Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Contaminated Soil Sampling and Preparation of Samples
2.3. Experimental Procedure
2.4. Kinetics of PAH degradation in soil
2.5. Gaussian Process Regression
3. Results and Discussion
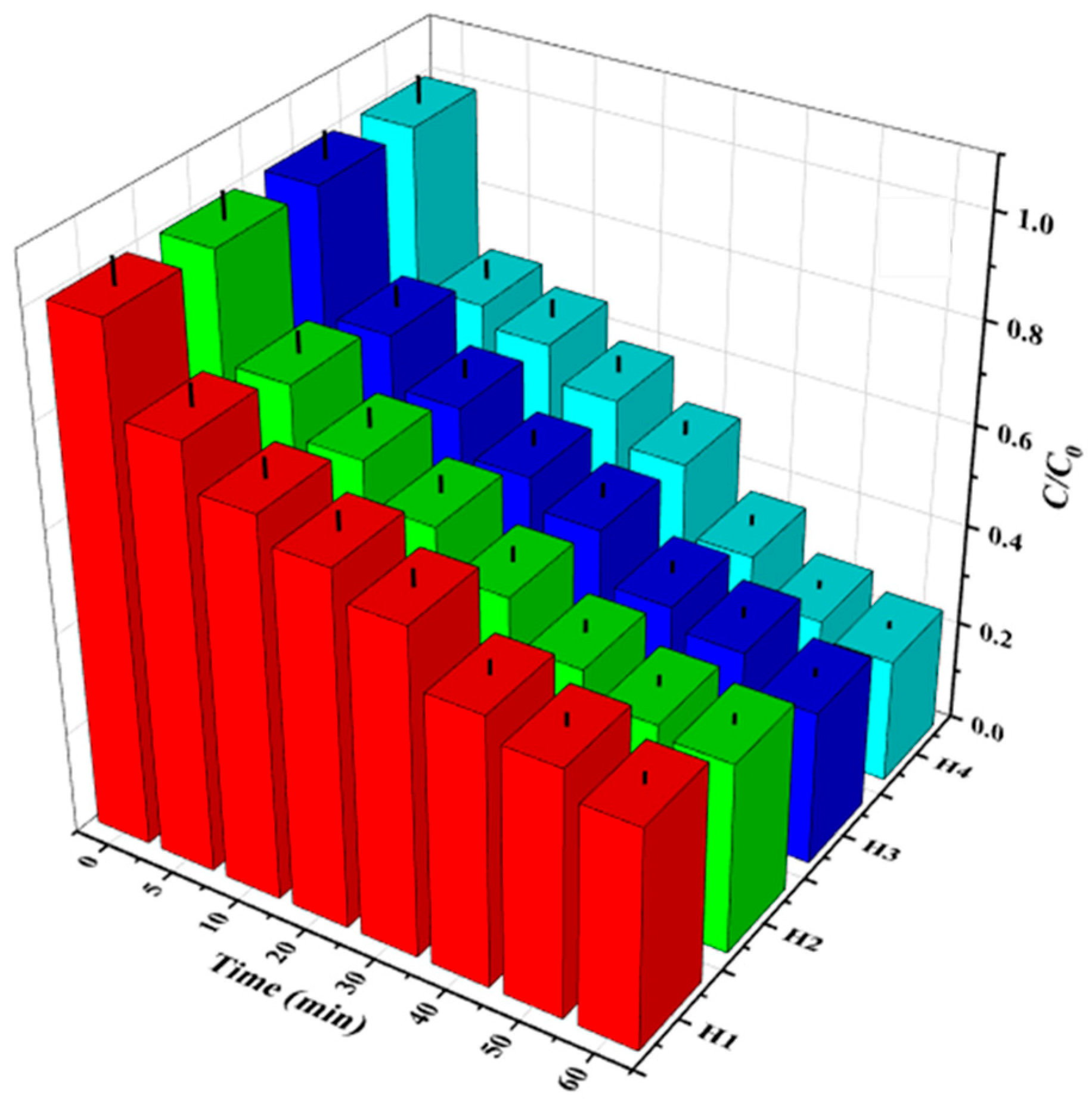
3.1. Effect of Oxidant Concentration
3.1.1. Effect of H2O2 Concentration
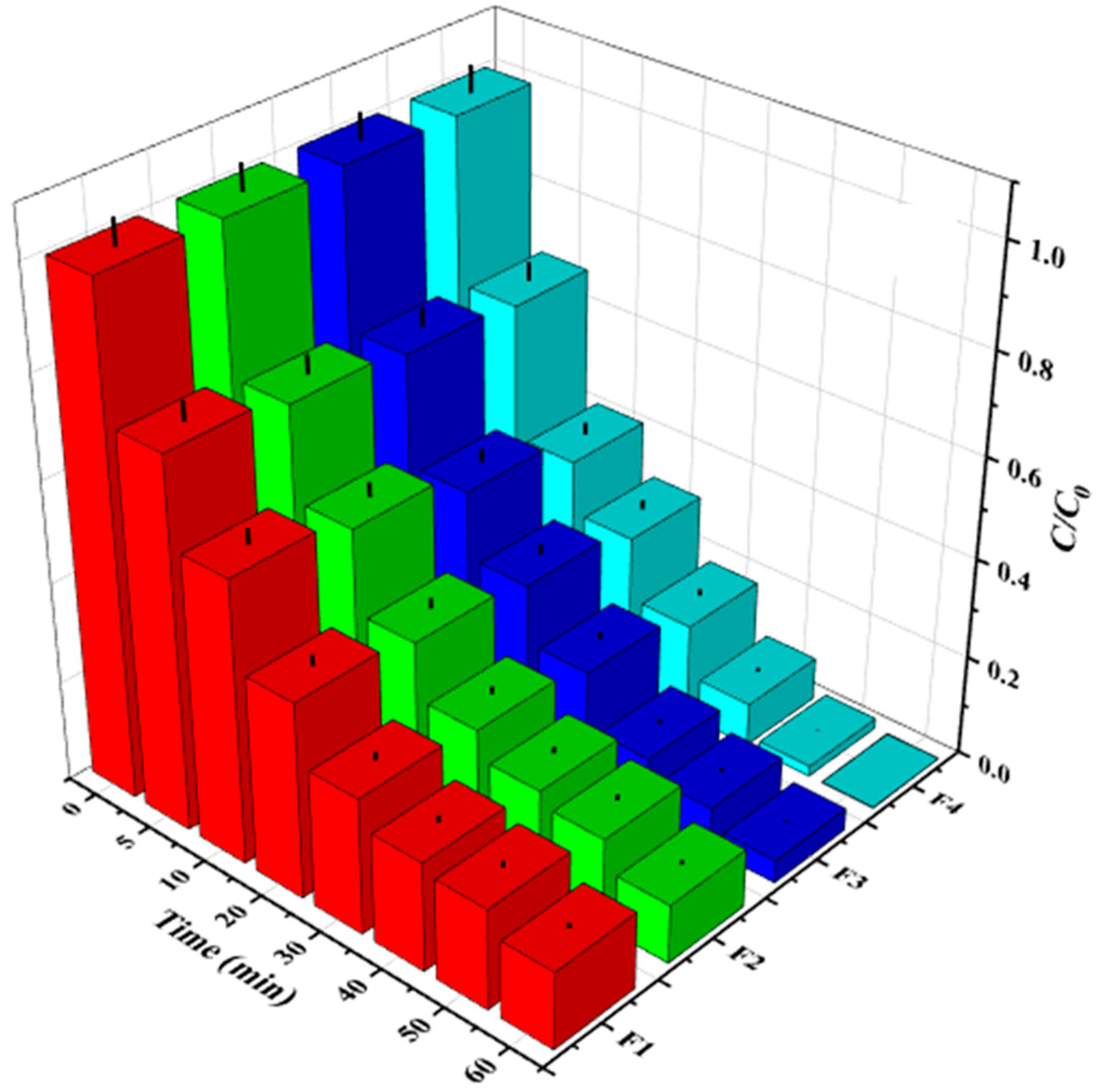
3.1.2. Effect of the Fenton Molar Ratio
3.2. Comparative Evaluation of Degradation Results Using H2O2 and H2O2/Fe2+
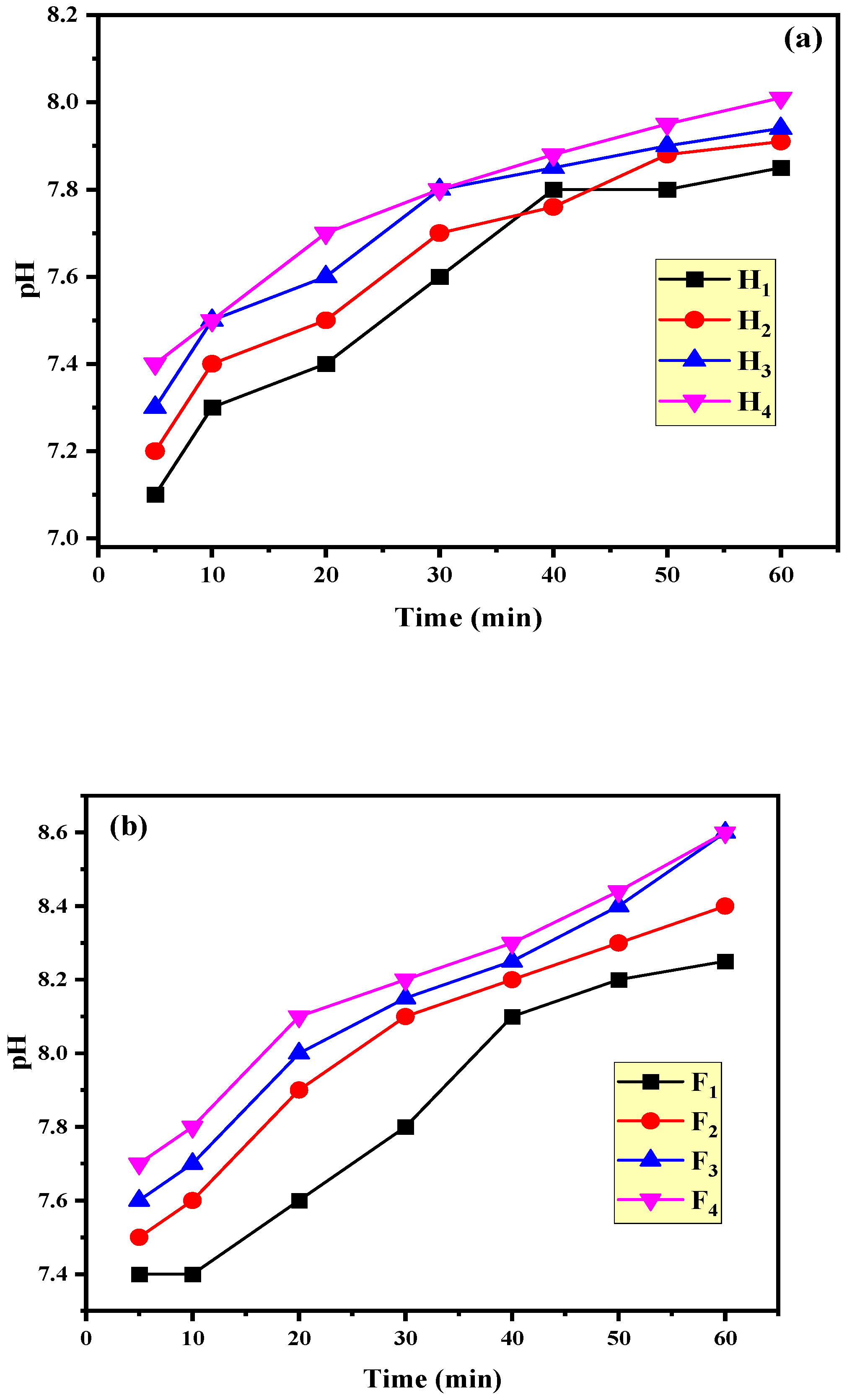
3.3. pH Evolution during the Oxidation Process
3.4. Impact of Fenton Oxidation on Treated Soil
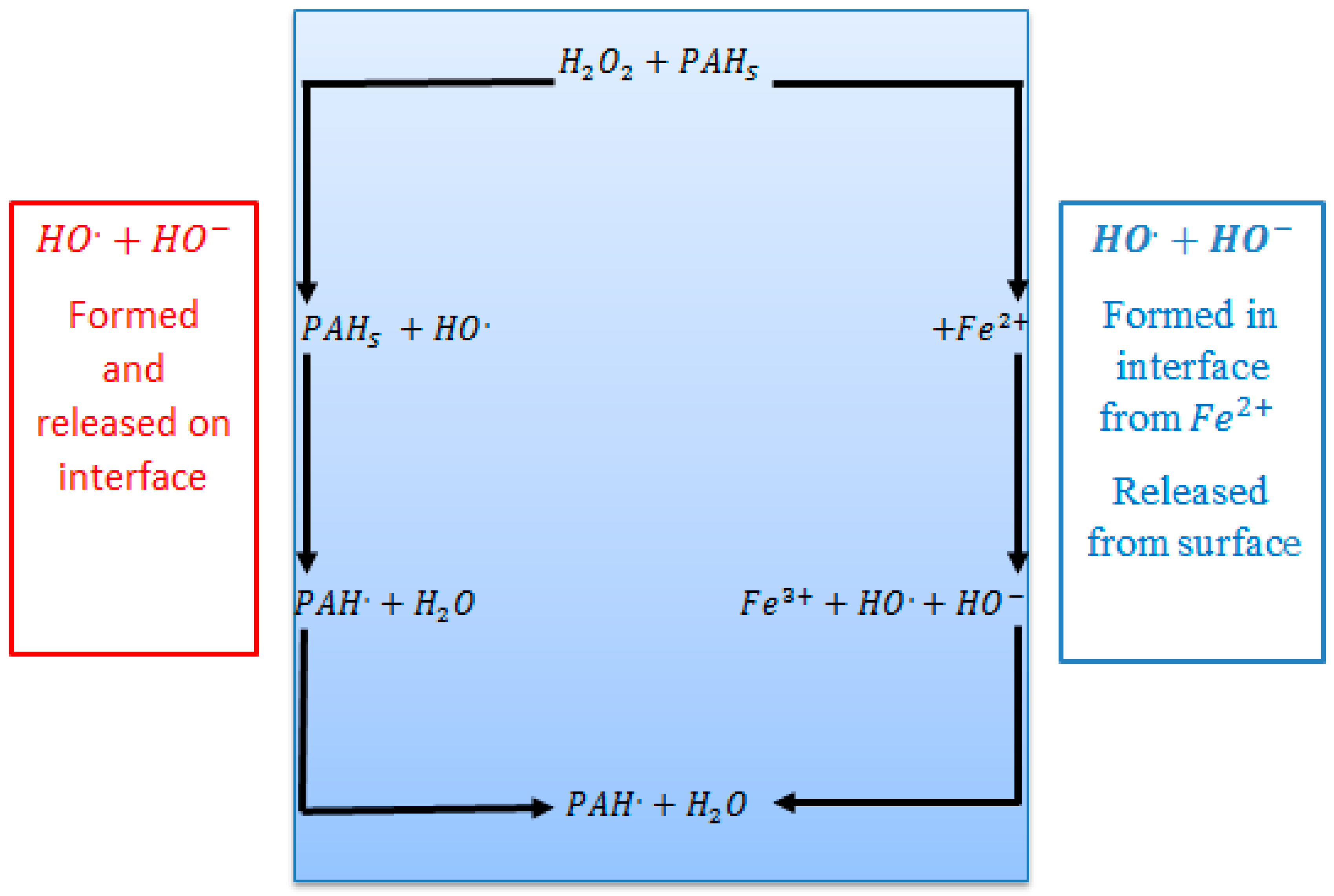
3.5. Proposed Mechanism of the Hydrogen Peroxide and Fenton Degradation
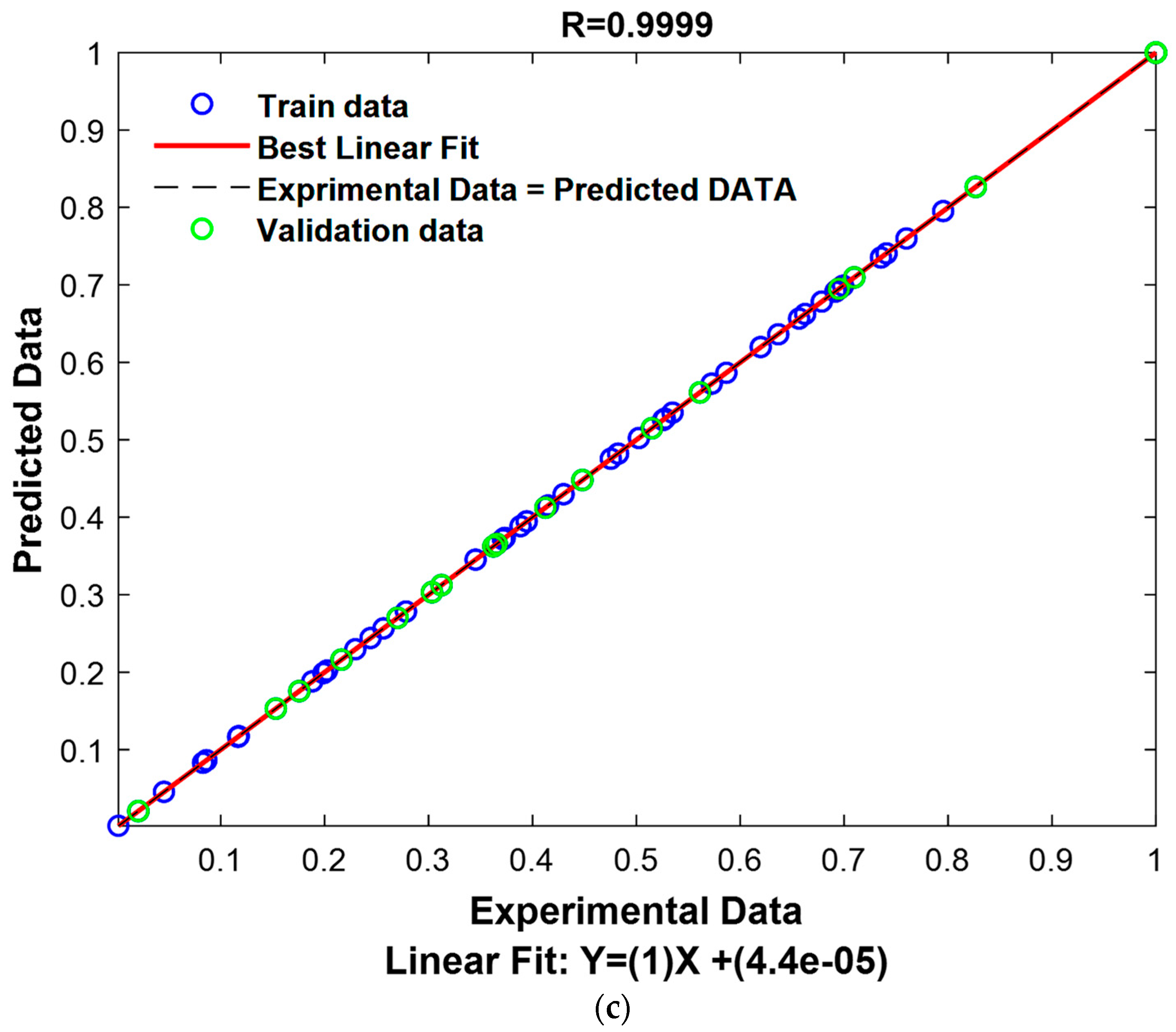
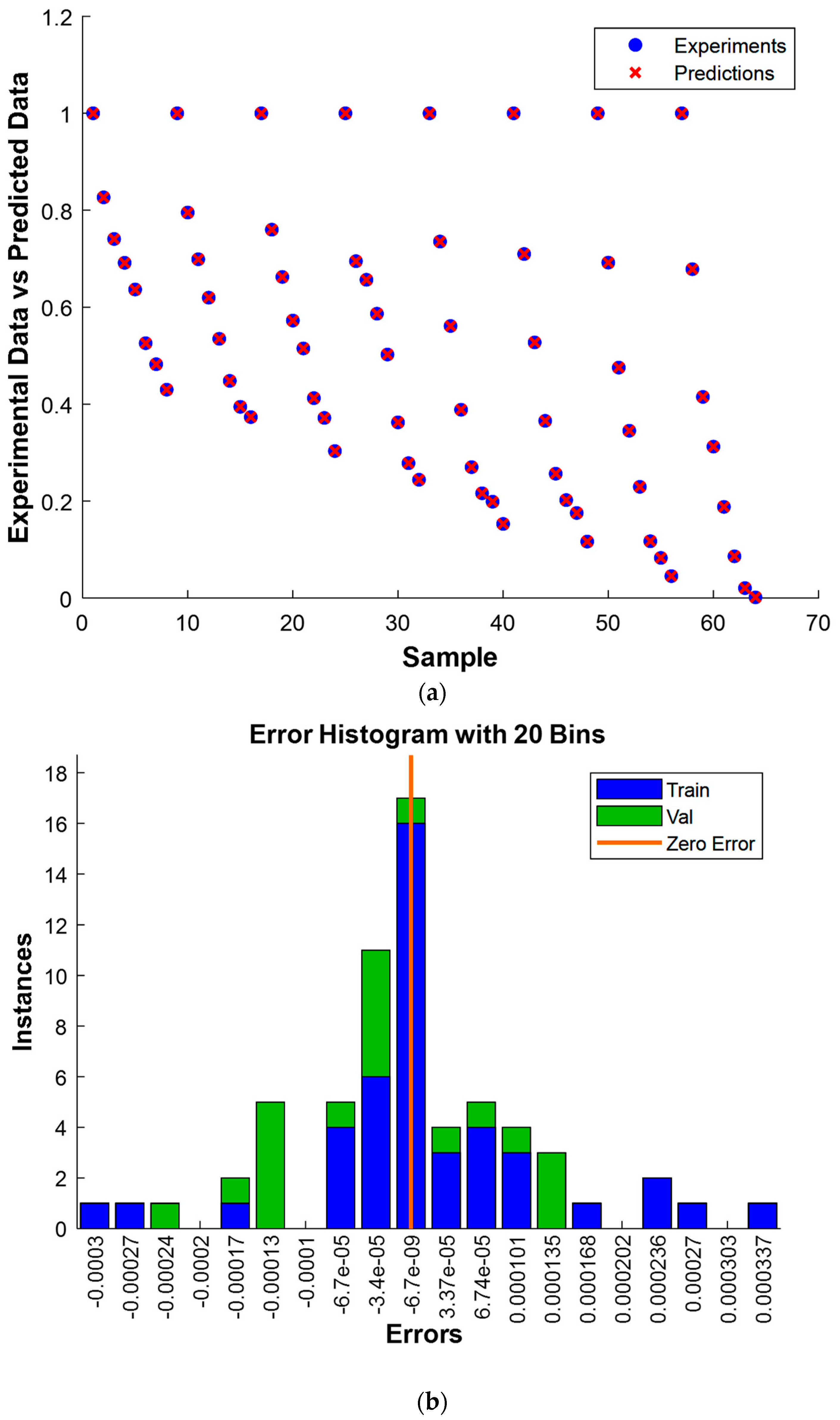
3.6. Gaussian Process Regression
3.6.1. Residual Analysis of Model GPR
3.6.2. Optimization and Validation of the Optimum Conditions
3.6.3. Interface for Optimization and Prediction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, X.; Xu, Y.; Li, G.; Zhang, Y.; Huang, T.; Hu, Z. Isolation, Characterization of Rhodococcus Sp. P14 Capable of Degrading High-Molecular-Weight Polycyclic Aromatic Hydrocarbons and Aliphatic Hydrocarbons. Mar. Pollut. Bull. 2011, 62, 2122–2128. [Google Scholar] [CrossRef]
- Gou, Y.; Zhao, Q.; Yang, S.; Wang, H.; Qiao, P.; Song, Y.; Cheng, Y.; Li, P. Removal of Polycyclic Aromatic Hydrocarbons (PAHs) and the Response of Indigenous Bacteria in Highly Contaminated Aged Soil after Persulfate Oxidation. Ecotoxicol. Environ. Saf. 2020, 190, 110092. [Google Scholar] [CrossRef]
- Li, G.; Huang, S.; Zhu, N.; Yuan, H.; Ge, D. Near-Infrared Responsive Upconversion Glass-Ceramic@ BiOBr Heterojunction for Enhanced Photodegradation Performances of Norfloxacin. J. Hazard. Mater. 2021, 403, 123981. [Google Scholar] [CrossRef] [PubMed]
- Madi, K.; Chebli, D.; Ait Youcef, H.; Tahraoui, H.; Bouguettoucha, A.; Kebir, M.; Zhang, J.; Amrane, A. Green Fabrication of ZnO Nanoparticles and ZnO/rGO Nanocomposites from Algerian Date Syrup Extract: Synthesis, Characterization, and Augmented Photocatalytic Efficiency in Methylene Blue Degradation. Catalysts 2024, 14, 62. [Google Scholar] [CrossRef]
- Gou, Y.; Yang, S.; Cheng, Y.; Song, Y.; Qiao, P.; Li, P.; Ma, J. Enhanced Anoxic Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) in Aged Soil Pretreated by Hydrogen Peroxide. Chem. Eng. J. 2019, 356, 524–533. [Google Scholar] [CrossRef]
- Lemaire, J.; Mora, V.; Faure, P.; Hanna, K.; Buès, M.; Simonnot, M.-O. Chemical Oxidation Efficiency for Aged, PAH-Contaminated Sites: An Investigation of Limiting Factors. J. Environ. Chem. Eng. 2019, 7, 103061. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Q.; Wu, B.; Peng, C.; Li, N.; Li, F.; Gu, Q. Treatment of PAH-Contaminated Soil Using Cement-Activated Persulfate. Environ. Sci. Pollut. Res. 2018, 25, 887–895. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, X.; Li, Z.; Gudda, F.O.; Waigi, M.G.; Wang, J.; Liu, H.; Gao, Y. Enhanced PAHs-Contaminated Site Soils Remediation by Mixed Persulfate and Calcium Peroxide. J. Environ. Manag. 2022, 306, 114363. [Google Scholar] [CrossRef]
- Li, Y.; Liao, X.; Huling, S.G.; Xue, T.; Liu, Q.; Cao, H.; Lin, Q. The Combined Effects of Surfactant Solubilization and Chemical Oxidation on the Removal of Polycyclic Aromatic Hydrocarbon from Soil. Sci. Total Environ. 2019, 647, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-Based Advanced Oxidation Processes (AOPs) for Organic-Contaminated Soil Remediation: A Review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Zhong, S.; Zhang, L. AOPs-Based Remediation of Petroleum Hydrocarbons-Contaminated Soils: Efficiency, Influencing Factors and Environmental Impacts. Chemosphere 2020, 246, 125726. [Google Scholar] [CrossRef]
- Alekseevich, V.M.; Viktorovich, A.O.; Rinatovich, T.A.; Vladimirovich, B.A.; Failevich, M.I. Study of Chemical Additives for Optimization of Binary Systems Used for Downhole Thermochemical Treatment of Heavy Oil. Processes 2023, 11, 2465. [Google Scholar]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y.; Wan, J.; Gong, X.; Zhu, Y. Degradation of Atrazine by a Novel Fenton-like Process and Assessment the Influence on the Treated Soil. J. Hazard. Mater. 2016, 312, 184–191. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl Radicals Based Advanced Oxidation Processes (AOPs) for Remediation of Soils Contaminated with Organic Compounds: A Review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Gan, S.; Yap, C.L.; Ng, H.K. Investigation of the Impacts of Ethyl Lactate Based Fenton Treatment on Soil Quality for Polycyclic Aromatic Hydrocarbons (PAHs)-Contaminated Soils. J. Hazard. Mater. 2013, 262, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, B.; Huon, S.; Steinmann, P.; Chabaux, F.; Velasquez, F.; Valles, V.; Arn, K.; Clauer, N.; Mariotti, A. Oxic–Anoxic Conditions in the Water Column of a Tropical Freshwater Reservoir (Pena-Larga Dam, NW Venezuela). Appl. Geochem. 2004, 19, 1295–1314. [Google Scholar] [CrossRef]
- Yang, K.; Nam, T.; Nam, K.; Kim, Y.-J. Characteristics of Heavy Metal Contamination by Anthropogenic Sources in Artificial Lakes of Urban Environment. KSCE J. Civ. Eng. 2016, 20, 121–128. [Google Scholar] [CrossRef]
- Xu, Y.; Che, T.; Li, Y.; Fang, C.; Dai, Z.; Li, H.; Xu, L.; Hu, F. Remediation of Polycyclic Aromatic Hydrocarbons by Sulfate Radical Advanced Oxidation: Evaluation of Efficiency and Ecological Impact. Ecotoxicol. Environ. Saf. 2021, 223, 112594. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, Q.; Zhao, L.; Wang, L.; Tang, J. Enhanced Degradation of Petroleum Hydrocarbons in Soil by FeS@ BC Activated Persulfate and Its Mechanism. Sep. Purif. Technol. 2022, 282, 120060. [Google Scholar] [CrossRef]
- Ranc, B.; Faure, P.; Croze, V.; Simonnot, M. Selection of Oxidant Doses for in Situ Chemical Oxidation of Soils Contaminated by Polycyclic Aromatic Hydrocarbons (PAHs): A Review. J. Hazard. Mater. 2016, 312, 280–297. [Google Scholar] [CrossRef]
- Ouriache, H.; Arrar, J.; Namane, A.; Bentahar, F. Treatment of Petroleum Hydrocarbons Contaminated Soil by Fenton like Oxidation. Chemosphere 2019, 232, 377–386. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M. A Critical Review of the Application of Chelating Agents to Enable Fenton and Fenton-like Reactions at High pH Values. J. Hazard. Mater. 2019, 362, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Hanna, K.; Haderlein, S. Fenton Oxidation to Remediate PAHs in Contaminated Soils: A Critical Review of Major Limitations and Counter-Strategies. Sci. Total Environ. 2016, 569, 179–190. [Google Scholar] [CrossRef]
- Ahile, U.J.; Wuana, R.A.; Itodo, A.U.; Sha’Ato, R.; Dantas, R.F. A Review on the Use of Chelating Agents as an Alternative to Promote Photo-Fenton at Neutral pH: Current Trends, Knowledge Gap and Future Studies. Sci. Total Environ. 2020, 710, 134872. [Google Scholar] [CrossRef]
- Satilmis, I.; Schrader, W. Studying the Fenton Treatment of Polycyclic Aromatic Compounds in a Highly Contaminated Soil with Different Modifiers by High Resolution Mass Spectrometry. J. Hazard. Mater. Adv. 2022, 8, 100200. [Google Scholar] [CrossRef]
- Checa-Fernandez, A.; Santos, A.; Romero, A.; Dominguez, C.M. Application of Chelating Agents to Enhance Fenton Process in Soil Remediation: A Review. Catalysts 2021, 11, 722. [Google Scholar] [CrossRef]
- Mechati, S.; Zamouche, M.; Tahraoui, H.; Filali, O.; Mazouz, S.; Bouledjemer, I.N.E.; Toumi, S.; Triki, Z.; Amrane, A.; Kebir, M. Modeling and Optimization of Hybrid Fenton and Ultrasound Process for Crystal Violet Degradation Using AI Techniques. Water 2023, 15, 4274. [Google Scholar] [CrossRef]
- Tahraoui, H.; Belhadj, A.-E.; Triki, Z.; Boudellal, N.R.; Seder, S.; Amrane, A.; Zhang, J.; Moula, N.; Tifoura, A.; Ferhat, R. Mixed Coagulant-Flocculant Optimization for Pharmaceutical Effluent Pretreatment Using Response Surface Methodology and Gaussian Process Regression. Process Saf. Environ. Prot. 2023, 169, 909–927. [Google Scholar] [CrossRef]
- Tahraoui, H.; Belhadj, A.-E.; Amrane, A.; Houssein, E.H. Predicting the Concentration of Sulfate Using Machine Learning Methods. Earth Sci. Inform. 2022, 15, 1023–1044. [Google Scholar] [CrossRef]
- Hamri, N.; Imessaoudene, A.; Hadadi, A.; Cheikh, S.; Boukerroui, A.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Tran, H.N.; Ezzat, A.O. Enhanced Adsorption Capacity of Methylene Blue Dye onto Kaolin through Acid Treatment: Batch Adsorption and Machine Learning Studies. Water 2024, 16, 243. [Google Scholar] [CrossRef]
- Bouchelkia, N.; Tahraoui, H.; Amrane, A.; Belkacemi, H.; Bollinger, J.-C.; Bouzaza, A.; Zoukel, A.; Zhang, J.; Mouni, L. Jujube Stones Based Highly Efficient Activated Carbon for Methylene Blue Adsorption: Kinetics and Isotherms Modeling, Thermodynamics and Mechanism Study, Optimization via Response Surface Methodology and Machine Learning Approaches. Process Saf. Environ. Prot. 2023, 170, 513–535. [Google Scholar] [CrossRef]
- Tahraoui, H.; Toumi, S.; Hassein-Bey, A.H.; Bousselma, A.; Sid, A.N.E.H.; Belhadj, A.-E.; Triki, Z.; Kebir, M.; Amrane, A.; Zhang, J. Advancing Water Quality Research: K-Nearest Neighbor Coupled with the Improved Grey Wolf Optimizer Algorithm Model Unveils New Possibilities for Dry Residue Prediction. Water 2023, 15, 2631. [Google Scholar] [CrossRef]
- Zamouche, M.; Chermat, M.; Kermiche, Z.; Tahraoui, H.; Kebir, M.; Bollinger, J.-C.; Amrane, A.; Mouni, L. Predictive Model Based on K-Nearest Neighbor Coupled with the Gray Wolf Optimizer Algorithm (KNN_GWO) for Estimating the Amount of Phenol Adsorption on Powdered Activated Carbon. Water 2023, 15, 493. [Google Scholar] [CrossRef]
- Aleboyeh, A.; Aleboyeh, H.; Moussa, Y. “Critical” Effect of Hydrogen Peroxide in Photochemical Oxidative Decolorization of Dyes: Acid Orange 8, Acid Blue 74 and Methyl Orange. Dye. Pigment. 2003, 57, 67–75. [Google Scholar] [CrossRef]
- Changyin, Z.; Fengxiao, Z.; Fuwang, W.; Juan, G.; Guangping, F.; Dongmei, Z.; Guodong, F. Comparison of Persulfate Activation and Fenton Reaction in Remediating an Organophosphorus Pesticides-Polluted Soil. Pedosphere 2017, 27, 465–474. [Google Scholar]
- Lemaire, J.; Laurent, F.; Leyval, C.; Schwartz, C.; Buès, M.; Simonnot, M.-O. PAH Oxidation in Aged and Spiked Soils Investigated by Column Experiments. Chemosphere 2013, 91, 406–414. [Google Scholar] [CrossRef]
- Biache, C.; Lorgeoux, C.; Andriatsihoarana, S.; Colombano, S.; Faure, P. Effect of Pre-Heating on the Chemical Oxidation Efficiency: Implications for the PAH Availability Measurement in Contaminated Soils. J. Hazard. Mater. 2015, 286, 55–63. [Google Scholar] [CrossRef]
- e Silva, P.T.D.S.; Da Silva, V.L.; de Barros Neto, B.; Simonnot, M.O. Phenanthrene and Pyrene Oxidation in Contaminated Soils Using Fenton’s Reagent. J. Hazard. Mater. 2009, 161, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.J. Polycyclic Aromatic Hydrocarbons Sorbed on Soils: A Short Review of Chemical Oxidation Based Treatments. J. Hazard. Mater. 2006, 138, 234–251. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, J.; Buès, M.; Kabeche, T.; Hanna, K.; Simonnot, M.-O. Oxidant Selection to Treat an Aged PAH Contaminated Soil by in Situ Chemical Oxidation. J. Environ. Chem. Eng. 2013, 1, 1261–1268. [Google Scholar] [CrossRef]
- Watts, R.J.; Stanton, P.C.; Howsawkeng, J.; Teel, A.L. Mineralization of a Sorbed Polycyclic Aromatic Hydrocarbon in Two Soils Using Catalyzed Hydrogen Peroxide. Water Res. 2002, 36, 4283–4292. [Google Scholar] [CrossRef] [PubMed]
- Vicente, F.; Rosas, J.; Santos, A.; Romero, A. Improvement Soil Remediation by Using Stabilizers and Chelating Agents in a Fenton-like Process. Chem. Eng. J. 2011, 172, 689–697. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lim, W.T.; Park, J.; Kim, Y. Effect of pH on Fenton and Fenton-like Oxidation. Environ. Technol. 2009, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Sandford, S.A.; Bernstein, M.P.; Materese, C.K. The Infrared Spectra of Polycyclic Aromatic Hydrocarbons with Excess Peripheral H Atoms (Hn-PAHs) and Their Relation to the 3.4 and 6.9 Μm PAH Emission Features. Astrophys. J. Suppl. Ser. 2013, 205, 8. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Singh, R.; Rastogi, S. Study of Vibrational Spectra of Polycyclic Aromatic Hydrocarbons with Phenyl Side Group. J. Mol. Spectrosc. 2023, 391, 111720. [Google Scholar] [CrossRef]
- Obinaju, B.E.; Martin, F.L. ATR-FTIR Spectroscopy Reveals Polycyclic Aromatic Hydrocarbon Contamination despite Relatively Pristine Site Characteristics: Results of a Field Study in the Niger Delta. Environ. Int. 2016, 89, 93–101. [Google Scholar] [CrossRef]
- Langhoff, S.R.; Bauschlicher, C.W.; Hudgins, D.M.; Sandford, S.A.; Allamandola, L.J. Infrared Spectra of Substituted Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. A 1998, 102, 1632–1646. [Google Scholar] [CrossRef]
- Karavoltsos, S.; Sakellari, A.; Bakeas, E.; Bekiaris, G.; Plavšić, M.; Proestos, C.; Zinelis, S.; Koukoulakis, K.; Diakos, I.; Dassenakis, M. Trace Elements, Polycyclic Aromatic Hydrocarbons, Mineral Composition, and FT-IR Characterization of Unrefined Sea and Rock Salts: Environmental Interactions. Environ. Sci. Pollut. Res. 2020, 27, 10857–10868. [Google Scholar] [CrossRef]
- Tian, H.; Li, C.; Wang, Z.; Zhao, S.; Xu, Y.; Wang, S. Polycyclic Aromatic Hydrocarbons Degradation Mechanisms in Methods Using Activated Persulfate: Radical and Non-Radical Pathways. Chem. Eng. J. 2023, 473, 145319. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Zhou, X.; Waigi, M.G.; Gudda, F.O.; Zhang, C.; Ling, W. Promoted Oxidation of Polycyclic Aromatic Hydrocarbons in Soils by Dual Persulfate/Calcium Peroxide System. Sci. Total Environ. 2021, 758, 143680. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Ning, X.; An, T.; Zhang, J.; Chen, C.; Ke, Y.; Wang, Y.; Zhang, Y.; Sun, J.; Liu, J. Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) in Textile Dyeing Sludge with Ultrasound and Fenton Processes: Effect of System Parameters and Synergistic Effect Study. J. Hazard. Mater. 2016, 307, 7–16. [Google Scholar] [CrossRef] [PubMed]





| Texture | pH | Electric Conductivity (mS/m) | Organic Matter (%) | Humidity | Density (g/cm3) |
|---|---|---|---|---|---|
| Sand 40% | |||||
| Silt 48% | 7.05 | 8.06 | 6.0 | 0.12 | 2.60 |
| Clay 12% | |||||
| Elements | Concentration (mg/kg Soil) | Elements | Concentration (mg/kg Soil) | |
|---|---|---|---|---|
| BTEX | Benzene | <0.01 | ||
| Ethylbenzene | <0.01 | |||
| Styrene | <0.01 | |||
| Toluene | 0.001 | |||
| Xylene | 0.001 | |||
| Total BTEX | 0.005 | |||
| PAHs | Naphtalene | 27.12 | Crysene | 16.00 |
| Acenaphtylene | 3.56 | Benzo(a)anthracene | 98.20 | |
| Acenaphtene | 91.22 | Benzo(b)fluoranthene | 102.60 | |
| Fluorene | 98.06 | Benzo(k)fluoranthene | 31.00 | |
| Phénanthrene | 250.03 | Benzo(a)pyrene | 44.37 | |
| Anthracene | 44.67 | Dibenzo(a,h)anthracene | 11.20 | |
| Fluoranthene | 182.67 | Benzo(g,h,i)perylene | 32.30 | |
| Pyrene | 139.80 | Indeno(1,2,3-cd)pyrene | 27.20 | |
| Total | 1200 | |||
| Oxidant | Tests | H2O2:Fe2+ Molar Ratio | Oxidant Concentration/M H2O2:Fe2+ |
|---|---|---|---|
| H2O2 | H1 | 25:0 | 5 |
| H2 | 50:0 | ||
| H3 | 100:0 | ||
| H4 | 200:0 | ||
| Fenton | F1 | 50:1 | 5:0.5 |
| F2 | 50:2 | 5:0.5 | |
| F3 | 100:1 | 5:0.5 | |
| F4 | 100:2 | 5:0.5 |
| 1st Order | 2nd Order | 1st Order | 2nd Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | k·min−1 | R2 | k·L/mol·min | R2 | k·min−1 | R2 | k·L/mol·min | ||
| H1 | 0.95 | 0.013 | 0.97 | 0.017 | F1 | 0.97 | 0.039 | 0.997 | 0.069 |
| H2 | 0.95 | 0.017 | 0.98 | 0.025 | F2 | 0.97 | 0.043 | 0.997 | 0.077 |
| H3 | 0.94 | 0.019 | 0.97 | 0.03 | F3 | 0.98 | 0.055 | 0.975 | 0.097 |
| H4 | 0.94 | 0.023 | 0.95 | 0.035 | F4 | 0.98 | 0.066 | 0.958 | 0.118 |
| Kernel Function | Basis Function | Kernel Scale | Sigma | Quantite | R | RMSE (1.0 e-03) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SigmaM | SigmaF | Train | Val | All | Train | Val | All | ||||
| ARD-Exponential | Constant | 18.8299 160.0958 51.8017 | 0.6258 | 0.0029 | 44 | 1.0000 | 0.9997 | 0.9999 | 0.1392 | 0.0212 | 0.1173 |
| X1 = 60. X2 = 200. and X3 = 2 | |
|---|---|
| C/C0 experimental values | 0.001 |
| C/C0 predicted values | 0 |
| Error | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smara, M.; Khalladi, R.; Moulai-Mostefa, N.; Madi, K.; Mansour, D.; Lekmine, S.; Benslama, O.; Tahraoui, H.; Zhang, J.; Amrane, A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes 2024, 12, 621. https://doi.org/10.3390/pr12030621
Smara M, Khalladi R, Moulai-Mostefa N, Madi K, Mansour D, Lekmine S, Benslama O, Tahraoui H, Zhang J, Amrane A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes. 2024; 12(3):621. https://doi.org/10.3390/pr12030621
Chicago/Turabian StyleSmara, Mahdia, Razika Khalladi, Nadji Moulai-Mostefa, Kamilia Madi, Dorsaf Mansour, Sabrina Lekmine, Ouided Benslama, Hichem Tahraoui, Jie Zhang, and Abdeltif Amrane. 2024. "Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling" Processes 12, no. 3: 621. https://doi.org/10.3390/pr12030621
APA StyleSmara, M., Khalladi, R., Moulai-Mostefa, N., Madi, K., Mansour, D., Lekmine, S., Benslama, O., Tahraoui, H., Zhang, J., & Amrane, A. (2024). Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes, 12(3), 621. https://doi.org/10.3390/pr12030621









