Utilization of Silicon Dust to Prepare Si3N4 Used for Steelmaking Additives: Thermodynamics and Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Raw Materials
2.2. Experimental Methods
2.3. Characterization and Analytical Methods
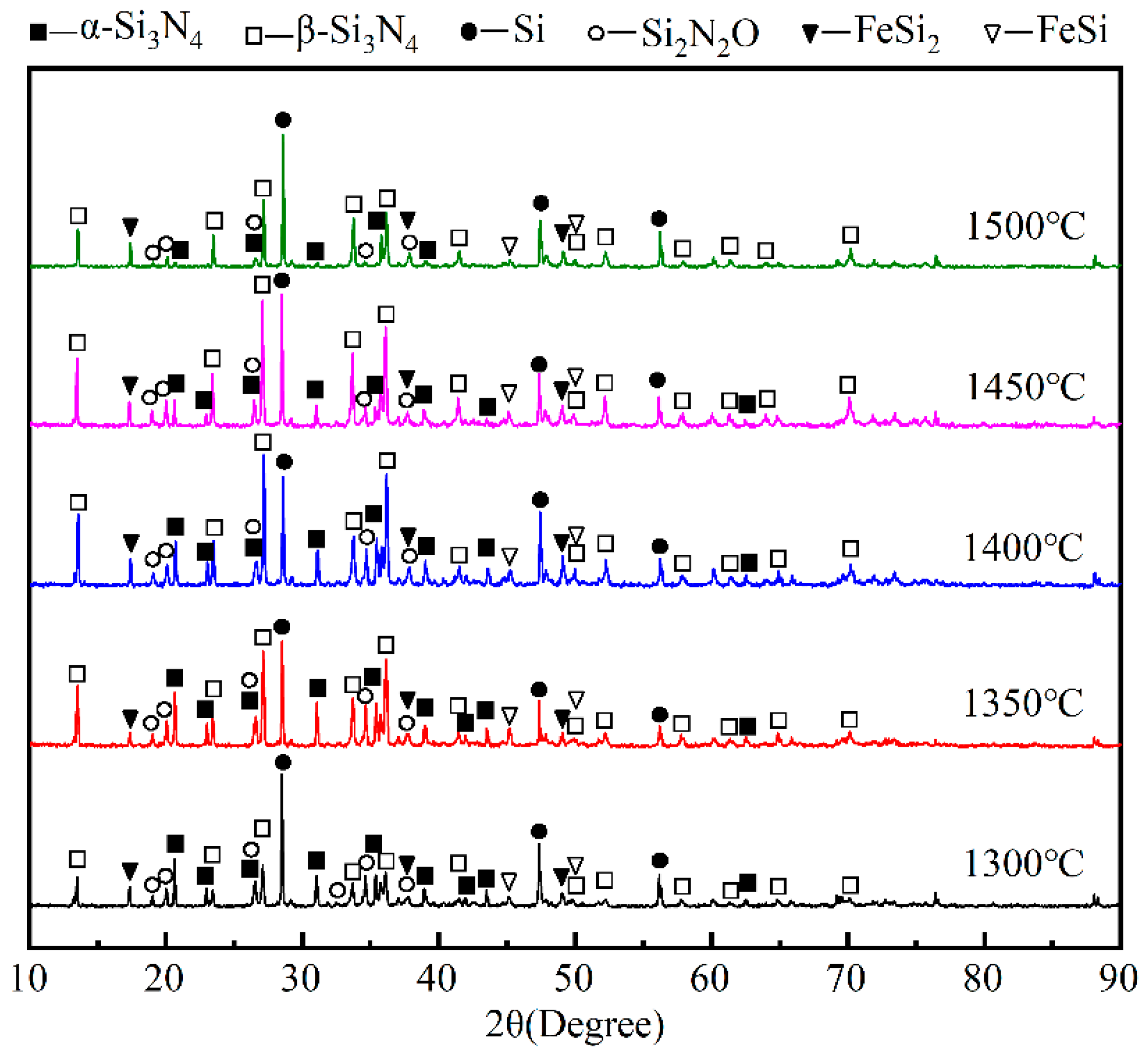
3. Results
4. Discussion
4.1. Thermodynamic Analysis of Silicon Dust Nitriding
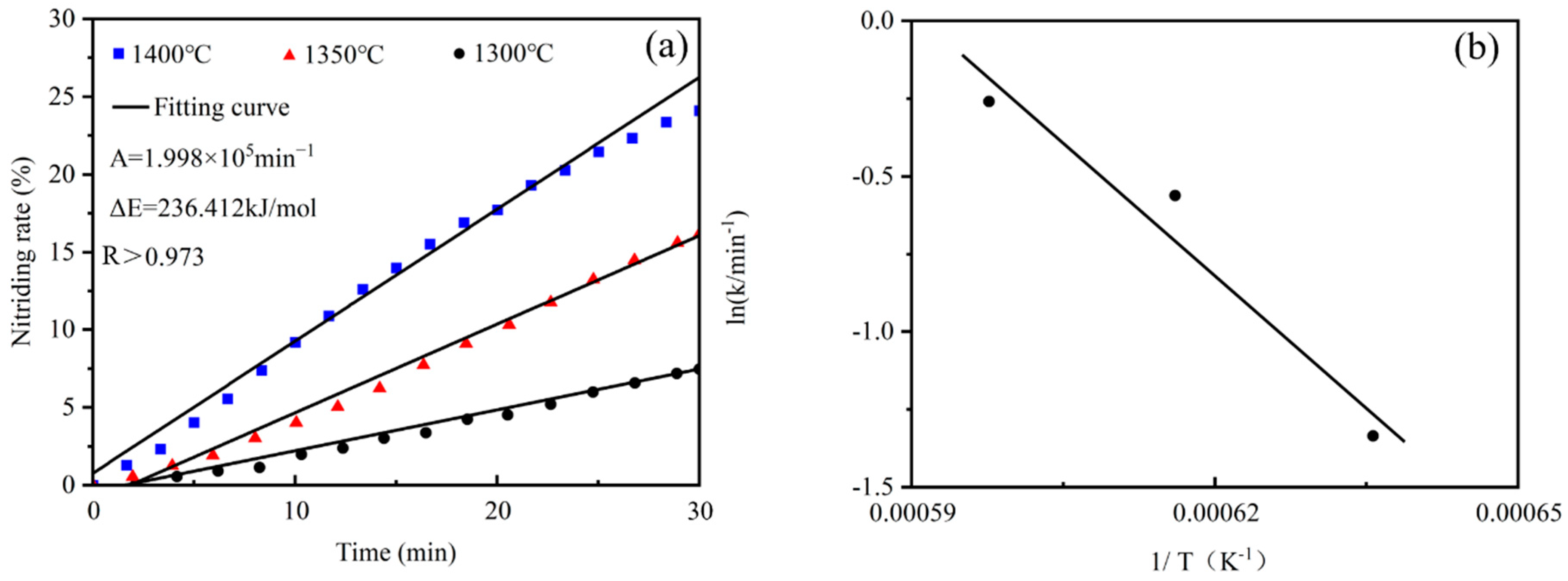
4.2. Kinetic Analysis of Silicon Dust Nitriding
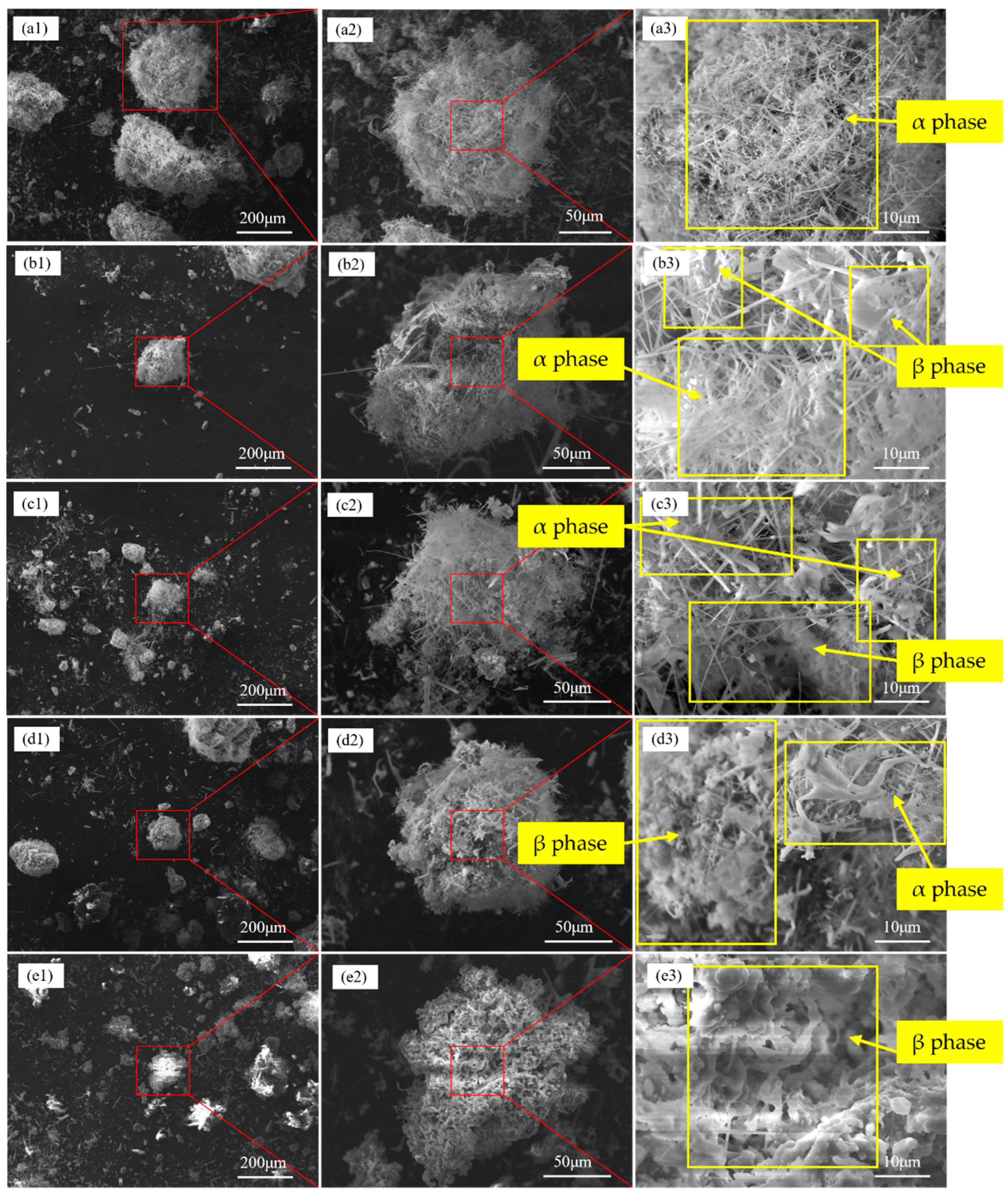
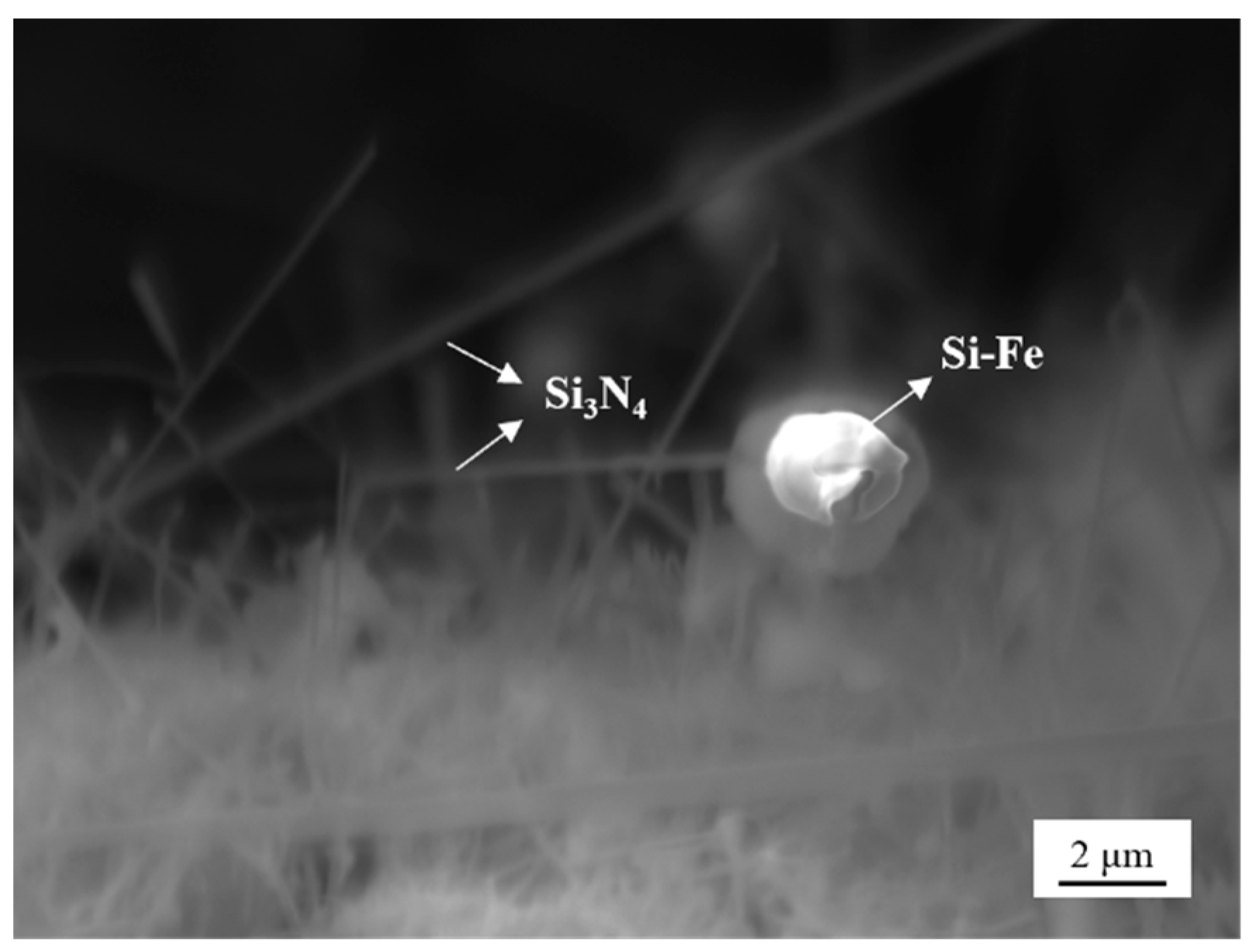
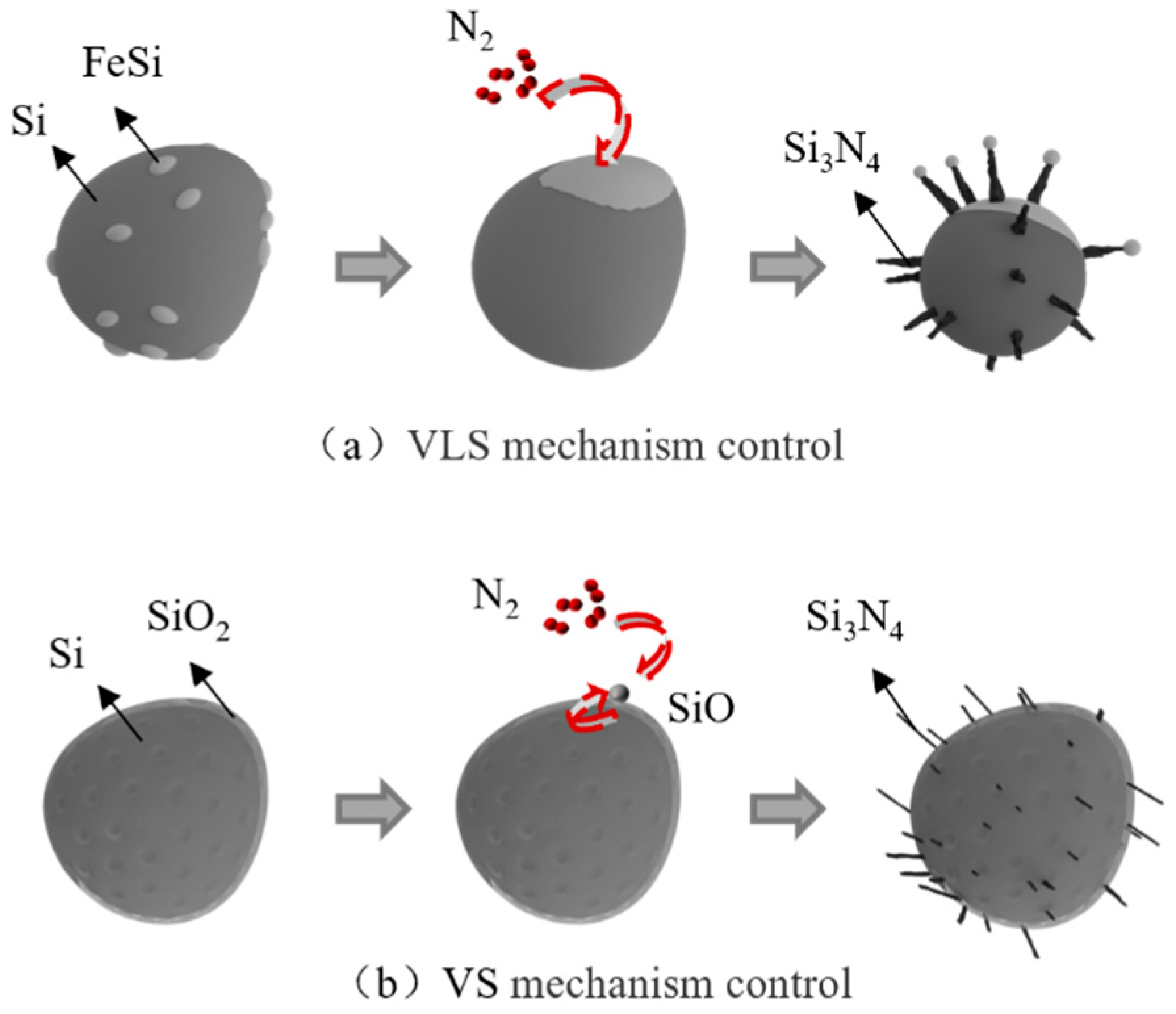
4.3. Nitriding Mechanism of Silicon Dust
5. Conclusions
- This study employs an isothermal thermogravimetric method to investigate the kinetics of the silicon dust nitriding reaction. The results show that when the silicon dust is held at temperatures of 1300, 1350, 1400, 1450, and 1500 °C for a duration of 3 h each, the weight gain rate is highest at 1350 °C (26.57%) and lowest at 1500 °C (16.97%). The reaction temperature of silicon dust nitriding is not the higher the better, but should be adjusted according to the specific reaction conditions.
- Silicon dust nitriding can be divided into two stages. For the first stage of the rate control step for the interfacial chemical reaction, the apparent activation energy is 2.36 × 105 kJ·mol−1, the reaction progress equation. The second stage of the rate control step for the diffusion control is due to the high temperature of the silicon dust occurring in the liquid-phase aggregation and solidification, and the diffusion of N2 has become the limiting link of the reaction rate.
- Silicon dust nitridation product is divided into spherical particles and massive particles, respectively, from the raw material of Si, Fe-aggregated particles and Si, SiO2-aggregated particles through the evolution of nitridation. Furthermore, the presence of Fe in the silicon dust as well as oxide layer on its surface can help to increase the rate of the reaction. Si particles growth primarily occurs during the vapor–liquid–solid (VLS) and the vapor–solid (VS) co-control process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Li, J.; Liu, H.; Ji, Y.; Zhong, Z.; Su, F. Recent advances in Rochow-Müller process research: Driving to molecular catalysis and to a more sustainable silicone industry. ChemCatChem 2019, 11, 2757–2769. [Google Scholar] [CrossRef]
- Hamawand, I.B.; Hanna, F.Z. Production of organosilane by the direct reaction of silicon with methyl chloride. Eng. Technol. J. 2007, 25, 1128–1138. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, Z.L.; Ji, Y.J. One-dimensional Cu-based catalysts with layered Cu–Cu2O–CuO walls for the Rochow reaction. Nano Res. 2016, 9, 1377–1399. [Google Scholar]
- Lu, J.P.; Yang, L.; Tan, S.M.; He, W.; Lu, X. A Kind of Silicon Dregs Resource Treatment Method. CN 113666376 A 19 November 2021. [Google Scholar]
- Fu, X.H.; Wang, F.H.; Zhou, Z.S. Synthesis of Tetrachlorosilane from Spent Silicon Contact Mass. Silicone Mater. 2011, 25, 397–403. [Google Scholar]
- Feng, L.; Shen, Y.B.; Gao, L. Comprehensive utilization of the spent silicon contact mass. New Chem. Mater. 2014, 42, 226–228. [Google Scholar]
- He, Q.; Yu, J.; Wang, Y. Silicon nanoparticles prepared from industrial wastes as high-performing anode materials for lithium-ion batteries. Solid State Ion. 2018, 325, 141. [Google Scholar] [CrossRef]
- Lu, X.; Gu, F.; Liu, Q. Ni–MnOx catalysts supported on Al2O3-modified Si waste with outstanding CO methanation catalytic performance. Ind. Eng. Chem. Res. 2015, 54, 12516–12524. [Google Scholar] [CrossRef]
- Wang, H.X.; Li, Z.B. Experimental study on the use of Si3N4 for the production of HRB400 grade III steel bars. Iron Stell 2007, 1, 14. [Google Scholar]
- Yang, C.L.; Tang, G.B.; Liu, X. Experimental study and trial production of low cost hot rolled rebar by adding Si3N4. Mater. Heat Treat. 2011, 40, 5. [Google Scholar]
- Buscarino, G.; Vaccaro, G.; Agnello, S. Variability of the Si-O-Si angle in amorphous-SiO2 probed by electron paramagnetic resonance and Raman spectroscopy. J. Non-Cryst. Solids 2009, 355, 18–21. [Google Scholar] [CrossRef]
- Avdyukova, N.V.; Korobeinicheva, I.K.; Shmidt, É.N. Use of the Raman spectra for determining tri-and tetrasubstituted C=C bonds in terpene compounds. Chem. Nat. Compd. 1974, 10, 694–695. [Google Scholar] [CrossRef]
- Ichiro, S.; Hirofumi, O.; Keijiro, T.; Akihiro, Y.; Etsuo, N.; Charmian, J. Raman scattering study of the interaction of 3-aminopropyltriethoxy silane on silica gel. Time-dependent conformational change of aminopropylsilyl segments. Vib. Spectrosc. 1997, 14, 125–132. [Google Scholar]
- Flörke, O.W.; Köhler, H.B.; Langer, K.; Tönges, I. Water inmicrocrystalline quartz of volcanic origin: Agates. Contrib. Mineral. Petrol. 1982, 80, 324–333. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, D.; Dong, J. Direct synthesis of methylchlorosilanes: Catalysts, mechanisms, reaction conditions, and reactor designs. Org. Process Res. Dev. 2022, 26, 2270. [Google Scholar] [CrossRef]
- Chang, F.W.; Liou, T.H.; Tsai, F.M. The nitridation kinetics of silicon powder compacts. Thermochimica Acta. 2000, 35, 71–80. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, G.H.; Chou, K.C. Preparation of α-Si3N4 by direct nitridation using polysilicon waste by diamond wire cutting. Int. J. Appl. Ceram. Technol. 2020, 17, 84–93. [Google Scholar] [CrossRef]
- Yu, C.H.; Chiu, K.A.; Do, T.H. Formation of Aligned α-Si3N4 Microfibers by Plasma Nitridation of Si (110) Substrate Coated with SiO2. Coatings 2021, 11, 1251. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Yang, Z. Preparation and Reaction Mechanism of Combustion Synthesis of α-Si3N4 Powder from Si Cutting Waste. Silicon 2023, 15, 3159–3163. [Google Scholar] [CrossRef]
- Li, Y.W.; Zhang, X.; Tian, H.B. Preparation of silicon nitride powder by direct nitridation process. Bull. Chin. Ceram. Soc. 2003, 22, 30–34. [Google Scholar]
- Yang, J.; Wu, P.; Wang, L. Study on rapid nitridation process of molten silicon by thermogravimetry and in situ Raman spectroscopy. J. Am. Ceram. Soc. 2022, 105, 5627. [Google Scholar] [CrossRef]
- Yao, G.S.; Li, Y.; Jiang, P. Formation mechanisms of Si3N4 and Si2N2O in silicon powdernitridation. Solid State Sci. 2017, 66, 50–56. [Google Scholar] [CrossRef]
- Jennings, H.M. Review on reactions between silicon and nitrogen-Part 1 Mechanisms. J. Mater. Sci. 1983, 18, 951–967. [Google Scholar] [CrossRef]
- Jennings, H.M.; Dalgleish, B.J.; Pratt, P.L. Reactions between silicon and nitrogen. J. Mater. Sci. 1988, 23, 2573–2583. [Google Scholar] [CrossRef]
- Jennings, H.M.; Richman, M.H. Structure, formation, mechanism and kinetics of reaction-bonded silicon nitride. J. Mater. Sci. 1976, 11, 2087–2098. [Google Scholar] [CrossRef]
- Han, L.; Wang, J.; Li, F. Low-temperature preparation of Si3N4 whiskers bonded/reinforced SiC porous ceramics via foam-gelcasting combined with catalytic nitridation. J. Eur. Ceram. Soc. 2018, 38, 1210. [Google Scholar] [CrossRef]
- Jin, X.; Ran, S.; Fang, Z. Recycling the diamond-wire saw silicon powder for preparing the regulable Si3N4 materials by non-catalytic nitridation. J. Clean. Prod. 2022, 371, 133656. [Google Scholar] [CrossRef]
- Jin, X.; Zhuang, Y.; Xing, P. Preparation of reactive sintering Si3N4-Si2N2O composites ceramics with diamond-wire saw powder waste as raw material. J. Hazard. Mater. 2020, 400, 123145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wang, Y.Y.; Peng, N. Isothermal reduction kinetics of zinc calcine under carbon monoxide. Trans. Nonferrous Met. Soc. China 2020, 30, 2274–2282. [Google Scholar] [CrossRef]
- Li, M.C.; Wang, L.; Yang, F. Reaction behavior of reduction of WO3 to WO2.72 under CO-CO2 mixed gases. Chin. J. Nonferrous Met. 2022, 32, 866–882. [Google Scholar]
- Jin, X.X.; Peng, F.Z.; Yan, X.K.; Jian, J.; Sheng, N.W.; Dong, H. Effect of Si3N4 diluent on direct nitridation of silicon powder. Ceram. Int. 2019, 45, 10943–10950. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, K.; Sun, S. Synthesis of Si3N4 nanowires by catalyst-free nitridation of (Si + SiO2) mixture. Micro Nano Lett. 2019, 14, 919–921. [Google Scholar] [CrossRef]
- Liu, Z.; Chai, Z.; Yu, C. Liquid-Phase-Assisted Catalytic Nitridation of Silicon and In Situ Growth of α-Si3N4. Materials 2022, 15, 6074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, F.; Dong, L. In situ combustion synthesis of spherical Si@Si3N4 granules. J. Am. Ceram. Soc. 2022, 105, 6529. [Google Scholar] [CrossRef]
- Lan, Y.; Huang, Y.; Li, X. Non-catalytic vapor synthesis of α-Si3N4 nano whiskers via direct nitridation of silicon powders. Ceram. Int. 2021, 47, 17789. [Google Scholar] [CrossRef]
- Wang, L.; He, G.; Yang, Z. Combustion synthesis of α-Si3N4 powders using in-situ nano-SiO2 coated Si and Si3N4 reactants—Science Direct. Ceram. Int. 2021, 47, 4854. [Google Scholar] [CrossRef]
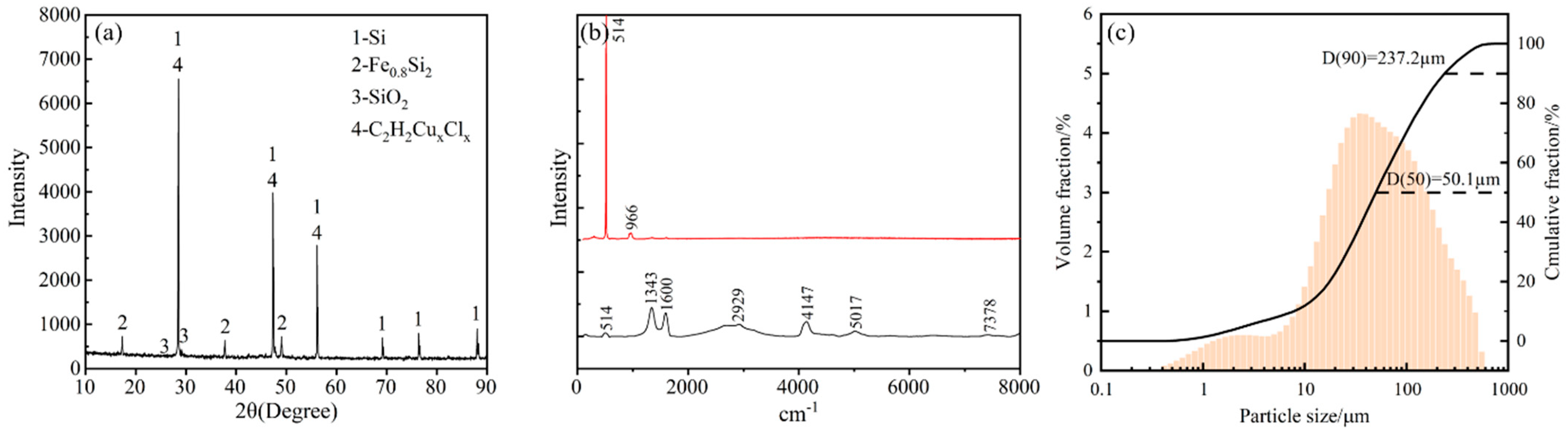

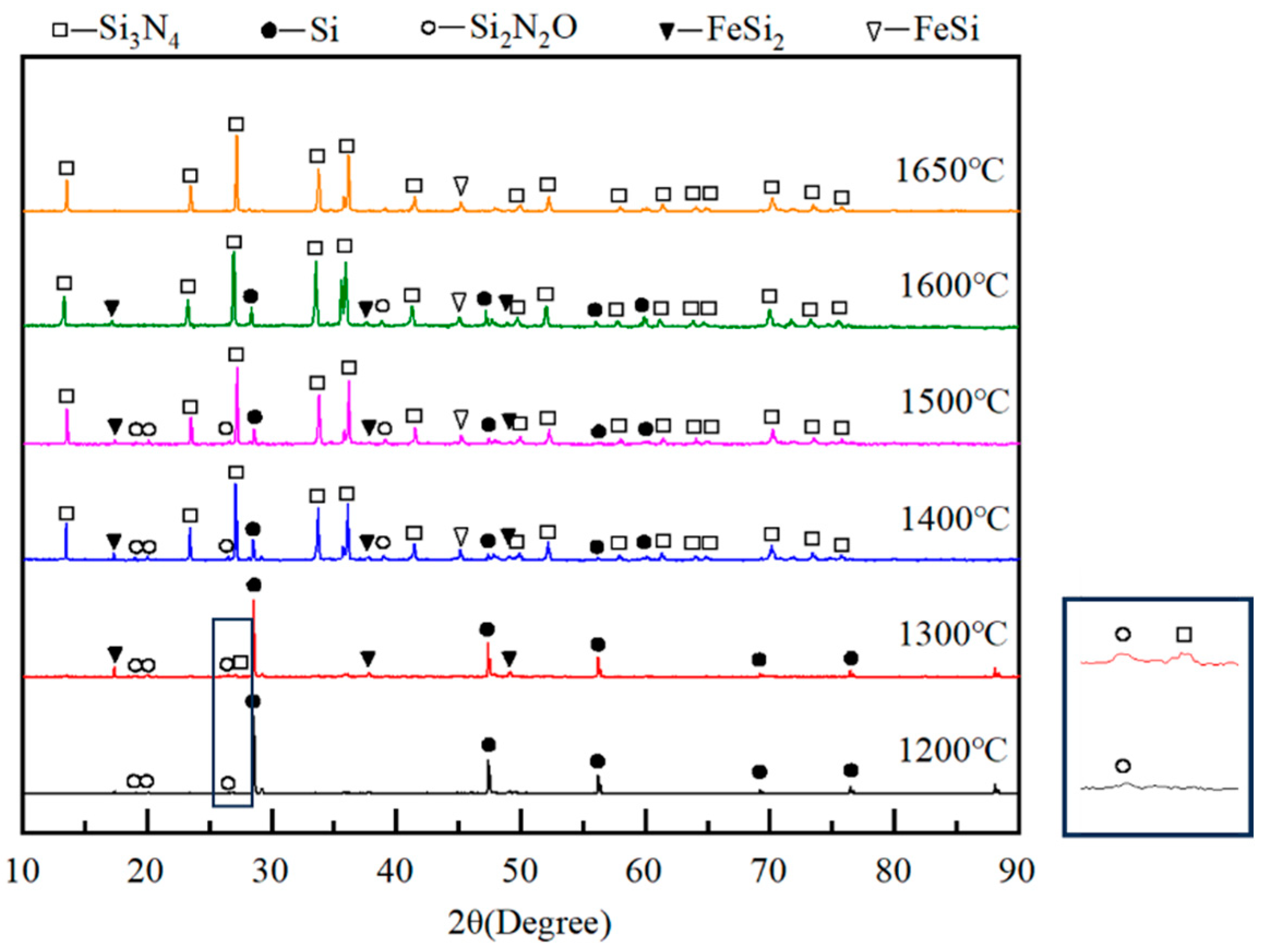
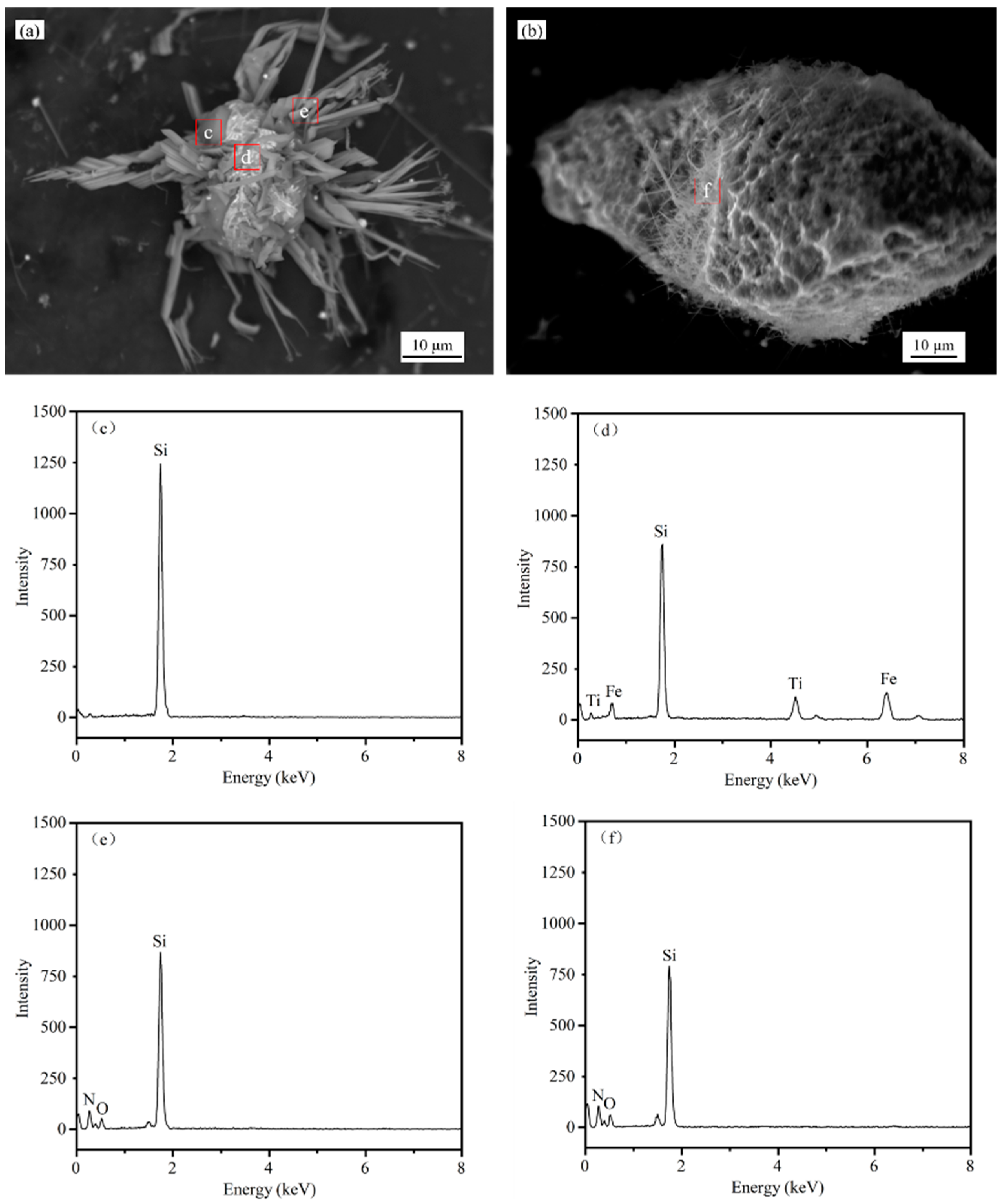
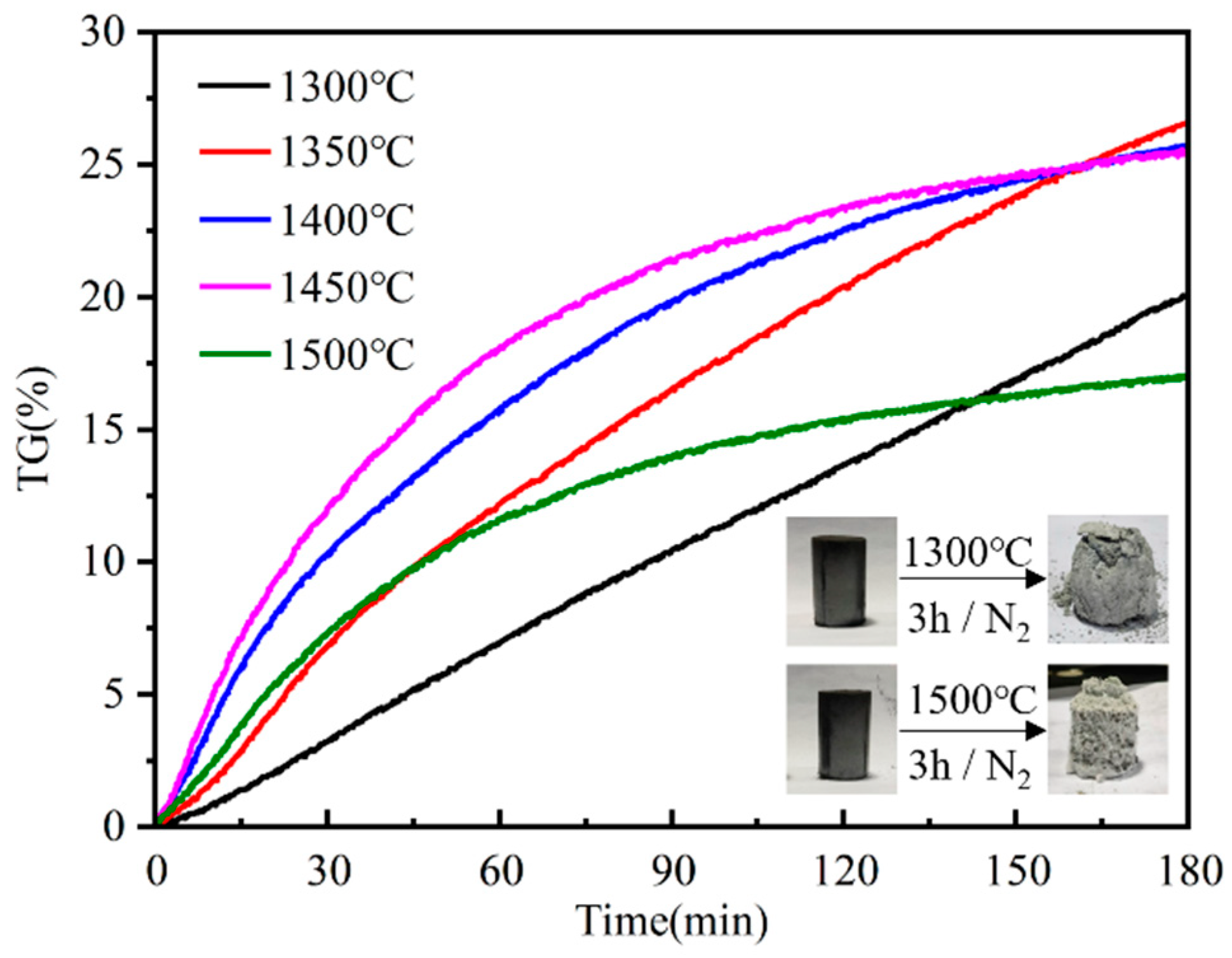


| Si | Fe | Cu | Al | Ti | Zr | V | Mn | O | Cl | C | Ca | P | S | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 68.56 | 9.52 | 3.39 | 1.25 | 1.05 | 0.131 | 0.127 | 0.114 | 7.90 | 4.29 | 2.60 | 0.636 | 0.144 | 0.054 | 0.039 |
| Model | Code | G(α) | Reaction Mechanism |
|---|---|---|---|
| Chemical reaction | F1 | −ln(1 − α) | 1-order reaction |
| F2 | (1 − α) − 1 | 2-order reaction | |
| F3 | (1 − α) − 2 | 3-order reaction | |
| Diffusion reaction | D1 | α2 | One-dimensional, Parabolic equation |
| D2 | (1 − α)ln(1 − α) + α | Two-dimensional, Valensi equation | |
| D3 | [1 − (1 − α)1/3]2 | Three-dimensional, Jander equation | |
| D4 | 1 − 2α/3 − (1 − α)2/3 | Three-dimensional, Ginstring-Brounshtein | |
| Interfacial reaction | R1 | α | One-dimensional |
| R2 | 1 − (1 − α)1/2 | Two-dimensional, contraction cylinder | |
| R3 | 1 − (1 − α)1/3 | Three-dimensional, contraction sphere | |
| Nucleation and growth | A2 | [−ln(1 − α)]1/2 | Aevrami-Erofeev equation I |
| A3 | [−ln(1 − α)]1/3 | Aevrami-Erofeev equation II |
| Corridor | Temperature (°C) | Time (min) |
|---|---|---|
| Phase I | 1300 | 0–180 |
| 1350 | 0–30 | |
| 1400 | 0–30 | |
| Phase II | 1350 | 30–180 |
| 1400 | 30–180 | |
| 1450 | 0–180 | |
| 1500 | 0–180 |
| Model | R1300 | R1350 (0–30) | R1400 (0–30) | RAVG1 | R1350 (30–180) | R1400 (30–180) | R1450 | R1500 | RAVG2 |
|---|---|---|---|---|---|---|---|---|---|
| F1 | 0.9980 | 0.9935 | 0.9985 | 0.9967 | 0.9996 | 0.9974 | 0.9796 | 0.9753 | 0.9880 |
| F2 | 0.9885 | 0.9910 | 0.9977 | 0.9924 | 0.9889 | 0.9997 | 0.9953 | 0.9866 | 0.9926 |
| F3 | 0.9715 | 0.9880 | 0.9962 | 0.9852 | 0.9652 | 0.9966 | 0.9995 | 0.9941 | 0.9888 |
| D1 | 0.9731 | 0.9458 | 0.9715 | 0.9635 | 0.9985 | 0.9991 | 0.9922 | 0.9947 | 0.9961 |
| D2 | 0.9652 | 0.9432 | 0.9693 | 0.9592 | 0.9949 | 0.9995 | 0.9957 | 0.9961 | 0.9962 |
| D3 | 0.9556 | 0.9404 | 0.9670 | 0.9544 | 0.9879 | 0.9987 | 0.9977 | 0.9971 | 0.9954 |
| D4 | 0.9621 | 0.9422 | 0.9686 | 0.9576 | 0.9929 | 0.9993 | 0.9966 | 0.9965 | 0.9963 |
| R1 | 0.9995 | 0.9955 | 0.9988 | 0.9979 | 0.9952 | 0.9892 | 0.9517 | 0.9604 | 0.9741 |
| R2 | 0.9997 | 0.9946 | 0.9987 | 0.9977 | 0.9993 | 0.9940 | 0.9671 | 0.9683 | 0.9822 |
| R3 | 0.9994 | 0.9942 | 0.9987 | 0.9974 | 0.9998 | 0.9953 | 0.9716 | 0.9707 | 0.9844 |
| A2 | 0.9868 | 0.9963 | 0.9896 | 0.9909 | 0.9967 | 0.9888 | 0.9299 | 0.9274 | 0.9607 |
| A3 | 0.9675 | 0.9871 | 0.9782 | 0.9776 | 0.9930 | 0.9844 | 0.8991 | 0.9003 | 0.9442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Xue, Z.; Song, S.; Cromarty, R.; Chen, Y. Utilization of Silicon Dust to Prepare Si3N4 Used for Steelmaking Additives: Thermodynamics and Kinetics. Processes 2024, 12, 301. https://doi.org/10.3390/pr12020301
Hu Q, Xue Z, Song S, Cromarty R, Chen Y. Utilization of Silicon Dust to Prepare Si3N4 Used for Steelmaking Additives: Thermodynamics and Kinetics. Processes. 2024; 12(2):301. https://doi.org/10.3390/pr12020301
Chicago/Turabian StyleHu, Qian, Zhengliang Xue, Shengqiang Song, Robert Cromarty, and Yiliang Chen. 2024. "Utilization of Silicon Dust to Prepare Si3N4 Used for Steelmaking Additives: Thermodynamics and Kinetics" Processes 12, no. 2: 301. https://doi.org/10.3390/pr12020301
APA StyleHu, Q., Xue, Z., Song, S., Cromarty, R., & Chen, Y. (2024). Utilization of Silicon Dust to Prepare Si3N4 Used for Steelmaking Additives: Thermodynamics and Kinetics. Processes, 12(2), 301. https://doi.org/10.3390/pr12020301






