Application of Pure and Modified Polyvinylidene Fluoride Materials for Wastewater Treatment Using UASB Reactor Technologies: A Review
Abstract
1. Introduction
2. Polymer Physical and Chemical Tests
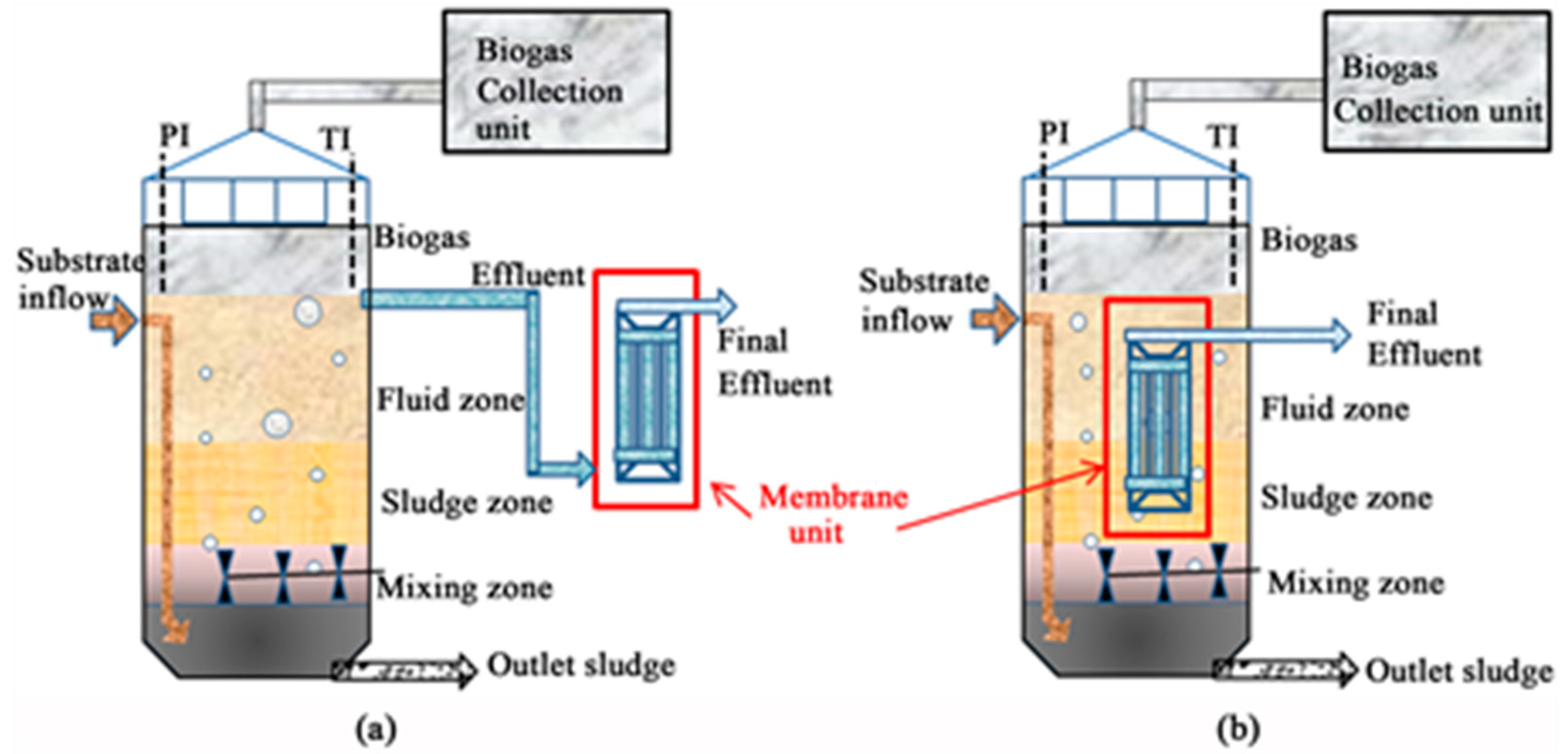
3. Typical AnPVDF/UASB Reactor Systems

| Configuration | Membrane Character | Scale (L) | Influent COD (mg/L) | Operation Conditions | COD Removal (%) | Ref. |
|---|---|---|---|---|---|---|
| Ext | HF 0.22 µm | 4 | 330–370 | T: 20 °C pH: n/a HRT: 12 h | 91.9 | [9] |
| Sub | HF 0.22 µm | 4 | 330–370 | T: 20 °C pH: n/a HRT: 12 h | 91.3 | [9] |
| Sub | FS 70,000 Da | 30 | 2600 | T: 20 and 55 °C pH: 7 HRT: n/a | 99.99 | [46] |
| Sub | HF 0.40 µm | 94 | 2200 | T: 37 °C pH: n/a HRT: 47 d | >98 | [47] |
| Sub | FS 0.10 µm | 1 | 100–800 | T: 15 °C pH: n/a HRT: 0.32 d | 86.2 | [48] |
| Sub | FS 140,000 Da | 80 | 342–527 | T: 30 °C pH: 7 HRT: 10 h | 90.0 | [41] |
| Ext | FS 0.40 µm | 80 | 11,224–12,898 | T: n/a pH: 7.1 HRT: 40 h | 90.0 | [42] |
4. Brief Comparison of a PVDF/UASB System and a Conventional UASB Reactor
5. Modification of PVDF Membrane to Reduce Fouling during Wastewater Treatment
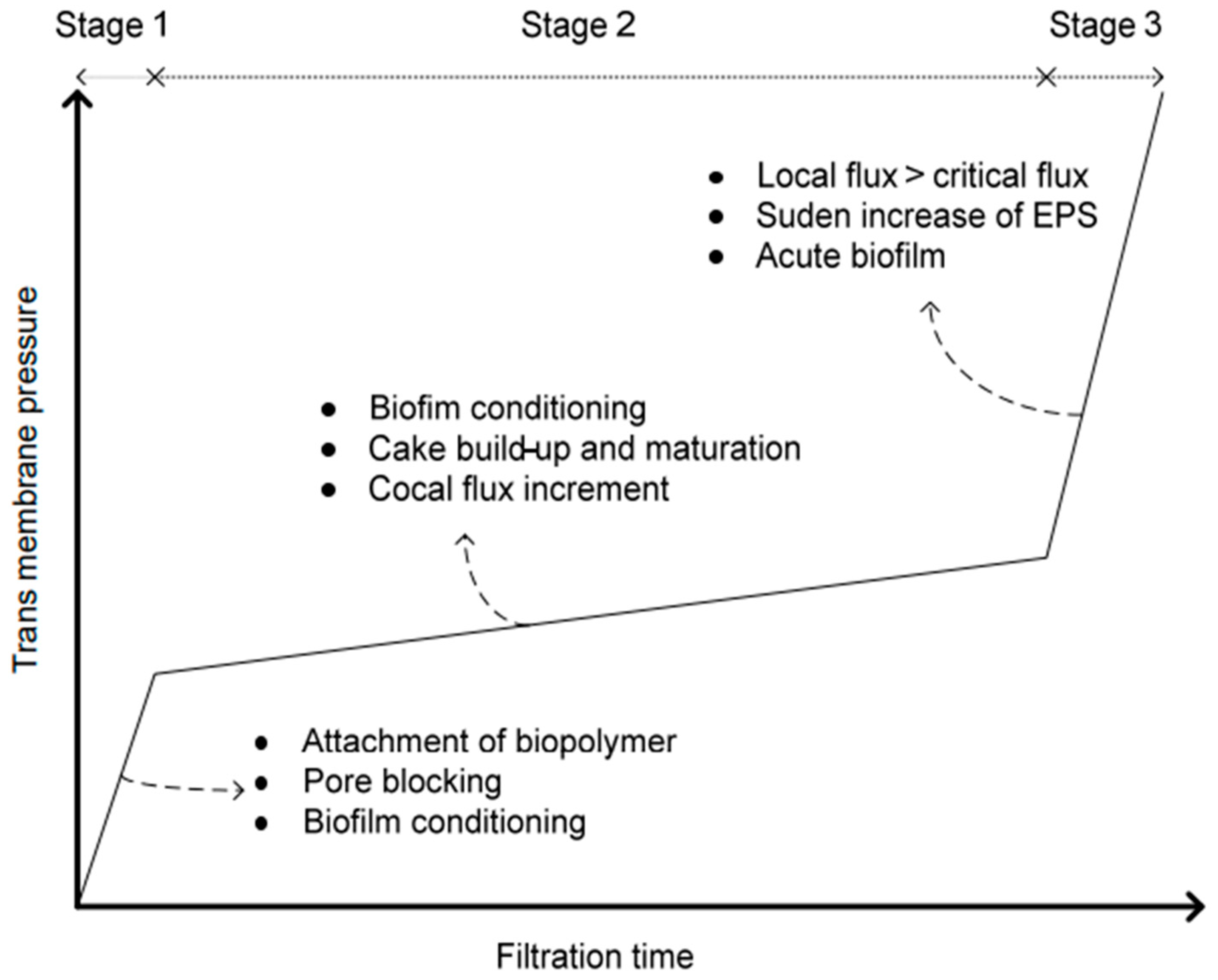
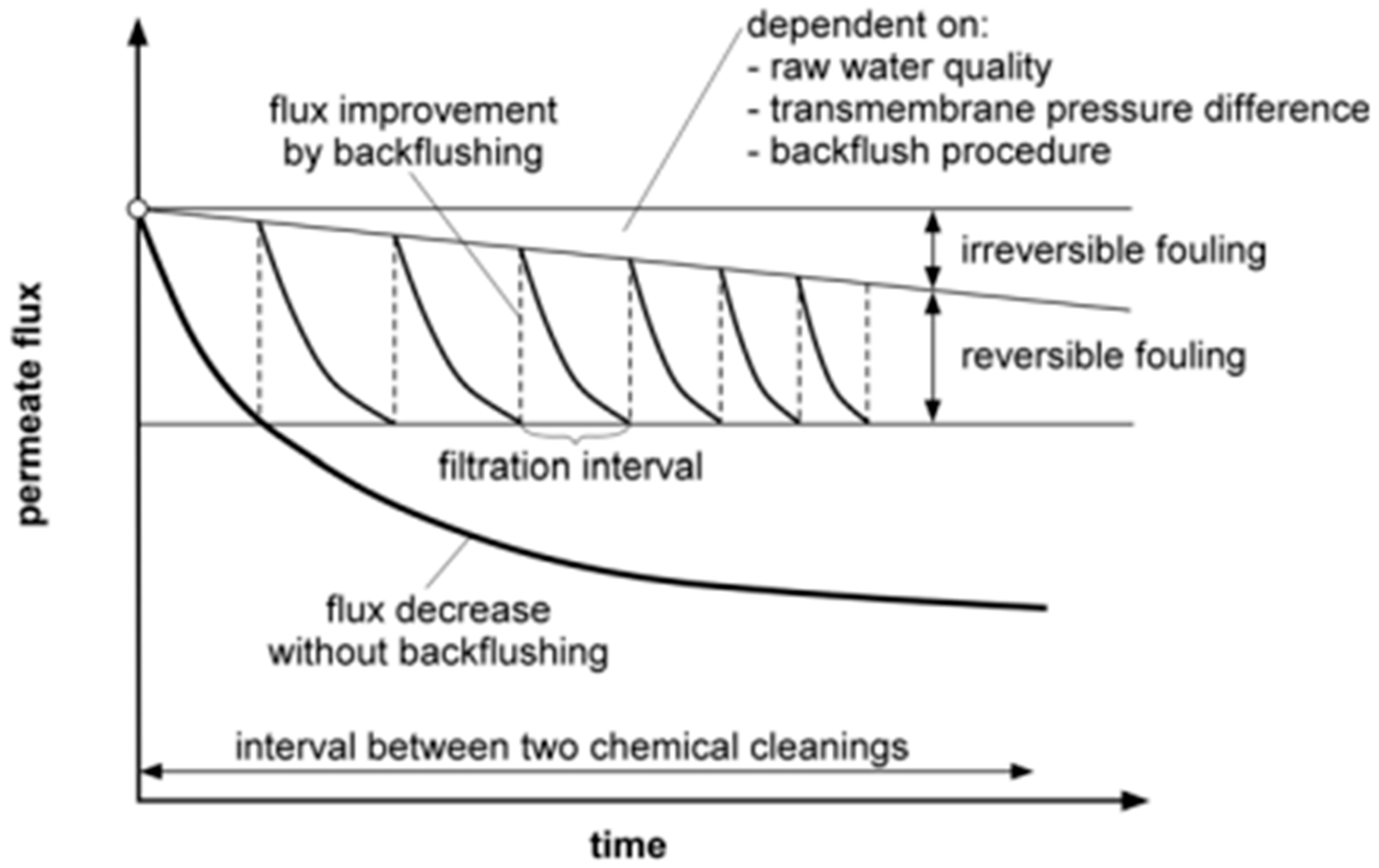
5.1. Fouling Process
5.2. Modification of PVDF
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Khateeb, M.; Hassan, G.K.; El Liethy, M.A.; El Khatib, K.M.; Abdel Shafy, H.I.; Hu, A.; Gad, M. Sustainable municipal wastewater treatment using an innovative integrated compact unit: Microbial communities, parasite removal, and techno economic analysis. Ann. Microbiol. 2023, 73, 35. [Google Scholar] [CrossRef]
- Arthur, P.M.; Konaté, Y.; Sawadogo, B.; Sagoe, G.; Dwumfour-Asare, B.; Ahmed, I.; Bayitse, R.; Ampomah-Benefo, K. Evaluating the Potential of Renewable Energy Sources in a Full-Scale Upflow Anaerobic Sludge Blanket Reactor Treating Municipal Wastewater in Ghana. Sustainability 2023, 15, 3743. [Google Scholar] [CrossRef]
- Vassalle, L.; Díez-Montero, R.; Machado, A.T.; Moreira, C.; Ferrer, I.; Mota, C.R.; Passos, F. Upflow anaerobic sludge blanket in microalgae-based sewage treatment: Codigestion for improving biogas production. Bioresour. Technol. 2020, 300, 122677. [Google Scholar] [CrossRef]
- Rattier, M.; Jimenez, J.A.; Miller, M.W.; Dhanasekar, A.; Willis, J.; Keller, J.; Batstone, D. Long-term comparison of pilot UASB and AnMBR systems treating domestic sewage at ambient temperatures. J. Environ. Chem. Eng. 2022, 10, 108489. [Google Scholar] [CrossRef]
- Ozgun, H.; Dereli, R.K.; Ersahin, M.E.; Kinaci, C.; Spanjers, N.; Van Lier, J.B. A review of anaerobic membrane bioreactors for municipal wastewater treatment: Integration options, limitations and expectations. Sep. Purif. Technol. 2013, 118, 89–104. [Google Scholar] [CrossRef]
- Ho, J.; Sung, S. Methanogenic activities in anaerobic membrane bioreactors (AnMBR) treating synthetic municipal wastewater. Bioresour. Technol. 2010, 101, 2191–2196. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Judd, S.J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2016, 305, 37–45. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H.; Chang, S.W.; Nguyen, D.D.; Nguyen, P.D.; Bui, X.T.; Wu, Y. Impact of reactor configurations on the performance of a granular. Int. Biodeterior. Biodegrad. 2017, 121, 131–138. [Google Scholar] [CrossRef]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.J.; McElroy, C.R.; Kendrick, E.; Goodship, V. On the Solubility and Stability of Polyvinylidene Fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef]
- Boutevin, B.; Pietrasanta, Y.; Robin, J.J. Synthese et application de copolymeres graffes a base de polyfluorrure de vinylidene-I. Euro Polym. 1991, 27, 815–820. [Google Scholar] [CrossRef]
- Wypych, W. Handbook of Material Weathering, 6th ed.; ChemTech Publishing: Toronto, ON, Canada, 2018. [Google Scholar]
- Scheirs, J.; Burks, S.; Locaspi, A. Developments in fluoropolymer coatings. Trends Polym. Sci. 1995, 3, 74–82. [Google Scholar]
- Bartoszek, E.J.; Perillon, J.L. Long-Life Coating with PVDF Polymers. Surf. Coat. J. 1995, 78, 371–376. [Google Scholar]
- Hopfstock, H. Paint removal composition and system. Paint. Ink Int. 1997, 10, 1–23. [Google Scholar]
- Benzinger, W.D.; Parekh, B.S.; Eichelberger, J.L. High temperature ultrafiltration with Kynar poly(vinylidene fluoride)membranes. Sep. Sci. Technol. 1980, 15, 1193–1204. [Google Scholar] [CrossRef]
- Zeus Technical Account. New Focus on PVDF; Zeus Industrial Products Inc.: Orangeburg, SC, USA, 2019. [Google Scholar]
- Hu, X.; An, A.K.J.; Chopra, S.S. Life Cycle Assessment of the Polyvinylidene Fluoride Polymer with Applications in Various Emerging Technologies. ACS Sustain. Chem. Eng. 2022, 10, 5708–5718. [Google Scholar] [CrossRef]
- Zapsas, G.; Patil, Y.; Gnanou, Y.; Ameduri, B.; Hadjichristidis, N. Poly(vinylidene fluoride)-based complex macromolecular architectures: From synthesis to properties and applications. Prog. Polym. Sci. 2020, 104, 101231. [Google Scholar] [CrossRef]
- Ameduri, B. From vinylidene fluoride (VDF) tothe applications of VDF-containing polymers and copolymers: Recent and future trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef]
- Guiot, J.; Ameduri, B.; Boutevin, B. Radical homopolymerization of vinylidene fluoride initiated by tert-butyl peroxypivalate. Investigation of the microstructure by 19F and 1H NMR spectroscopies and mechanisms. Macromolecules 2002, 35, 8694–8697. [Google Scholar] [CrossRef]
- Pladis, P.; Alexopoulos, A.H.; Kiparissides, C.J. Mathematical modeling and simulation of vinylidene fluoride emulsuin polymerization. Ind. Eng. Chem. 2014, 53, 7352–7364. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, X.; Sun, Y.; Bai, R. Anticorrosion Performance of PVDF Membranes Modified by Blending PTFE Nanoemulsion and Prepared through Usual Non-Solvent-Induced Phase Inversion Method. Membranes 2021, 11, 420. [Google Scholar] [CrossRef]
- Park, G.C.; Kim, D. Porous PTFE reinforced SPEEK proton exchange membranes for enhanced mechanical, dimensional, and electrochemical stability. Polymer 2021, 218, 123506. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A comparative analysis of the basic properties and applications of poly (vinylidene fuoride) (PVDF) and poly (methyl methacrylate) (PMMA). Polym. Bull. 2022, 79, 5635–5665. [Google Scholar] [CrossRef]
- Osman, R.M.; Hodaifa, G. An overview of anaerobic membrane bioreactors: Current developments, fouling problems, and future prospects. J. Environ. Chem. Eng. 2023, 11, 111482. [Google Scholar] [CrossRef]
- Daud, M.K.; Rizvi, H.; Akram, M.F.; Ali, S.; Rizwan, M.; Nafees, M.; Jin, Z.S. Review of Upflow Anaerobic Sludge Blanket Reactor Technology: Effect of Different Parameters and Developments for Domestic Wastewater Treatment. J. Chem. 2018, 2018, 1596319. [Google Scholar] [CrossRef]
- Martinez-Sosa, D.; Helmreich, B.; Netter, T.; Paris, S.; Bischof, F.; Horn, H. Pilot-scale anaerobic submerged membrane bioreactor (AnSMBR) treating municipal wastewater: The fouling phenomenon and long-term operation. Water Sci. Technol. 2011, 64, 1804–1811. [Google Scholar] [CrossRef]
- Ellouze, M.; Saddoud, A.; Dhouib, A.; Sayadi, S. Assessment of the impact of excessive chemical additions to municipal wastewaters and comparison of three technologies in the removal performance of pathogens and toxicity. Microbiol. Res. 2009, 164, 138–148. [Google Scholar] [CrossRef]
- Kocadagistan, E.; Topcu, N. Treatment investigation of the Erzurum City municipal wastewaters with anaerobic membrane bioreactors. Desalination 2007, 216, 367–376. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wu, Z.; Lu, F.; Tong, J.; Zang, L. Formation of dynamic membrane in an anaerobic membrane bioreactor for municipal wastewater treatment. Chem. Eng. J. 2010, 165, 175–183. [Google Scholar] [CrossRef]
- Jain, M. Anaerobic Membrane Bioreactor as Highly Efficient and Reliable Technology for Wastewater Treatment—A Review. Adv. Chem. Eng. Sci. 2018, 8, 82–100. [Google Scholar] [CrossRef]
- Seghezzo, L. Anaerobic Treatment of Domestic Wastewater in Subtropical Regions; Wageningen University and Research: Wageningen, The Netherlands, 2004. [Google Scholar]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a Unique Biological Group. Microb. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef]
- Gujer, W.; Zehnder, A.J. Conversion processes in anaerobic digestion. Water Sci. Technol. 1983, 15, 127–167. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.; Wang, Q.; Wu, Z.; Ma, J. Disintegration and acidification of MBR sludge under alkaline conditions. Chem. Eng. J. 2013, 231, 206–213. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Zhou, Q.; Song, Z. The effect of pH on anaerobic fermentation of primary sludge at room temperature. J. Hazard. Mater. 2009, 172, 196–201. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, X.; Fu, B.; Chen, J.; Yu, H. Acidogenic fermentation of proteinaceous sewage sludge: Effect of pH. Water Res. 2012, 46, 799–807. [Google Scholar] [CrossRef]
- TBW GmbH. Anaerobic Treatment of Municipal Wastewater in UASB-Reactors; Technical Information W6e, gtz; TBW GmbH: Frankfurt, Germany, 2001. [Google Scholar]
- Chen, R.; Nie, Y.; Kato, H.; Wu, J.; Utashiro, T.; Lu, J.; Yue, S.; Jiang, H.; Zhang, L.; Li, Y.Y. Methanogenic degradation of toilet-paper cellulose upon sewage. Bioresour. Technol. 2017, 228, 69–76. [Google Scholar] [CrossRef]
- Gouveia, J.; Plaza, G.; Garralon, G.; Fdz-Polanco, F.; Pena, M. Long-term operation of a pilot scale anaerobic membrane bioreactor. Bioresour. Technol. 2015, 185, 225–233. [Google Scholar] [CrossRef]
- Lin, H.; Chen, J.; Wang, F.; Ding, L.; Hong, H. Feasibility evaluation of submerged anaerobic membrane bioreactor for municipal secondary wastewater treatment. Desalination 2011, 280, 120–126. [Google Scholar] [CrossRef]
- Umaiyakunjaram, R.; Shanmugam, P. Study on submerged anaerobic membrane bioreactor (SAMBR) treating high suspended solids raw tannery wastewater for biogas production. Bioresour. Technol. 2016, 216, 785–792. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Amin, S.K.; Abdallah, H.M.; Roushdy, M.H.; El-Sherbiny, C.A. An Overview of Production and Development of Ceramic Membranes. Int. J. Appl. Eng. Res. 2016, 11, 7708–7721. [Google Scholar]
- Lin, H.J.; Xie, K.; Mahendran, B.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Sludge properties and their effects on membrane fouling in submerged anaerobic membrane bioreactors (SAnMBRs). Water Res. 2009, 43, 3827–3837. [Google Scholar] [CrossRef] [PubMed]
- Berkessa, Y.W.; Yan, B.; Li, T.; Tan, M.; She, Z.; Jegatheesan, V.; Jiang, H.; Zhang, Y. Novel anaerobic membrane bioreactor (AnMBR) design for wastewater treatment at long HRT and high solid concentration. Bioresour. Technol. 2018, 250, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, E.; Yu, Y.; Tabraiz, S.; Yakubu, A.; Curtis, T.P.; Dolfing, J. High rate domestic wastewater treatment at 15 °C using anaerobic reactors inoculated with cold-adapted ediments/soils–shaping robust methanogenic communities. Environ. Sci. Water Res. Technol. 2019, 5, 70–82. [Google Scholar] [CrossRef]
- Hu, A.Y.; Stuckey, D.C. Activated Carbon Addition to a Submerged Anaerobic Membrane Bioreactor: Effect on Performance, Transmembrane Pressure, and Flux. J. Environ. Eng. 2007, 133, 73–80. [Google Scholar] [CrossRef]
- Xiong, J.; Yu, S.; Hu, Y.; Yang, Y.; Wang, X.C. Applying a dynamic membrane filtration (DMF) process for domestic wastewater preconcentration: Organics recovery and bioenergy production potential analysis. Sci. Total Environ. 2018, 680, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tan, X.; Liu, Y. Single-step fabrication of ceramic hollow fibers for oxygen permeation. J. Membr. Sci. 2006, 15, 1–5. [Google Scholar] [CrossRef]
- Bilad, M.R.; Baten, M.; Pollet, A.; Courtin, C.; Wouters, J.; Verbiest, T.; Vankelecom, I.F.J. A novel In-situ Enzymatic Cleaning Method for Reducing Membrane Fouling in Membrane Bioreactors (MBRs). Indones. J. Sci. Technol. 2016, 1, 1–22. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Drews, A.; Kraume, M. Process Improvement by Application of Membrane Bioreactors. Chem. Eng. Resour. 2005, 83, 276–284. [Google Scholar] [CrossRef]
- Spettmann, D.; Eppmann, S.; Flemming, H.C.; Wingender, J. Simultaneous visualisation of biofouling, organic and inorganic particle fouling on separation membranes. Water Sci. Technol. 2007, 55, 207–210. [Google Scholar] [CrossRef]
- Komlenic, R. Rethinking the causes of membrane biofouling. Filtr. Sep. 2010, 47, 26–28. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, H.; Yang, F.; Liu, L. Characterization of cake layer in submerged membrane bioreactor. Environ. Sci. Technol. 2007, 41, 4065–4070. [Google Scholar] [CrossRef]
- Erkan, H.S.; Turan, N.B.; Engin, G.O. Membrane Bioreactors for Wastewater Treatment. Compr. Anal. Chem. 2018, 81, 151–200. [Google Scholar]
- Ji, J.; Liu, F.; Hashim, N.A.; Abed, M.M.; Li, K. Poly(vinylidene fluoride) (PVDF) membranes for fluid separation. React. Funtional Polym. 2015, 86, 134–153. [Google Scholar] [CrossRef]
- Shen, S.; Hao, Y.; Zhang, Y.; Zhang, G.; Zhou, X.; Bai, R.B. Enhancing the antifouling properties of poly(vinylidene fluoride) (PVDF) membrane through a novel blending and surface-grafting modification approach. ACS Omega 2018, 3, 17403–17415. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Loo, H.E.; Bai, R.B. A novel membrane showing both hydrophilic and oleophobic surface properties and its non-fouling performance for potential water treament applications. J. Membr. Sci. 2013, 436, 47–56. [Google Scholar] [CrossRef]
- Abed, M.R.; Kumbharkar, S.C.; Groth, A.M.; Li, K. Economical production of PVDF-g-POEM for use as a blend in preparation of PVDF based hydrophilic hollow fibre membranes. Sep. Purif. Technol. 2013, 106, 47–55. [Google Scholar] [CrossRef]
- Hashim, N.A.; Liu, F.; Li, K. A simplified method for preparation of hydrophilic PVDF membranes from an amphiphilic graft copolymer. J. Membr. Sci. 2009, 345, 134–141. [Google Scholar] [CrossRef]
- Liu, B.C.; Chen, C.; Li, T.; Crittenden, J.; Chen, Y.S. High performance ultrafiltration mebrane composed of PVDF blended with its derivative copolymer PVDF-g-PEGMA. J. Membr. Sci. 2013, 445, 66–75. [Google Scholar] [CrossRef]
- Chen, C.; Tang, L.; Liu, B.; Zhang, X.; Crittenden, J.; Chen, K.L.; Chen, Y. Forming mechanism study of unique pillar pillar-like and defect-free PVDF ultrafiltration membranes with high flux. J. Membr. Sci. 2015, 487, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Chen, C.; Liu, B.; Crittenden, J.C. PVDF ultrafiltration membranes of controlled performance via blending PVDF-g-PEGMA copolymer synthesized under different reaction times. Front. Environ. Sci. Eng. China 2018, 12, 3. [Google Scholar] [CrossRef]
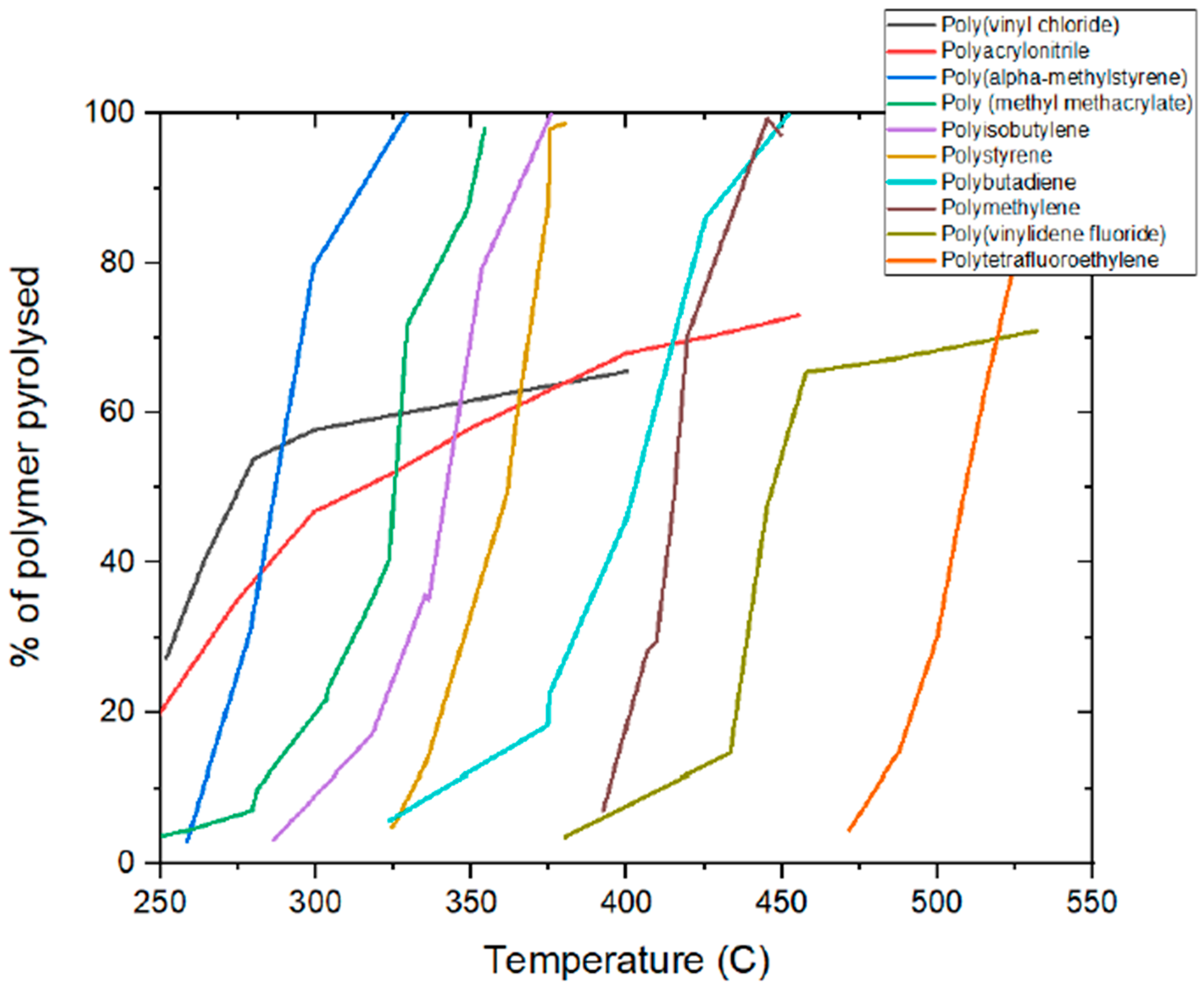

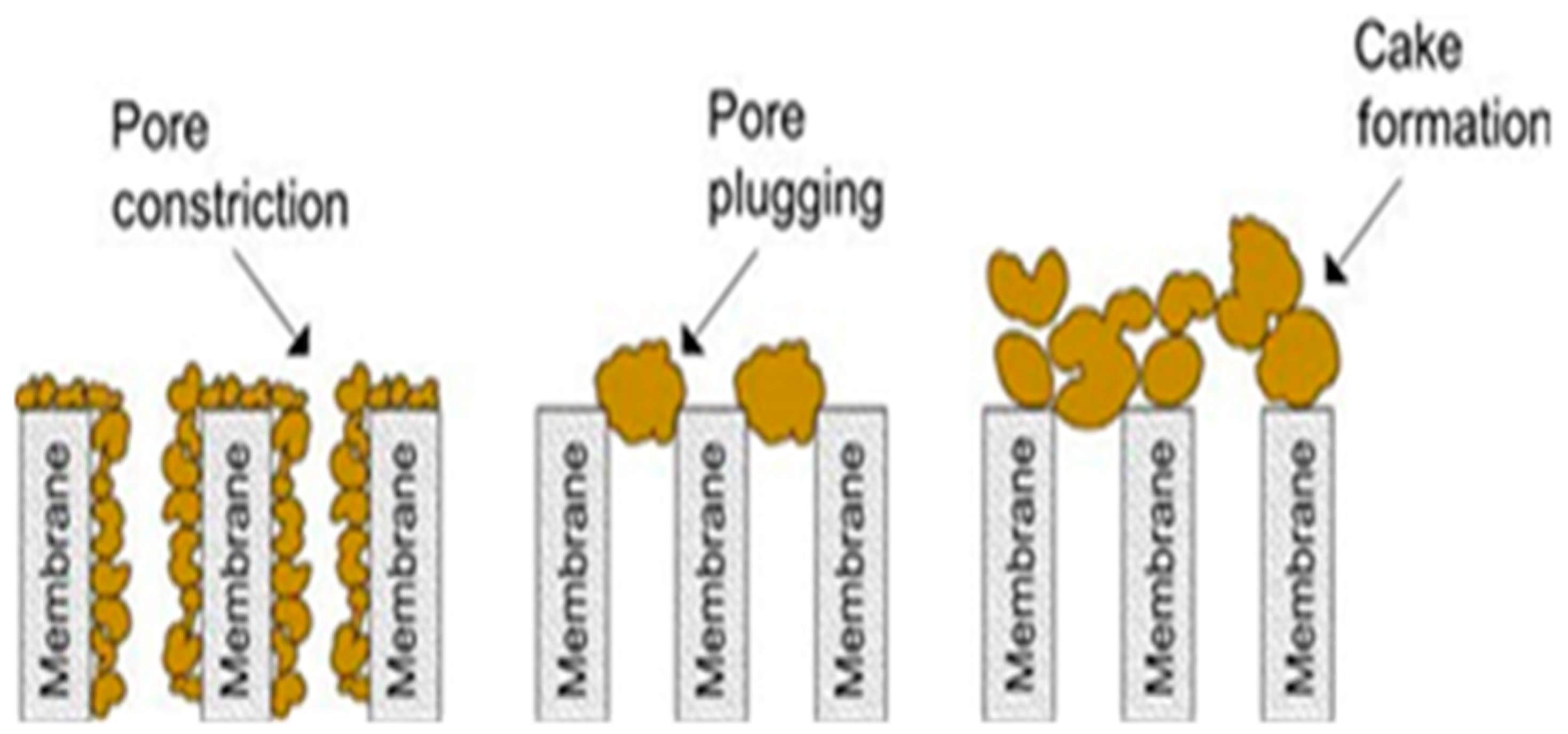
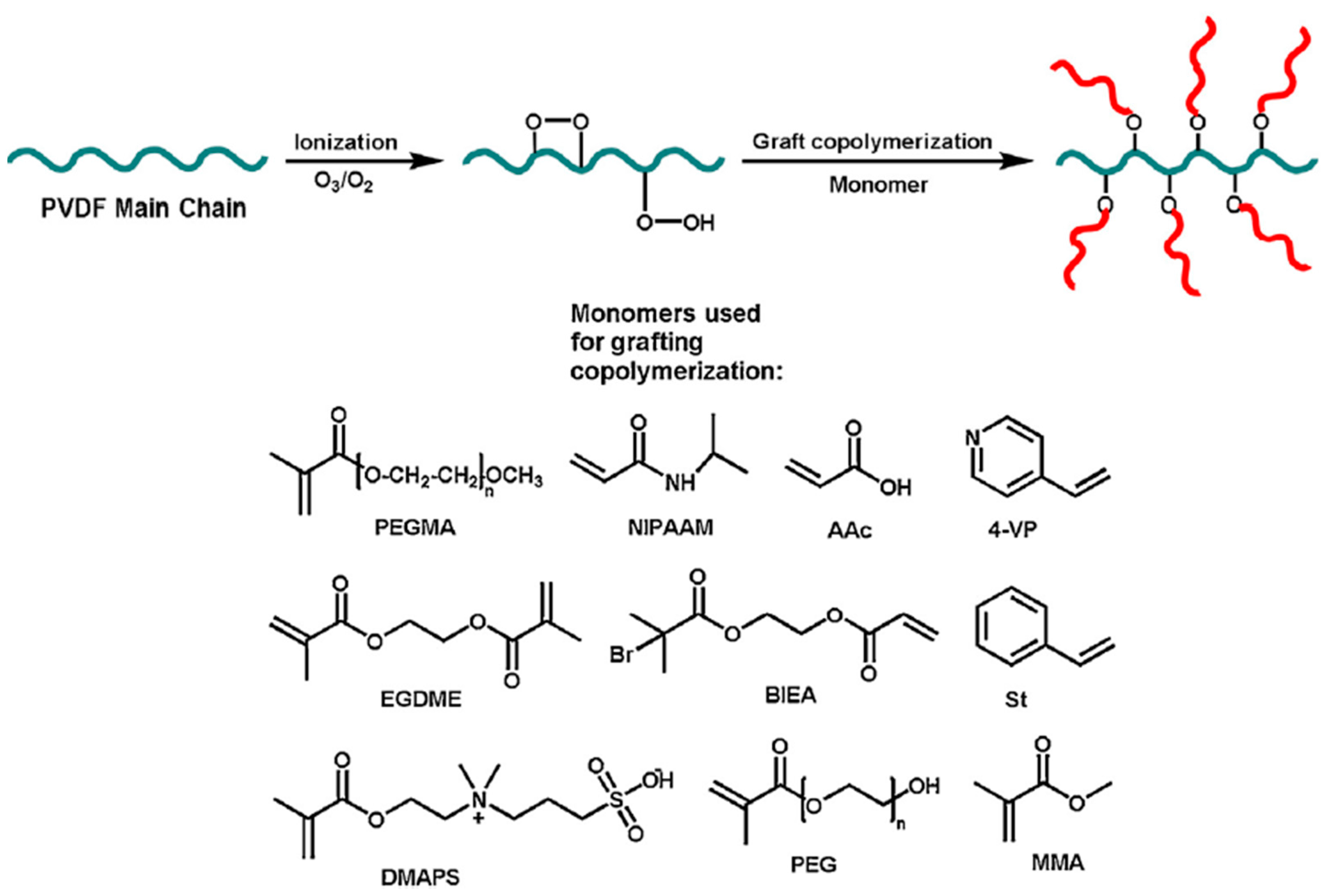

| Material and Exposure Conditions | Gross Retention |
|---|---|
| PVDF film/Florida, 20 years PVDF film/Weather-Ometer, 20,000 h | 58% 92% |
| Polytetrafluoroethylene (PTFE)/Florida, 10 years | 59% |
| Polyimide/Florida, 2000 h Polyimide/Weather-Ometer, 700 h | 25% 25% |
| Polyamide Kevlorage/Florida, 24 months PA-6/Florida, 3.3 years | N/A 47% |
| Advantages |
| Demands less reactor volume because it offers a good removal efficiency, even at a high OLR and a low temperature; |
| Owing to the local availability of building materials and other elements, the construction is straightforward; |
| A broad range of applications, from very tiny to extremely large, and a robustness in treatment efficiency; |
| Methane and hydrogen gases are produced as energy. In order to lower operating expenses, the energy generated might be used to heat the boilers. Reduced energy consumption in the absence of an external temperature control system; |
| Reduced carbon dioxide emissions as a result of lower energy consumption and the creation of extra energy in the form of biogas, which can power the system; |
| Minimal sludge generation in contrast to aerobic processes. The resultant sludge is stable, has strong dewatering properties, and may be kept in storage for a long time before being utilized as an inoculum to seed UASB reactors; |
| Short startup period (about one week) using granular anaerobic sludge as the seed; |
| Able to sustain shock loads from organic sources; |
| The capacity to treat sewage because there are macro- and micronutrients available, and the pH stays stable without the need for chemical additions. |
| Disadvantages |
| Post-treatment is necessary because aside from helminths’ eggs, which are successfully ensnared in the sludge, pathogens are not entirely eliminated; |
| In situations where activated sludge is not readily available, a lengthy beginning period is necessary because of the slow growth rate of microorganisms; |
| Anaerobic digestion produces H2S, which can cause corrosion, odor, and toxicity; |
| It is possible for a sizable amount of biogas to dissolve in the effluent, which needs to be recovered. |
| Production | ||
|---|---|---|
| Methane *1 (m3CH4/kgCODremoved) | Electrical demand (kWhel/kgCODremoved) | Energy (kWhel/kgCODremoved) |
| 0.2 | 0.6 | 0.08 |
| Configuration | Membrane Character | Scale (L) | Influent COD (mg/L) | Operation Conditions | Methane Yield (mLCH4/gCODremoval) | Ref. |
|---|---|---|---|---|---|---|
| Ext | HF 0.22 µm | 4 | 330–370 | T: 20 °C pH: n/a HRT: 12 h | 160.5 | [9] |
| Sub | HF 0.22 µm | 4 | 330–370 | T: 20 °C pH: n/a HRT: 12 h | 156.3 | [9] |
| n/a | HF 0.22 µm | 3 | 330–370 | T: 20 °C pH: n/a HRT: 12 h | 133.3 | [40] |
| ES | HF 0.045 µm | 310 | 621–1163 | T: 6–30 °C pH: n/a HRT: 10–13 h | 235.0 | [41] |
| Sub | FS 140,000 Da | 80 | 342–527 | T: 30 °C pH: 7 HRT: 10 h | 260.0 | [42] |
| Ext | FS 0.40 µm | 80 | 11,224–12,898 | T: n/a pH: 7.1 HRT: 40 h | 160.0 | [43] |
| Reactor System | Comparative Measure | ||
|---|---|---|---|
| PVDF/UASB UASB | Percentage of CH4 in effluent | Percentage of CH4 production | CH4 difference in effluent |
| 8.9 | 26.4 with recirculation compared to 13.7 without for PVDF/UASB systems | 2–3% higher in membrane module than in UASB reactor | |
| 16.3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikosana, M.L.; Khoabane Sikhwivhilu, K.; Moutloali, R.; Madyira, D. Application of Pure and Modified Polyvinylidene Fluoride Materials for Wastewater Treatment Using UASB Reactor Technologies: A Review. Processes 2024, 12, 734. https://doi.org/10.3390/pr12040734
Sikosana ML, Khoabane Sikhwivhilu K, Moutloali R, Madyira D. Application of Pure and Modified Polyvinylidene Fluoride Materials for Wastewater Treatment Using UASB Reactor Technologies: A Review. Processes. 2024; 12(4):734. https://doi.org/10.3390/pr12040734
Chicago/Turabian StyleSikosana, Mmontshi Lebohang, Keneiloe Khoabane Sikhwivhilu, Richard Moutloali, and Daniel Madyira. 2024. "Application of Pure and Modified Polyvinylidene Fluoride Materials for Wastewater Treatment Using UASB Reactor Technologies: A Review" Processes 12, no. 4: 734. https://doi.org/10.3390/pr12040734
APA StyleSikosana, M. L., Khoabane Sikhwivhilu, K., Moutloali, R., & Madyira, D. (2024). Application of Pure and Modified Polyvinylidene Fluoride Materials for Wastewater Treatment Using UASB Reactor Technologies: A Review. Processes, 12(4), 734. https://doi.org/10.3390/pr12040734










