Adhesion Behavior of Underground Coal Dust with Fused Silica: Effects of Relative Humidity and Particle Size
Abstract
1. Introduction
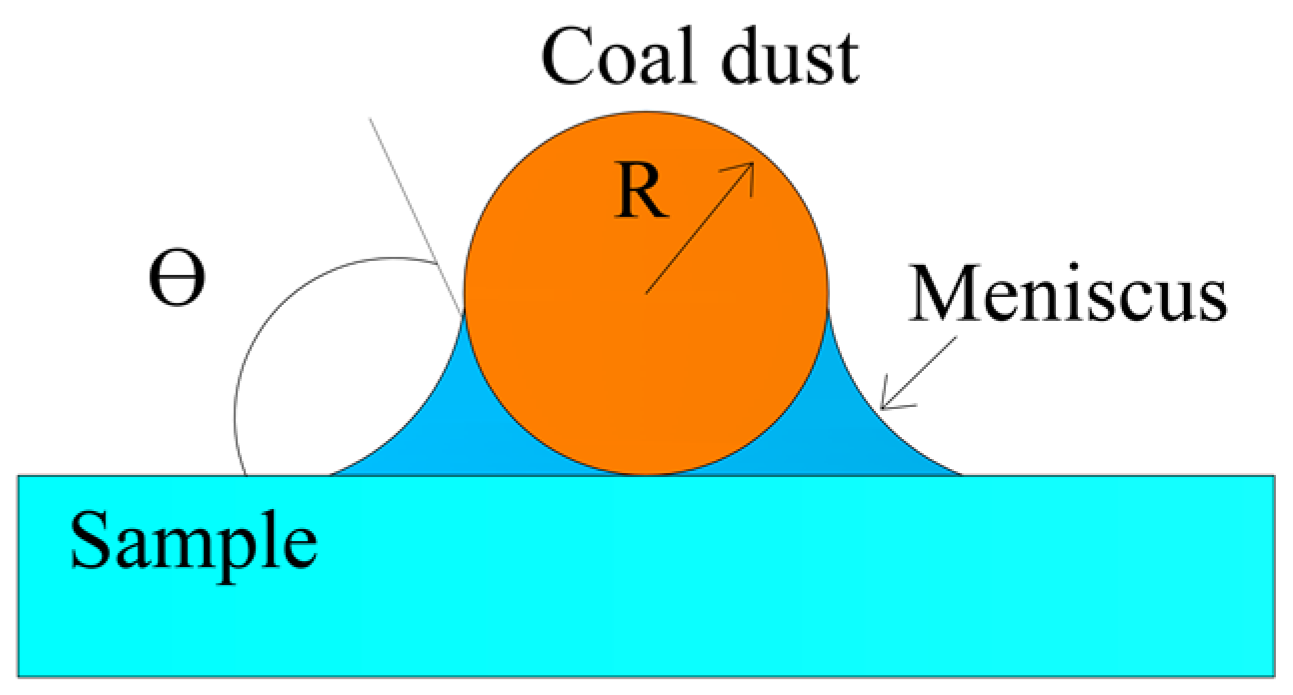
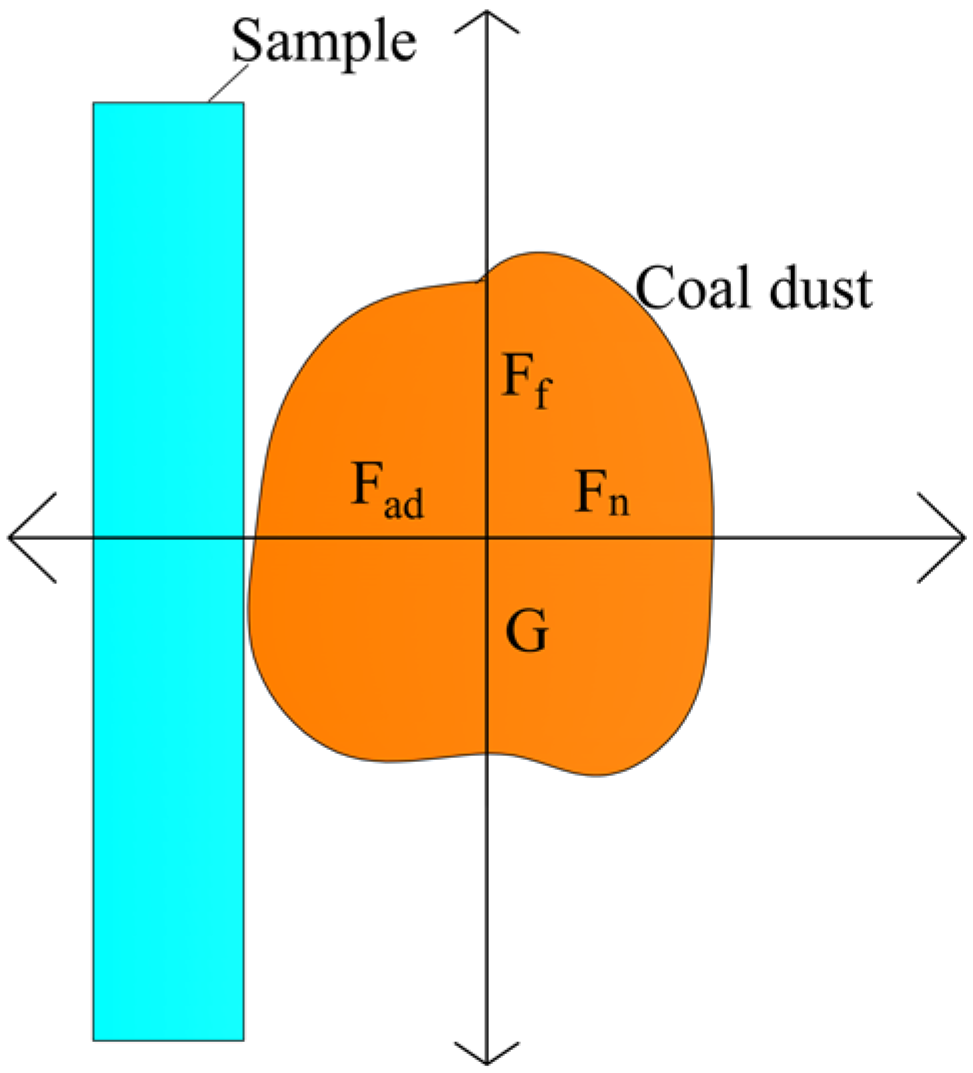
2. Theory and Background
3. Materials and Methods
3.1. Samples and Preparation
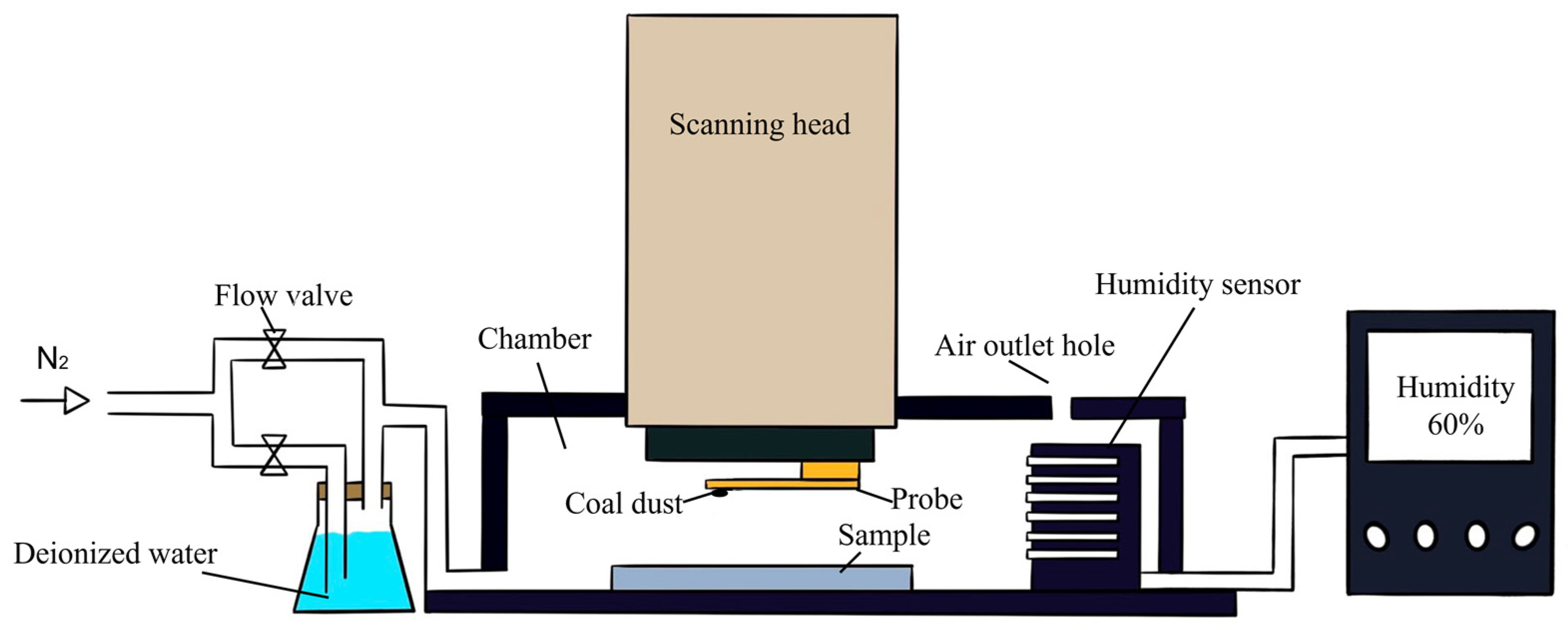
3.2. Method of Single Coal Dust Adhesion Force Test
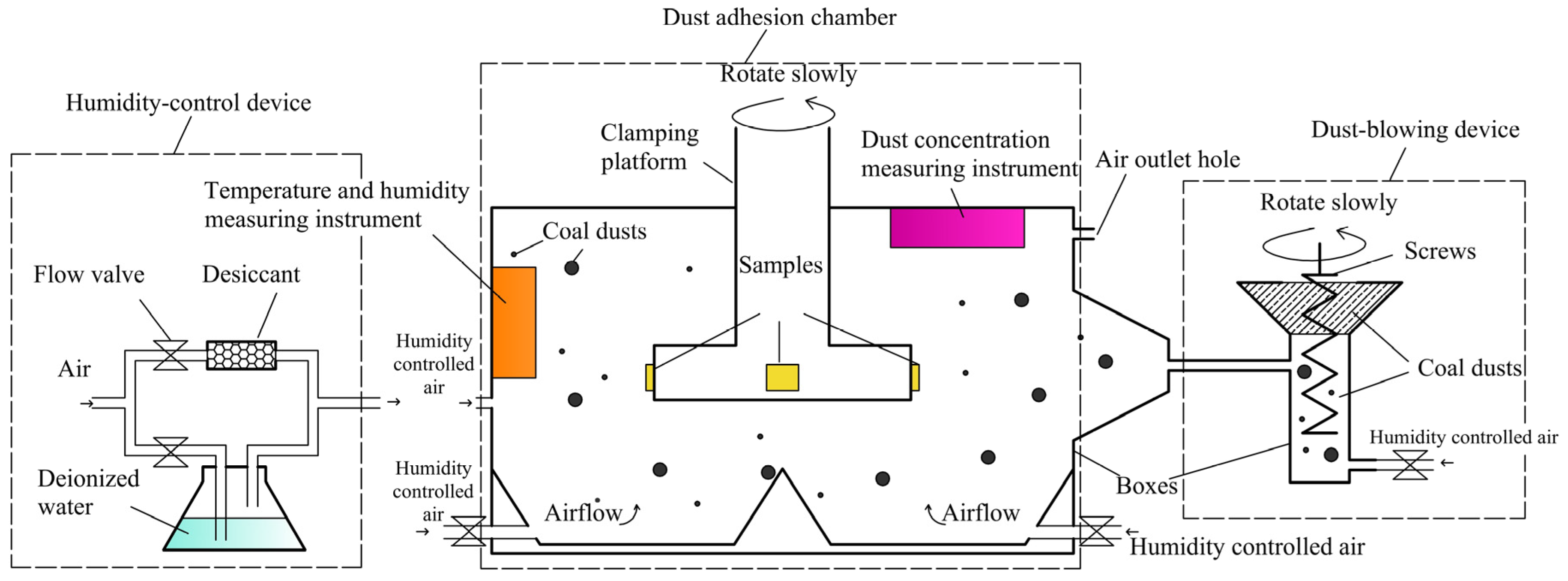
3.3. Experimental Method of Bulk Coal Dust Adhesion
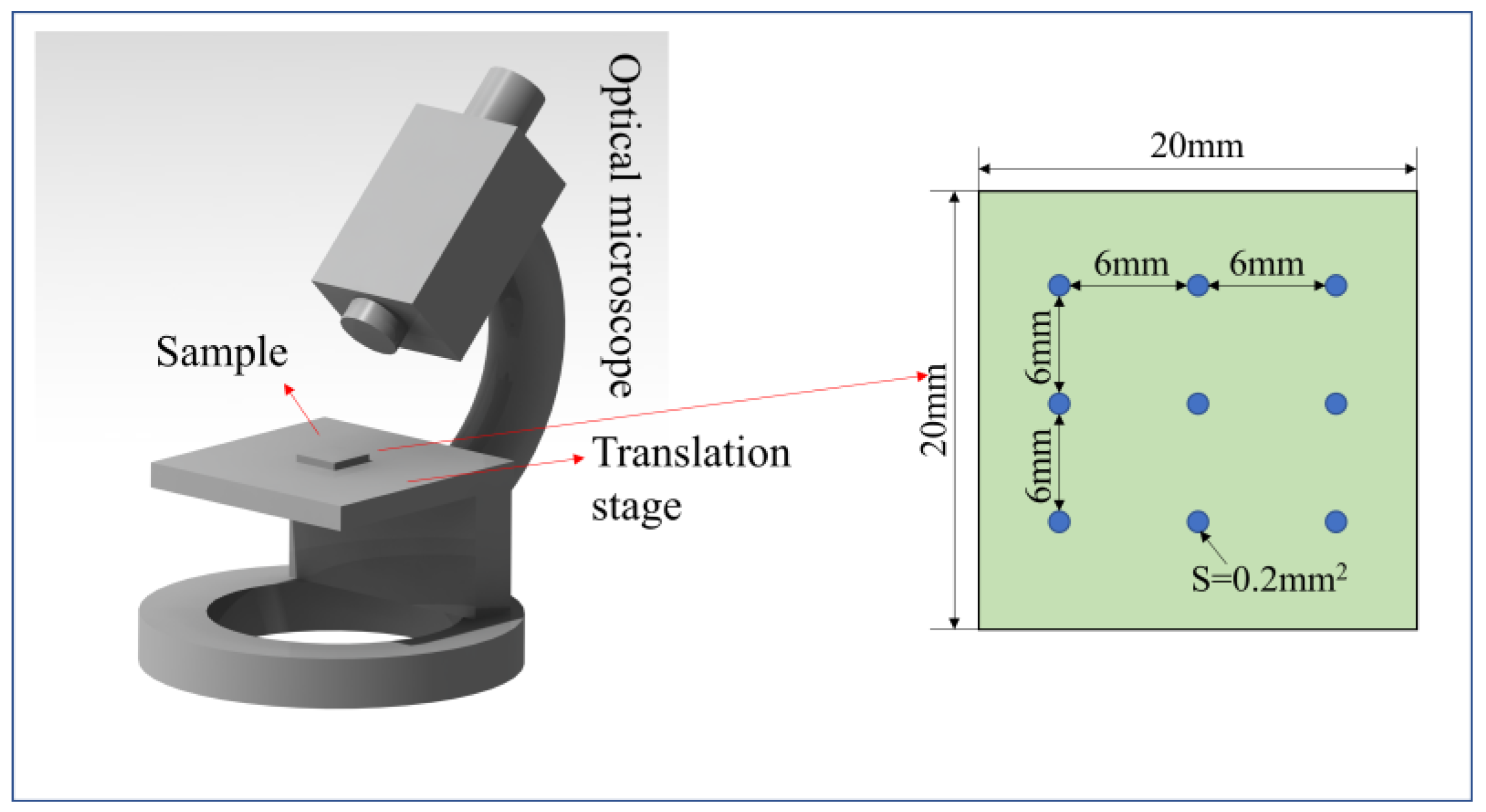
3.4. Characterization Techniques
4. Results and Discussion
4.1. Theoretical Calculation of Adhesion Forces between Coal Dust Particles and Fused Silica
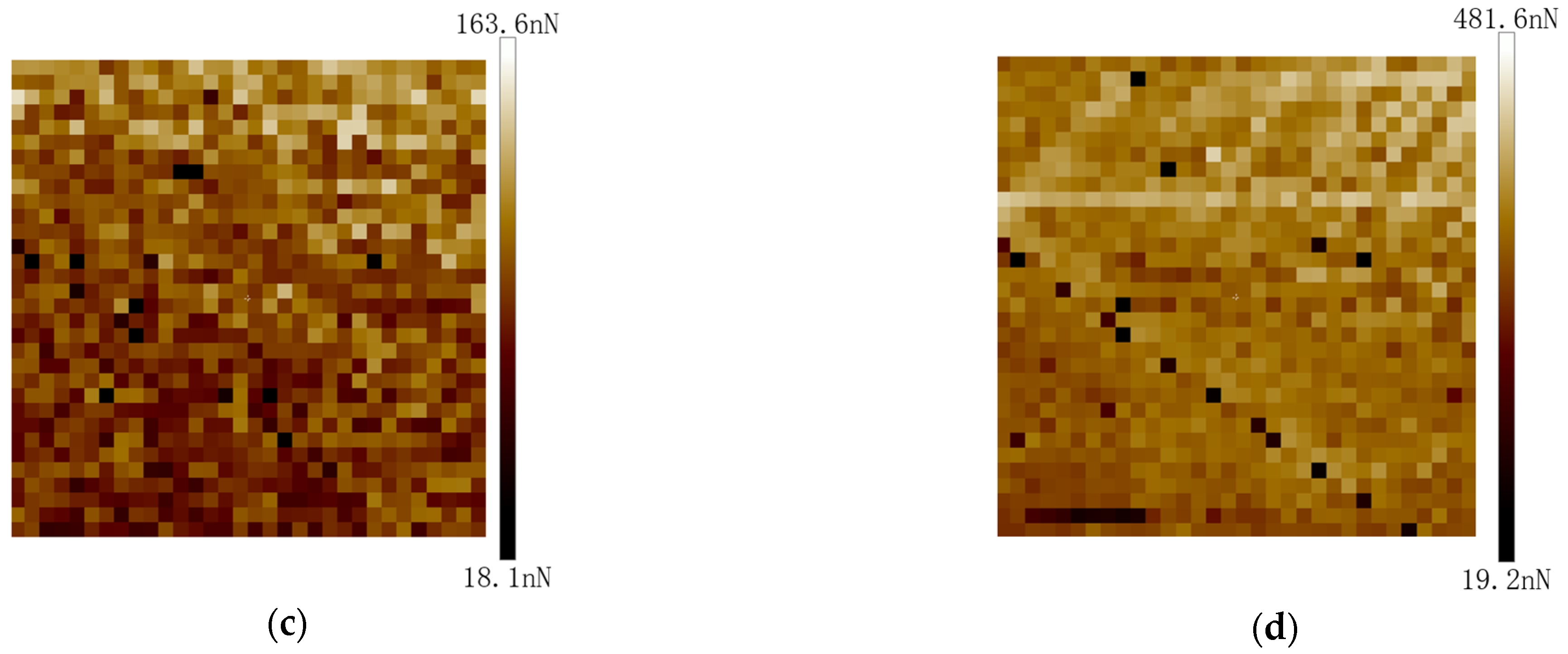
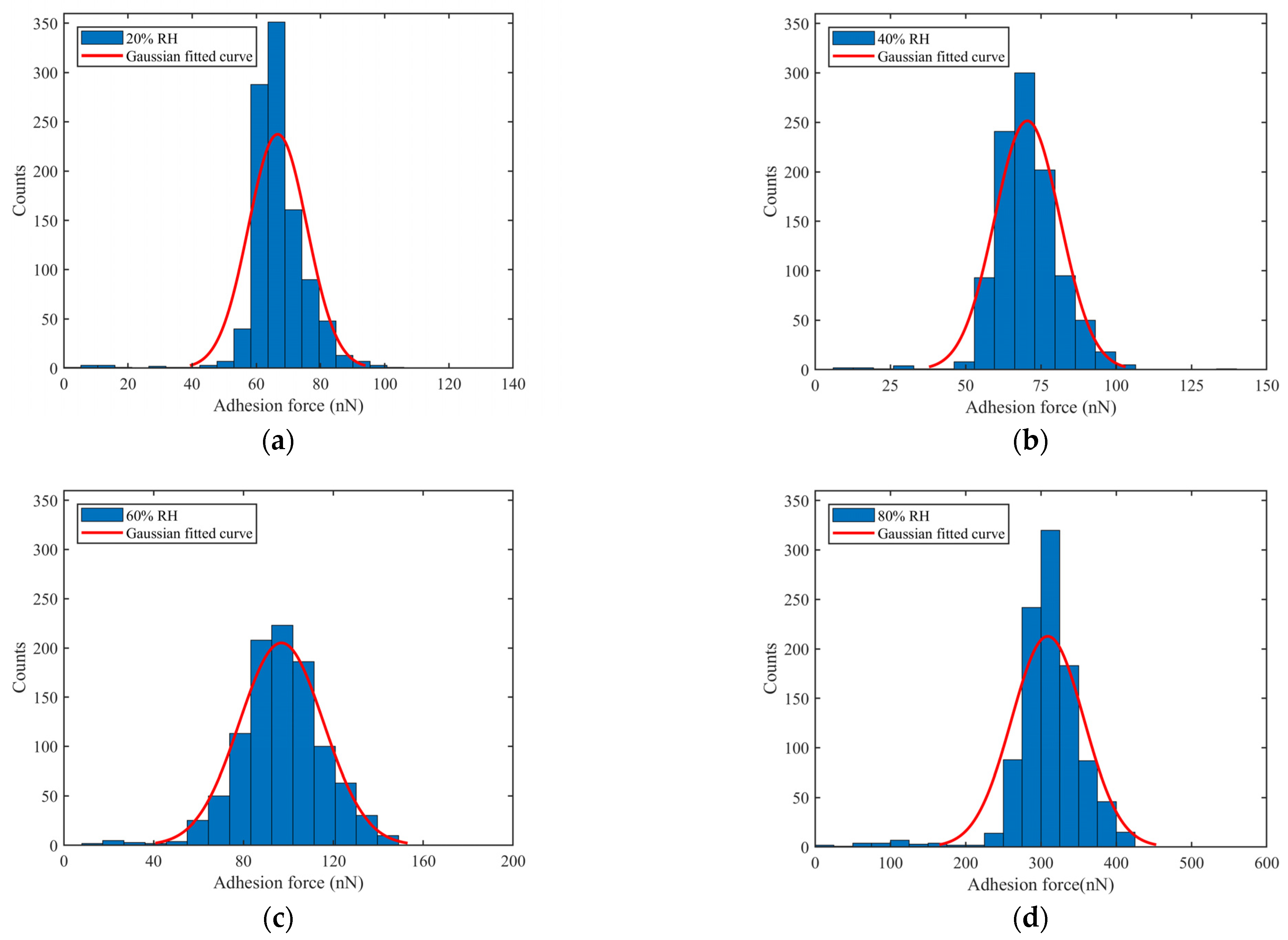
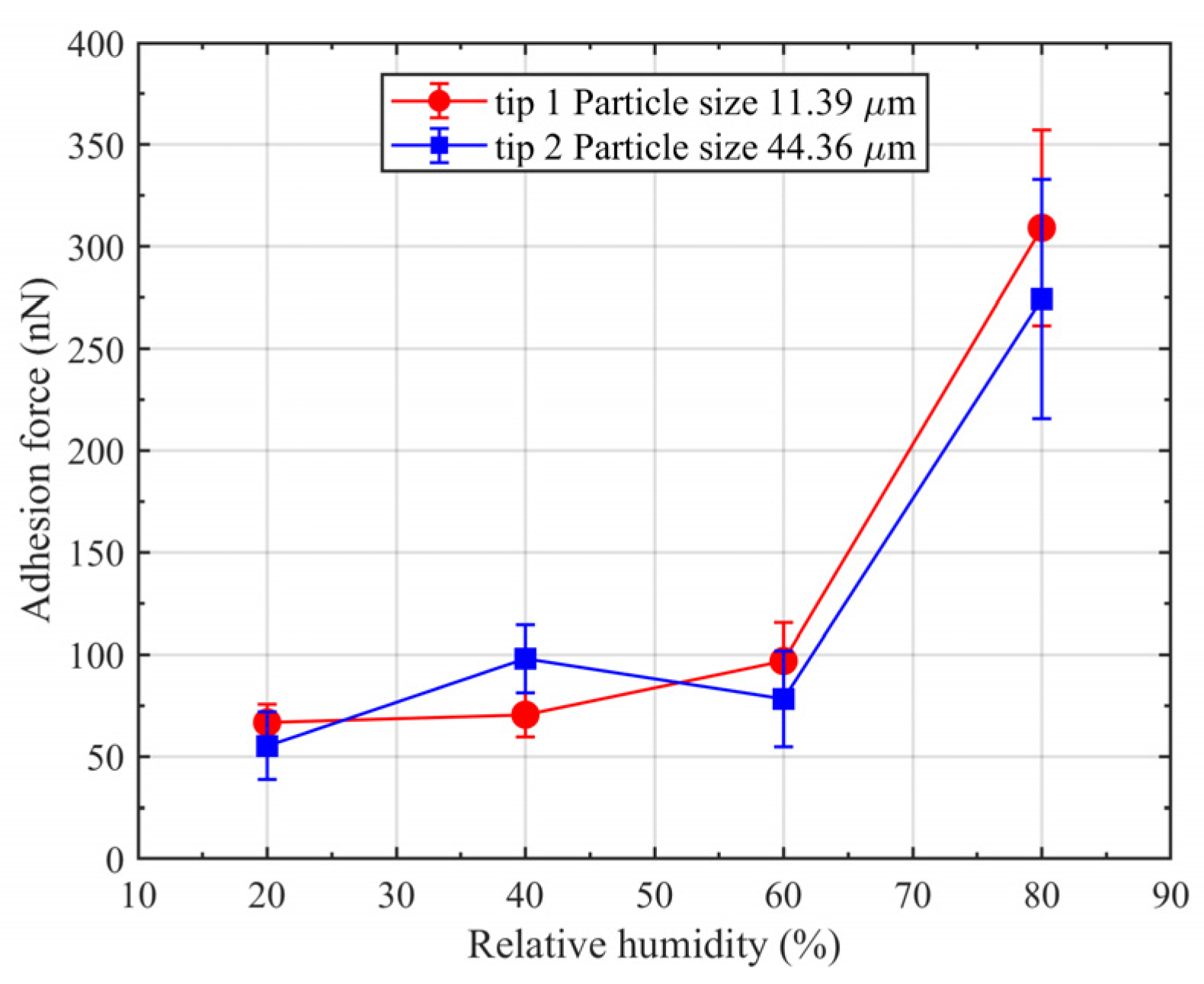
4.2. Measurement of Adhesion Force between Coal Dust Particles and Fused Silica Using AFM
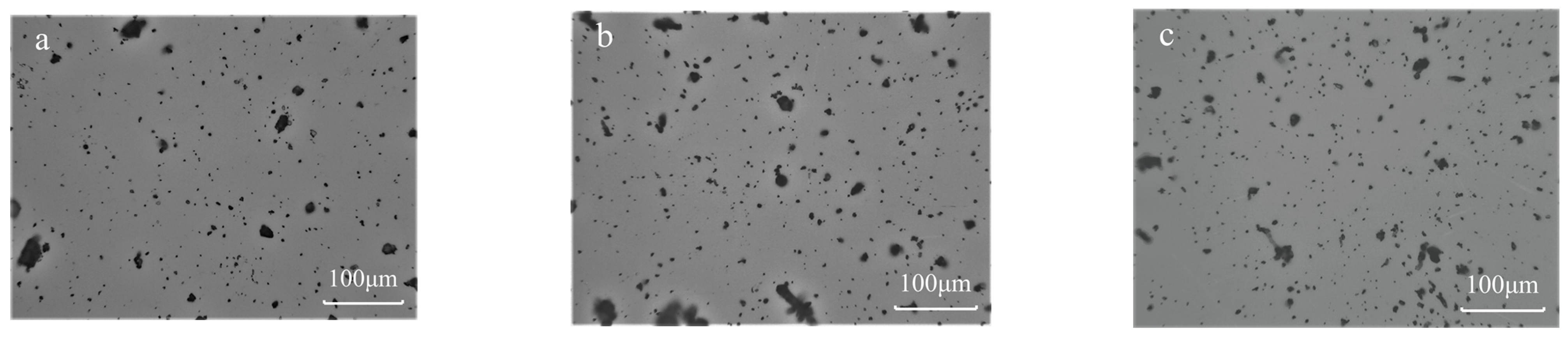
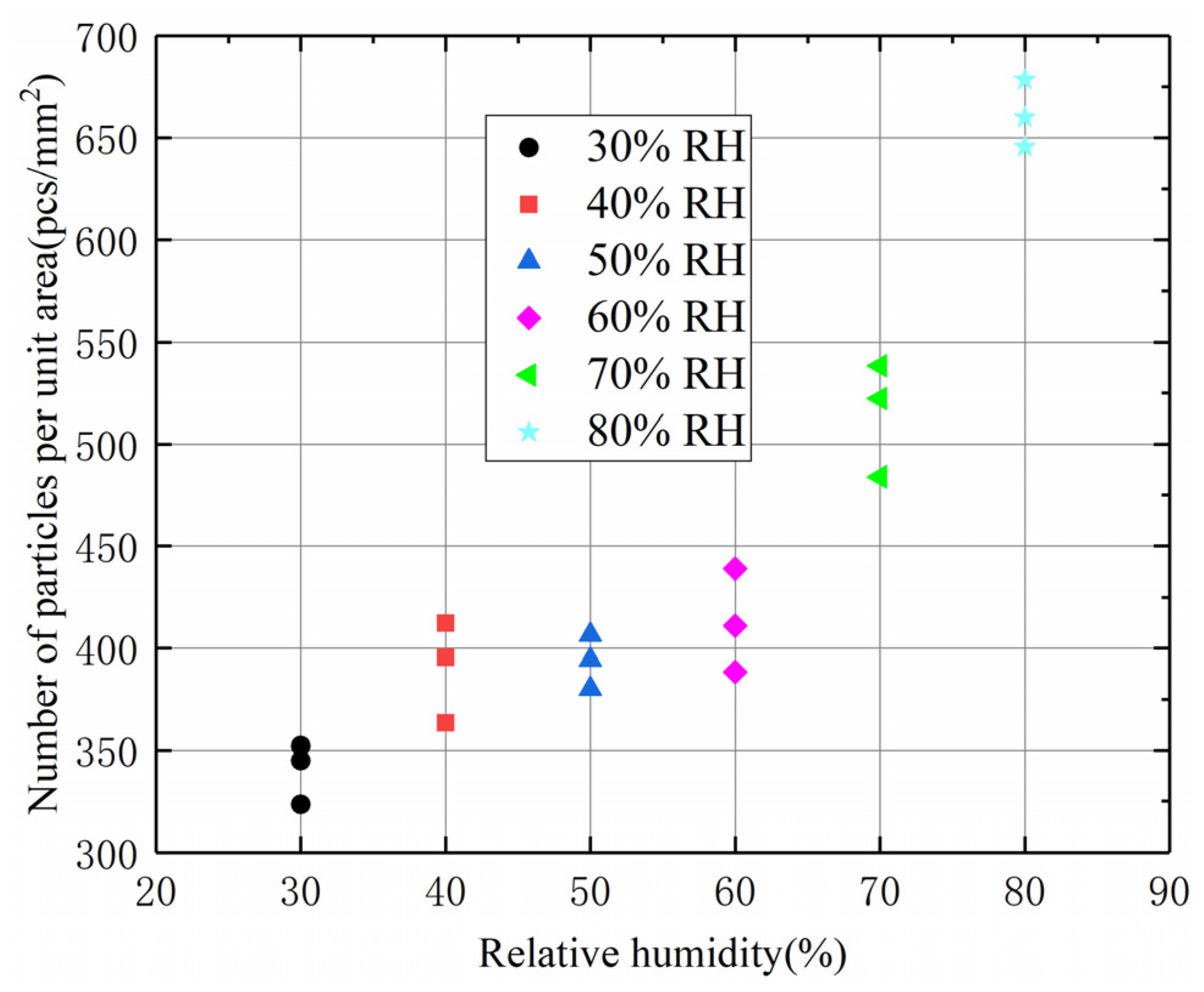
4.3. Effect of RH on the Number of Particles and Particle Size Distribution of Coal Dust Adhesion
5. Conclusions
- (1)
- The adhesion forces in this study consisted mainly of van der Waals and capillary forces. The adhesion force increased with increasing RH; adhesion was dominated by van der Waals forces at a lower RH and capillary forces at a higher RH. The amount of coal dust adhering to the fused silica surface increased with RH due to the increase in adhesion force.
- (2)
- As the size of the coal dust particles increases, the increase in gravitational force is much faster than that of the adhesion force, which made the particles with larger size less prone to adhering. Therefore, the coal dust adhering to the surface of fused silica was dominated by small-sized (lesser than 50 μm) particles.
- (3)
- The proportion of large-sized (larger than 50 μm) particles adhering to the surface increased with the increase in RH. This is due to the increase in the adhesion force with RH, which leads to an increase in the frictional force between the particles and fused silica glass, allowing the particles with larger size to adhere.
- (4)
- Due to the irregular shape and rough surface of the coal dust, the actual contact area between the coal dust and the fused silica is greatly reduced, resulting in the theoretical values of the van der Waals forces and capillary forces being much larger than the experimental values.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dávila-Pulido, G.I.; González-Ibarra, A.A.; Garza-García, M. A brief review on coal reserves, production and possible non-power uses: The case of Mexico. Heliyon 2023, 9, e16043. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Zhang, Z.M.; Guo, Z.H.; Su, C.; Sun, L.H. Energy structure transformation in the context of carbon neutralization: Evolutionary game analysis based on inclusive development of coal and clean energy. J. Clean. Prod. 2023, 398, 136626. [Google Scholar] [CrossRef]
- Kang, H.; Gao, F.; Xu, G.; Ren, H. Mechanical behaviors of coal measures and ground control technologies for China’s deep coal mines-A review. J. Rock Mech. Geotech. Eng. 2023, 15, 37–65. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Y.; Ren, H. Intelligent and ecological coal mining as well as clean utilization technology in China: Review and prospects. Int. J. Min. Sci. Technol. 2019, 29, 161–169. [Google Scholar] [CrossRef]
- Qian, J.; Liu, Z.; Lin, S.; Liu, H.; Ali, M.; Kim, W. Re-explosion hazard potential of solid residues and gaseous products of coal dust explosion. Adv. Powder Technol. 2023, 34, 104129. [Google Scholar] [CrossRef]
- Ji, Y.; Ren, T.; Wynne, P.; Wan, Z.; Ma, Z.; Wang, Z. A comparative study of dust control practices in Chinese and Australian longwall coal mines. Int. J. Min. Sci. Technol. 2016, 26, 199–208. [Google Scholar] [CrossRef]
- Gao, M.; Li, H.; Zhao, Y.; Liu, Y.; Zhou, W.; Li, L.; Xie, J.; Deng, J. Mechanism of micro-wetting of highly hydrophobic coal dust in underground mining and new wetting agent development. Int. J. Min. Sci. Technol. 2023, 33, 31–46. [Google Scholar] [CrossRef]
- Xi, Z.; Xia, T.; Shen, L.; Suo, L. Synthesis of cardanol grafted hydrophilic polymers and its mechanism of coal dust inhibition. Fuel 2023, 345, 128112. [Google Scholar] [CrossRef]
- Moutinho, H.; Jiang, C.-S.; To, B.; Perkins, C.; Muller, M.; Al-Jassim, M.; Simpson, L. Adhesion mechanisms on solar glass: Effects of relative humidity, surface roughness, and particle shape and size. Sol. Energy Mater. Sol. Cells 2017, 172, 145–153. [Google Scholar] [CrossRef]
- Quan, Y.; Zhang, L. Experimental investigation of the anti-dust effect of transparent hydrophobic coatings applied for solar cell covering glass. Sol. Energy Mater. Sol. Cells 2017, 160, 382–389. [Google Scholar] [CrossRef]
- Ermis, E.; Farnish, R.J.; Berry, R.J.; Bradley, M.S.A. Centrifugal tester versus a novel design to measure particle adhesion strength and investigation of effect of physical characteristics (size, shape, density) of food particles on food surfaces. J. Food Eng. 2011, 104, 518–524. [Google Scholar] [CrossRef]
- Petean PG, C.; Aguiar, M.L. Determining the adhesion force between particles and rough surfaces. Powder Technol. 2015, 274, 67–76. [Google Scholar] [CrossRef]
- Ma, W.B.; Qi, C.L.; Liu, Q.; Ding, Y.H.; Zhu, W. Adhesion force measurements between deep-sea soil particles metals by in situ AFM. Appl. Clay Sci. 2017, 148, 118–122. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Cai, Q.; Zhu, W.; Yang, C.; Guo, S. Influence of surface roughness on the adhesion force between the titanium plate and deep-sea sediment. Mar. Georesour. Geotechnol. 2021, 39, 1516–1524. [Google Scholar] [CrossRef]
- Sun, Q.; Peng, W.; Hai, X.; Yu, S. Adhesion study between micron-scale graphite particles and rough walls using the finite element method. Adv. Powder Technol. 2021, 32, 1951–1962. [Google Scholar] [CrossRef]
- Tan, C.L.C.; Gao, S.; Wee, B.S.; Asa-Awuku, A.; Thio, B.J.R. Adhesion of Dust Particles to Common Indoor Surfaces in an Air-Conditioned Environment. Aerosol Sci. Technol. 2014, 48, 541–551. [Google Scholar] [CrossRef]
- Bowling, R.A. A theoretical review of partical adhesion. In Particles on Surfaces 1; Springer: Boston, MA, USA, 1988. [Google Scholar]
- Rimai, D.S.; DeMejo, L.P. Physical Interactions Affecting the Adhesion of Dry Particles. Annu. Rev. Mater. Sci. 1996, 26, 21–41. [Google Scholar] [CrossRef]
- Walton, O.R. Review of Adhesion Fundamentals for Micron-Scale Particles. KONA Powder Part. J. 2008, 26, 129–141. [Google Scholar] [CrossRef]
- Gao, T.; Ye, J.; Zhang, K.; Liu, X.; Zhang, Y.; Liu, K. Role of capillary adhesion in the friction peak during the tacky transition. Friction 2022, 10, 1208–1216. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Johnson, D.; Ackermann, L.; Figgis, B.; Ayoub, M. Evaluation of the adhesion forces between dust particles and photovoltaic module surfaces. Sol. Energy Mater. Sol. Cells 2019, 191, 413–421. [Google Scholar] [CrossRef]
- Cleaver, J.A.S.; Tyrrell, J.W.G. The Influence of Relative Humidity on Particle Adhesion-a Review of Previous Work and the Anomalous Behavior of Soda-lime Glass. KONA Powder Part. J. 2004, 22, 9–22. [Google Scholar] [CrossRef]
- Fukunishi, A.; Mori, Y. Adhesion force between particles and substrate in a humid atmosphere studied by atomic force microscopy. Adv. Powder Technol. Int. J. Soc. Powder Technol. Jpn. 2006, 17, 567–580. [Google Scholar] [CrossRef]
- Jones, R.; Pollock, H.M.; Cleaver, J.A.; Hodges, C.S. Adhesion Forces between Glass and Silicon Surfaces in Air Studied by AFM: Effects of Relative Humidity, Particle Size, Roughness, and Surface Treatment. Langmuir 2002, 18, 8045–8055. [Google Scholar] [CrossRef]
- Xiao, X.; Qian, L. Investigation of Humidity-Dependent Capillary Force. Langmuir 2000, 16, 8153–8158. [Google Scholar] [CrossRef]
- Chau, A.; Régnier, S.; Delchambre, A.; Lambert, P. Theoretical and experimental study of the influence of AFM tip geometry and orientation on capillary force. J. Adhes. Sci. Technol. 2010, 24, 2499–2510. [Google Scholar] [CrossRef]
- Xia, W.; Ni, C.; Xie, G. The influence of surface roughness on wettability of natural/gold-coated ultra-low ash coal particles. Powder Technol. 2016, 288, 286–290. [Google Scholar] [CrossRef]
- Johnson, K.L.; Kendall, K.; Roberts, A.D. Surface Energy and the Contact of Elastic Solids. Proc. R. Soc. Lond. A Math. Phys. Sci. 1971, 324, 301–313. [Google Scholar]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Heim, L.-O.; Blum, J.; Preuss, M.; Butt, H.-J. Adhesion and Friction Forces between Spherical Micrometer-Sized Particles. Phys. Rev. Lett. 1999, 83, 3328–3331. [Google Scholar] [CrossRef]
- Popov, V.L.; Li, Q.; Lyashenko, I.A.; Pohrt, R. Adhesion and friction in hard and soft contacts: Theory and experiment. Friction 2021, 9, 1688–1706. [Google Scholar] [CrossRef]
- Rimai, D.S.; Demejo, L.P.; Bowen, R.C. Mechanics of particle adhesion. J. Adhes. Sci. Technol. 1994, 8, 1333–1355. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X. Model for an elastic-plastic spherical contact with the DMT adhesion. J. Adhes. Sci. Technol. 2023, 1–12. [Google Scholar] [CrossRef]
- Winterton, R.H.S. Van der Waals forces. Contemp. Phys. 1970, 11, 559–574. [Google Scholar] [CrossRef]
- Rabinovich, Y.I.; Adler, J.J.; Esayanur, M.S.; Ata, A.; Singh, R.K.; Moudgil, B.M. Capillary forces between surfaces with nanoscale roughness. Adv. Colloid Interface Sci. 2002, 96, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Bergström, L. Hamaker constants of inorganic materials. Adv. Colloid Interface Sci. 1997, 70, 125–169. [Google Scholar] [CrossRef]
- Brambilla, S.; Speckart, S.; Brown, M.J. Adhesion and aerodynamic forces for the resuspension of non-spherical particles in outdoor environments. J. Aerosol Sci. 2017, 112, 52–67. [Google Scholar] [CrossRef]
- Wang, J.; Qian, J.; Gao, H. Effects of Capillary Condensation in Adhesion between Rough Surfaces. Langmuir 2009, 25, 11727–11731. [Google Scholar] [CrossRef] [PubMed]






| Case | Element Content (At%) | ||||
|---|---|---|---|---|---|
| C | O | Si | Al | S | |
| Tip1 | 64.02 | 29.37 | 3.68 | 2.75 | 0.18 |
| Tip2 | 65.20 | 28.40 | 3.90 | 2.22 | 0.28 |
| Case | Force (nN) | |
|---|---|---|
| Tip1 (11.39 μm) | Tip2 (44.36 μm) | |
| Dry air | 1625 | 6331 |
| Humid air | 203 | 790 |
| RH (%) | Force (nN) | |
|---|---|---|
| Tip1 (11.39 μm) | Tip2 (44.36 μm) | |
| 60 | 1193 | 4649 |
| 70 | 1793 | 6982 |
| 80 | 2318 | 9026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Yang, Y.; Shang, H. Adhesion Behavior of Underground Coal Dust with Fused Silica: Effects of Relative Humidity and Particle Size. Processes 2024, 12, 735. https://doi.org/10.3390/pr12040735
Zhou S, Yang Y, Shang H. Adhesion Behavior of Underground Coal Dust with Fused Silica: Effects of Relative Humidity and Particle Size. Processes. 2024; 12(4):735. https://doi.org/10.3390/pr12040735
Chicago/Turabian StyleZhou, Shujun, Yue Yang, and Hongfei Shang. 2024. "Adhesion Behavior of Underground Coal Dust with Fused Silica: Effects of Relative Humidity and Particle Size" Processes 12, no. 4: 735. https://doi.org/10.3390/pr12040735
APA StyleZhou, S., Yang, Y., & Shang, H. (2024). Adhesion Behavior of Underground Coal Dust with Fused Silica: Effects of Relative Humidity and Particle Size. Processes, 12(4), 735. https://doi.org/10.3390/pr12040735






