Effect of Recycling on Thermomechanical Properties of Zein and Soy Protein Isolate Bioplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterisation
2.3.1. Dynamic Mechanical Analysis (DMA)
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Uniaxial Tensile Tests
2.3.4. Water Uptake Capacity (WUC)
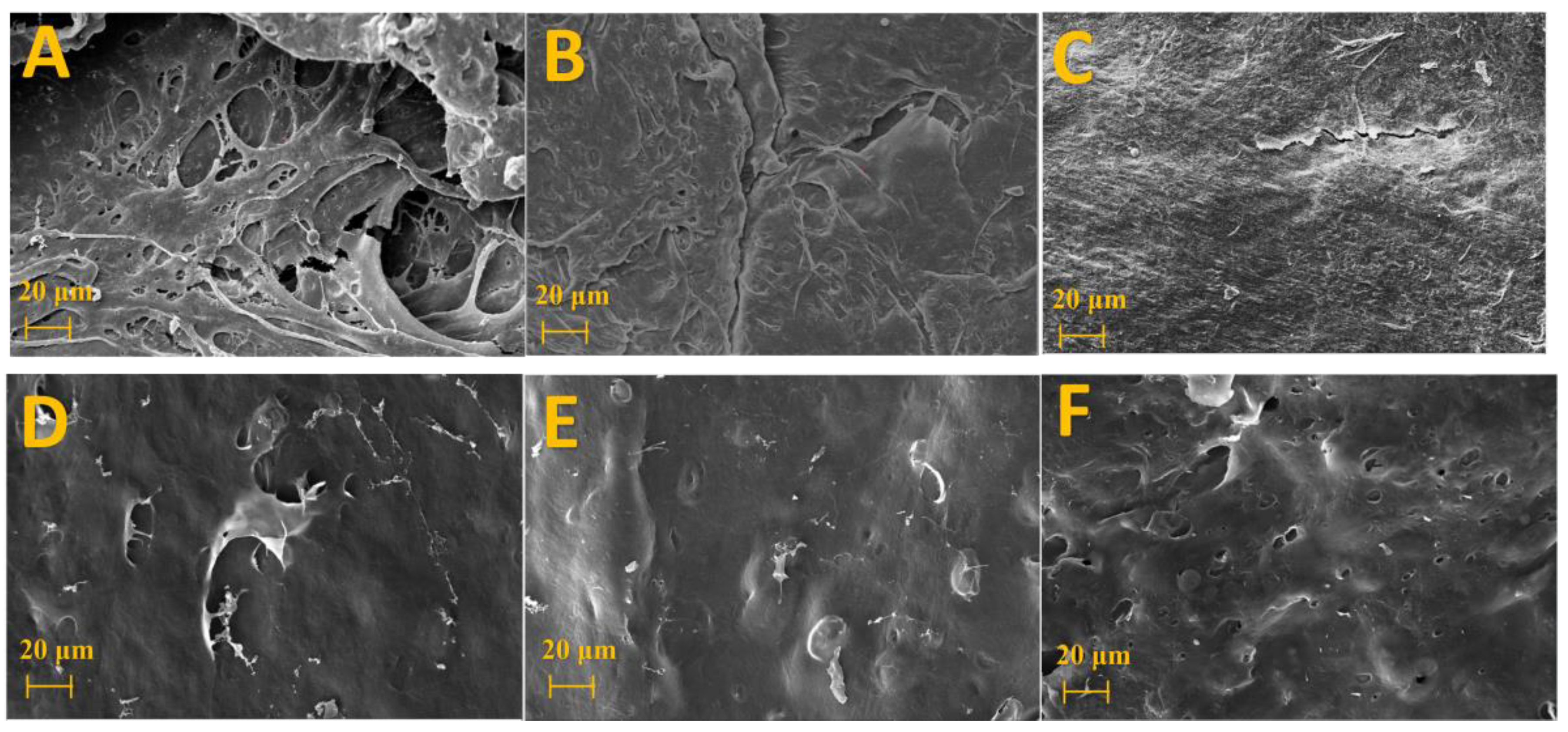
2.3.5. Scanning Electron Microscopy (SEM)
2.4. Statistical Analysis
3. Results
3.1. Characterisation of Blends
3.2. Characterisation of Bioplastics
4. General Discussion of the Results Obtained
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Bioplastics. Bioplastics, Facts and Figures. 2019. Available online: https://docs.european-bioplastics.org/publications/EUBP_Facts_and_figures.pdf (accessed on 1 December 2023).
- Coppola, G.; Gaudio, M.T.; Lopresto, C.G.; Calabro, V.; Curcio, S.; Chakraborty, S. Bioplastic from Renewable Biomass: A Facile Solution for a Greener Environment. Earth Syst. Environ. 2021, 5, 231–251. [Google Scholar] [CrossRef]
- Washam, C. Plastic go green. ChemMatters 2010, 10–12. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Achilias, D. Material Recycling: Trends and Perspectives; Intech Open: London, UK, 2012. [Google Scholar]
- Baillie, C.; Matovic, D.; Thamae, T.; Vaja, S. Waste-based composites—Poverty reducing solutions to environmental problems. Resour. Conserv. Recycl. 2011, 55, 973–978. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Beke, B.V. Recycling of Polymers: A Review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, Q.; Li, H.; Wu, P.; Waterhouse, G.I.N.; Li, Y.; Ai, S. A pectocellulosic bioplastic from fruit processing waste: Robust, biodegradable, and recyclable. Chem. Eng. J. 2023, 463, 142452. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Di Bartolo, A.; Infurna, G.; Dintcheva, N.T. A Review of Bioplastics and Their Adoption in the Circular Economy. Polymers 2021, 13, 1229. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crop. Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Lawton, J.W. Zein: A History of Processing and Use. Cereal Chem. 2002, 79, 1–18. [Google Scholar] [CrossRef]
- Alsadat-Seyedbokaei, F.; Felix, M.; Bengoechea, C. Zein as a Basis of Recyclable Injection Moulded Materials: Effect of Formulation and Processing Conditions. Polymers 2023, 15, 3841. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bagnani, M.; Soon, W.L.; Mezzenga, R. Turning Food Protein Waste into Sustainable Technologies. Chem. Rev. 2023, 123, 2112–2154. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Crop Production Analysis; FAOSTAT: Rome, Italy, 2018. [Google Scholar]
- Yamada, M.; Morimitsu, S.; Hosono, E.; Yamada, T. Preparation of bioplastic using soy protein. Int. J. Biol. Macromol. 2020, 149, 1077–1083. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Bioplastics and Petroleum-based Plastics: Strengths and Weaknesses. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Dey, A.; Rahman, M.; Gupta, A.; Yodo, N.; Lee, C.W. A Performance Study on 3D-Printed Bioplastic Pots from Soybean By-Products. Sustainability 2023, 15, 10535. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Shahabi, N.; Fallah, A.A.; Mahmoudi, E.; Al-Musawi, M.H.; Ghorbani, M. Soy protein isolate/kappa-carrageenan/cellulose nanofibrils composite film incorporated with zenian essential oil-loaded MOFs for food packaging. Int. J. Biol. Macromol. 2023, 250, 126176. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Thermomechanical properties and water uptake capacity of soy protein-based bioplastics processed by injection molding. J. Appl. Polym. Sci. 2016, 133, 43524. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Choi, W.S.; Han, J.H. Physical and mechanical properties of pea-protein-based edible films. J. Food Sci. 2001, 66, 319–322. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Q.; Zhang, Q. Rheological and mechanical properties of bioplastics based on gluten- and glutenin-rich fractions. J. Cereal Sci. 2009, 50, 376–380. [Google Scholar] [CrossRef]
- Félix, M.; Lucio-Villegas, A.; Romero, A.; Guerrero, A. Development of rice protein bio-based plastic materials processed by injection molding. Ind. Crop. Prod. 2016, 79, 152–159. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Protein/glycerol blends and injection-molded bioplastic matrices: Soybean versus egg albumen. J. Appl. Polym. Sci. 2016, 133, 42980. [Google Scholar] [CrossRef]
- Adilah, Z.M.; Hanani, Z.N. Active packaging of fish gelatin films with Morinda citrifolia oil. Food Biosci. 2016, 16, 66–71. [Google Scholar] [CrossRef]
- Pol, H.; Dawson, P.; Acton, J.; Ogale, A. Soy Protein Isolate/Corn-Zein Laminated Films: Transport and Mechanical Properties. J. Food Sci. 2002, 67, 212–217. [Google Scholar] [CrossRef]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of bioplastic materials: From rapeseed oil industry by products to added-value biodegradable biocomposite materials. Ind. Crop. Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Ramos, M.; Bengoechea, C.; Martínez, I.; Romero, A. Effect of blend mixing and formulation on thermophysical properties of gluten-based plastics. J. Cereal Sci. 2020, 96, 103090. [Google Scholar] [CrossRef]
- Corradini, E.; Mattoso, L.H.C.; Guedes, C.G.F.; Rosa, D.S. Mechanical, thermal and morphological properties of poly(ε-caprolactone)/zein blends. Polym. Adv. Technol. 2004, 15, 340–345. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Irissin-Mangata, J.; Bauduin, G.; Boutevin, B.; Gontard, N. New plasticizers for wheat gluten films. Eur. Polym. J. 2001, 37, 1533–1541. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Sandía, J.A.; Cordobés, F.; Guerrero, A. Development of novel soy-protein-based superabsorbent matrixes through the addition of salts. J. Appl. Polym. Sci. 2019, 136, 47012. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Controlled Release of Zinc from Soy Protein-Based Matrices to Plants. Agronomy 2021, 11, 580. [Google Scholar] [CrossRef]
- Ulrichs, T.; Drotleff, A.M.; Ternes, W. Determination of heat-induced changes in the protein secondary structure of reconstituted livetins (water-soluble proteins from hen’s egg yolk) by FTIR. Food Chem. 2015, 172, 909–920. [Google Scholar] [CrossRef]
- Derenne, A.; Derfoufi, K.M.; Cowper, B.; Delporte, C.; Butré, C.I.; Goormaghtigh, E. Analysis of Glycoproteins by ATR-FTIR Spectroscopy: Comparative Assessment BT. In Mass Spectrometry of Glycoproteins: Methods and Protocols; Delobel, A., Ed.; Springer: New York, NY, USA, 2021; pp. 361–374. [Google Scholar]
- Ni, N.; Zhang, D.; Dumont, M.-J. Synthesis and characterization of zein-based superabsorbent hydrogels and their potential as heavy metal ion chelators. Polym. Bull. 2018, 75, 31–45. [Google Scholar] [CrossRef]
- Masanabo, M.A.; Ray, S.S.; Emmambux, M.N. Properties of thermoplastic maize starch-zein composite films prepared by extrusion process under alkaline conditions. Int. J. Biol. Macromol. 2022, 208, 443–452. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Guerrero, P.; de la Caba, K.; Bengoechea, C.; Guerrero, A. Biorefinery concept in the meat industry: From slaughterhouse biowastes to superaborbent materials. Chem. Eng. J. 2023, 471, 144564. [Google Scholar] [CrossRef]
- Tian, H.; Wu, W.; Guo, G.; Gaolun, B.; Jia, Q.; Xiang, A. Microstructure and properties of glycerol plasticized soy protein plastics containing castor oil. J. Food Eng. 2012, 109, 496–500. [Google Scholar] [CrossRef]
- Lukubira, S.; Ogale, A. Thermoformable Anhydride–Glycerol Modified Meat and Bone Meal Bioplastics. J. Polym. Environ. 2015, 23, 517–525. [Google Scholar] [CrossRef]
- Ma, F.; Huang, H.-Y.; Zhou, L.; Yang, C.; Zhou, J.-H.; Liu, Z.-M. Study on the conformation changes of Lysozyme induced by Hypocrellin A: The mechanism investigation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1159–1165. [Google Scholar] [CrossRef]
- Ogale, A.; Cunningham, P.; Dawson, P.; Acton, J. Viscoelastic, Thermal, and Microstructural Characterization of Soy Protein Isolate Films. J. Food Sci. 2000, 65, 672–679. [Google Scholar] [CrossRef]
- Leroy, E.; Petit, I.; Audic, J.L.; Colomines, G.; Deterre, R. Rheological characterization of a thermally unstable bioplastic in injection molding conditions. Polym. Degrad. Stab. 2012, 97, 1915–1921. [Google Scholar] [CrossRef]
- Bier, J.M.; Verbeek, C.J.; Lay, M.C. Thermal transitions and structural relaxations in protein-based thermoplastics. Macromol. Mater. Eng. 2014, 299, 524–539. [Google Scholar] [CrossRef]
- Fan, F.; Roos, Y.H. Glass Transition-Associated Structural Relaxations and Applications of Relaxation Times in Amorphous Food Solids: A Review. Food Eng. Rev. 2017, 9, 257–270. [Google Scholar] [CrossRef]
- Yang, J.; Dong, X.; Wang, J.; Ching, Y.C.; Liu, J.; Li, C.; Baikeli, Y.; Li, Z.; Al-Hada, N.M.; Xu, S. Synthesis and properties of bioplastics from corn starch and citric acid-epoxidized soybean oil oligomers. J. Mater. Res. Technol. 2022, 20, 373–380. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Pelagio, M.J.; Bengoechea, C.; Guerrero, A. Plasma based superabsorbent materials modulated through chemical cross-linking. J. Environ. Chem. Eng. 2021, 9, 105017. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Bengoechea, C.; Rodríguez, N.; Guerrero, A. Development of green superabsorbent materials from a by-product of the meat industry. J. Clean. Prod. 2019, 223, 651–661. [Google Scholar] [CrossRef]
- Hu, G.; Yu, D. Tensile, thermal and dynamic mechanical properties of hollow polymer particle-filled epoxy syntactic foam. Mater. Sci. Eng. A 2011, 528, 5177–5183. [Google Scholar] [CrossRef]
- Deng, S.; Hou, M.; Ye, L. Temperature-dependent elastic moduli of epoxies measured by DMA and their correlations to mechanical testing data. Polym. Test. 2007, 26, 803–813. [Google Scholar] [CrossRef]
- Fang, Q.; Zhu, M.; Yu, S.; Sui, G.; Yang, X. Studies on soy protein isolate/polyvinyl alcohol hybrid nanofiber membranes as multi-functional eco-friendly filtration materials. Mater. Sci. Eng. B 2016, 214, 1–10. [Google Scholar] [CrossRef]
- Parris, N.; Dickey, L.C. Extraction and Solubility Characteristics of Zein Proteins from Dry-Milled Corn. J. Agric. Food Chem. 2001, 49, 3757–3760. [Google Scholar] [CrossRef]
- Morán, J.; Alvarez, V.; Petrucci, R.; Kenny, J.; Vazquez, A. Mechanical properties of polypropylene composites based on natural fibers subjected to multiple extrusion cycles. J. Appl. Polym. Sci. 2007, 103, 228–237. [Google Scholar] [CrossRef]
- Marandi, G.B.; Mahdavinia, G.R.; Ghafary, S. Swelling behavior of novel protein-based superabsorbent nanocomposite. J. Appl. Polym. Sci. 2011, 120, 1170–1179. [Google Scholar] [CrossRef]
- Baier, S.K.; Decker, E.A.; McClements, D. Impact of glycerol on thermostability and heat-induced gelation of bovine serum albumin. Food Hydrocoll. 2004, 18, 91–100. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Partal, P.; Garcia-Morales, M.; Gallegos, C. Development of highly-transparent protein/starch-based bioplastics. Bioresour. Technol. 2010, 101, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Omrani-Fard, H.; Abbaspour-Fard, M.H.; Khojastehpour, M.; Dashti, A. Gelatin/Whey Protein- Potato Flour Bioplastics: Fabrication and Evaluation. J. Polym. Environ. 2020, 28, 2029–2038. [Google Scholar] [CrossRef]
- Verbeek, C.J.R.; Van Den Berg, L.E. Development of Proteinous Bioplastics Using Bloodmeal. J. Polym. Environ. 2011, 19, 1–10. [Google Scholar] [CrossRef]
- Domenek, S.; Feuilloley, P.; Gratraud, J.; Morel, M.-H.; Guilbert, S. Biodegradability of wheat gluten based bioplastics. Chemosphere 2004, 54, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Vefago, L.H.M.; Avellaneda, J. Recycling concepts and the index of recyclability for building materials. Resour. Conserv. Recycl. 2013, 72, 127–135. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Schmid, M.; Nerney, O.M.; Wildner, J.; Smykala, L.; Lazzeri, A.; Cinelli, P. Processing and Validation of Whey-Protein-Coated Films and Laminates at Semi-Industrial Scale as Novel Recyclable Food Packaging Materials with Excellent Barrier Properties. Adv. Mater. Sci. Eng. 2013, 2013, 496207. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of Whey-Protein-Coated Films and Laminates as Novel Recyclable Food Packaging Materials with Excellent Barrier Properties. Int. J. Polym. Sci. 2012, 2012, 562381. [Google Scholar] [CrossRef]
- Zheng, G.; Kang, X.; Ye, H.; Fan, W.; Sonne, C.; Lam, S.S.; Liew, R.K.; Xia, C.; Shi, Y.; Ge, S. Recent advances in functional utilisation of environmentally friendly and recyclable high-performance green biocomposites: A review. Chin. Chem. Lett. 2023; in press. [Google Scholar] [CrossRef]
- Bian, H.; Luo, J.; Wang, R.; Zhou, X.; Ni, S.; Shi, R.; Fang, G.; Dai, H. Recyclable and Reusable Maleic Acid for Efficient Production of Cellulose Nanofibrils with Stable Performance, ACS Sustain. Chem. Eng. 2019, 7, 24. [Google Scholar] [CrossRef]
- Pelegrini, K.; Maraschin, T.G.; Brandalise, R.N.; Piazza, D. Study of the degradation and recyclability of polyethylene and polypropylene present in the marine environment. J. Appl. Polym. Sci. 2019, 136, 48215. [Google Scholar] [CrossRef]
- Bauer, A.-S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.S.; Varzakas, T.; Krauter, V. Recyclability and Redesign Challenges in Multilayer Flexible Food Packaging—A Review. Foods 2021, 10, 2702. [Google Scholar] [CrossRef]

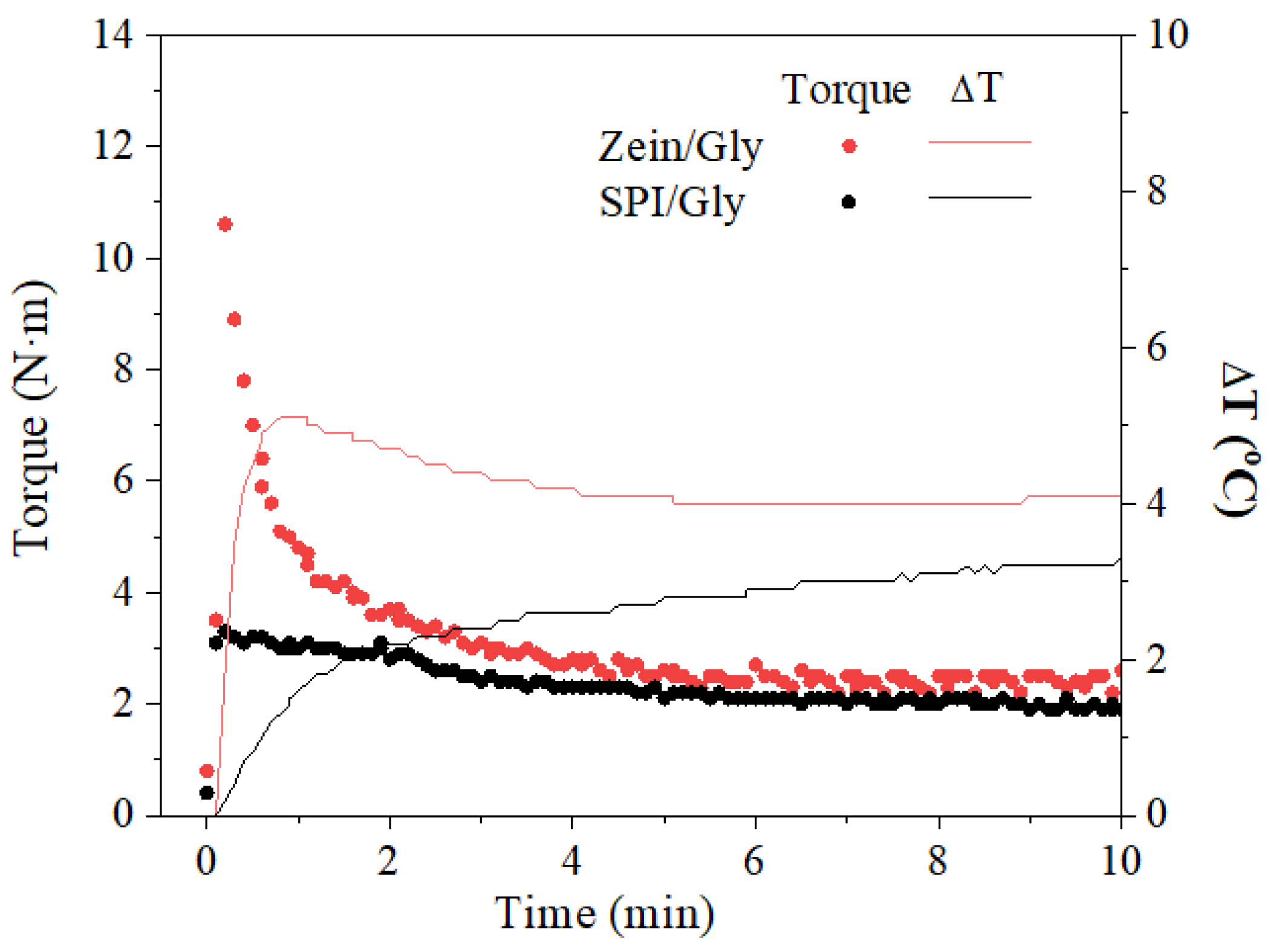

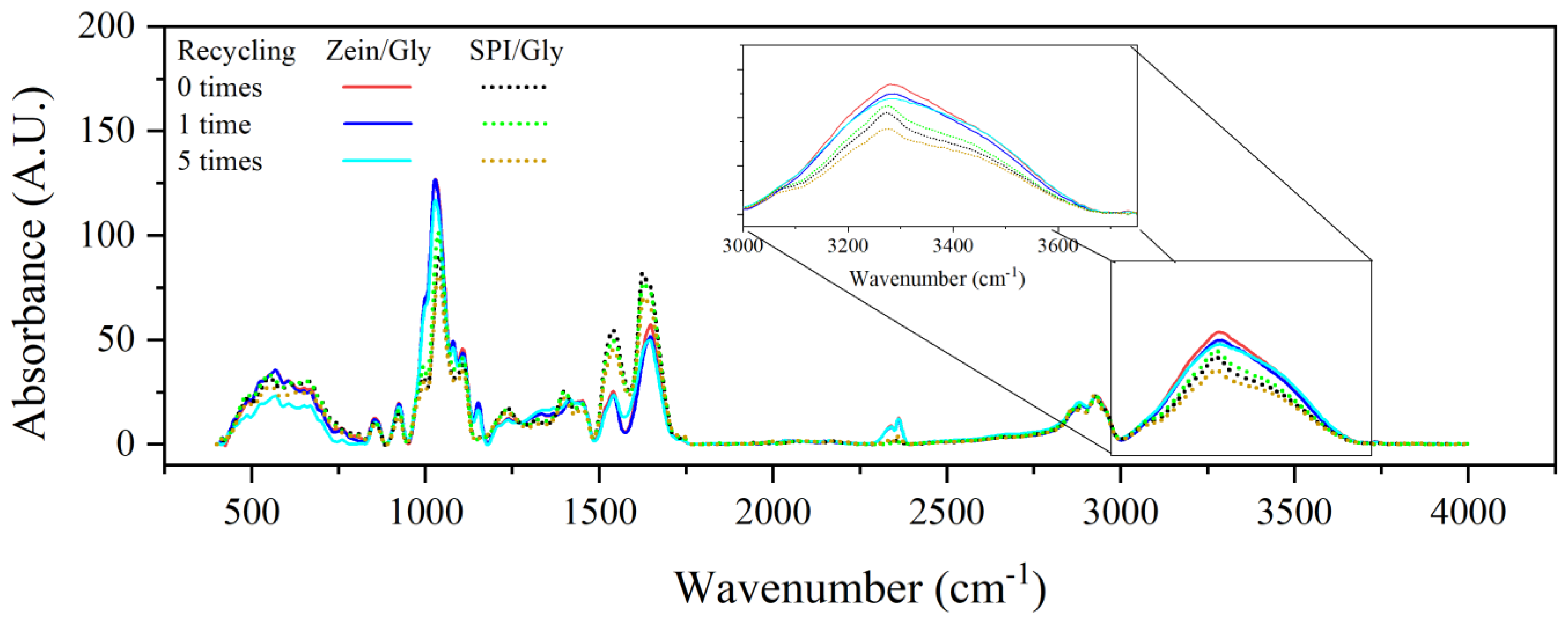

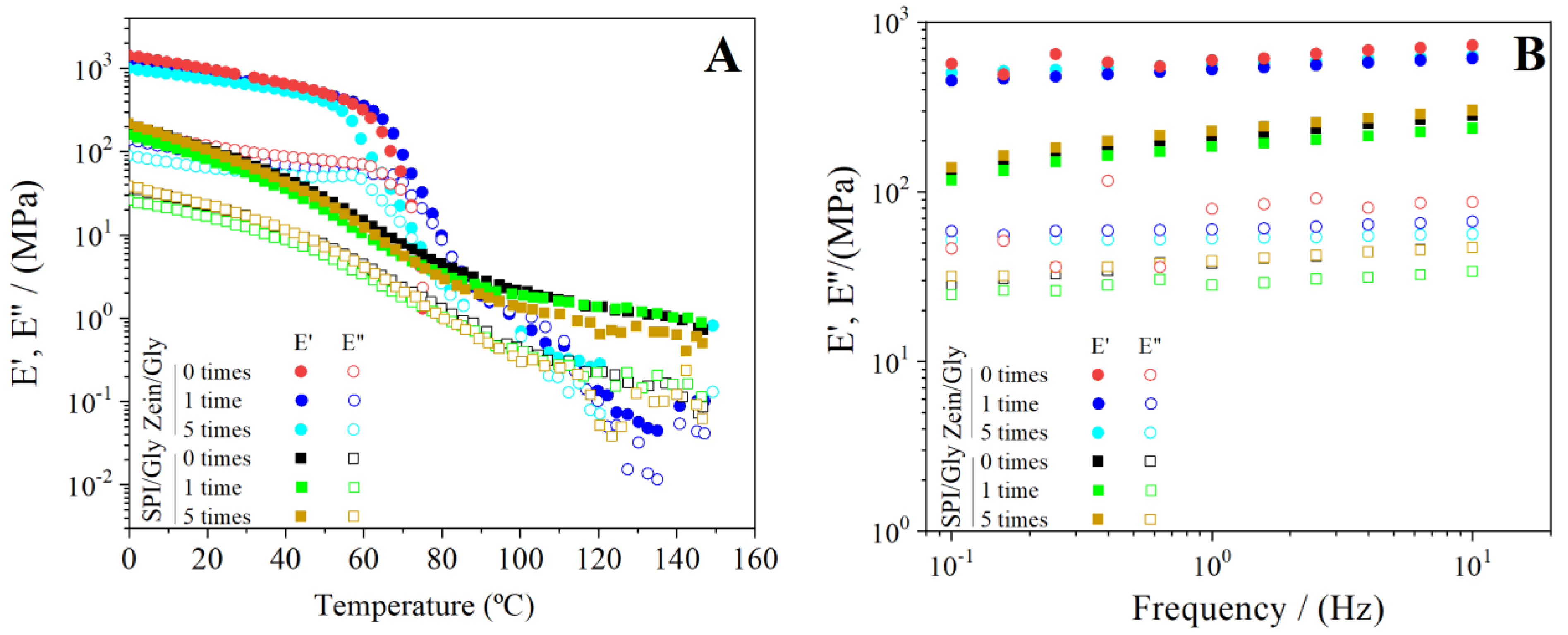

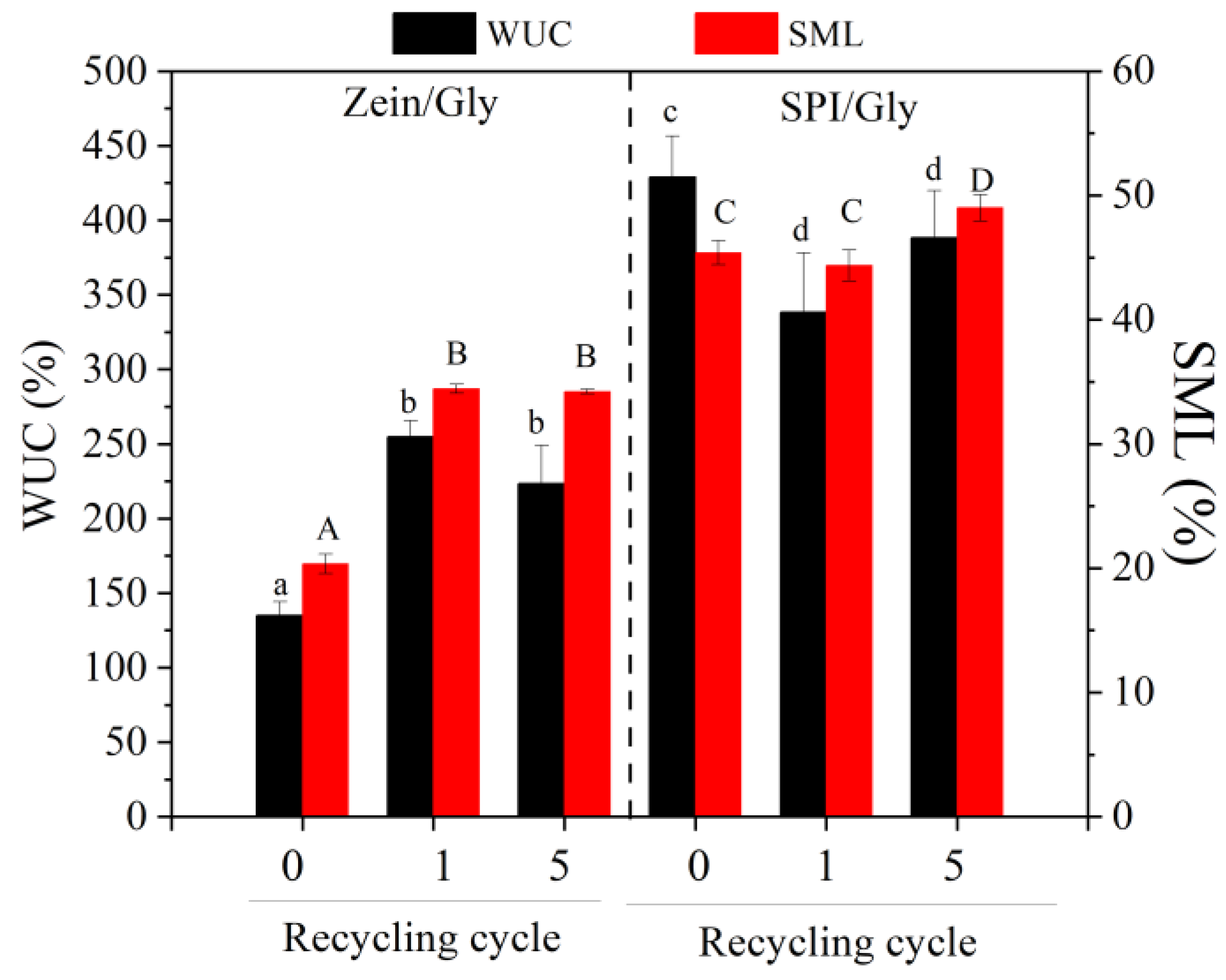
| Processing Conditions | Zein/Gly | SPI/Gly |
|---|---|---|
| Cylinder temperature (°C) | 150 | 40 |
| Mould temperature (°C) | 40 | 150 |
| Injection pressure (bar) | 500 | 500 |
| Holding pressure (bar) | 200 | 200 |
| Injection time (s) | 10 | 10 |
| Holding time (s) | 170 | 170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsadat-Seyedbokaei, F.; Felix, M.; Bengoechea, C. Effect of Recycling on Thermomechanical Properties of Zein and Soy Protein Isolate Bioplastics. Processes 2024, 12, 302. https://doi.org/10.3390/pr12020302
Alsadat-Seyedbokaei F, Felix M, Bengoechea C. Effect of Recycling on Thermomechanical Properties of Zein and Soy Protein Isolate Bioplastics. Processes. 2024; 12(2):302. https://doi.org/10.3390/pr12020302
Chicago/Turabian StyleAlsadat-Seyedbokaei, Fahimeh, Manuel Felix, and Carlos Bengoechea. 2024. "Effect of Recycling on Thermomechanical Properties of Zein and Soy Protein Isolate Bioplastics" Processes 12, no. 2: 302. https://doi.org/10.3390/pr12020302







