Study on the Wetting Mechanisms of Different Coal Ranks Based on Molecular Dynamics
Abstract
:1. Introduction
2. Coal Molecular Model
3. Molecular Simulation Section
3.1. Coal Molecular Simulation
3.2. Molecular Dynamics Simulation of Coal–Water Interaction
4. Results and Discussion
4.1. Simulation Results and Energy Changes
4.2. Hydrogen Bonds
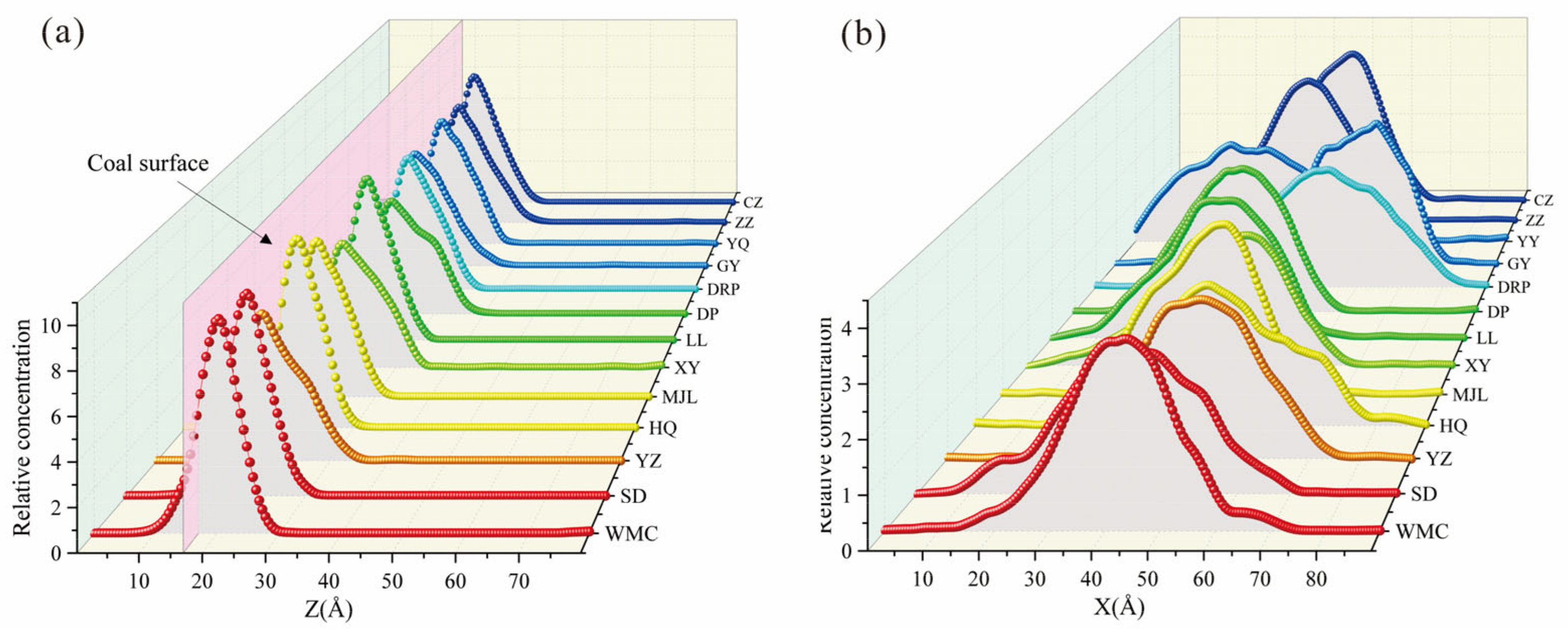
4.3. Relative Concentration Analysis
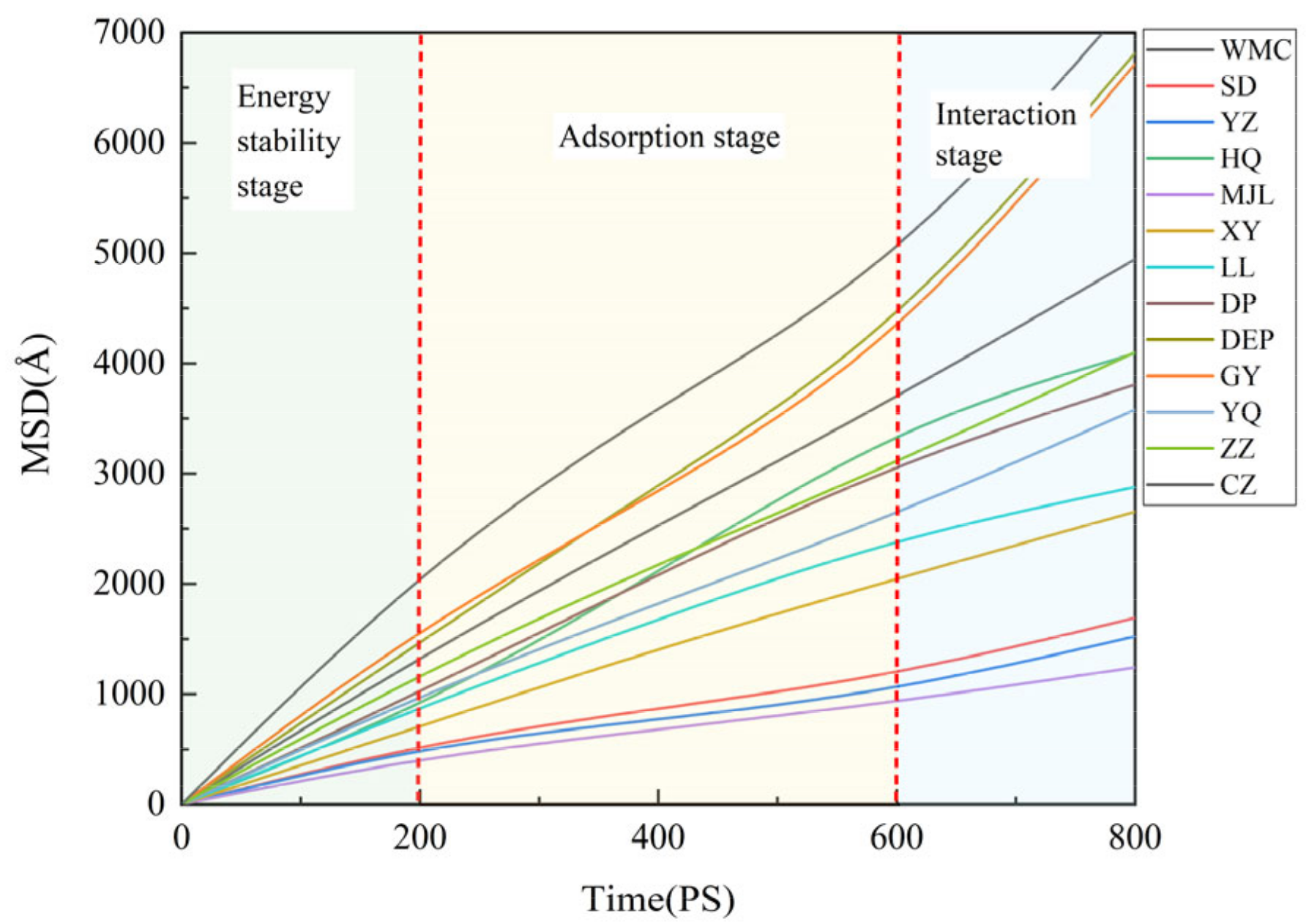
4.4. Dynamic Characteristics of Water Molecules
4.5. The Effects of Structural Parameters on Wettability
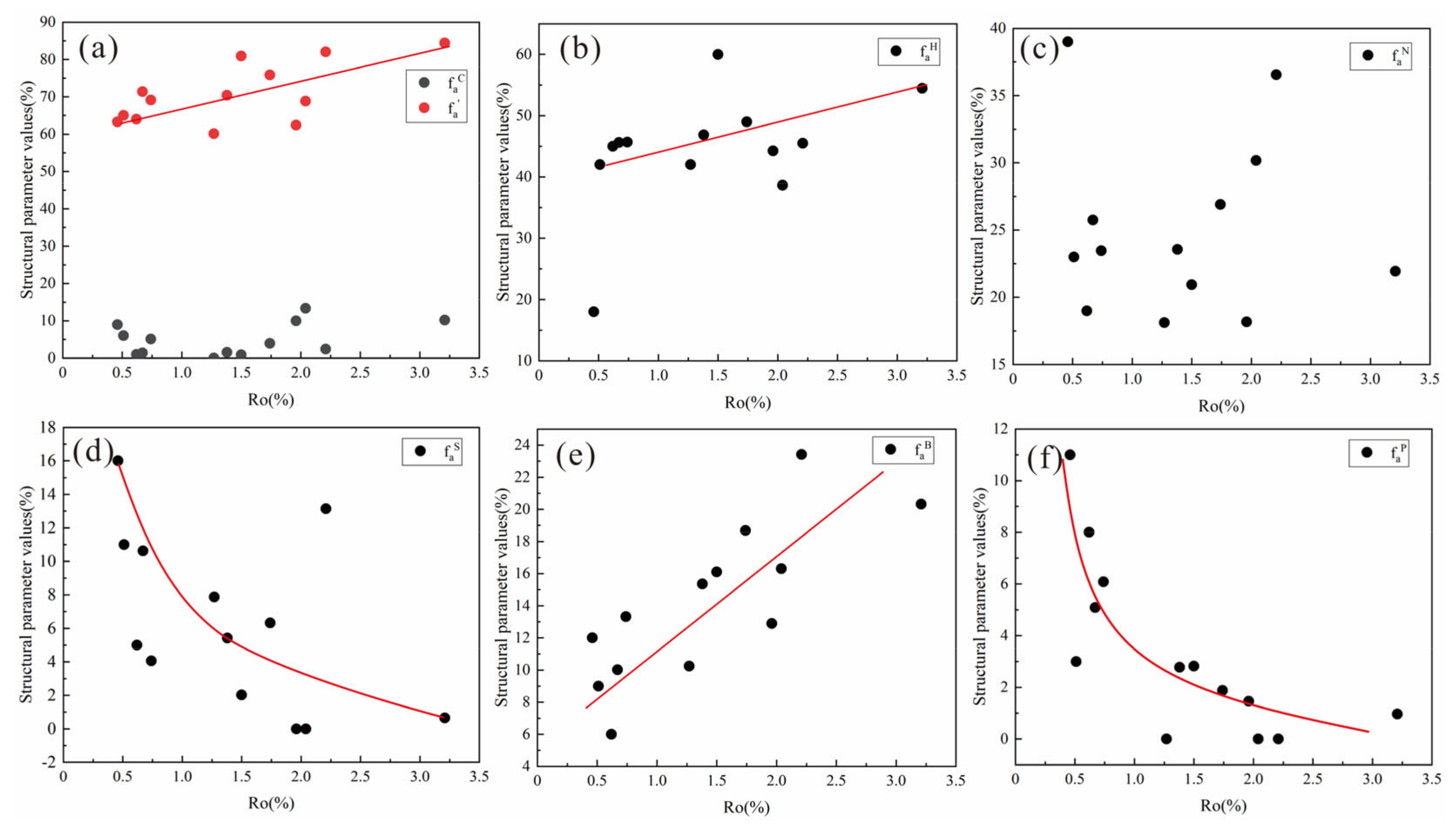
4.5.1. Analysis of Coal Quality Elements
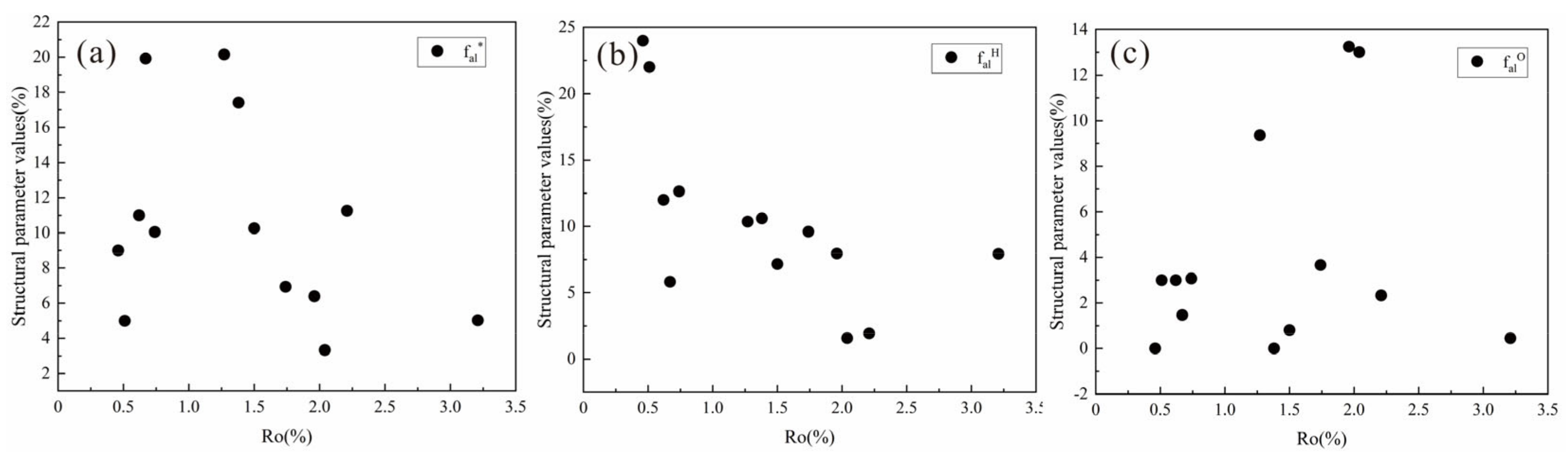
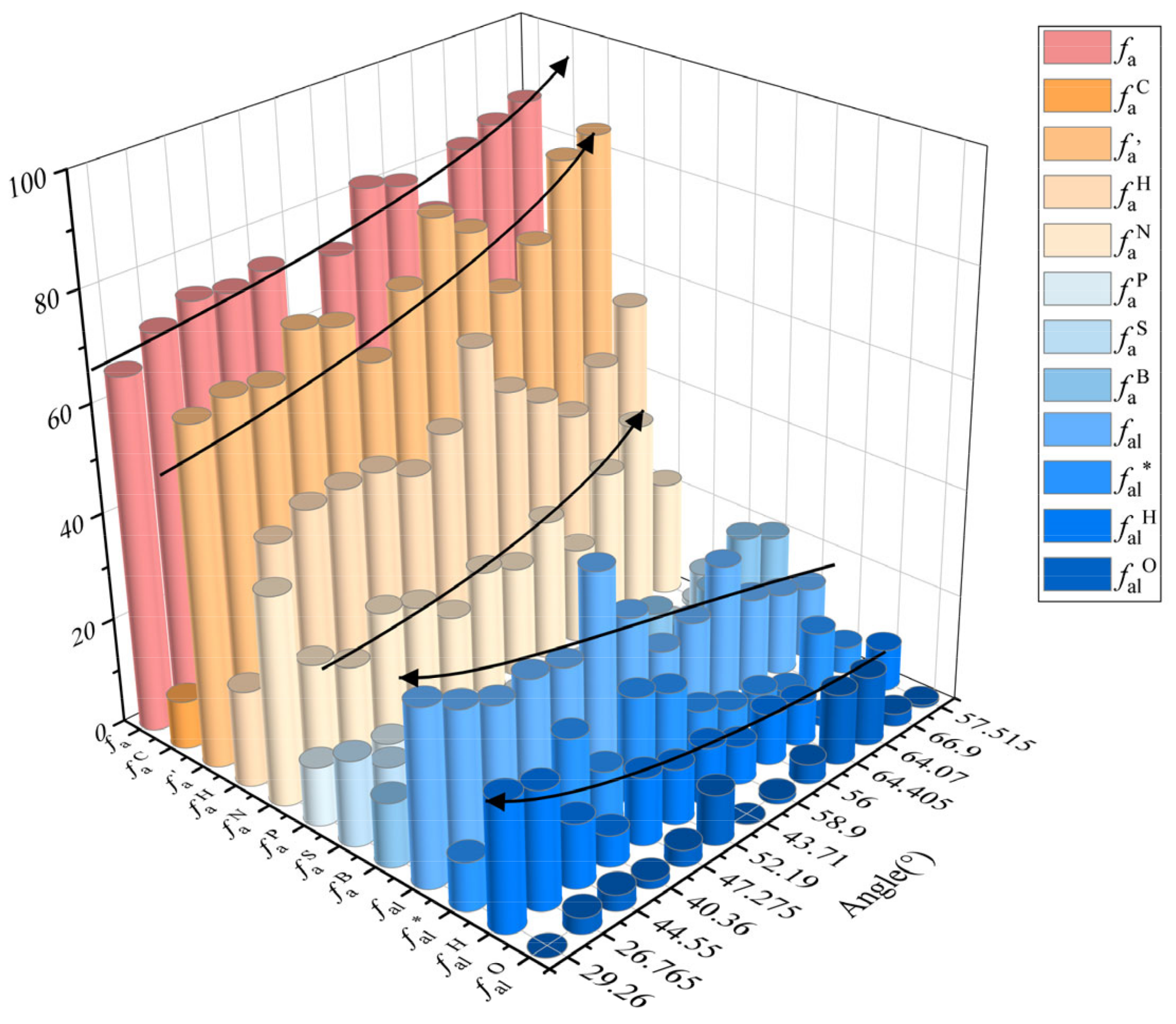
4.5.2. Analysis of 13C-NMR Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, X.X.; Yao, Y.B.; Liu, D.M.; Zhou, Y.F. Investigations of CO2-Water Wettability of Coal: NMR Relaxation Method. Int. J. Coal Geol. 2018, 188, 38–50. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yao, Y.B.; Derek, E.; Liu, D.M.; Cai, Y.D. Dynamic Fluid Interactions During CO2-ECBM and CO2 Sequestration in Coal Seams. Part 2: CO2-H2O Wettability. Fuel 2020, 279, 118560. [Google Scholar] [CrossRef]
- Sun, X.X.; Yao, Y.B.; Chen, J.Y.; Xie, S.S.; Li, C.C. Determination of Coal Wettability by Using Low-field Nuclear Magnetic Resonance. Geoscience 2015, 29, 190–197. [Google Scholar]
- Yao, Y.B.; Sun, X.X.; Wan, L. Micro-mechanism of geological sequestration of CO2 in coal seam. Coal Geol. Explor. 2023, 51, 146–157. [Google Scholar]
- Zhang, X.H.; Xu, C.C.; Yan, G.Q.; Huang, Q.M. Influencing Factors Analysis of Different Types of Coal Wettability. Saf. Coal Mines 2015, 46, 156–162. [Google Scholar]
- Li, J.Y.; Li, K.Q. Influence factors of coal surface wettability. J. China Coal Soc. 2016, 41, 448–453. [Google Scholar]
- Li, S.G.; Guo, D.D.; Bai, Y.; Yan, M.; Lin, H.F.; Shi, Y. Effect of SDBS of different mass fractions on coal’s wettabilityby molecular simulation. China Saf. Sci. J. 2020, 30, 21–27. [Google Scholar]
- Xu, Y.; Li, P.; Sun, Z.G. Research on Factors Affecting Wettability of Coal Dust with Different Degrees of Metamorphism. Coal Mine Mach. 2022, 43, 65–68. [Google Scholar]
- Lin, X.; Liu, Z.; Geng, N.; Hu, P.; Gu, Q. Optimization and Application of Water Injection Process in Gas-Bearing Coal Seam. Processes 2023, 11, 3003. [Google Scholar] [CrossRef]
- Gao, M.Z.; Wang, M.Y.; Xie, J.; Gao, Y.N.; Deng, G.D.; Yang, B.G.; Wang, F.; Hao, H.C.; Xie, H.P. In-situ disturbed mechanical behavior of deep coal rock. J. China Coal Soc. 2020, 45, 2691–2703. [Google Scholar]
- Li, M.; Xu, H.Q.; Shu, X.Q. Discussion on the application of chemical dust suppressants in suppressing coal dust. China Coal 2007, 33, 46–50. [Google Scholar]
- Shi, X.X.; Jiang, Z.A.; Deng, Y.F.; Xing, J.J. Study of fully mechanized mining face dust pollution status. J. Saf. Sci. Technol. 2008, 4, 67–70. [Google Scholar]
- Yuan, L. Scientific conception of coal mine dust control and occupational safety. J. China Coal Soc. 2020, 45, 1–7. [Google Scholar]
- Wang, J. Study on Physicochemical Properties and Dustfall Experiments of Coal Dust with Different Particle Sizes. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, 2019. [Google Scholar]
- Li, J.Y.; Lu, Y.P.; Sun, C.X.; Shi, Y. Experimental study of the wetting characteristics of coal dust. China Energy Environ. Prot. 2015, 6, 36–38. [Google Scholar]
- Zhang, J.G.; Li, H.M.; Liu, Y.T.; Li, X.Y.; Xie, J.; Dai, Z.X.; Ye, S.Q.; Li, L.M.; Zhou, W.Q.; Zhao, Y.; et al. Micro-wetting characteristics of coal dust and preliminary study on the development of dust suppressant in Pingdingshan mining area. J. China Coal Soc. 2021, 46, 812–825. [Google Scholar]
- Yan, M.; Yue, M.; Lin, H.F.; Yan, D.J.; Wei, J.N.; Qin, X.Y.; Zhang, J. Experimental study on the influence of middle and low rank coal functional groups on coal wettability. Coal Sci. Technol. 2023, 51, 103–113. [Google Scholar]
- Zhou, G.; Cheng, W.M.; Xu, C.C.; Nie, W. Characteristic analysis of 13C-NMR for the wettability difference of coal dust with diverse degrees of metamorphism. J. China Coal Soc. 2015, 40, 2849–2855. [Google Scholar]
- Zhou, G.; Xue, J.; Cheng, W.M.; Nie, W.; Wang, H. Effect of stacking structure on the wettability of coal dust based on X-ray diffraction experiment. Chin. J. Eng. 2015, 37, 1535–1541. [Google Scholar]
- Xia, Y.C.; Yang, Z.L.; Zhang, R.; Xing, Y.W.; Gui, X.H. Enhancement of the Surface Hydrophobicity of Low-Rank Coal by Adsorbing DTAB: An Experimental and Molecular Dynamics Simulation Study. Fuel 2019, 239, 145–152. [Google Scholar] [CrossRef]
- Van Niekerk, D.; Mathews, J.P. Molecular Dynamic Simulation of Coal-Solvent Interactions in Permian-Aged South African Coals. Fuel Process. Technol. 2011, 92, 729–734. [Google Scholar] [CrossRef]
- Liu, L.Y.; Qiao, E.L.; Liang, S.; Min, F.F.; Xue, C.G. Effect of Hydration Layer on the Adsorption of Dodecane Collector on Low-Rank Coal: A Molecular Dynamics Simulation Study. Processes 2020, 8, 1207. [Google Scholar] [CrossRef]
- Cheng, G.; Peng, Y.J.; Lu, Y.; Zhang, M. Adsorption of Multi-Collector on Long-Flame Coal Surface Via Density Functional Theory Calculation and Molecular Dynamics Simulation. Processes 2023, 11, 2775. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.M.; Lei, J.M.; Xie, J.; Li, L.M.; Gan, Y.; Deng, J.; Qi, R.; Liu, Y.L. Study on the Surface Wetting Mechanism of Bituminous Coal Based on the Microscopic Molecular Structure. RSC Adv. 2023, 13, 5933–5945. [Google Scholar] [CrossRef] [PubMed]
- Li, E.Z.; Lu, Y.; Cheng, F.Q.; Wang, X.M.; Miller, J.D. Effect of Oxidation on the Wetting of Coal Surfaces by Water: Experimental and Molecular Dynamics Simulation Studies. Physicochem. Probl. Miner. Process. 2018, 54, 1039–1051. [Google Scholar]
- Meng, J.Q.; Wang, L.J.; Zhang, S.; Lyu, Y.P.; Xia, J.K. Effect of Anionic/Nonionic Surfactants on the Wettability of Coal Surface. Chem. Phys. Lett. 2021, 785, 139130. [Google Scholar] [CrossRef]
- Xia, Y.C.; Rong, G.Q.; Xing, Y.W.; Gui, X.H. Synergistic Adsorption of Polar and Nonpolar Reagents on Oxygen-Containing Graphite Surfaces: Implications for Low-Rank Coal Flotation. J. Colloid Interface Sci. 2019, 557, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, F.G.; Xiang, J.H. Macromolecular structure and formation mechanism of raw coal in coal seam 11 of Wumuchang district, Inner Mongolia. J. Fuel Chem. Technol. 2013, 41, 1294–1302. [Google Scholar]
- Bai, P.P.; Liang, Z.Y.; Su, X.P. Simulation study of the molecular structure of Shendong coal. Coal Chem. Ind. 2021, 49, 78–90. [Google Scholar]
- Xiang, J.H.; Zeng, F.G.; Liang, H.Z.; Sun, B.L.; Zhang, L.; Li, M.F.; Jia, J.B. Model construction of the macro molecular structure of Yanzhou coal and its molecular simulation. J. Fuel Chem. Technol. 2011, 39, 481–488. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zeng, F.G.; Xiang, J.H.; Deng, X.P.; Xiang, X.H. Macromolecular model construction and molecular simulation of organic matter in Majiliang vitrain. CIESC J. 2020, 71, 1802–1811. [Google Scholar]
- Ma, Y.P. Construction of Liulin 3# Coal Supramolecular Structure Model and Study of Adsorption Property. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2018. [Google Scholar]
- Li, P.P. Molecular Structure Model Construction and Molecular Simulation of No.2 Coal in Duerping Mine. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2014. [Google Scholar]
- Xiang, J.H.; Zeng, F.G.; Li, B.; Zhang, L.; Li, M.F.; Liang, H.Z. Construction of macromolecular structural model of anthracite from Chengzhuang coal mine and its molecular simulation. J. Fuel Chem. Technol. 2013, 41, 391–399. [Google Scholar] [CrossRef]
- Feng, Z.Z. Construction of Molecular Model of Anthracite Dust Phase and Study on Its Wettability. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2021. [Google Scholar]
- Zhang, Q.; Chen, X.Y.; Wang, H.T.; Xu, C.H. Exploration on Molecular Dynamics Simulation Methods of Microscopic Wetting Process for Coal Dust. Int. J. Coal Sci. Technol. 2021, 8, 205–216. [Google Scholar] [CrossRef]
- Zhang, L. Wettablility Alteration of Lignite Achieved by Nonionic Surfactants with Different Structures: A Molecular Dynamics Simulation Study. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2018. [Google Scholar]
- Xia, Y.C. Molecular Simulations on Adsorption of Water onto Lignite Surface and Its Wettablility Modificaton. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2018. [Google Scholar]
- Burdelnaya, N.; Bushnev, D.; Mokeev, M.; Dobrodumov, A. Experimental Study of Kerogen Maturation by Solid-State 13C NMR Spectroscopy. Fuel 2014, 118, 308–315. [Google Scholar] [CrossRef]

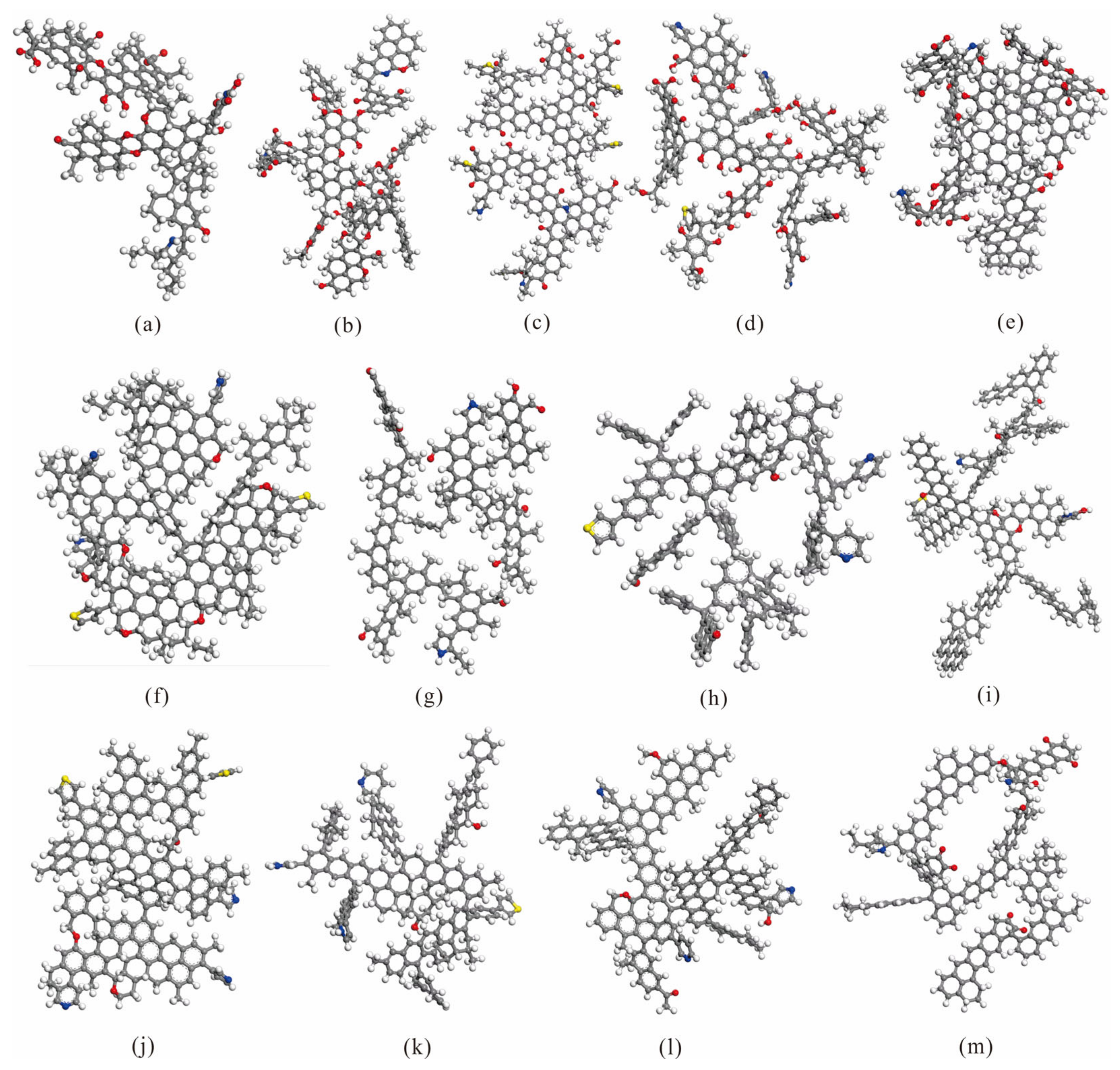
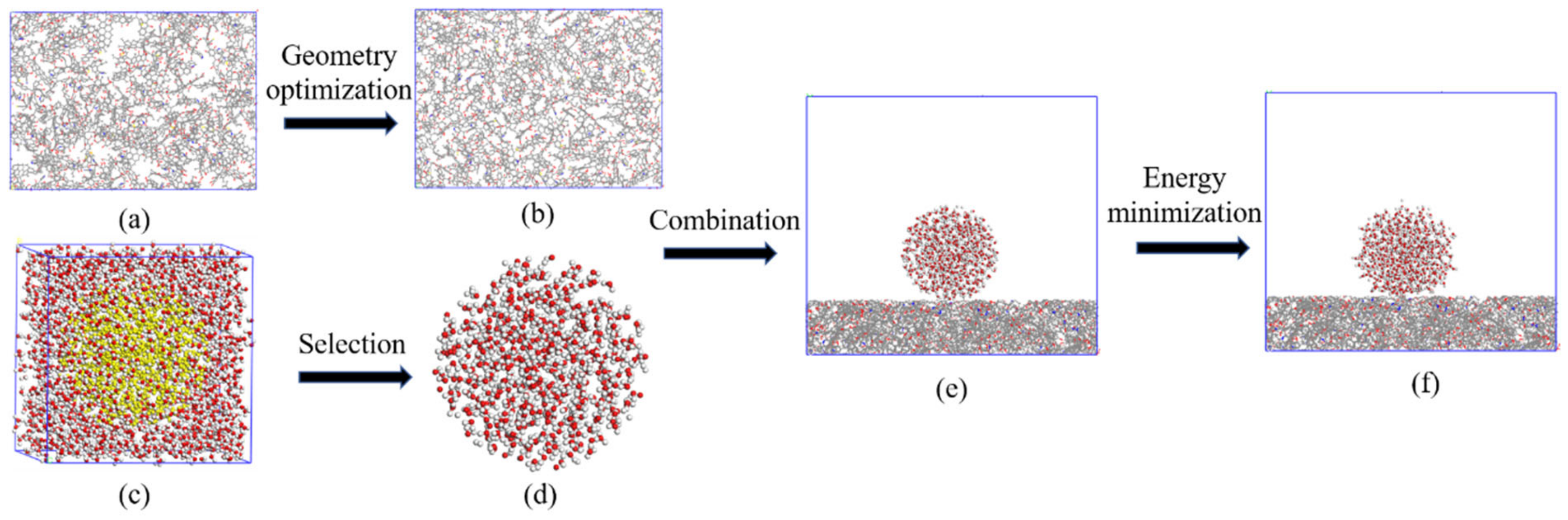
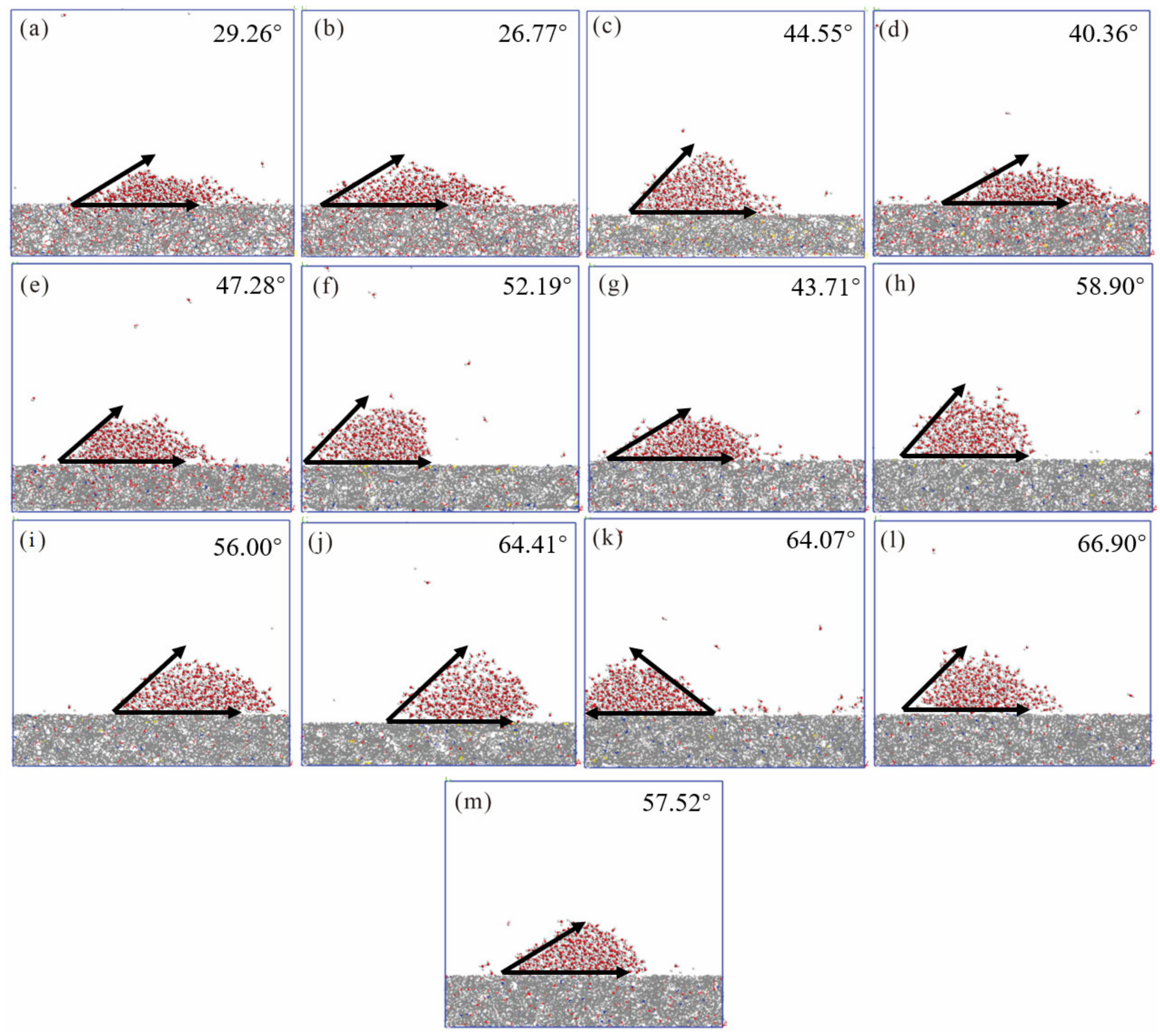




| Number | Coal Sample | RO(%) | Coal Rank | Proximate Analysis (%) | Ultimate Analysis (%) | Atomic Ratio | Density (g/cm3) | Angle (°) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | C | H | O | N | S | H/C | O/C | ||||||
| 1 | WMC [25] | 0.46 | lignite | 5.14 | 16.15 | 33.15 | 77.79 | 4.47 | 16.02 | 1.29 | 0.43 | 0.69 | 0.15 | 1.39 | 29.26 |
| 2 | SD [26] | 0.51 | long-flame coal | 9.77 | 1.77 | 41.17 | 77.93 | 4.71 | 16.18 | 4.71 | 0.18 | 0.73 | 0.16 | 1.17 | 26.77 |
| 3 | YZ [27] | 0.62 | long-flame coal | 1.92 | 14.32 | 46.23 | 80.17 | 5.61 | 8.12 | 1.43 | 4.68 | 0.84 | 0.08 | 1.17 | 44.55 |
| 4 | HQ | 0.67 | long-flame coal | 2.96 | 36.66 | 28.47 | 75.69 | 5.35 | 16.55 | 1.68 | 0.73 | 0.85 | 0.16 | 1.30 | 40.36 |
| 5 | MJL [28] | 0.74 | gas coal | 1.70 | 20.30 | 38.18 | 81.69 | 5.09 | 11.14 | 1.35 | 0.73 | 0.85 | 0.16 | 1.30 | 40.36 |
| 6 | XY | 1.27 | fat coal | 0.73 | 5.63 | 28.15 | 83.54 | 4.93 | 2.63 | 1.43 | 1.80 | 0.71 | 0.02 | 1.30 | 40.36 |
| 7 | LL [29] | 1.38 | coking coal | 4.68 | 3.39 | 28.94 | 79.30 | 5.73 | 8.92 | 1.36 | 0.69 | 0.87 | 0.08 | 1.22 | 43.71 |
| 8 | DP | 1.50 | coking coal | 0.60 | 4.17 | 18.80 | 90.38 | 4.76 | 2.09 | 1.48 | 1.30 | 0.63 | 0.02 | 1.35 | 58.90 |
| 9 | DEP [30] | 1.74 | lean coal | 0.62 | 8.61 | 17.04 | 90.13 | 4.56 | 3.32 | 1.44 | 0.55 | 0.61 | 0.03 | 1.35 | 56.00 |
| 10 | GY | 1.96 | lean coal | 0.56 | 6.89 | 17.22 | 84.18 | 4.30 | 1.40 | 1.26 | 1.93 | 0.61 | 0.01 | 1.30 | 64.41 |
| 11 | YQ | 2.04 | meager coal | 1.50 | 3.18 | 8.28 | 89.25 | 3.44 | 1.49 | 1.48 | 1.11 | 0.46 | 0.01 | 1.35 | 64.07 |
| 12 | ZZ | 2.21 | anthracite | 0.86 | 10.61 | 10.84 | 91.31 | 3.58 | 3.12 | 1.62 | 0.37 | 0.47 | 0.03 | 1.35 | 66.90 |
| 13 | CZ [31] | 3.21 | anthracite | 0.74 | 14.76 | 11.30 | 89.22 | 3.51 | 5.61 | 1.10 | 0.56 | 0.47 | 0.05 | 1.48 | 57.52 |
| Number | Coal Sample | HBs | Oxygen-Containing Functional Groups | ||||
|---|---|---|---|---|---|---|---|
| -C=O | -COOH | -OH | -C-O-C- | -CHO | |||
| 1 | WMC [25] | 94 | 3 | 2 | 7 | 4 | 0 |
| 2 | SD [26] | 82 | 9 | 0 | 9 | 13 | 0 |
| 3 | YZ [27] | 29 | 8 | 6 | 3 | 0 | 0 |
| 4 | HQ | 85 | 2 | 2 | 28 | 2 | 0 |
| 5 | MJL [28] | 54 | 4 | 3 | 9 | 3 | 0 |
| 6 | XY | 10 | 0 | 0 | 1 | 5 | 0 |
| 7 | LL [29] | 34 | 5 | 0 | 4 | 0 | 0 |
| 8 | DP | 4 | 0 | 0 | 2 | 0 | 1 |
| 9 | DEP [30] | 12 | 1 | 0 | 1 | 5 | 0 |
| 10 | GY | 6 | 1 | 0 | 1 | 1 | 0 |
| 11 | YQ | 22 | 1 | 0 | 2 | 0 | 0 |
| 12 | ZZ | 11 | 1 | 0 | 1 | 3 | 0 |
| 13 | CZ [31] | 15 | 7 | 0 | 1 | 1 | 0 |
| Correlation | Angle | HBs | -C=O | -COOH | -OH | -C-O-C- | -CHO |
|---|---|---|---|---|---|---|---|
| Angle | 1 | ||||||
| HBs | −0.87909 | 1 | |||||
| -C=O | −0.57683 | 0.398672 | 1 | ||||
| -COOH | −0.36544 | 0.331269 | 0.41051 | 1 | |||
| -OH | −0.52507 | 0.742642 | 0.097232 | 0.28167 | 1 | ||
| -C-O-C- | −0.54787 | 0.454336 | 0.31011 | −0.20406 | 0.145618 | 1 | |
| -CHO | 0.20335 | −0.28858 | −0.3118 | −0.16457 | −0.13333 | −0.23895 | 1 |
| Number | Coal Sample | 13C-NMR Parameters (%) | XBP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | WMC | 66.00 | 9.00 | 63.27 | 18.00 | 39.00 | 11.00 | 16.00 | 12.00 | 30.55 | 9.00 | 24.00 | 0 | 0.13 |
| 2 | SD | 71.00 | 6.00 | 65.00 | 42.00 | 23.00 | 3.00 | 11.00 | 9.00 | 29.00 | 5.00 | 22.00 | 3.00 | 0.16 |
| 3 | YZ | 74.00 | 1.00 | 64.00 | 45.00 | 19.00 | 8.00 | 5.00 | 6.00 | 26.00 | 11.00 | 12.00 | 3.00 | 0.10 |
| 4 | HQ | 72.78 | 1.40 | 71.37 | 45.63 | 25.75 | 5.09 | 10.63 | 10.02 | 27.22 | 19.92 | 5.83 | 1.47 | 0.16 |
| 5 | MJL | 74.24 | 5.10 | 69.14 | 45.68 | 23.46 | 6.08 | 4.06 | 13.32 | 25.76 | 10.05 | 12.64 | 3.07 | 0.24 |
| 6 | XY | 60.13 | 0 | 60.13 | 42.00 | 18.12 | 0 | 7.88 | 10.25 | 39.87 | 20.15 | 10.36 | 9.36 | 0.21 |
| 7 | LL | 71.99 | 1.55 | 70.44 | 46.88 | 23.56 | 2.78 | 5.42 | 15.36 | 28.01 | 17.41 | 10.60 | 0 | 0.28 |
| 8 | DP | 81.77 | 0.86 | 80.92 | 59.98 | 20.94 | 2.82 | 2.02 | 16.10 | 18.23 | 10.26 | 7.16 | 0.81 | 0.25 |
| 9 | DEP | 79.81 | 3.93 | 75.88 | 48.98 | 26.90 | 1.88 | 6.33 | 18.69 | 20.19 | 6.93 | 9.60 | 3.66 | 0.33 |
| 10 | GY | 72.41 | 9.98 | 62.42 | 44.25 | 18.17 | 1.46 | 0 | 12.89 | 27.59 | 6.39 | 7.95 | 13.25 | 0.26 |
| 11 | YQ | 82.08 | 13.36 | 68.82 | 38.65 | 30.17 | 0 | 0 | 16.31 | 17.92 | 3.33 | 1.58 | 13.01 | 0.30 |
| 12 | ZZ | 84.47 | 2.42 | 82.05 | 45.49 | 36.56 | 0 | 13.14 | 23.42 | 15.53 | 11.26 | 1.94 | 2.33 | 0.40 |
| 13 | CZ | 86.59 | 10.18 | 84.43 | 54.47 | 21.94 | 0.97 | 0.65 | 20.32 | 13.41 | 5.03 | 7.93 | 0.45 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, S.; Tang, S.; Zhang, S.; Li, J. Study on the Wetting Mechanisms of Different Coal Ranks Based on Molecular Dynamics. Processes 2024, 12, 455. https://doi.org/10.3390/pr12030455
Zhang C, Zhang S, Tang S, Zhang S, Li J. Study on the Wetting Mechanisms of Different Coal Ranks Based on Molecular Dynamics. Processes. 2024; 12(3):455. https://doi.org/10.3390/pr12030455
Chicago/Turabian StyleZhang, Chen, Songhang Zhang, Shuheng Tang, Shouren Zhang, and Jianxin Li. 2024. "Study on the Wetting Mechanisms of Different Coal Ranks Based on Molecular Dynamics" Processes 12, no. 3: 455. https://doi.org/10.3390/pr12030455
APA StyleZhang, C., Zhang, S., Tang, S., Zhang, S., & Li, J. (2024). Study on the Wetting Mechanisms of Different Coal Ranks Based on Molecular Dynamics. Processes, 12(3), 455. https://doi.org/10.3390/pr12030455






