Biosyst. Eng. 2018, 41, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Abeln, F.; Chuck, C.J. The History, State of the Art and Future Prospects for Oleaginous Yeast Research. Microb. Cell Fact. 2021, 20, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.J.S.; Bonturi, N.; Kerkhoven, E.J.; Miranda, E.A.; Lahtvee, P.J. C/N Ratio and Carbon Source-Dependent Lipid Production Profiling in Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Sun, C.; Zhang, C.; Liu, H.; Cui, Q.; Song, X.; Wang, S. A Phospholipid:Diacylglycerol Acyltransferase Is Involved in the Regulation of Phospholipids Homeostasis in Oleaginous Aurantiochytrium sp. Biotechnol. Biofuels Bioprod. 2023, 16. [Google Scholar] [CrossRef]
- Beopoulos, A.; Nicaud, J.M.; Gaillardin, C. An Overview of Lipid Metabolism in Yeasts and Its Impact on Biotechnological Processes. Appl. Microbiol. Biotechnol. 2011, 90, 1193–1206. [Google Scholar] [CrossRef]
- Takaku, H.; Matsuzawa, T.; Yaoi, K.; Yamazaki, H. Lipid Metabolism of the Oleaginous Yeast Lipomyces starkeyi. Appl. Microbiol. Biotechnol. 2020, 104, 6141–6148. [Google Scholar] [CrossRef]
- Klug, L.; Daum, G. Yeast Lipid Metabolism at a Glance. FEMS Yeast Res. 2014, 14, 369–388. [Google Scholar] [CrossRef]
- Naveira-Pazos, C.; Robles-Iglesias, R.; Fernández-Blanco, C.; Veiga, M.C.; Kennes, C. State-of-the-Art in the Accumulation of Lipids and Other Bioproducts from Sustainable Sources by Yarrowia Lipolytica. Rev. Environ. Sci. Biotechnol. 2023, 22, 1131–1158. [Google Scholar] [CrossRef]
- Davies, R.J.; Holdsworth, J.E.; Reader, S.L. Microbiology Biotechnology The Effect of Low Oxygen Uptake Rate on the Fatty Acid Profile of the Oleaginous Yeast Apiotrichum Curvature; Springer: Cham, Switzerland, 1990; Volume 33. [Google Scholar]
- Hu, M.; Qiu, L.; Wang, Y. The PHO Pathway Involved in Phosphate Metabolism in Yeast for Efficient Phosphorus Removal. In Proceedings of the E3S Web of Conferences, Guilin, China, 10–12 August 2018; EDP Sciences: Les Ulis, France, 2018; Volume 53. [Google Scholar]
- De Oliva-Neto, P.; Dorta, C.; Flavia, A.; Carvalho, A.; Gomes De Lima, V.M. The Brazilian Technology of Fuel Ethanol Fermentation-Yeast Inhibition Factors and New Perspectives to Improve the Technology. Mater. Process. Energy Commun. Curr. Res. Technol. Dev. 2013, 1, 371–379. [Google Scholar]
- Almuhayawi, M.S.; Hassan, E.A.; Almasaudi, S.; Zabermawi, N.; Azhar, E.I.; Najjar, A.; Alkuwaity, K.; Abujamel, T.S.; Alamri, T.; Harakeh, S. Biodiesel Production through Rhodotorula toruloides Lipids and Utilization of De-Oiled Biomass for Congo Red Removal. Sustainability 2023, 15, 13412. [Google Scholar] [CrossRef]
- Fickers, P.; Cheng, H.; Lin, C.S.K. Sugar Alcohols and Organic Acids Synthesis in Yarrowia lipolytica: Where Are We? Microorganisms 2020, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Calvey, C.H.; Su, Y.K.; Willis, L.B.; McGee, M.; Jeffries, T.W. Nitrogen Limitation, Oxygen Limitation, and Lipid Accumulation in Lipomyces starkeyi. Bioresour. Technol. 2016, 200, 780–788. [Google Scholar] [CrossRef]
- Xu, X.H.; Liu, Z.X.; Shi, X.Y.; Miao, C.; Sheng, S.; Xu, Y.; Wu, F.A.; Wang, J. Fed-Batch Fermentation of Yarrowia lipolytica Using Defatted Silkworm Pupae Hydrolysate: A Dynamic Model-Based Approach for High Yield of Lipid Production. Waste Biomass Valorization 2018, 9, 2399–2411. [Google Scholar] [CrossRef]
- Demerdash, M.; Nasr, A.A. Batch and Repeated Fed Batch Cultivation of Candida utilis on Corn Meal Hydrolysate for Cellular Biomass Production. J. Food Dairy Sci. 2000, 25, 3461–3472. [Google Scholar] [CrossRef]
- Bao, W.; Li, Z.; Wang, X.; Gao, R.; Zhou, X.; Cheng, S.; Men, Y.; Zheng, L. Approaches to Improve the Lipid Synthesis of Oleaginous Yeast Yarrowia lipolytica: A Review. Renew. Sustain. Energy Rev. 2021, 149, 111386. [Google Scholar] [CrossRef]
- Fei, Q.; Liu, Y.; Meruvu, H.; Jiao & Rongzhan, Z. Advanced Fermentation Strategies to Enhance Lipid Production from Lignocellulosic Biomass. In Emerging Technologies for Biorefineries, Biofuels, and Value-Added Commodities; Liu, Z.-H., Ragauskas, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 229–243. ISBN 978-3-030-65584-6. [Google Scholar]
- Grubišić, M.; Mihajlovski, K.; Gruičić, A.M.; Beluhan, S.; Šantek, B.; Šantek, M.I. Strategies for Improvement of Lipid Production by Yeast Trichosporon oleaginosus from Lignocellulosic Biomass. J. Fungi 2021, 7, 934. [Google Scholar] [CrossRef]
- Rivera, E.C.; Yamakawa, C.K.; Herrera Garcia, M.; Geraldo, V.C.; Rossell, C.E.V.; Filho, R.M.; Bonomi, A. A Procedure for Estimation of Fermentation Kinetic Parameters in Fed-Batch Bioethanol Production Process with Cell Recycle. Chem. Eng. 2013, 32, 1369–1374. [Google Scholar]
- Mendes, T.A.d.O.; Pinto, L.M.; Mendes, D.d.S.O.; Malta, H.L.; Oliveira, E.d.S. Aumento Na Produção de Biomassa de Levedura Em Propagador Aerado Por Processo Descontínuo e Semicontínuo Para Produção de Cachaça. Braz. J. Food Technol. 2013, 16, 81–89. [Google Scholar] [CrossRef]
- Rajpurohit, H.; Eiteman, M.A. Nutrient-Limited Operational Strategies for the Microbial Production of Biochemicals. Microorganisms 2022, 10, 2226. [Google Scholar] [CrossRef] [PubMed]
- Monge, O.; Certucha Barragn, M.T.; Almendariz Tapi, F.J. Microbial Biomass in Batch and Continuous System. In Biomass Now—Sustainable Growth and Use; InTech Open: London, UK, 2013. [Google Scholar]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic Diversity in the Macromolecular Composition of Microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical Composition and Nutritional Properties of Freshwater and Marine Microalgal Biomass Cultured in Photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- de Oliveira, M.A.C.L.; Monteiro, M.P.C.; Robbs, P.G.; Leite, S.G.F. Growth and Chemical Composition of Spirulina maxima and Spirulina platensis Biomass at Different Temperatures. Aquac. Int. 1999, 7, 261–275. [Google Scholar] [CrossRef]
- El-fayoumy, E.A.; Ali, H.E.A.; Elsaid, K.; Elkhatat, A.; Al-Meer, S.; Rozaini, M.Z.H.; Abdullah, M.A. Co-Production of High Density Biomass and High-Value Compounds via Two-Stage Cultivation of Chlorella vulgaris Using Light Intensity and a Combination of Salt Stressors. Biomass Convers. Biorefin 2024, 14, 22673–22686. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Maximizing Biomass and Lipid Production in Heterotrophic Culture of Chlorella vulgaris: Techno-Economic Assessment. Recent. Pat. Food Nutr. Agric. 2019, 10, 115–123. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, J.; Fa, Y.; Liu, X.; Lindblad, P. Enhancing Microalgal Lipid Accumulation for Biofuel Production. Front. Microbiol. 2022, 13, 1024441. [Google Scholar] [CrossRef]
- Zou, S.; Lu, Y.; Ma, H.; Li, Y.; Chen, G.; Han, D.; Hu, Q. Microalgal Glycerol-3-Phosphate Acyltransferase Role in Galactolipids and High-Value Storage Lipid Biosynthesis. Plant Physiol. 2023, 192, 426–441. [Google Scholar] [CrossRef]
- Shi, T.-Q.; Wang, L.-R.; Zhang, Z.-X.; Sun, X.-M.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef]
- Gao, B.; Hong, J.; Chen, J.; Zhang, H.; Hu, R.; Zhang, C. The Growth, Lipid Accumulation and Adaptation Mechanism in Response to Variation of Temperature and Nitrogen Supply in Psychrotrophic Filamentous Microalga Xanthonema hormidioides (Xanthophyceae). Biotechnol. Biofuels Bioprod. 2023, 16, 12. [Google Scholar] [CrossRef]
- Alkhamis, Y.A.; Mathew, R.T.; Nagarajan, G.; Rahman, S.M.; Rahman, M. pH Induced Stress Enhances Lipid Accumulation in Microalgae Grown under Mixotrophic and Autotrophic Condition. Front. Energy Res. 2022, 10, 1033068. [Google Scholar] [CrossRef]
- Roostaei, J.; Zhang, Y.; Gopalakrishnan, K.; Ochocki, A.J. Mixotrophic Microalgae Biofilm: A Novel Algae Cultivation Strategy for Improved Productivity and Cost-Efficiency of Biofuel Feedstock Production. Sci. Rep. 2018, 8, 12528. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.T.; Mori, K.; Toyama, T. Enhanced Biomass and Lipid Production Capacity of Chlamydomonas reinhardtii under Mixotrophic Cultivation Using Sewage Effluent and Waste Molasses. Jpn. J. Water Treat. Biol. 2020, 56, 57–66. [Google Scholar] [CrossRef]
- Iasimone, F.; Panico, A.; De Felice, V.; Fantasma, F.; Iorizzi, M.; Pirozzi, F. Effect of Light Intensity and Nutrients Supply on Microalgae Cultivated in Urban Wastewater: Biomass Production, Lipids Accumulation and Settleability Characteristics. J. Environ. Manag. 2018, 223, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, I.O.; Ikwebe, J.; Ogbonna, J.C.; Eze, C.N.; Ndrimbula, J.B. Effects of Light Intensity and Photoperiod on Growth, Lipid Accumulation and Fatty Acid Composition of Desmodesmus subspicatus LC172266 under Photoautotrophic Cultivation. Niger. J. Biotechnol. 2022, 38, 1–13. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M. Production of Chlorella vulgaris Biomass in Tubular Photobioreactors during Different Culture Conditions. Appl. Sci. 2021, 11, 3106. [Google Scholar] [CrossRef]
- Arcila, J.S.; Céspedes, D.; Buitrón, G. Influence of Wavelength Photoperiods and N/P Ratio on Wastewater Treatment with Microalgae–Bacteria. Water Sci. Technol. 2021, 84, 712–724. [Google Scholar] [CrossRef]
- Ho, S.-H.; Nakanishi, A.; Ye, X.; Chang, J.-S.; Chen, C.-Y.; Hasunuma, T.; Kondo, A. Dynamic Metabolic Profiling of the Marine Microalga Chlamydomonas sp. JSC4 and Enhancing Its Oil Production by Optimizing Light Intensity. Biotechnol. Biofuels 2015, 8, 48. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, J.; Lu, H.; Huang, R.; Zhou, J.; Cen, K. Simultaneous Enhancement of Microalgae Biomass Growth and Lipid Accumulation under Continuous Aeration with 15% CO2. RSC Adv. 2015, 5, 50851–50858. [Google Scholar] [CrossRef]
- Pedersen, T.C.; Gardner, R.D.; Gerlach, R.; Peyton, B.M. Assessment of Nannochloropsis gaditana Growth and Lipid Accumulation with Increased Inorganic Carbon Delivery. J. Appl. Phycol. 2018, 30, 2155–2166. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and Novel Strategies for Enhancing Lipid Accumulation and Quality in Microalgae. Renew. Sustain. Energy Rev. 2016, 55, 1–16. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Vaidyanathan, S. Influence of Gas Management on Biochemical Conversion of CO2 by Microalgae for Biofuel Production. Appl. Energy 2020, 261, 114420. [Google Scholar] [CrossRef]
- Omar Faruque, M.; Ilyas, M.; Mozahar Hossain, M.; Abdur Razzak, S. Influence of Nitrogen to Phosphorus Ratio and CO2 Concentration on Lipids Accumulation of Scenedesmus dimorphus for Bioenergy Production and CO2 Biofixation. Chem. Asian J. 2020, 15, 4307–4320. [Google Scholar] [CrossRef] [PubMed]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of Microalgae with High Lipid Content and Their Potential as Sources of Nutraceuticals. Phytochem. Rev. 2023, 22, 833–860. [Google Scholar] [CrossRef]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A Comprehensive Review on Carbon Source Effect of Microalgae Lipid Accumulation for Biofuel Production. Sci. Total Environ. 2022, 806, 151387. [Google Scholar] [CrossRef]
- Fistarol, G.O. Viability of Using Flue Gases as Carbon Source for Microalgae Cultivation. Int. J. Green. Technol. 2018, 2, 13–19. [Google Scholar] [CrossRef][Green Version]
- Yu, X.; Guo, W.; Hu, Z.; Li, P.; Zhang, Z.; Cheng, J.; Song, C.; Ye, Q. Flue Gas CO2 Supply Methods for Microalgae Utilization: A Review. Clean. Energy Sci. Technol. 2023, 1. [Google Scholar] [CrossRef]
- Pavlou, A.; Penloglou, G.; Kiparissides, C. Evaluation of Tolerant to CO2 Excess Microalgae for the Production of Multiple Biochemicals in a 3G Biorefinery. Sustainability 2023, 15, 3889. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Wang, Q.; Yang, X.; Xie, H.; Zhao, Z.K. Efficient Conversion of Biomass into Lipids by Using the Simultaneous Saccharification and Enhanced Lipid Production Process. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef]
- Saisriyoot, M.; Thanapimmetha, A.; Suwaleerat, T.; Chisti, Y.; Srinophakun, P. Biomass and Lipid Production by Rhodococcus opacus PD630 in Molasses-Based Media with and without Osmotic-Stress. J. Biotechnol. 2019, 297, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Xia, L. An Oleaginous Endophyte Bacillus subtilis HB1310 Isolated from Thin-Shelled Walnut and Its Utilization of Cotton Stalk Hydrolysate for Lipid Production. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Saravanabhupathy, S.; Singh, S.; Daverey, A.; Dutta, K. Multi-Objective Optimization for Biomass and Lipid Production by Oleaginous Bacteria Using Vegetable Waste as Feedstock. Environ. Eng. Res. 2022, 27. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Chatzifragkou, A.; Philippoussis, A.; Aggelis, G. Suitability of Low-Cost Sugars as Substrates for Lipid Production by the Fungus Thamnidium elegans. Energy Fuels 2010, 24, 4078–4086. [Google Scholar] [CrossRef]
- Ali, T.H.; El-Ghonemy, D.H. Optimization of Culture Conditions for the Highest Lipid Production from Some Oleaginous Fungi for Biodiesel Preparation. Asian J. Appl. Sci. 2014, 2, 5. [Google Scholar]
- Dzurendova, S.; Zimmermann, B.; Tafintseva, V.; Kohler, A.; Ekeberg, D.; Shapaval, V. The Influence of Phosphorus Source and the Nature of Nitrogen Substrate on the Biomass Production and Lipid Accumulation in Oleaginous Mucoromycota Fungi. Appl. Microbiol. Biotechnol. 2020, 104, 8065–8076. [Google Scholar] [CrossRef]
- Dobrowolski, A.; Drzymała, K.; Rzechonek, D.A.; Mituła, P.; Mirończuk, A.M. Lipid Production from Waste Materials in Seawater-Based Medium by the Yeast Yarrowia lipolytica. Front. Microbiol. 2019, 10, 547. [Google Scholar] [CrossRef]
- Galafassi, S.; Cucchetti, D.; Pizza, F.; Franzosi, G.; Bianchi, D.; Compagno, C. Lipid Production for Second Generation Biodiesel by the Oleaginous Yeast Rhodotorula graminis. Bioresour. Technol. 2012, 111, 398–403. [Google Scholar] [CrossRef]
- Wang, G.Y.; Chi, Z.; Song, B.; Wang, Z.P.; Chi, Z.M. High Level Lipid Production by a Novel Inulinase-Producing Yeast Pichia guilliermondii Pcla22. Bioresour. Technol. 2012, 124, 77–82. [Google Scholar] [CrossRef]
- Chang, W.; Li, Y.; Qu, Y.; Liu, Y.; Zhang, G.; Zhao, Y.; Liu, S. Mixotrophic Cultivation of Microalgae to Enhance the Biomass and Lipid Production with Synergistic Effect of Red Light and Phytohormone IAA. Renew. Energy 2022, 187, 819–828. [Google Scholar] [CrossRef]
- Duong, V.T.; Ahmed, F.; Thomas-Hall, S.R.; Quigley, S.; Nowak, E.; Schenk, P.M. High Protein- and High Lipid-Producing Microalgae from Northern Australia as Potential Feedstock for Animal Feed and Biodiesel. Front. Bioeng. Biotechnol. 2015, 3, 53. [Google Scholar] [CrossRef]
- Tang, C.; Dai, D.; Li, S.; Qv, M.; Liu, D.; Li, Z.; Huang, L.Z.; Zhu, L. Responses of Microalgae under Different Physiological Phases to Struvite as a Buffering Nutrient Source for Biomass and Lipid Production. Bioresour. Technol. 2023, 384, 129352. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Gulfraz, M.; Raja, G.K.; Inam-ul-Haq, M.; Awais, M.; Mustafa, M.S.; Khan, S.U.; Tlili, I.; Shadloo, M.S. Screening of Native Hyper-Lipid Producing Microalgae Strains for Biomass and Lipid Production. Renew. Energy 2020, 160, 1295–1307. [Google Scholar] [CrossRef]
- Xue, S.J.; Chi, Z.; Zhang, Y.; Li, Y.F.; Liu, G.L.; Jiang, H.; Hu, Z.; Chi, Z.M. Fatty Acids from Oleaginous Yeasts and Yeast-like Fungi and Their Potential Applications. Crit. Rev. Biotechnol. 2018, 38, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Chapkin, R.S. Importance of Dietary γ-Linolenic Acid in Human Health and Nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef]
- Yang, B.; Gao, H.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, Y.Q.; Chen, H.; Chen, W. Bacterial Conjugated Linoleic Acid Production and Their Applications. Prog. Lipid Res. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Venkatakrishnan, P.; Palanisamy, P. Synthesis and Characterization of Myristic Acid—Infused Al2O3/CuO/MWCNT Nanocomposites for Energy Storage and Cooling Applications. Phys. Scr. 2024, 99. [Google Scholar] [CrossRef]
- Basene, Y.; Gothalwal, R. Effective Use and Transformation of Organic/Agriculture Waste for Energy Recovery along with Zero Carbon Footprint. Energy 2022, 10, 2307–2316. [Google Scholar] [CrossRef]
- Andersson, V.; Heyne, S.; Harvey, S.; Berntsson, T. Integration of Algae-Based Biofuel Production with an Oil Refinery: Energy and Carbon Footprint Assessment. Int. J. Energy Res. 2020, 44, 10860–10877. [Google Scholar] [CrossRef]
- Al Azad, S.; Madadi, M.; Song, G.; Sun, C.; Sun, F. New Trends in Microbial Lipid-Based Biorefinery for Fermentative Bioenergy Production from Lignocellulosic Biomass. Biofuel Res. J. 2024, 11, 2040–2064. [Google Scholar] [CrossRef]
- Baral, N.R.; Banerjee, D.; Mukhopadhyay, A.; Simmons, B.A.; Singer, S.W.; Scown, C.D. Integration of Genome-Scale Metabolic Model with Biorefinary Process Model Reveals Market-Competitive Carbon-Negative Sustainable Aviation Fuel Utilizing Microbial Cell Mass Lipids and Biogenic CO2. Bioresources 2024, 19, 4056–4086. [Google Scholar] [CrossRef]
- Jones, A.D.; Boundy-Mills, K.L.; Barla, G.F.; Kumar, S.; Ubanwa, B.; Balan, V. Microbial Lipid Alternatives to Plant Lipids. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1995, pp. 1–32. [Google Scholar]
- Xu, Q.; Tang, Q.; Xu, Y.; Wu, J.; Mao, X.; Li, F.; Wang, S.; Wang, Y. Biotechnology in Future Food Lipids: Opportunities and Challenges. Annu. Rev. Food Sci. Technol. 2023, 14, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, C.; Zissis, G.; Buso, D. Influence of Different Abiotic Factors on Lipid Production by Microalgae—A Review. OCL—Oilseeds Fats Crops Lipids 2021, 28, 57. [Google Scholar] [CrossRef]
- Lee, J.-E.; Vadlani, P.V.; Min, D. Sustainable Production of Microbial Lipids from Lignocellulosic Biomass Using Oleaginous Yeast Cultures. J. Sustain. Bioenergy Syst. 2017, 7, 36–50. [Google Scholar] [CrossRef]
- Gallego-García, M.; Susmozas, A.; Negro, M.J.; Moreno, A.D. Challenges and Prospects of Yeast-Based Microbial Oil Production within a Biorefinery Concept. Microb. Cell Fact. 2023, 22, 246. [Google Scholar] [CrossRef] [PubMed]
- Christophe, G.; Kumar, V.; Nouaille, R.; Gaudet, G.; Fontanille, P.; Pandey, A.; Soccol, C.R.; Larroche, C. Recent Developments in Microbial Oils Production: A Possible Alternative to Vegetable Oils for Biodiesel Without Competition with Human Food? Braz. Arch. Biol. Technol. 2012, 55, 29–46. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.C.; Ma, C. Elevating Biotransamiantion of Pineapple Peel-Derived Furfural into Furfurylamine by Recombinant Escherichia coli Carrying Pyruvate Decarboxylase and Mutant ω-Transaminase with Low Loading of Amine Donor. Food Biosci. 2024, 62, 105164. [Google Scholar] [CrossRef]
- Wedan, R.J.; Longenecker, J.Z.; Nowinski, S.M. Mitochondrial Fatty Acid Synthesis Is an Emergent Central Regulator of Mammalian Oxidative Metabolism. Cell Metab. 2024, 36, 36–47. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, M.; Chen, H.; Lu, X.; Tian, Y.; Hu, P.; Zhao, Q.; Li, P.; Li, C.; Ji, X.; et al. Metabolic Engineering of Yarrowia lipolytica for High-Level Production of Squalene. Bioresour. Technol. 2024, 394, 130233. [Google Scholar] [CrossRef]
- Han, Z.; Zong, Y.; Zhang, X.; Gong, D.; Wang, B.; Prusky, D.; Sionov, E.; Xue, H.; Bi, Y. Erg4 Is Involved in Ergosterol Biosynthesis, Conidiation and Stress Response in Penicillium Expansum. J. Fungi 2023, 9, 568. [Google Scholar] [CrossRef]
- Park, J.K.; Jeon, J.M.; Yang, Y.H.; Kim, S.H.; Yoon, J.J. Efficient Polyhydroxybutyrate Production Using Acetate by Engineered Halomonas sp. JJY01 Harboring Acetyl-CoA Acetyltransferase. Int. J. Biol. Macromol. 2024, 254, 127475. [Google Scholar] [CrossRef]
- Baik, Y.; Lee, K.; Choi, M. Catalytic Conversion of Triglycerides into Diesel, Jet Fuel, and Lube Base Oil. Chin. J. Catal. 2024, 58, 15–24. [Google Scholar] [CrossRef]
- Worland, A.M.; Han, Z.; Maruwan, J.; Wang, Y.; Du, Z.Y.; Tang, Y.J.; Su, W.W.; Roell, G.W. Elucidation of Triacylglycerol Catabolism in Yarrowia lipolytica: How Cells Balance Acetyl-CoA and Excess Reducing Equivalents. Metab. Eng. 2024, 85, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Kurt, E.; LBassi, T.; Sa, L.; Xie, D. Biotechnological Production of Omega-3 Fatty Acids: Current Status and Future Perspectives. Front. Microbiol. 2023, 14, 1280296. [Google Scholar] [CrossRef] [PubMed]
- Eilam, Y.; Khattib, H.; Pintel, N.; Avni, D. Microalgae—Sustainable Source for Alternative Proteins and Functional Ingredients Promoting Gut and Liver Health. Glob. Chall. 2023, 7, 2200177. [Google Scholar] [CrossRef]
- Calarnou, L.; Vigouroux, E.; Thollas, B.; Le Grand, F.; Mounier, J. Screening for the Production of Polyunsaturated Fatty Acids and Cerebrosides in Fungi. J. Appl. Microbiol. 2024, 135, 30. [Google Scholar] [CrossRef]
- Jiru, T.M.; Groenewald, M.; Pohl, C.; Steyn, L.; Kiggundu, N.; Abate, D. Optimization of Cultivation Conditions for Biotechnological Production of Lipid by Rhodotorula kratochvilovae (Syn, Rhodosporidium kratochvilovae) SY89 for Biodiesel Preparation. 3 Biotech 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Gorte, O.; Kugel, M.; Ochsenreither, K. Optimization of Carbon Source Efficiency for Lipid Production with the Oleaginous Yeast Saitozyma podzolica DSM 27192 Applying Automated Continuous Feeding. Biotechnol. Biofuels 2020, 13, 181. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Saini, R.; Hegde, K.; Brar, S.K.; Lefebvre, A.; Avalos Ramirez, A. Carbon/Nitrogen Ratio as a Tool to Enhance the Lipid Production in Rhodosporidium toruloides-1588 Using C5 and C6 Wood Hydrolysates. J. Clean. Prod. 2023, 384, 135687. [Google Scholar] [CrossRef]
- Ye, Z.; Sun, T.; Hao, H.; He, Y.; Liu, X.; Guo, M.; Chen, G. Optimising Nutrients in the Culture Medium of Rhodosporidium toruloides Enhances Lipids Production. AMB Express 2021, 11. [Google Scholar] [CrossRef]
- Gao, B.; Wang, F.; Huang, L.; Liu, H.; Zhong, Y.; Zhang, C. Biomass, Lipid Accumulation Kinetics, and the Transcriptome of Heterotrophic Oleaginous Microalga Tetradesmus bernardii under Different Carbon and Nitrogen Sources. Biotechnol. Biofuels 2021, 14. [Google Scholar] [CrossRef]
- Konzock, O.; Zaghen, S.; Fu, J.; Kerkhoven, E.J. Urea Is a Drop-in Nitrogen Source Alternative to Ammonium Sulphate in Yarrowia lipolytica. iScience 2022, 25, 105703. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, K.; Sundararaju, P.; Srinivasan, N.; Uthandi, S. Bioconversion of Sago Processing Wastewater into Biodiesel: Optimization of Lipid Production by an Oleaginous Yeast, Candida tropicalis ASY2 and Its Transesterification Process Using Response Surface Methodology. Microb. Cell Fact. 2021, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, A.; Bhran, A.; Rasmey, A.H.; Mikky, Y. Optimization of Cultural Conditions for Lipid Accumulation by Aspergillus Wentii Ras101 and Its Transesterification to Biodiesel: Application of Response Surface Methodology. 3 Biotech 2018, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A Comprehensive Classification System for Lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Yellapu, S.K.; Bharti; Kaur, R.; Kumar, L.R.; Tiwari, B.; Zhang, X.; Tyagi, R.D. Recent Developments of Downstream Processing for Microbial Lipids and Conversion to Biodiesel. Bioresour. Technol. 2018, 256, 515–528. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Morales-Palomo, S.; González-Fernández, C. Microbial Lipids from Organic Wastes: Outlook and Challenges. Bioresour. Technol. 2021, 323, 124612. [Google Scholar] [CrossRef]
- Joseph Antony Sundarsingh, T.; Ameen, F.; Ranjitha, J.; Raghavan, S.; Shankar, V. Engineering Microbes for Sustainable Biofuel Production and Extraction of Lipids—Current Research and Future Perspectives. Fuel 2024, 355, 129532. [Google Scholar] [CrossRef]
- Min, D.B.; Ellefson, W.C. Food Analysis, 4th ed.; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; Volume 2, ISBN 978-1-4419-1477-4. [Google Scholar]
- Breil, C.; Abert Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and Folch Methods for Solid–Liquid–Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents. Int. J. Mol. Sci. 2017, 18, 708. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Porto de Souza Vandenberghe, L.; Karp, S.G.; Murawski de Mello, A.F.; Thomaz Soccol, V.; Soccol, C.R. Microbial Lipid Production from Soybean Hulls Using Lipomyces starkeyi LPB53 in a Circular Economy. Bioresour. Technol. 2023, 372, 128650. [Google Scholar] [CrossRef]
- Ponnumsamy, V.K.; Al-Hazmi, H.E.; Shobana, S.; Dharmaraja, J.; Jadhav, D.A.; Piechota, G.; Igliński, B.; Kumar, V.; Bhatnagar, A.; Chae, K.J.; et al. A Review on Homogeneous and Heterogeneous Catalytic Microalgal Lipid Extraction and Transesterification for Biofuel Production. Chin. J. Catal. 2024, 59, 97–117. [Google Scholar] [CrossRef]
- Enomoto, A.; Fukushima, H.; Nagai, K.; Hakoda, M.; Nakamura, K. Disruption of Microbial Cells by the Flash Discharge of High-Pressure Carbon Dioxide. Biosci. Biotechnol. Biochem. 1994, 58, 1297–1301. [Google Scholar] [CrossRef]
- Silva, J.d.M.E.; Martins, L.H.d.S.; Moreira, D.K.T.; Silva, L.d.P.; Barbosa, P.d.P.M.; Komesu, A.; Ferreira, N.R.; Oliveira, J.A.R.d. Microbial Lipid Based Biorefinery Concepts: A Review of Status and Prospects. Foods 2023, 12, 2074. [Google Scholar]
- Middelberg, A.P.J. Process-Scale Disruption of Microorganisms. Biotechnol. Adv. 1995, 13, 491–551. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.; Zhao, S.; Yang, X.; Xu, W.; Guo, M.; Xu, E.; Ding, T.; Ye, X.; Liu, D. Ultrasound-Assisted Extraction of Lipids as Food Components: Mechanism, Solvent, Feedstock, Quality Evaluation and Coupled Technologies—A Review. Trends Food Sci. Technol. 2022, 122, 83–96. [Google Scholar] [CrossRef]
- Marques, F.; Pinho, M.; Guerra, I.M.S.; Conde, T.A.; Silva, J.; Cardoso, H.; Martins, M.; Abreu, M.H.; Cerqueira, M.Â.; Domingues, M.R. Unlocking Functional Lipid Ingredients from Algae by Food-Grade Biosolvents and Ultrasound-Assisted Extraction for Nutritional Applications. LWT 2024, 200. [Google Scholar] [CrossRef]
- Mienis, E.; Vandamme, D.; Foubert, I. Ultrasound Assisted Extraction of Nannochloropsis: Effects on Lipid Extraction Efficiency and Lipid Stability. Algal Res. 2024, 80. [Google Scholar] [CrossRef]
- Karimi Sani, I.; Mehrnoosh, F.; Rasul, N.H.; Hassani, B.; Mohammadi, H.; Gholizadeh, H.; Sattari, N.; Kaveh, M.; Khodaei, S.M.; Alizadeh Sani, M.; et al. Pulsed Electric Field-Assisted Extraction of Natural Colorants; Principles and Applications. Food Biosci. 2024, 61, 104746. [Google Scholar] [CrossRef]
- Ferraz, L.P.; Silva, E.K. Pulsed Electric Field-Assisted Extraction Techniques for Obtaining Vegetable Oils and Essential Oils: Recent Progress and Opportunities for the Food Industry. Sep. Purif. Technol. 2025, 354, 128833. [Google Scholar] [CrossRef]
- Straessner, R.; Nikolausz, M.; Silve, A.; Nazarova, N.; Wuestner, R.; Papachristou, I.; Akaberi, S.; Leber, K.; Mueller, G.; Frey, W. Holistic Exploitation of Pulsed Electric Field (PEF)-Treated and Lipid Extracted Microalgae Auxenochlorella protothecoides, Utilizing Anaerobic Digestion (AD). Algal Res. 2023, 69. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Filho, R.M. Recent Advances in Lipid Extraction Using Green Solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Duarte, S.H.; dos Santos, P.; Michelon, M.; Oliveira, S.M.d.P.; Martínez, J.; Maugeri, F. Recovery of Yeast Lipids Using Different Cell Disruption Techniques and Supercritical CO2 Extraction. Biochem. Eng. J. 2017, 125, 230–237. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of Supercritical CO2 in Lipid Extraction—A Review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.J.; Guo, H.W.; Wang, Y.J.; Ji, R.; Kang, L.L.; Wang, Y.T.; Guo, X.; Li, J.G.; Jiang, L.Q.; et al. A Review of the Strategy to Promote Microalgae Value in CO2 Conversion-Lipid Enrichment-Biodiesel Production. J. Clean. Prod. 2024, 436, 140538. [Google Scholar] [CrossRef]
- Santana, A.; Jesus, S.; Larrayoz, M.A.; Filho, R.M. Supercritical Carbon Dioxide Extraction of Algal Lipids for the Biodiesel Production. Procedia Eng. 2012, 42, 1755–1761. [Google Scholar]
- Gomez-Gomez, A.; Brito-de la Fuente, E.; Gallegos, C.; Garcia-Perez, J.V.; Benedito, J. Combination of Supercritical CO2 and High-Power Ultrasound for the Inactivation of Fungal and Bacterial Spores in Lipid Emulsions. Ultrason. Sonochem. 2021, 76, 105636. [Google Scholar] [CrossRef]
- Vasilakis, G.; Gamraoui, A.; Karayannis, D.; Giannakis, N.; Chatti, A.; Politis, I.; Diamantopoulou, P.; Papanikolaou, S. Mycelial Mass, Microbial Lipids and γ-Linolenic Acid (GLA) by Cunninghamella elegans Cultivated on Agro-Industrial Residues. Resour. Chem. Mater. 2024. [Google Scholar] [CrossRef]
- Das, S.; Das, S.; Ghangrekar, M.M. Enzymatic Cell Disruption Followed by Application of Imposed Potential for Enhanced Lipid Extraction from Wet Algal Biomass Employing Photosynthetic Microbial Fuel Cell. Bioresour. Technol. 2022, 363. [Google Scholar] [CrossRef]
- Carruthers, D.N.; Lee, T.S. Translating Advances in Microbial Bioproduction to Sustainable Biotechnology. Front. Bioeng. Biotechnol. 2022, 10, 968437. [Google Scholar] [CrossRef]
- Long, X.; He, N.; He, Y.; Jiang, J.; Wu, T. Biosurfactant Surfactin with PH-Regulated Emulsification Activity for Efficient Oil Separation When Used as Emulsifier. Bioresour. Technol. 2017, 241, 200–206. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Q.; Wang, X.; Li, Y.; Zhou, Q. Surfactants Selectively Reallocated the Bacterial Distribution in Soil Bioelectrochemical Remediation of Petroleum Hydrocarbons. J. Hazard. Mater. 2018, 344, 23–32. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial Biosurfactants: A Review of Recent Environmental Applications. Bioeng. 2022, 13, 12365–12391. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Hayashi, J.A. Structure of a Rhamnolipid from Pseudomonas aeruginosa. Arch. Biochem. Biophys. 1965, 111, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Peñalver, P.; Rodríguez, A.; Daverey, A.; Font, X.; Gea, T. Use of Wastes for Sophorolipids Production as a Transition to Circular Economy: State of the Art and Perspectives. Rev. Environ. Sci. Biotechnol. 2019, 18, 413–435. [Google Scholar] [CrossRef]
- Pi, Y.; Bao, M.; Liu, Y.; Lu, T.; He, R. The Contribution of Chemical Dispersants and Biosurfactants on Crude Oil Biodegradation by Pseudomonas sp. LSH-7′. J. Clean. Prod. 2017, 153, 74–82. [Google Scholar] [CrossRef]
- Grand View Research Biosurfactants Market Size, Share & Trends Analysis Report By Product (Rhamnolipids, Sophorolipids, MES, APG, Sorbitan Esters, Sucrose Esters), By Application (Household), By Region And Segment Forecasts, 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/biosurfactants-industry (accessed on 17 October 2024).
- Heider, S.A.E.; Peters-Wendisch, P.; Netzer, R.; Stafnes, M.; Brautaset, T.; Wendisch, V.F. Production and Glucosylation of C50 and C40 Carotenoids by Metabolically Engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2014, 98, 1223–1235. [Google Scholar] [CrossRef]
- Barretto, D.A.; Vootla, S.K. In Vitro Anticancer Activity of Staphyloxanthin Pigment Extracted from Staphylococcus gallinarum KX912244, a Gut Microbe of Bombyx mori. Indian. J. Microbiol. 2018, 58, 146–158. [Google Scholar] [CrossRef]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.W. Recent Development in the Production Strategies of Microbial Carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef]
- Cardoso, L.A.C.; Karp, S.G.; Vendruscolo, F.; Kanno, K.Y.F.; Zoz, L.I.C.; Carvalho, J.C. Biotechnological Production of Carotenoids and Their Applications in Food and Pharmaceutical Products. In Carotenoids; Cvetkovic, D.J., Nikolic, G.S., Eds.; InTechOpen: Rijeka, Croatia, 2017; Volume 1, pp. 125–142. [Google Scholar]
- Grand View Research Carotenoids Market Size, Share & Trends Analysis Report By Product, By Application (Food, Supplements, Feed, Pharmaceuticals, Cosmetics), By Source, By Region, And Segment Forecasts, 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/carotenoids-market (accessed on 17 October 2024).
- Germany’s Union for the Promotion of Oil and Protein Plants World Production of Biodiesel Rises to Record Level–Demand for Soybeans Drives Biodiesel Fuel Production in North and South America. Available online: https://www.ufop.de/english/news/chart-week/#kw23_2024 (accessed on 17 October 2024).
- Pandey, A.K.; Sirohi, R.; Gaur, V.K.; Pandey, K.; Udayan, A.; Sharma, P.; Pilli, S.; Kim, S.H.; Pandey, A. Sustainable Technologies for Biodiesel Production from Microbial Lipids. In Biomass, Biofuels, Biochemicals Circular Bioeconomy: Technologies for Biofuels and Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–66. [Google Scholar] [CrossRef]
- Radlein, D.; Quignard, A. Une Bréve Revue Historique de La Pyrolyse Rapide de La Biomasse. Oil Gas Sci. Technol. 2013, 68, 765–783. [Google Scholar] [CrossRef]
- Béligon, V.; Christophe, G.; Fontanille, P.; Larroche, C. Microbial Lipids as Potential Source to Food Supplements. Curr. Opin. Food Sci. 2016, 7, 35–42. [Google Scholar] [CrossRef]
- Birch, E.E.; Garfield, S.; Castañeda, Y.; Hughbanks-Wheaton, D.; Uauy, R.; Hoffman, D. Visual Acuity and Cognitive Outcomes at 4 Years of Age in a Double-Blind, Randomized Trial of Long-Chain Polyunsaturated Fatty Acid-Supplemented Infant Formula. Early Hum. Dev. 2007, 83, 279–284. [Google Scholar] [CrossRef]
- Horrobin, D.F. Nutritional and Medical Importance of Gamma-Linolenic Acid. Prog. Lipid Res. 1992, 31, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N. Polyhydroxybutyrate (PHB) Production by Bacteria and Its Application as Biodegradable Plastic in Various Industries. Acad. J. Polym. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In Vivo and Post-Synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef] [PubMed]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving Biological Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) Co-Polymer: A Critical Review. Rev. Environ. Sci. Biotechnol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Valdés, G.; Mendonça, R.T.; Aggelis, G. Lignocellulosic Biomass as a Substrate for Oleaginous Microorganisms: A Review. Appl. Sci. 2020, 10, 7698. [Google Scholar] [CrossRef]
- El Kantar, S.; Khelfa, A.; Vorobiev, E.; Koubaa, M. Strategies for Increasing Lipid Accumulation and Recovery from Y. Lipolytica: A Review. OCL—Oilseeds Fats Crops Lipids 2021, 28, 51. [Google Scholar]
- Li, Y.P.; Ahmadi, F.; Kariman, K.; Lackner, M. Recent Advances and Challenges in Single Cell Protein (SCP) Technologies for Food and Feed Production. NPJ Sci. Food 2024, 8, 66. [Google Scholar] [CrossRef]
- Němcová, A.; Szotkowski, M.; Samek, O.; Cagáňová, L.; Sipiczki, M.; Márová, I. Use of Waste Substrates for the Lipid Production by Yeasts of the Genus Metschnikowia—Screening Study. Microorganisms 2021, 9, 2295. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Lin, Q.-L.; Jin, X.-C.; Wang, X.-L.; Zhao, Y. Screening and Fermentation Optimization of Microbial Lipid-Producing Molds from Forest Soils. Afr. J. Microbiol. Res. 2010, 4, 1462–1468. [Google Scholar]
- Hashem, A.H.; Abu-Elreesh, G.; El-Sheikh, H.H.; Suleiman, W.B. Isolation, Identification, and Statistical Optimization of a Psychrotolerant Mucor racemosus for Sustainable Lipid Production. Biomass Convers. Biorefin 2023, 13, 3415–3426. [Google Scholar] [CrossRef]
- Li, Y.; Ghasemi Naghdi, F.; Garg, S.; Adarme-Vega, T.C.; Thurecht, K.J.; Ghafor, W.A.; Tannock, S.; Schenk, P.M. A Comparative Study: The Impact of Different Lipid Extraction Methods on Current Microalgal Lipid Research. Microb. Cell Fact. 2014, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, X.; Turcotte, F.; Deschênes, J.S.; Tremblay, R.; Jolicoeur, M. Current Lipid Extraction Methods Are Significantly Enhanced Adding a Water Treatment Step in Chlorella protothecoides. Microb. Cell Fact. 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wei, C.; Yan, Q.; Shan, X.; Wu, M.; Zhao, X.; Song, Y. Optimization of a Novel Lipid Extraction Process from Microalgae. Sci. Rep. 2021, 11, 20221. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22I, 13643. [Google Scholar] [CrossRef]
- Retra, K.; Bleijerveld, O.B.; Van Gestel, R.A.; Tielens, A.G.M.; Van Hellemond, J.J.; Brouwers, J.F. A Simple and Universal Method for the Separation and Identification of Phospholipid Molecular Species. Rapid Commun. Mass. Spectrom. 2008, 22, 1853–1862. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L.; Lombardo, M. Promising Sources of Plant-Derived Polyunsaturated Fatty Acids: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 1683. [Google Scholar] [CrossRef]
- Priefert, H.; Schneider, J.; Windau, J. Method for Producing a Biomass with an Increased Content of Polyunsaturated Fatty Acids. Patent Application No. US20220017929A1, 7 November 2022. [Google Scholar]
- Kumar, G.R.K.; Subhash, V.G.; Musale, A.S.; Govindachary, S.; Prasad, V.; Gupta, S.D. Inducing Lipid in Fungi Rapidly Comprises Exposing the Fungi to Infrared Radiation. Patent Application No. 202021039478, 2022. [Google Scholar]
- Huang, Q.; Huang, J.; Zhang, S.; Xu, S.; Shen, X. Method for Improving Oil Yield of Microalgae in Photoheterotrophic System. Patent Application No. CN113512567A, 29 June 2021. [Google Scholar]
- Soccol, C.R.; Colonia, B.S.O.; De Melo Pereira, G.V.; Karp, S.G. Producing Traustochitrid Biomass Rich in Docosahexaenoic Acid Used in Animal Feed, by Using Sugarcane Molasses, Yeast Cream and Cornstarch as Substrates for Culture Medium, Artificial Sea Water and Monosodium Glutamate by Employing Auratiochytrium. Patent Application No. 870230058262, 2022. [Google Scholar]
- Petrie, J.R.; Singh, S.P.; El Tahchy, A.; Shrestha, P.; Devilla, R.A.; Nguyen, H. Composition Useful e.g., for Producing Food-like Aroma and Flavor When Heated, Comprises Mortierella Species Biomass, Branched Chain Fatty Acids, Sugars, Sugar Alcohols, Sugar Acids or Sugar Derivatives and Amino Acids or Thiamine. Patent Application No. WO2024050590A1, 14 March 2024. [Google Scholar]
- Ranjitha, J.; Vijayalakshmi, S.; Donatus, M.S.; Anand, M. Producing Biodiesel from Microalgae, Comprises e.g., Isolation of Oleaginous Microalgal Species from Agro-Industrial Waste Water, and Cultivation of Oleaginous Microalgal Species Using Media Optimized Heterotrophic Growth. Patent Application No. 202041037060, 2020. [Google Scholar]
- Cucchetti, D.; Frattini, A.; Del Seppia, A.; Prando, T. Producing Lipids in Presence of Oleaginous Yeast by Fermentation, Used to Produce Biofuels in Diesel Engines for Automotive or Aviation, Comprises Growing Oleaginous Cell Biomass, and Producing Lipid. Patent Application No. WO2023180925A1, 21 March 2023. [Google Scholar]
- Soccol, C.R.; De Melo Pereira, G.V.; De Carvalho Neto, D.P. Cosmetic Composition for Hair, Comprises Microbial Lipids from Dry and Enriched Biomass, Distilled Water as Vehicle, Surfactant, Conditioning Agent, Foam Active Stabilizer, Sequestrant Component, Wetting Component, and Thickening Component. Patent Application No. 870190128208, 2021. [Google Scholar]
- Mcnamara, H.M.; Ticku, S.; Heller, D.; Moevus, C.; Zhang, B. Refined, Bleached, and/or Deodorized Microbial Oil Used in Personal Care Composition, Is Produced by Oleaginous Yeast. Patent Application No. WO2022104063A2, 12 November 2022. [Google Scholar]
- Mcnamara, H.M.; Moevus, C.; Li, J.; Chapeaux, A. Isolating Bioproduct from Yeast Involves Treating Yeast Cells with Beta-1,3-Glucomannanase, Separating Lipid Phase from Aqueous Phase of Enzymatically Lysed Sample via Solvent or Non-Solvent Extraction, and Isolating Bioproduct from Sample. Patent Application No. WO2023220060A1, 9 May 2023. [Google Scholar]
- Ribeiro De Lima Pereira, E.; Dantas Calixto, C.; Barbosa Da Silva Araújo, V.; Da Costa Sassi, C.F.; Emerenciano Das Chagas, B.M.; De Lira, E.B.; Alves Alcântara, M.; Barboza Da Silva, V.M.; De Athayde Filho, P.F.; Sassi, R. Extracting Bioactive Compounds from Microalgae Involves Selecting Dry Microalgal Biomass in the Form of a Powder, Preferably from Scenedesmus quadricauda, Obtaining Saponifiable Compounds, Comprising Fatty and Unsaponifiable Acids. Patent Application No. 870190015157, 2020. [Google Scholar]
- Ratledge, C. Microbial Oils: An Introductory Overview of Current Status and Future Prospects. OCL—Oilseeds Fats Crops Lipids 2013, 20, D602. [Google Scholar] [CrossRef]
- Soccol, C.R.; Dalmas Neto, C.J.; Soccol, V.T.; Sydney, E.B.; da Costa, E.S.F.; Medeiros, A.B.P.; Vandenberghe, L.P.d.S. Pilot Scale Biodiesel Production from Microbial Oil of Rhodosporidium toruloides DEBB 5533 Using Sugarcane Juice: Performance in Diesel Engine and Preliminary Economic Study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals. Available online: https://www.undp.org/sustainable-development-goals (accessed on 31 October 2024).
- Qin, L.; Qin, Y.; Cui, N.; Han, X.; Lu, H.; Yang, T.; Liang, W. Photosynthetic Microalgae Microbial Fuel Cells for Bioelectricity Generation and Microalgae Lipid Recovery Using Gd-Co@N-CSs/NF as Cathode. Chem. Eng. J. 2024, 490. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).