Hyperspectral and Microtomographic Analyses to Evaluate the Stability of Quercetin and Calcium Effervescent Tablets Exposed to Heat and Ultraviolet Radiation †
Abstract
1. Introduction
2. Materials and Methods
2.1. Analyzed Tablets
- Preparation 1—composed of citric acid, sodium carbonates, calcium carbonate, sorbitols, corn starch, quercetin, polyvinylpyrrolidone, polyethylene glycol, acesulfame K, aspartame;
- Preparation 2—composed of citric acid, calcium carbonate, sorbitol, sodium bicarbonate, extract from leaves of the common pachnotica (Perilla frutescens), lemon flavor, quercetin, sweetener carrier: polyethylene glycol, sodium saccharin, vitamin D3, riboflavin;
- Preparation 3—composed of citric acid, sodium bicarbonate, calcium carbonate, sorbitol, corn starch, sodium cyclamate, quercetin, flavors: aspartame, acesulfame K, sodium selenate; folic acid, vitamin B12.
2.2. Evaluation of the Effervescent Tablets
2.3. Photodegradation under Simulated Aging
2.4. Hyperspectral Imaging
2.5. X-ray Microtomography
2.5.1. Analysis of the Density of the Inner Structure Using X-ray Microtomography
2.5.2. Porosity Analysis Using X-ray Microtomography
2.6. Statistical Analyses
3. Results
3.1. Characteristics of the Analysed Effervescent Tablets
3.2. Hyperspectral Analysis
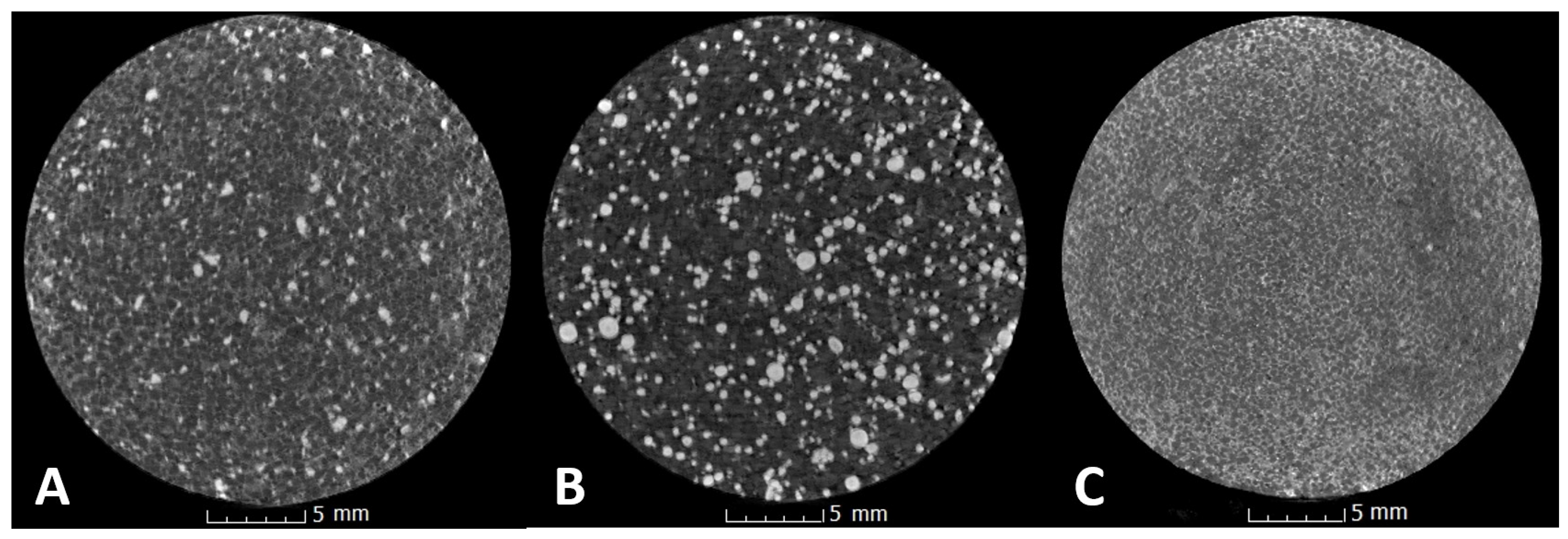
3.3. X-ray Microtomography Analyses
3.3.1. Measurement of Density of Analyzed Tablets
3.3.2. Measurement of Porosity of Analyzed Tablets
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jamrógiewicz, M.; Karczewska-Kaliszak, A.; Milewska, K. Light-sensitive medicinal compounds—The latest review of studies and strategies in proceedings. Farm. Pol. 2019, 75, 575–583. (In Polish) [Google Scholar] [CrossRef]
- Ramos, P.; Broncel, M. Influence of Storage Conditions on the Stability of Gum Arabic and Tragacanth. Molecules 2022, 27, 1510. [Google Scholar] [CrossRef]
- Gul, W.; Basheer, S.; Karim, F.; Ayub, S. Effect of Acid. Base. Temperature and U.V. Light on Amlodipine Besylate. Int. J. Adv. Res. Chem. Sci. 2015, 2, 21–24. [Google Scholar]
- Mira, J.J.; Lorenzo, S.; Guilabert, M.; Navarro, I.; Pérez-Jover, V. A Systematic Review of Patient Medication Error on Self-Administering Medication at Home. Expert Opin. Drug Saf. 2015, 14, 815–838. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Ibrahim, H.K.; Ghorab, M.M. Effervescent tablet formulation for enhanced patient compliance and the therapeutic effect of risperidone. Drug Deliv. 2016, 23, 297–306. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin. Inflammation and Immunity. Nutrients 2016, 18, 167. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Fornal, E. The Effects of Quercetin Supplementation on Blood Pressure—Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 101350. [Google Scholar] [CrossRef] [PubMed]
- Özsoy Gökbilen, S.; Becer, E.; Vatansever, H.S. Senescence-mediated anticancer effects of quercetin. Nutr. Res. 2022, 104, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Hayes, A.W.; Pressman, P.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Quercetin induces its chemoprotective effects via hormesis. Food Chem. Toxicol. 2024, 184, 114419. [Google Scholar] [CrossRef] [PubMed]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Jafarinia, M.; Sadat Hosseini, M.; Kasiri, N.; Fazel, N.; Fathi, F.; Ganjalikhani Hakemi, M.; Eskandari, N. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Dębińska, A.; Sozańska, B. Dietary Polyphenols-Natural Bioactive Compounds with Potential for Preventing and Treating Some Allergic Conditions. Nutrients 2023, 15, 4823. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Shirai, M.; Inaba, Y.; Takara, T. Effects of repeated oral intake of a quercetin-containing supplement on allergic reaction: A randomized, placebo-controlled, double-blind parallel-group study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4331–4345. [Google Scholar] [CrossRef] [PubMed]
- Cesarone, M.R.; Belcaro, G.; Hu, S.; Dugall, M.; Hosoi, M.; Ledda, A.; Feragalli, B.; Maione, C.; Cotellese, R. Supplementary prevention and management of asthma with quercetin phytosome: A pilot registry. Minerva Med. 2019, 110, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Beto, J.A. The role of calcium in human aging. Clin. Nutr. Res. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Xing, T.; Hu, Y.; Wang, B.; Zhu, J. Role of oral calcium supplementation alone or with vitamin D in preventing post-thyroidectomy hypocalcaemia: A meta-analysis. Medicine 2019, 98, e14455. [Google Scholar] [CrossRef] [PubMed]
- Fardellone, P.; Brazier, M.; Kamel, S.; Guéris, J.; Graulet, A.M.; Liénard, J.; Sebert, J.L. Biochemical effects of calcium supplementation in postmenopausal women: Influence of dietary calcium intake. Am. J. Clin. Nutr. 1998, 67, 1273–1278. [Google Scholar] [CrossRef]
- Jin, X.; Shen, J.; Liu, T.; Zhou, R.; Huang, X.; Wang, T.; Wu, W.; Wang, M.; Xie, R.; Yuan, J. The significance of short-term preoperative calcium and activated vitamin D3 supplementation in thyroidectomy: A randomized trial and prospective study. Endocr. Connect. 2023, 13, e230377. [Google Scholar] [CrossRef]
- Sittitrai, P.; Ruenmarkkaew, D.; Klibngern, H.; Ariyanon, T.; Hanprasertpong, N.; Boonyaprapa, S.; Sreesawat, M. Perioperative versus postoperative calcium and vitamin D supplementation to prevent symptomatic hypocalcemia after total thyroidectomy: A randomized placebo controlled trial. Int. J. Surg. 2023, 109, 13–20. [Google Scholar] [CrossRef]
- Woo Kinshella, M.L.; Sarr, C.; Sandhu, A.; Bone, J.N.; Vidler, M.; Moore, S.E.; Elango, R.; Cormick, G.; Belizan, J.M.; Hofmeyr, G.J.; et al. PRECISE Network. Calcium for pre-eclampsia prevention: A systematic review and network meta-analysis to guide personalised antenatal care. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1833–1843. [Google Scholar] [CrossRef]
- Cormick, G.; Betran, A.P.; Romero, I.B.; Cormick, M.S.; Belizán, J.M.; Bardach, A.; Ciapponi, A. Effect of Calcium Fortified Foods on Health Outcomes: A Systematic Review and Meta-Analysis. Nutrients. 2021, 3, 316. [Google Scholar] [CrossRef]
- Zhang, D.; Zhong, X.; Cheng, C.; Su, Z.; Xue, Y.; Liu, Y.; Zhang, Y.; Feng, M.; Xu, Z.; Zhao, T.; et al. Effect of Vitamin D and/or Calcium Supplementation on Pancreatic β-Cell Function in Subjects with Prediabetes: A Randomized. Controlled Trial. J. Agric. Food Chem. 2023, 71, 347–357. [Google Scholar] [CrossRef]
- Liu, Y.; Le, S.; Liu, Y.; Jiang, H.; Ruan, B.; Huang, Y.; Ao, X.; Shi, X.; Fu, X.; Wang, S. The effect of calcium supplementation in people under 35 years old: A systematic review and meta-analysis of randomized controlled trials. elife 2022, 11, e79002. [Google Scholar] [CrossRef]
- Darwin, A.H.; Carroll, M.P.; Galvis Noda, S.D.; Perez Perez, S.F.; Mhaskar, R.S.; Spoto-Cannons, A.C.; Lockey, R.F. Calcium and vitamin D intake in allergic versus non-allergic children and corresponding parental attitudes towards dairy products. World Allergy Organ. J. 2021, 14, 100579. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Maternal consumption of dairy products. calcium. and vitamin D during pregnancy and infantile allergic disorders. Ann. Allergy Asthma Immunol. 2014, 113, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Schaepman-Strub, G.; Schaepman, M.E.; Painter, T.H.; Dangel, S.; Martonchik, J.V. Reflectance quantities in optical remote sensing—Definitions and case studies. Remote Sens. Environ. 2006, 103, 27–42. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Yang, W.; Ning, Z.; Dong, H. Review on the Application of Hyperspectral Imaging Technology of the Exposed Cortex in Cerebral Surgery. Front. Bioeng. Biotechnol. 2022, 10, 906728. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, O.; Gomez, R.B.; Chainani, A.; Roper, W.E. Hyperspectral imaging applied to medical diagnoses and food safety. In Geo-Spatial and Temporal Image and Data Exploitation III; SPIE: Bellingham, WA, USA, 2023; Volume 5097, pp. 215–221. [Google Scholar] [CrossRef]
- Sarić, R.; Nguyen, V.D.; Burge, T.; Berkowitz, O.; Trtílek, M.; Whelan, J.; Lewsey, M.G.; Čustović, E. Applications of hyperspectral imaging in plant phenotyping. Trends Plant Sci. 2022, 27, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, J.; Goudarzi, N.M.; Eeckhaut, G.; Schrijnemakers, K.; Cnudde, V.; Boone, M.N. Characterization of Pharmaceutical Tablets by X-ray Tomography. Pharmaceuticals 2023, 16, 733. [Google Scholar] [CrossRef]
- da Silva, Í.B. X-ray Computed microtomography technique applied for cementitious materials: A review. Micron 2018, 107, 1. [Google Scholar] [CrossRef]
- Landis, E.N.; Keane, D.T. X-ray microtomography. Mater. Charact. 2010, 61, 1305. [Google Scholar] [CrossRef]
- Dave, V.S.; Shahin, H.I.; Youngren-Ortiz, S.R.; Chougule, M.B.; Haware, R.V. Emerging technologies for the non-invasive characterization of physical-mechanical properties of tablets. Int. J. Pharm. 2017, 532, 299–312. [Google Scholar] [CrossRef]
- Meisner, M.; Duda, P.; Szulc-Musioł, B.; Sarecka-Hujar, B. X-ray Microtomography as a Non-Invasive Method for Evaluating the Stability of Commercial Effervescent Tablets. Eng. Proc. 2023, 37, 94. [Google Scholar] [CrossRef]
- Sarecka-Hujar, B.; Szulc-Musioł, B.; Meisner, M.; Duda, P. The Use of Novel. Rapid Analytical Tools in the Assessment of the Stability of Tablets—A Pilot Analysis of Expired and Unexpired Tablets Containing Nifuroxazide. Processes 2022, 10, 1934. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 10th ed.; EDQM Council of Europe: Strasbourg, France, 2022.
- Meisner, M.; Duda, P.; Szulc-Musioł, B.; Sarecka-Hujar, B. Characteristics of Commercial Effervescent Tablets Using Selected Pharmacopeial and Novel Analytical Methods. Appl. Sci. 2023, 13, 3171. [Google Scholar] [CrossRef]
- Vanbillemont, B.; Everaert, H.; De Beer, T. New advances in the characterization of lyophi-lised orally disintegrating tablets. Int. J. Pharm. 2020, 579, 119153. [Google Scholar] [CrossRef] [PubMed]
- Miguélez-Morán, A.M.; Wu, C.-Y.; Dong, H.; Seville, J.P.K. Characterisation of density distri-butions in roller-compacted ribbons using micro-indentation and X-ray micro-computed tomography. Eur. J. Pharm. Biopharm. 2009, 72, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wiedey, R.; Kleinebudde, P. The Density distribution in Ribbons from Roll Compaction. Chem. Ing. Tech. 2017, 89, 1017–1024. [Google Scholar] [CrossRef]
- Akseli, I.; Iyer, S.; Lee, H.P.; Cuitiño, A.M. A quantitative correlation of the effect of density distributions in roller-compacted ribbons on the mechanical properties of tablets using ul-trasonics and X-ray tomography. AAPS Pharm. Sci. Tech. 2011, 12, 834–853. [Google Scholar] [CrossRef]
- Mazel, V.; Diarra, H.; Malvestio, J.; Tchoreloff, P. Lamination of biconvex tablets: Numerical and experimental study. Int. J. Pharm. 2018, 542, 66–71. [Google Scholar] [CrossRef]
- Palanisamy, P.; Abhishekh, R.; Kumar, D. Formulation and evaluation of effervescent tablets of aceclofenac. Int. Res. J. Pharm. 2011, 2, 185–190. [Google Scholar] [CrossRef]
- Aslani, A.; Fattahi, F. Formulation. characterization and physicochemical evaluation of ranitidine effervescent tablets. Adv. Pharm. Bull. 2013, 3, 315–322. [Google Scholar] [CrossRef]
- Dubray, C.; Maincent, P.; Milon, J.Y. From the pharmaceutical to the clinical: The case for effervescent paracetamol in pain management. A narrative review. Curr. Med. Res. Opin. 2021, 37, 1039–1048. [Google Scholar] [CrossRef]
- Sandler, N.; Reiche, K.; Heinamaki, J.; Yliruusi, J. Effect of Moisture on Powder Flow Properties of Theophylline. Pharmaceutics 2010, 2, 275–290. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Torres, M.P.R.; Raiser, A.L.; Ribeiro, E.B.; Andrighetti, C.R.; Valladão, D.M.S. Effervescent vitamin C tablets and its quality control. Sci. Elec. Arch. 2020, 13, 73–79. [Google Scholar] [CrossRef]
- Sudhir, K.; Pagire, S.; Seaton, C.; Anant Paradkar, A. Improving Stability of Effervescent Products by Co-Crystal Formation: A Novel Application of Crystal Engineered Citric Acid. Cryst. Growth Des. 2020, 20, 4839–4844. [Google Scholar] [CrossRef]
- Sun, C.C. Microstructure of Tablet-Pharmaceutical Significance, Assessment, and Engineering. Pharm. Res. 2017, 34, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, H.; Chang, T.; Zhang, J.; Cui, H.L. Terahertz detection of porosity and porous microstructure in pharmaceutical tablets: A review. Int. J. Pharm. 2020, 591, 120006. [Google Scholar] [CrossRef]
- Anuschek, M.; Bawuah, P.; Zeitler, J.A. Terahertz time-domain spectroscopy for powder compact porosity and pore shape measurements: An error analysis of the anisotropic bruggeman model. Int. J. Pharm. 2021, 3, 100079. [Google Scholar] [CrossRef]
- Moradikouchi, A.; Sparén, A.; Folestad, S.; Stake, J.; Rodilla, H. Terahertz frequency domain sensing for fast porosity measurement of pharmaceutical tablets. Int. J. Pharm. 2022, 618, 121579. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Wang, H.J.; Wang, J.Y. Preparation and evaluation of captopril loaded gastro-retentive zein based porous floating tablets. Int. J. Pharm. 2020, 579, 119185. [Google Scholar] [CrossRef]
- Donoso, M.; Kildsig, D.O.; Ghaly, E.S. Prediction of tablet hardness and porosity using near-infrared diffuse reflectance spectroscopy as a nondestructive method. Pharm. Dev. Technol. 2003, 8, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Notenboom, K.; Beers, E.; van Riet-Nales, D.A.; Egberts, T.C.; Leufkens, H.G.; Jansen, P.A.; Bouvy, M.L. Practical problems with medication use that older people experience: A qualitative study. J. Am. Geriatr. Soc. 2014, 62, 2339–2344. [Google Scholar] [CrossRef]
- Kollerup, M.G.; Curtis, T.; Schantz Laursen, B. Visiting nurses’ posthospital medication management in home health care: An ethnographic study. Scand. J. Caring. Sci. 2018, 32, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.L.; Hor, C.Y.; Ong, S.T.; Roslan, M.F.; Beh, X.Y.; Permal, D.; Rama, S. Home medication management problems and associated factors among psychiatric patients using home care pharmacy services at government hospitals in western Malaysia. BMC Health Serv. Res. 2022, 22, 726. [Google Scholar] [CrossRef]
- Dijkstra, N.E.; Sino, C.G.M.; Schuurmans, M.J.; Schoonhoven, L.; Heerdink, E.R. Medication self-management: Considerations and decisions by older people living at home. Res. Social. Adm. Pharm. 2022, 18, 2410–2423. [Google Scholar] [CrossRef] [PubMed]
- Funk, O.G.; Yung, R.; Arrighi, S.; Lee, S. Medication Storage Appropriateness in US Households. Innov. Pharm. 2021, 12, 10. [Google Scholar] [CrossRef]
- Hewson, C.; Shen, C.C.; Strachan, C.; Norris, P. Personal medicines storage in New Zealand. J. Prim. Health Care 2013, 5, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Vlieland, N.D.; van den Bemt, B.J.F.; Bekker, C.L.; Bouvy, M.L.; Egberts, T.C.G.; Gardarsdottir, H. Older Patients’ Compliance with Drug Storage Recommendations. Drugs Aging 2018, 35, 233–241. [Google Scholar] [CrossRef] [PubMed]


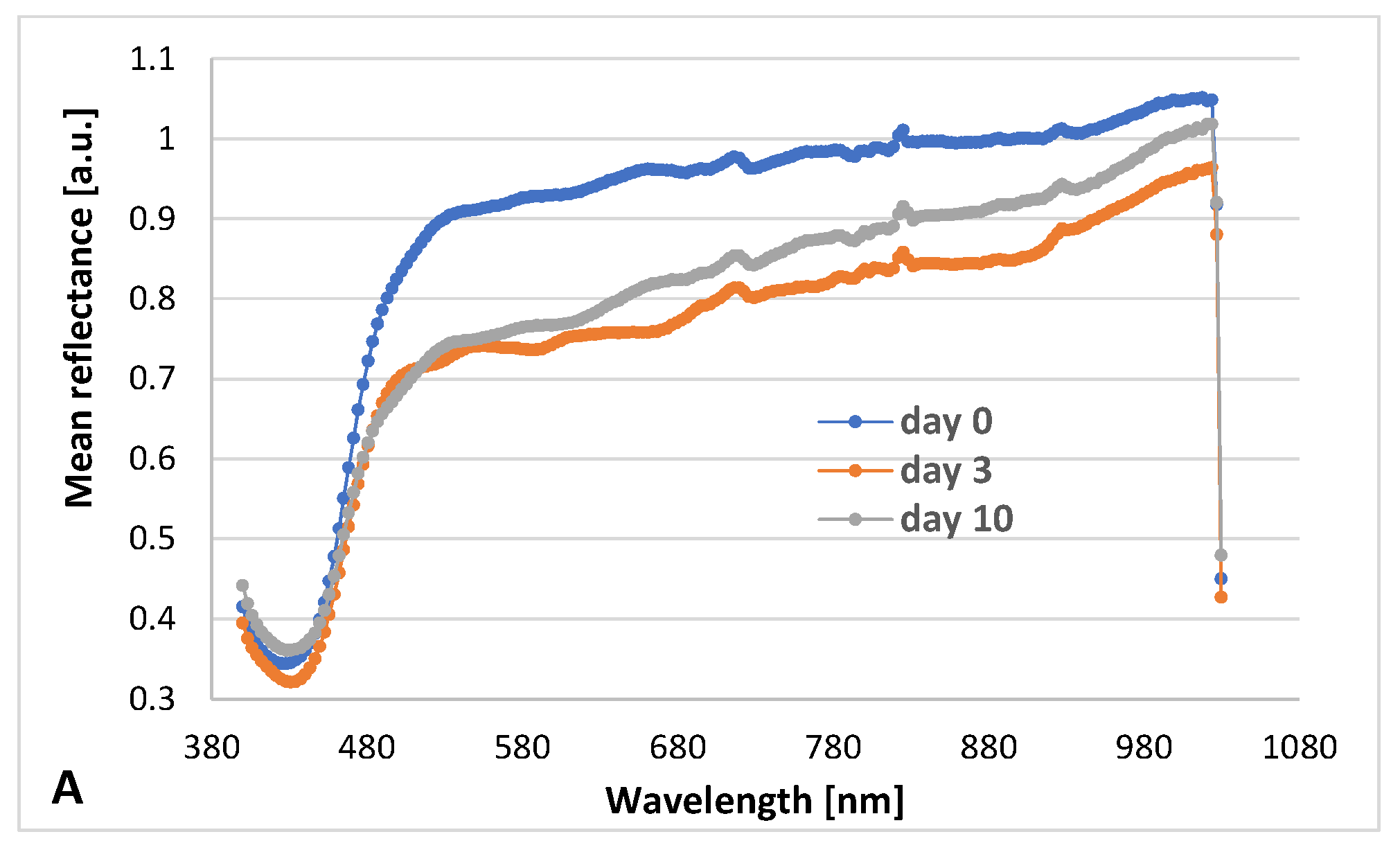
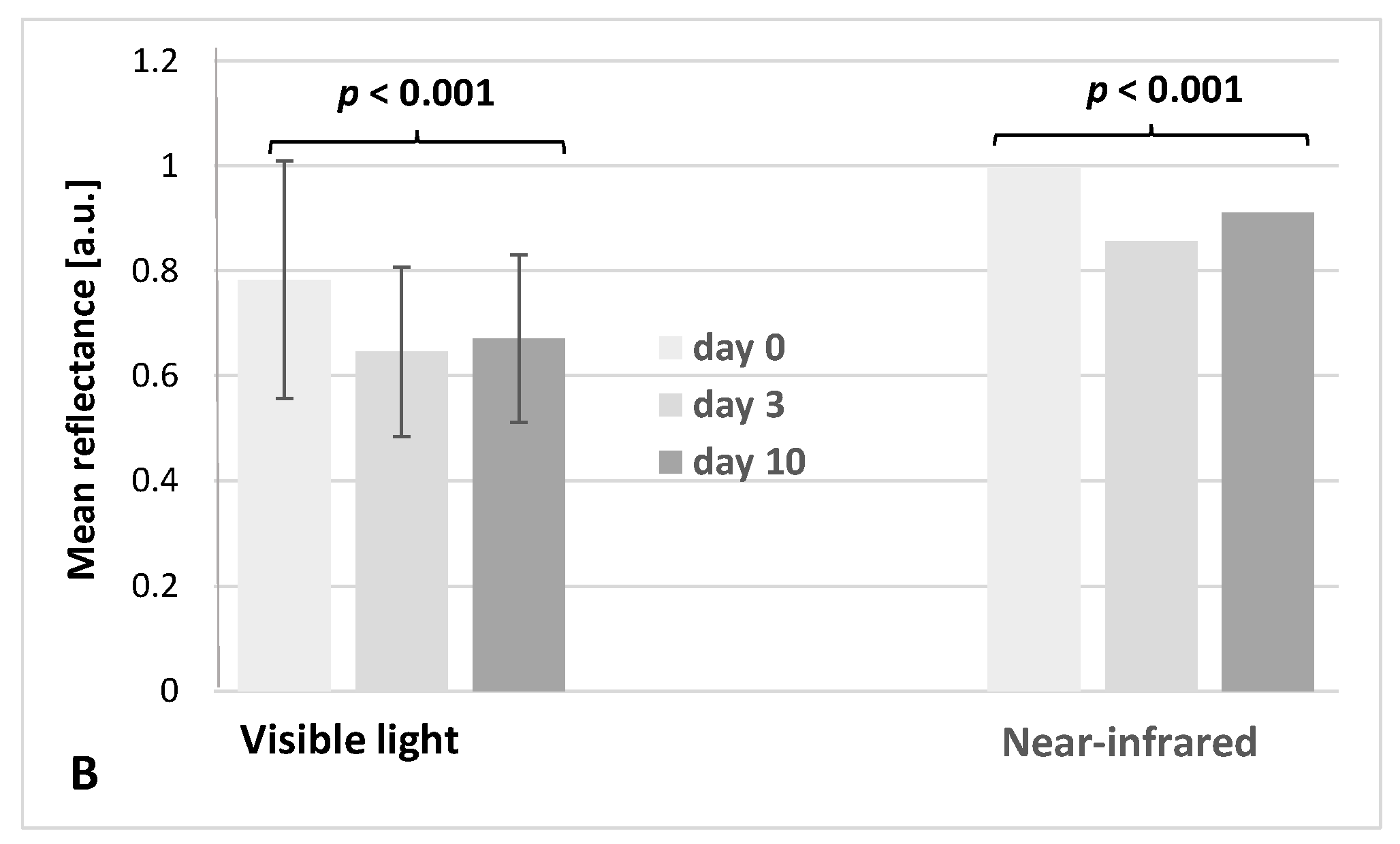
| Preparation | Duration of Experiment [Days] | Weight [g] n = 20, M ± SD | Force Needed to Crush the Tablet [N] n = 5, M ± SD | Hardness Factor [N/m2] n = 5, M ± SD | Disintegration Time [min] n = 6, M ± SD |
|---|---|---|---|---|---|
| Preparation 1 | day 0 | 4.210 ± 0.056 | 48.860 ± 2.971 | 35.13 × 104 ± 1.23 × 104 | 1.08 ± 0.05 |
| day 3 | 4.192 ± 0.069 | 52.480 ± 7.174 | 37.57 × 104 ± 3.64 × 104 | 1.19 ± 0.01 | |
| day 10 | 4.168 ± 0.036 | 62.240 ± 10.013 | 44.58 × 104 ± 5.04 × 104 | 1.35 ± 0.01 | |
| p | 0.132 | 0.145 | 0.040 1 | 0.002 2 | |
| Preparation 2 | day 0 | 3.990 ± 0.049 | 97.540 ± 19.403 | 68.24 × 104 ± 10.48 × 104 | 1.15 ± 0.01 |
| day 3 | 3.991 ± 0.031 | 97.380 ± 6.997 | 69.61 × 104 ± 3.42 × 104 | 1.17 ± 0.01 | |
| day 10 | 4.025 ± 0.046 | 126.260 ± 9.455 | 89.93 × 104 ± 3.42 × 104 | 1.29 ± 0.01 | |
| p | 0.519 | 0.017 3 | 0.012 4 | <0.001 5 | |
| Preparation 3 | day 0 | 4.227 ± 0.011 | 85.210 ± 2.330 | 59.34 × 104 ±1.75 × 104 | 1.05 ± 0.00 |
| day 3 | 4.174 ± 0.015 | 137.74 ± 3.691 | 97.51 × 104 ± 2.08 × 104 | 1.01 ± 0.01 | |
| day 10 | 4.183 ± 0.033 | 145.24 ± 9.633 | 102.38 × 104 ± 4.89 × 104 | 1.05 ± 0.02 | |
| p | <0.001 6 | <0.001 7 | <0.001 8 | 0.053 |
| Preparation | Duration of Experiment [Days] | Density (g/cm3) n = 9, M ± SD | Porosity (%) n = 9, M ± SD |
|---|---|---|---|
| Preparation 1 | day 0 | 1.501 ± 0.023 | 0.2070 ± 0.0525 |
| day 3 | 1.495 ± 0.025 | 0.0927 ± 0.0591 | |
| day 10 | 1.511 ± 0.031 | 0.1355 ± 0.0321 | |
| p | <0.001 2 | < 0.001 4 | |
| Preparation 2 | day 0 | 1.429 ± 0.049 | 1.1700 ± 0.1793 |
| day 3 | 1.420 ± 0.055 | 0.4365 ± 0.0625 | |
| day 10 | 1.445 ± 0.048 | 0.8523 ± 0.1037 | |
| p | 0.001 1 | <0.001 5 | |
| Preparation 3 | day 0 | 1.452 ± 0.030 | 0.0003 ± 0.0006 |
| day 3 | 1.440 ± 0.030 | 0.0007 ± 0.0008 | |
| day 10 | 1.450 ± 0.028 | 0.0003 ± 0.0007 | |
| p | <0.001 3 | <0.001 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulc-Musioł, B.; Duda, P.; Meisner, M.; Sarecka-Hujar, B. Hyperspectral and Microtomographic Analyses to Evaluate the Stability of Quercetin and Calcium Effervescent Tablets Exposed to Heat and Ultraviolet Radiation. Processes 2024, 12, 531. https://doi.org/10.3390/pr12030531
Szulc-Musioł B, Duda P, Meisner M, Sarecka-Hujar B. Hyperspectral and Microtomographic Analyses to Evaluate the Stability of Quercetin and Calcium Effervescent Tablets Exposed to Heat and Ultraviolet Radiation. Processes. 2024; 12(3):531. https://doi.org/10.3390/pr12030531
Chicago/Turabian StyleSzulc-Musioł, Beata, Piotr Duda, Michał Meisner, and Beata Sarecka-Hujar. 2024. "Hyperspectral and Microtomographic Analyses to Evaluate the Stability of Quercetin and Calcium Effervescent Tablets Exposed to Heat and Ultraviolet Radiation" Processes 12, no. 3: 531. https://doi.org/10.3390/pr12030531
APA StyleSzulc-Musioł, B., Duda, P., Meisner, M., & Sarecka-Hujar, B. (2024). Hyperspectral and Microtomographic Analyses to Evaluate the Stability of Quercetin and Calcium Effervescent Tablets Exposed to Heat and Ultraviolet Radiation. Processes, 12(3), 531. https://doi.org/10.3390/pr12030531







