H2 Reduction of Na2SO4 to Na2S Based on Dilute-Phase Fluidization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
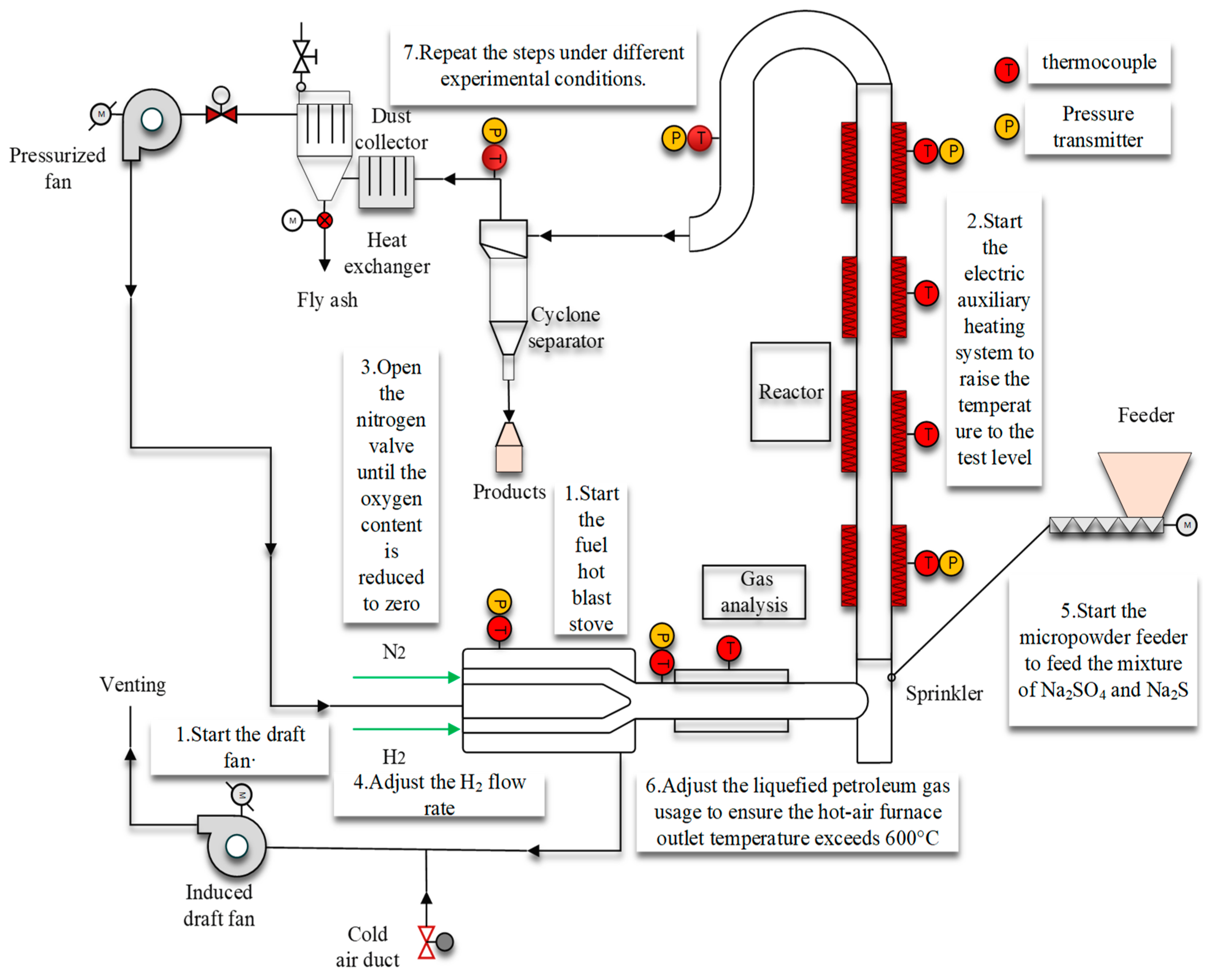
2.2. Experimental Methods
2.3. Material Characterization
3. Results and Discussion
3.1. Eutectic Characteristics of the Na2SO4 and Na2S Mixture
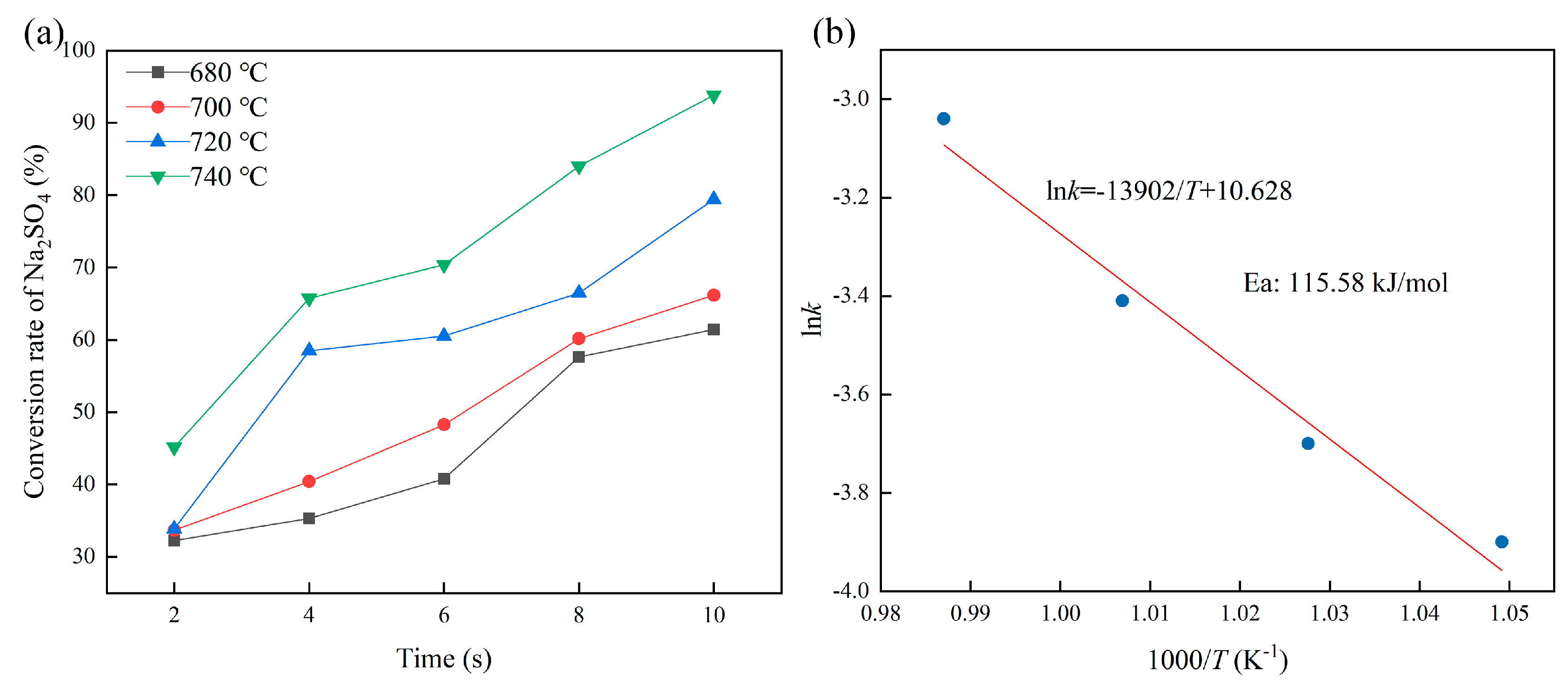
3.2. Characteristics of the H2 Reduction of Na2SO4 in a Mixed-Material System
4. Conclusions
- The mixture of Na2S and Na2SO4 exhibited low-temperature eutectic characteristics. The system remained in a solid phase at temperatures below 700 °C. The eutectic temperature of the system increased with an increase in the proportion of Na2S at temperatures above 700 °C, reducing the generation of the liquid phase and alleviating fusion and clogging.
- In the dilute-phase fluidization system, the samples with 80% Na2S and Na2SO4 as the reactant suppressed the formation of the liquid phase and accelerated the reduction process. After reduction roasting for 10 s, the characteristic diffraction peaks of Na2SO4 almost disappeared, indicating that the reduction was nearly complete. At this point, the Na2S content increased to 98.74%, achieving a high reduction rate of 93.7%.
- The microstructural morphologies of the products revealed their kinetic characteristics. The addition of 80% Na2S to the reactants accelerated the reduction rate and promoted the uniformity and homogeneity of the transformed products.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kostick, D.S. Sodium sulfate. In US Geological Survey Minerals Yearbook; U.S. Geological Survey (USGS): Reston, VA, USA, 2013; pp. 150–151. [Google Scholar]
- Du, Z.; Liu, F.; Liu, J.; Pan, F.; Fan, C.; Zhang, J. A green process for producing Na2S from waste Na2SO4 through hydrogen agglomerate fluidized bed reduction of baso4. J. Clean Prod. 2022, 355, 131816. [Google Scholar] [CrossRef]
- Smith, W.H.; Birnbaum, J.; Wolden, C.A. Production and purification of anhydrous sodium sulfide. J. Sulfur Chem. 2021, 42, 426–442. [Google Scholar] [CrossRef]
- Çelikler, C.; Varol, M. Investigation of control methods for agglomeration and slagging during combustion of olive cake in a bubbling fluidized bed combustor. J. Clean Prod. 2021, 320, 128841. [Google Scholar] [CrossRef]
- Hua, W.; Xu, X.; Zhang, X.; Yan, H.; Zhang, J. Progress in corrosion and anti-corrosion measures of phase change materials in thermal storage and management systems. J. Energy Storage 2022, 56, 105883. [Google Scholar] [CrossRef]
- Shen, X.; Shao, H.; Wang, Z.; Zhai, Y. Preparation of Na2S from Na2SO4 using co. Adv. Mater. Res. 2014, 1004, 706. [Google Scholar]
- Fu, H.; Chen, C.; Li, J.; Lan, Y.; Wang, L.; Yuan, J. Influence of na2s on the corrosion behavior of q345 steel in sodium aluminate solution. Mater. Res. Express 2019, 6, 1065a–1069a. [Google Scholar] [CrossRef]
- Pereira, R.J.L.; Hu, W.; Metcalfe, I.S. Impact of gas–solid reaction thermodynamics on the performance of a chemical looping ammonia synthesis process. Energy Fuels 2022, 36, 9757–9767. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Hou, X.; Chen, Y.; Fang, Z.; Chen, J.; Liang, T.; Chou, K.; Nickel, K.G. Progress in cognition of gas-solid interface reaction for non-oxide ceramics at high temperature. Crit. Rev. Solid State Mat. Sci. 2021, 46, 218–250. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Weng, S.; Wu, S.; Liu, Q.; Cao, M.; Li, Y.; Wang, Z.; Zhu, L.; Xiao, R. Spontaneous gas–solid reaction on sulfide electrolytes for high-performance all-solid-state batteries. Energy Environ. Sci. 2023, 16, 1091–1099. [Google Scholar] [CrossRef]
- Osman, M.; Khan, M.N.; Zaabout, A.; Cloete, S.; Amini, S. Review of pressurized chemical looping processes for power generation and chemical production with integrated CO2 capture. Fuel Process. Technol. 2021, 214, 106684. [Google Scholar] [CrossRef]
- Simoncelli, M.; Marzari, N.; Mauri, F. Wigner formulation of thermal transport in solids. Phys. Rev. X 2022, 12, 41011. [Google Scholar] [CrossRef]
- Yang, Z.; He, Z.; Zhang, X.; Jiang, X.; Jin, Z.; Li, J.; Ma, L.; Wang, H.; Chang, Y. In-Situ gas flow separation between biochar and the heat carrier in a circulating fluidized bed reactor for biomass pyrolysis. Chem. Eng. J. 2023, 472, 145099. [Google Scholar] [CrossRef]
- Qie, Z.; Alhassawi, H.; Sun, F.; Gao, J.; Zhao, G.; Fan, X. Characteristics and applications of micro fluidized beds (mfbs). Chem. Eng. J. 2022, 428, 131330. [Google Scholar] [CrossRef]
- Di Renzo, A.; Scala, F.; Heinrich, S. Recent advances in fluidized bed hydrodynamics and transport phenomena—Progress and understanding. Processes 2021, 9, 639. [Google Scholar] [CrossRef]
- Leubner, I.H. Particle nucleation and growth models. Curr. Opin. Colloid Interface Sci. 2000, 5, 151–159. [Google Scholar] [CrossRef]
- Szabó, R.; Lente, G. General nucleation-growth type kinetic models of nanoparticle formation: Possibilities of finding analytical solutions. J. Math. Chem. 2021, 59, 1808–1821. [Google Scholar] [CrossRef]
- Ahn, B.; Bosetti, L.; Mazzotti, M. Secondary nucleation by interparticle energies. II. Kinetics. Cryst. Growth Des. 2021, 22, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.J.; Cassar, D.R.; Deceanne, A.V.; Kirchner, K.A.; Mckenzie, M.E.; Zanotto, E.D.; Mauro, J.C. Energy landscape modeling of crystal nucleation. Acta Mater. 2021, 217, 117163. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Goonetilleke, E.C.; Huang, X. Temperature-dependent kinetic pathways of heterogeneous ice nucleation competing between classical and non-classical nucleation. Nat. Commun. 2021, 12, 4954. [Google Scholar] [CrossRef] [PubMed]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Pérez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. Ictac kinetics committee recommendations for analysis of thermal decomposition kinetics. Thermochim. Acta 2023, 719, 179384. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. Gas–solid fluidization of cohesive powders. Chem. Eng. Res. Des. 2018, 133, 347–387. [Google Scholar] [CrossRef]
- Geng, P.; Qin, G.; Zhou, J.; Li, T.; Ma, N. Characterization of microstructures and hot-compressive behavior of gh4169 superalloy by kinetics analysis and simulation. J. Mater. Process. Technol. 2021, 288, 116879. [Google Scholar] [CrossRef]





| Material Ratio | Atmosphere | T/°C | Viscosity/pa·s | V0/m·s−1 |
|---|---|---|---|---|
| Na2S:Na2SO4 = 8:2 | H2 | 680 | 1.92 × 10−5 | 0.53 |
| Na2S:Na2SO4 = 8:2 | H2 | 700 | 1.93 × 10−5 | 0.52 |
| Na2S:Na2SO4 = 8:2 | H2 | 720 | 1.97 × 10−5 | 0.51 |
| Na2S:Na2SO4 = 8:2 | H2 | 740 | 1.99 × 10−5 | 0.51 |
| Samples | Initial Mixing Ratio (wt%) | Melt State during Roasting under N2 Flow for 30 min at Different Temperatures 1 | |||||
|---|---|---|---|---|---|---|---|
| Na2S | Na2SO4 | 680 °C | 700 °C | 720 °C | 740 °C | 760 °C | |
| 1 | 0 | 100 | S | S | S | S | S |
| 2 | 20 | 80 | S | S | S | SL | L |
| 3 | 30 | 70 | S | S | L | L | L |
| 4 | 40 | 60 | S | S | L | L | L |
| 5 | 50 | 50 | S | S | L | L | L |
| 6 | 60 | 40 | S | SL | SL | L | L |
| 7 | 70 | 30 | S | S | S | SL | L |
| 8 | 80 | 20 | S | S | S | S | L |
| 9 | 100 | 0 | S | S | S | S | S |
| T (°C) | t (s) | Raw Materials (wt%) | Reduction Product (wt%) | ||
|---|---|---|---|---|---|
| Na2SO4 | Na2S | Na2S | Na2SO4 | ||
| 680 | 2 | 20 | 80 | 85.60 | 14.40 |
| 4 | 20 | 80 | 86.25 | 13.75 | |
| 6 | 20 | 80 | 87.41 | 12.59 | |
| 8 | 20 | 80 | 91.00 | 9.00 | |
| 10 | 20 | 80 | 91.80 | 8.20 | |
| 700 | 2 | 20 | 80 | 85.90 | 14.10 |
| 4 | 20 | 80 | 87.33 | 12.67 | |
| 6 | 20 | 80 | 89.00 | 11.00 | |
| 8 | 20 | 80 | 91.53 | 8.47 | |
| 10 | 20 | 80 | 92.81 | 7.19 | |
| 720 | 2 | 20 | 80 | 86.49 | 13.51 |
| 4 | 20 | 80 | 91.52 | 8.48 | |
| 6 | 20 | 80 | 91.94 | 8.06 | |
| 8 | 20 | 80 | 93.15 | 6.85 | |
| 10 | 20 | 80 | 95.80 | 4.20 | |
| 740 | 2 | 20 | 80 | 88.79 | 11.21 |
| 4 | 20 | 80 | 93.00 | 7.00 | |
| 6 | 20 | 80 | 93.95 | 6.05 | |
| 8 | 20 | 80 | 96.73 | 3.27 | |
| 10 | 20 | 80 | 98.74 | 1.26 | |
| T (°C) | |||||
|---|---|---|---|---|---|
| k (min−1) | m | R2 | k (min−1) | R2 | |
| 680 | 0.04305 | 0.62152 | 0.91004 | 0.02115 | 0.91646 |
| 700 | 0.04536 | 0.56597 | 0.97741 | 0.02329 | 0.98301 |
| 720 | 0.05845 | 0.47181 | 0.92981 | 0.03088 | 0.91831 |
| 740 | 0.08715 | 0.48173 | 0.98867 | 0.05056 | 0.96535 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.; Chen, Y.; Zhao, B.; Chen, C.; Huang, S.; Peng, S. H2 Reduction of Na2SO4 to Na2S Based on Dilute-Phase Fluidization. Processes 2024, 12, 776. https://doi.org/10.3390/pr12040776
He F, Chen Y, Zhao B, Chen C, Huang S, Peng S. H2 Reduction of Na2SO4 to Na2S Based on Dilute-Phase Fluidization. Processes. 2024; 12(4):776. https://doi.org/10.3390/pr12040776
Chicago/Turabian StyleHe, Fang, Yanxin Chen, Bo Zhao, Chang Chen, Song Huang, and Shoubin Peng. 2024. "H2 Reduction of Na2SO4 to Na2S Based on Dilute-Phase Fluidization" Processes 12, no. 4: 776. https://doi.org/10.3390/pr12040776
APA StyleHe, F., Chen, Y., Zhao, B., Chen, C., Huang, S., & Peng, S. (2024). H2 Reduction of Na2SO4 to Na2S Based on Dilute-Phase Fluidization. Processes, 12(4), 776. https://doi.org/10.3390/pr12040776






