Inhibition Localized Corrosion of N80 Petroleum Pipeline Steel in NaCl-Na2S Solution Using an Imidazoline Quaternary Ammonium Salt
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen and Corrosion Medium Preparation
2.2. Simulated Occluded Cell for Corrosion Experiment
- η—Corrosion inhibition efficiency, %;
- Icorr—Corrosion current density in blank solution, μA·cm−2;
- I′corr—Corrosion current density after addition of corrosion inhibitor, μA·cm−2.
- η—Corrosion inhibition efficiency, %;
- Rp—Polarization resistance after addition of corrosion inhibitor, Ω·cm−2;
- Rp0—Polarization resistance in blank solution, Ω·cm−2.
3. Results and Discussion
3.1. The Solution State Inside and Outside the Occluded Area without Adding Corrosion Inhibitor
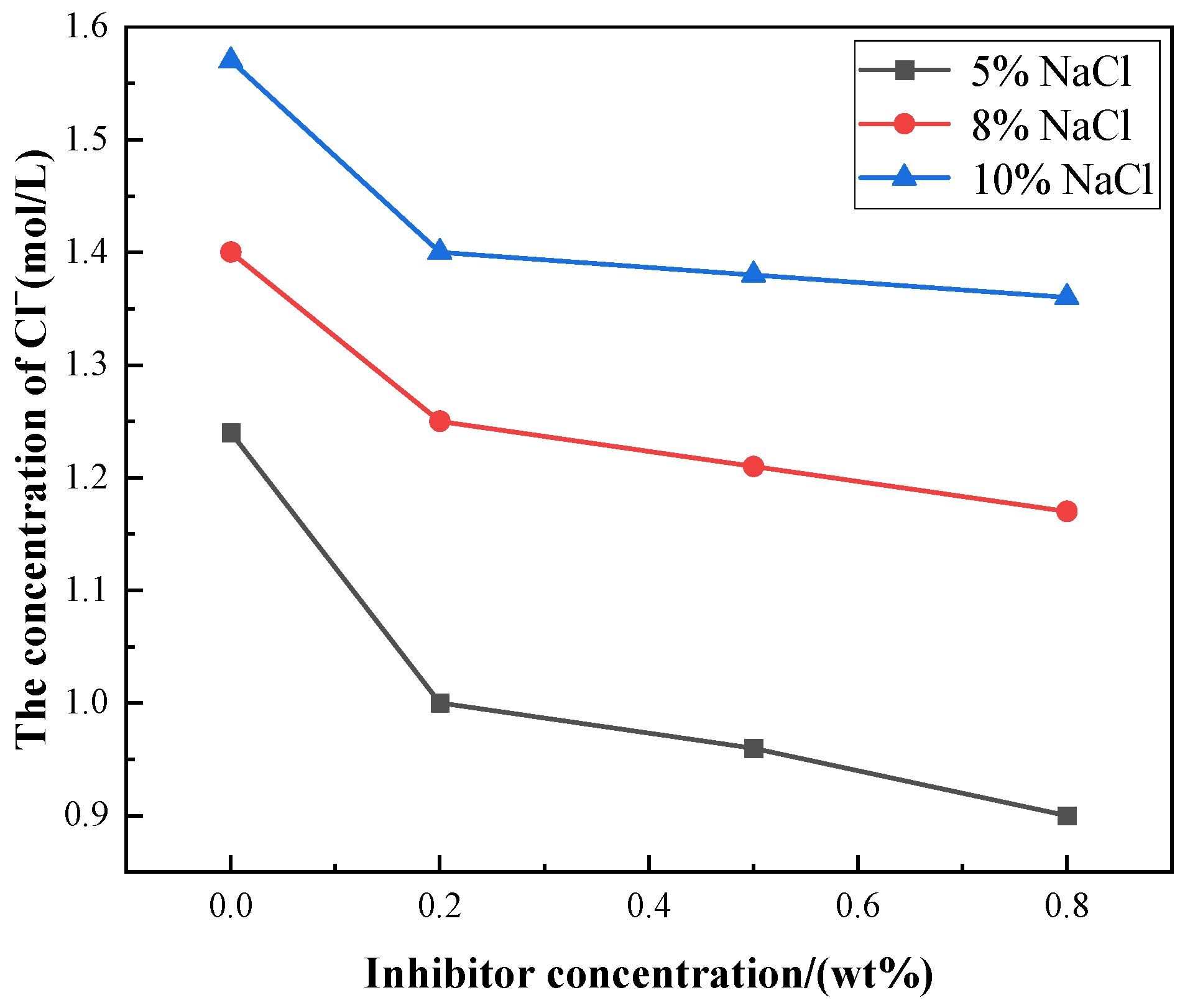
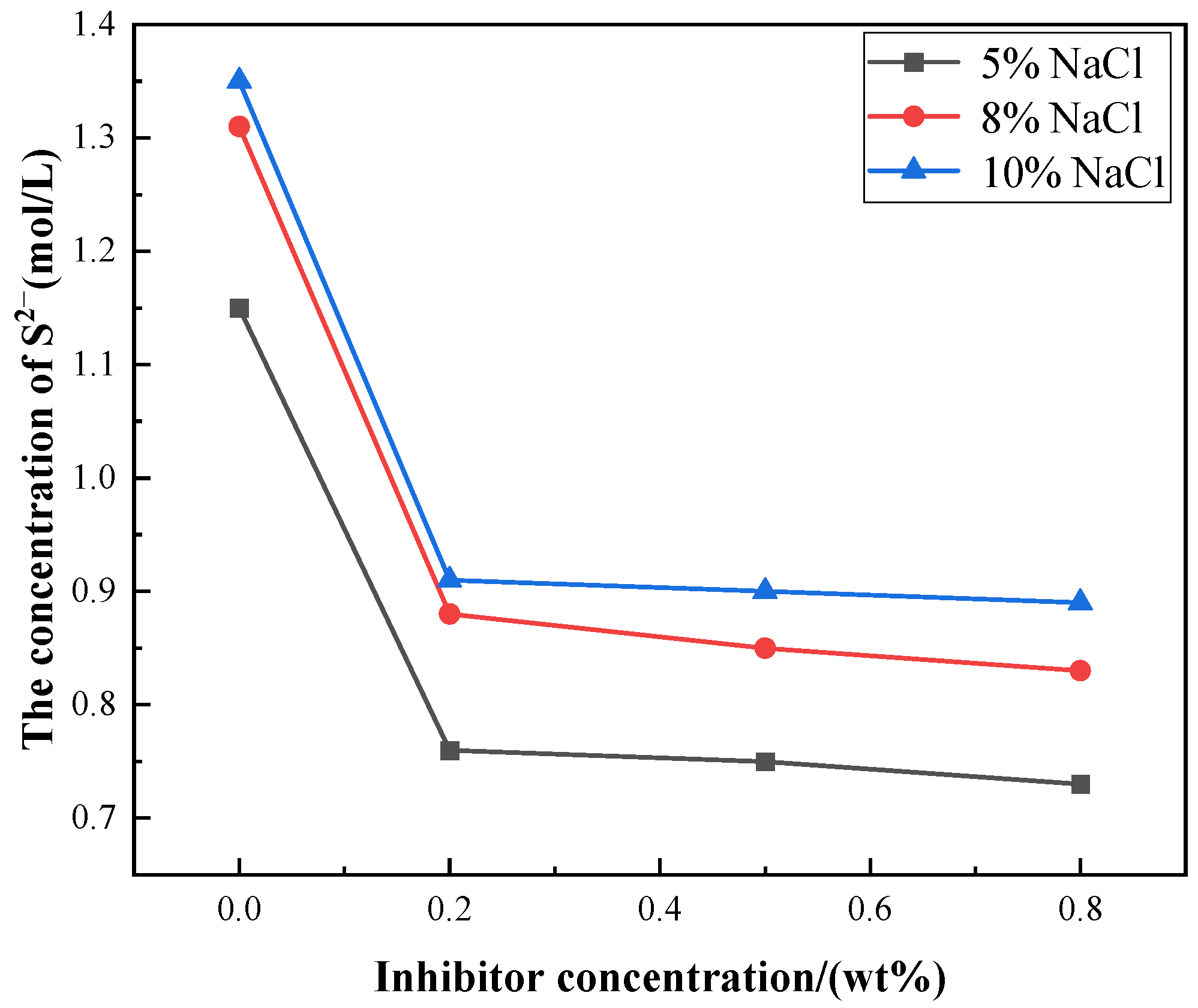
3.2. Changes of Cl−, S2− Concentration and pH Value in the Occluded Area with Different Amounts of Corrosion Inhibitors
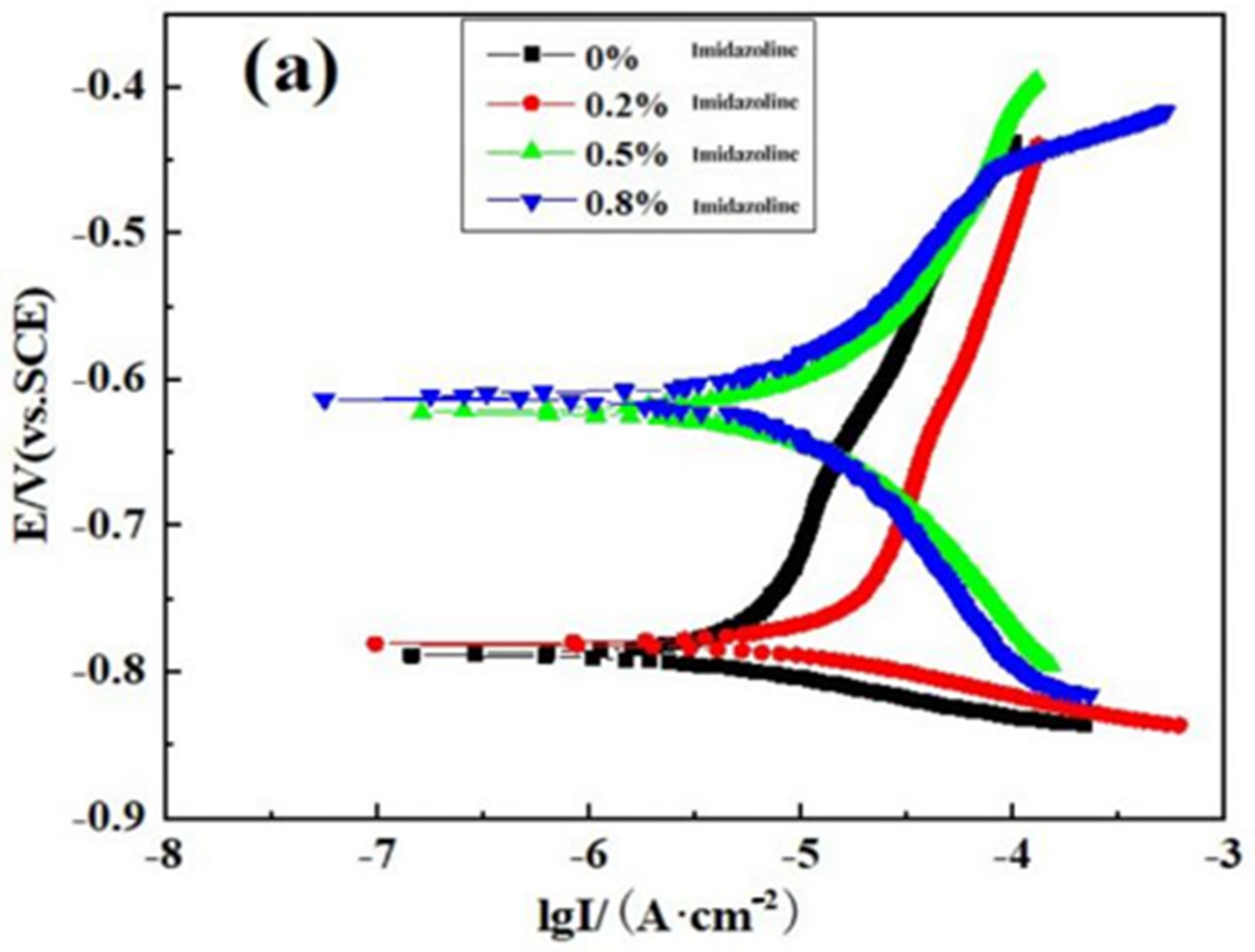
3.3. Polarization Curve Analysis
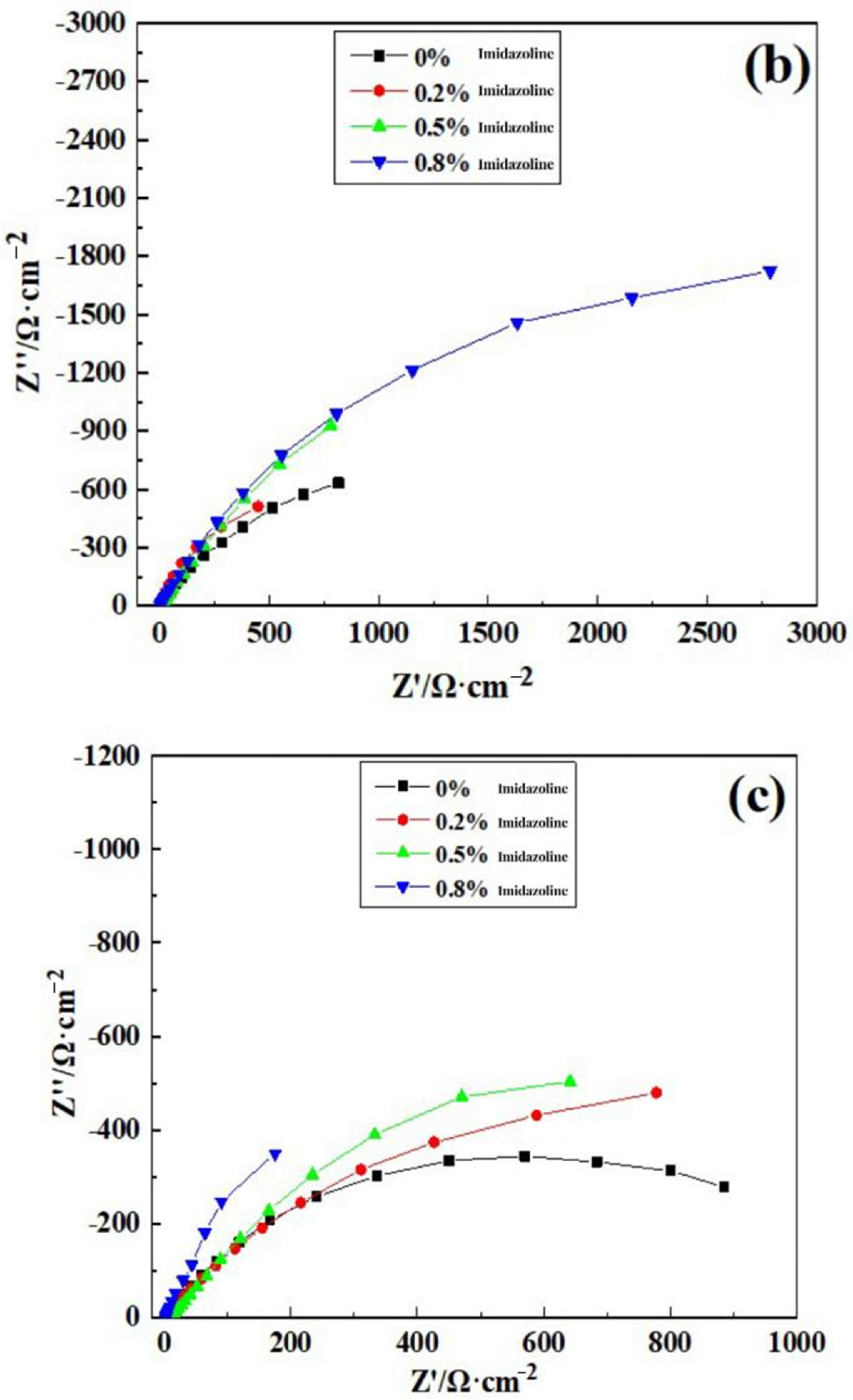
3.4. Electrochemical Impedance Spectroscopy Analysis
4. Conclusions
- (1)
- In the three solution systems, after the anode polarization of the occluded cell, the solution in the occluded area is acidified, and the pH value decreases sharply; the migration of Cl− and S2− is enhanced and enriched in the occluded area.
- (2)
- An imidazoline quaternary ammonium salt corrosion inhibitor can reduce the migration of small-radius anions (Cl− and S2−) to the occluded area and inhibit the acidification of the solution in the occluded area simultaneously. As a result, the dissolution of metals in the occluded area is prevented, owing to the decrease in the corrosivity of the solution in the occluded area as a result of the change in the chemical state of the occluded area.
- (3)
- In the three corrosion solution systems of 2% Na2S + 5% NaCl, 2% Na2S + 8% NaCl, and 2% Na2S + 10% NaCl, the imidazoline quaternary ammonium salt corrosion inhibitor can form an adsorption film layer on the metal surface to increase the polarization resistance and reduce the corrosion rate. The addition of a corrosion inhibitor can significantly increase the anode polarization kinetic constant, which can effectively inhibit the local corrosion of N80 steel in these corrosion systems.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.-V.; Voldsund, M.; Breuhaus, P.; Elmegaard, B. Energy efficiency measures for offshore oil and gas platforms. Energy 2016, 117, 325–340. [Google Scholar] [CrossRef]
- Lv, G.; Li, Q.; Wang, S.; Li, X. Key techniques of reservoir engineering and injection–production process for CO2 flooding in China’s SINOPEC Shengli Oilfield. J. CO2 Util. 2015, 11, 31–40. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Wang, Y.; Qi, G.; Yu, T.; Xin, X.; Zhao, B.; Chen, G. Oil-Soluble Exogenous Catalysts and Reservoir Minerals Synergistically Catalyze the Aquathermolysis of Heavy Oil. Molecules 2023, 28, 6766. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Wu, X.; Sun, Z.; Yu, C.; Ge, Y.; Yang, X.; Wen, L.; Ni, C.; Fu, X.; Zhang, J. Status and prospects of exploration and exploitation key technologies of the deep petroleum resources in onshore China. J. Nat. Gas Geosci. 2018, 3, 25–35. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Q.; Li, Q.; Huang, H.; Ni, W.; Wang, Q.; Xin, X.; Zhao, B.; Chen, G. Preparation of Multifunctional Surfactants Derived from Sodium Dodecylbenzene Sulfonate and Their Use in Oil-Field Chemistry. Molecules 2023, 28, 3640. [Google Scholar] [CrossRef] [PubMed]
- Xiao-hui, H. Study on Corrosion Behavior of Coiled Tubing in East Sichuan Gas Field. In Proceedings of the 2011 International Conference of Environmental Science and Engineering, Bali Island, Indonesia, 1–3 April 2011. [Google Scholar]
- Xu, X.; Shi, W.; Carr, T.R.; Zhai, G.; Wang, R.; Zhang, X.; Liu, K. Mesozoic horizontal stress in the East Sichuan Fold-and-thrust Belt, South China: Insights for Lower Paleozoic shale gas retention. J. Nat. Gas Sci. Eng. 2021, 95, 104154. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Obot, I.B. Corrosion challenges in petroleum refinery operations: Sources, mechanisms, mitigation, and future outlook. J. Saudi Chem. Soc. 2021, 25, 101370. [Google Scholar] [CrossRef]
- Jin, J.; Sun, J.; Lv, K.; Hou, Q.; Guo, X.; Liu, K.; Deng, Y.; Song, L. Catalytic pyrolysis of oil shale using tailored Cu@zeolite catalyst and molecular dynamic simulation. Energy 2023, 278, 127858. [Google Scholar] [CrossRef]
- Gamburg, Y.D.; Zangari, G. The Structure of the Metal-Solution Interface. In Theory and Practice of Metal Electrodeposition; Gamburg, Y.D., Zangari, G., Eds.; Springer: New York, NY, USA, 2011; pp. 27–51. [Google Scholar]
- Zhang, H.-h.; Pang, X.; Zhou, M.; Liu, C.; Wei, L.; Gao, K. The behavior of pre-corrosion effect on the performance of imidazoline-based inhibitor in 3 wt.% NaCl solution saturated with CO2. Appl. Surf. Sci. 2015, 356, 63–72. [Google Scholar] [CrossRef]
- Zhao, J.; Gu, F.; Zhao, T.; Jiang, R. Corrosion inhibition performance of imidazoline derivatives with different pedant chains under three flow rates in high-pressure CO2 environment. Res. Chem. Intermed. 2016, 42, 5753–5764. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, L.; He, Z.; Xu, X.; Hu, J.; Chen, Q.; Zhang, R.; Pu, J.; Guo, L. Synergistic Effect of Imidazoline Derivative and Benzimidazole as Corrosion Inhibitors for Q235 Steel: An Electrochemical, XPS, FT-IR and MD Study. Arab. J. Sci. Eng. 2022, 47, 7123–7134. [Google Scholar] [CrossRef]
- Gharbi, K.; Chouicha, S.; Kelland, M.A. Field test investigation of the performance of corrosion inhibitors: A case study. J. Pet. Explor. Prod. Technol. 2021, 11, 3879–3888. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Jafar Mazumder, M.A.; Ali, S.A.; Aljeaban, N.A.; Alharbi, B.G. Design and synthesis of a novel corrosion inhibitor embedded with quaternary ammonium, amide and amine motifs for protection of carbon steel in 1 M HCl. J. Mol. Liq. 2020, 317, 113917. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Ghaffari, S.; Hajizadeh, A. Film former corrosion inhibitors for oil and gas pipelines—A technical review. J. Nat. Gas Sci. Eng. 2018, 58, 92–114. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Wang, X.; Cui, Z.; Cui, H.; Li, Y. The stress corrosion cracking behavior of N80 carbon steel under a crevice in an acidic solution containing different concentrations of NaCl. Corros. Sci. 2023, 216, 111068. [Google Scholar] [CrossRef]
- Zhang, H. Study on the Influence of Galvanic Corrosion on Ship Structural Materials. IOP Conf. Ser. Earth Environ. Sci. 2019, 252, 022015. [Google Scholar] [CrossRef]
- Sriplai, N.; Sombatmankhong, K. Corrosion inhibition by imidazoline and imidazoline derivatives: A review. Corros. Rev. 2023, 41, 237–262. [Google Scholar] [CrossRef]
- Jin, J.; Sun, J.; Lv, K.; Guo, X.; Hou, Q.; Liu, J.; Wang, J.; Bai, Y.; Huang, X. Oxygen vacancy BiO2−x/Bi2WO6 synchronous coupling with Bi metal for phenol removal via visible and near-infrared light irradiation. J. Colloid Interf. Sci. 2021, 605, 342–353. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, L.; Yang, Z.; Ma, Q.; He, D.; Xu, K.; Zhang, H.; Zhang, P.; Sun, W.; Liu, G. Effect of ZnO as corrosion product on corrosion behavior of zinc-iron corrosion protection systems. Corros. Sci. 2024, 227, 111802. [Google Scholar] [CrossRef]
- GB/T 15453-2018; Determination of Chloride in Water for Industrial Circulating Cooling System and Boiler. State Administration for Market Regulation, Standardization Administration of China: Beijing, China, 2018.
- GB/T 223.68-1997; Methods for Chemical Analysis of Iron, Steel and Alloy—The Potassium Iodate Titration Method after Combustion in the Pipe Furnace for the Determination of Sulfur Content. CSBTS (State Bureau of Technical Supervision): Beijing, China, 1997.
- Chen, F.-Y.; Wu, Z.-Y.; Adler, Z.; Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- Yi, Y.; Weinberg, G.; Prenzel, M.; Greiner, M.; Heumann, S.; Becker, S.; Schlögl, R. Electrochemical corrosion of a glassy carbon electrode. Catal. Today 2017, 295, 32–40. [Google Scholar] [CrossRef]
- Kim, D.-J.; Kwon, H.-C.; Kim, H.P. Effects of the solution temperature and the pH on the electrochemical properties of the surface oxide films formed on Alloy 600. Corros. Sci. 2008, 50, 1221–1227. [Google Scholar] [CrossRef]
- Popov, K.I.; Djokić, S.S.; Nikolić, N.D.; Jović, V.D. The Cathodic Polarization Curves in Electrodeposition of Metals. In Morphology of Electrochemically and Chemically Deposited Metals; Popov, K.I., Djokić, S.S., Nikolić, N.D., Jović, V.D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–23. [Google Scholar]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Mechanistic aspect of near-neutral pH stress corrosion cracking of pipelines under cathodic polarization. Corros. Sci. 2012, 55, 54–60. [Google Scholar] [CrossRef]
- Ma, C.; Lin, W. Normal dispersion effects on the nonlinear focus. J. Opt. Soc. Am. B 2016, 33, 1055–1059. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Sun, L.; Fan, H. Effects of pH and Cl− concentration on corrosion behavior of the galvanized steel in simulated rust layer solution. Corros. Sci. 2012, 65, 520–527. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Praveen, B.M.; Hebbar, N.; Venkatesha, T.V.; Tandon, H.C. Inhibition study of mild steel corrosion in 1 M hydrochloric acid solution by 2-chloro 3-formyl quinoline. Int. J. Ind. Chem. 2016, 7, 9–19. [Google Scholar] [CrossRef]
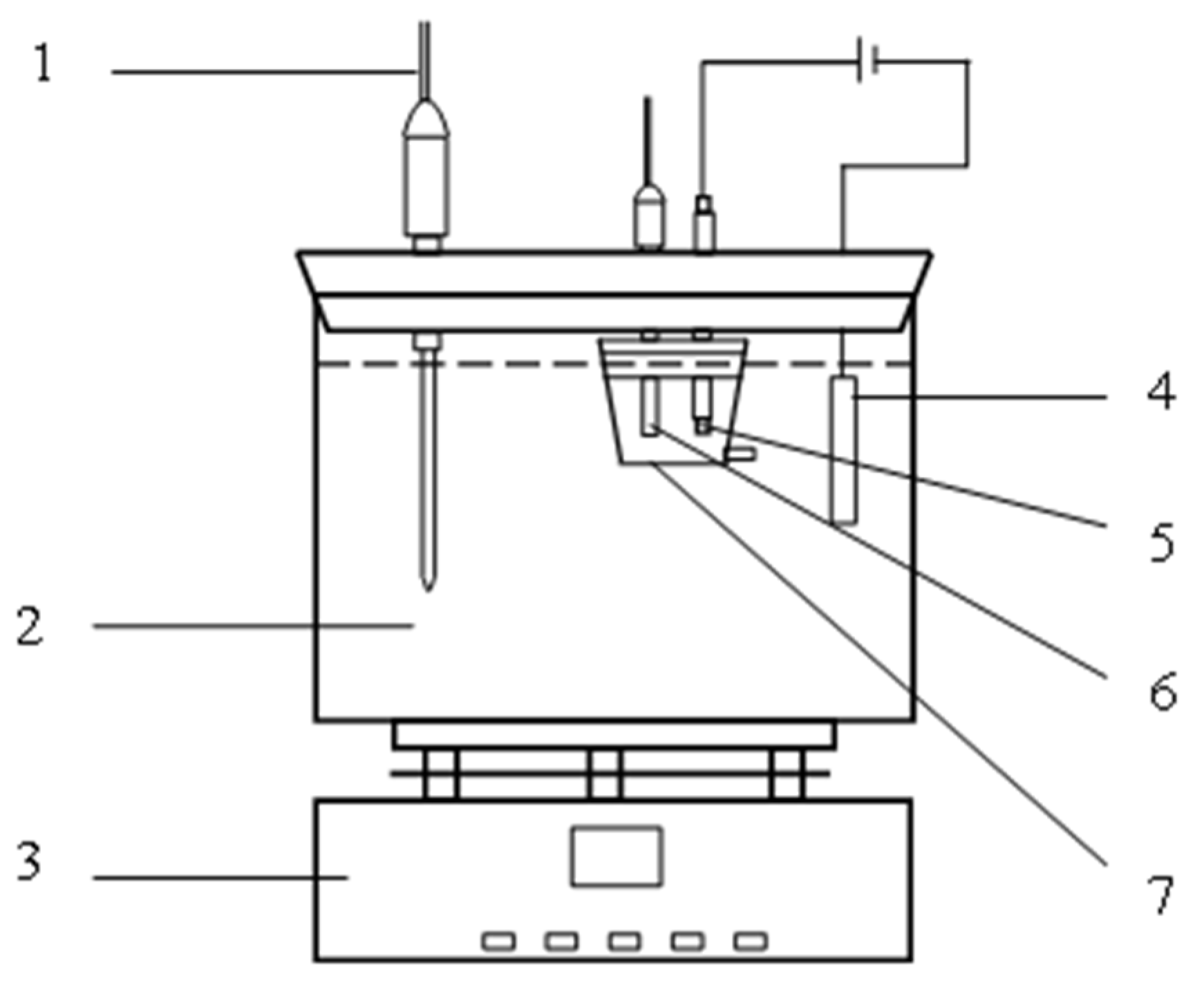




| Anodic Current Density (mA·cm−2) | Solution System | Solution Position | Cl− Concentration (mol·L−1) | S2− Concentration (mol·L−1) | pH |
|---|---|---|---|---|---|
| I = 1 | 2% Na2S + 5% NaCl | inside | 1.2411 | 1.1440 | 4.66 |
| outside | 1.1081 | 0.9948 | 13.05 | ||
| 2% Na2S + 8% NaCl | inside | 1.3981 | 1.3206 | 4.82 | |
| outside | 1.2388 | 1.1005 | 12.28 | ||
| 2% Na2S + 10% NaCl | inside | 1.5708 | 1.3448 | 5.95 | |
| outside | 1.1501 | 1.1023 | 12.92 |
| Current (mA·cm−2) | Solution System | Inhibitor Concentration (wt%) | Cl− Concentration (mol·L−1) | S2− Concentration (mol·L−1) | pH |
|---|---|---|---|---|---|
| I = 1 | 2% Na2S + 5% NaCl | 0 | 1.2411 | 1.1440 | 4.66 |
| 0.2 | 0.9967 | 0.7768 | 5.09 | ||
| 0.5 | 0.9546 | 0.7533 | 5.38 | ||
| 0.8 | 0.9022 | 0.7380 | 5.83 | ||
| 2% Na2S + 8% NaCl | 0 | 1.3981 | 1.3206 | 4.82 | |
| 0.2 | 1.2420 | 0.8806 | 5.23 | ||
| 0.5 | 1.2100 | 0.8442 | 5.46 | ||
| 0.8 | 1.1500 | 0.8311 | 6.08 | ||
| 2% Na2S + 10% NaCl | 0 | 1.5708 | 1.3448 | 5.95 | |
| 0.2 | 1.4057 | 0.9067 | 6.21 | ||
| 0.5 | 1.3655 | 0.8956 | 6.42 | ||
| 0.8 | 1.3430 | 0.8812 | 7.05 |
| Solution System | Temperature (°C) | Inhibitor Concentration (wt%) | βa (mV) | βc (mV) | E0 (mV) | I0 (A/cm2) | Inhibition Efficiency η |
|---|---|---|---|---|---|---|---|
| 2% Na2S + 5% NaCl | 50 | 0 | 191.56 | 32.73 | −7.885 × 102 | 2.56 × 10−5 | --- |
| 0.2 | 322.43 | 35.88 | −7.819 × 102 | 8.85 × 10−6 | 76% | ||
| 0.5 | 119.69 | 126.94 | −6.223 × 102 | 5.78 × 10−6 | 88% | ||
| 0.8 | 326.02 | 232.31 | −6.133 × 102 | 5.33 × 10−6 | 93% | ||
| 2% Na2S + 8% NaCl | 50 | 0 | 196.65 | 36.531 | −8.875 × 102 | 1.24 × 10−5 | --- |
| 0.2 | 490.59 | 28.46 | −8.845 × 102 | 4.92 × 10−6 | 60% | ||
| 0.5 | 255.08 | 31.38 | −8.277 × 102 | 4.62 × 10−6 | 63% | ||
| 0.8 | 160.34 | 71.63 | −7.736 × 102 | 1.94 × 10−6 | 84% | ||
| 2% Na2S + 10% NaCl | 50 | 0 | 213.04 | 65.87 | −8.656 × 102 | 8.13 × 10−5 | --- |
| 0.2 | 112.07 | 67.40 | −7.783 × 102 | 1.94 × 10−5 | 65% | ||
| 0.5 | 175.60 | 118.81 | −7.276 × 102 | 9.80 × 10−6 | 77% | ||
| 0.8 | 181.48 | 111.04 | −6.829 × 102 | 5.54 × 10−6 | 79% |
| Solution System | Temperature (°C) | Corrosion Inhibitor Concentration (wt%) | Cdl | Rp | Inhibition Efficiency η |
|---|---|---|---|---|---|
| 2% Na2S + 5% NaCl | 50 | 0% | 9.41 × 10−4 | 408 | --- |
| 0.2% | 4.40 × 10−4 | 832 | 51% | ||
| 0.5% | 3.44 × 10−4 | 1158 | 65% | ||
| 0.8% | 1.57 × 10−4 | 1615 | 75% | ||
| 2% Na2S + 8% NaCl | 50 | 0% | 2.81 × 10−4 | 483 | --- |
| 0.2% | 3.31 × 10−4 | 943 | 49% | ||
| 0.5% | 7.28 × 10−4 | 993 | 51% | ||
| 0.8% | 8.02 × 10−3 | 2101 | 77% | ||
| 2% Na2S + 10% NaCl | 50 | 0% | 1.96 × 10−3 | 140 | --- |
| 0.2% | 3.04 × 10−4 | 262 | 47% | ||
| 0.5% | 2.17 × 10−2 | 332 | 58% | ||
| 0.8% | 1.82 × 10−3 | 594 | 76% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Du, T.; Cui, G.; He, H.; Wu, P.; Li, Y. Inhibition Localized Corrosion of N80 Petroleum Pipeline Steel in NaCl-Na2S Solution Using an Imidazoline Quaternary Ammonium Salt. Processes 2024, 12, 491. https://doi.org/10.3390/pr12030491
Li S, Du T, Cui G, He H, Wu P, Li Y. Inhibition Localized Corrosion of N80 Petroleum Pipeline Steel in NaCl-Na2S Solution Using an Imidazoline Quaternary Ammonium Salt. Processes. 2024; 12(3):491. https://doi.org/10.3390/pr12030491
Chicago/Turabian StyleLi, Shanjian, Te Du, Guotao Cui, Haoxuan He, Panfeng Wu, and Yongfei Li. 2024. "Inhibition Localized Corrosion of N80 Petroleum Pipeline Steel in NaCl-Na2S Solution Using an Imidazoline Quaternary Ammonium Salt" Processes 12, no. 3: 491. https://doi.org/10.3390/pr12030491
APA StyleLi, S., Du, T., Cui, G., He, H., Wu, P., & Li, Y. (2024). Inhibition Localized Corrosion of N80 Petroleum Pipeline Steel in NaCl-Na2S Solution Using an Imidazoline Quaternary Ammonium Salt. Processes, 12(3), 491. https://doi.org/10.3390/pr12030491






