Automatic Control of Nucleation and Crystal Growth Using Online Raman Analyzer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Main Reagents
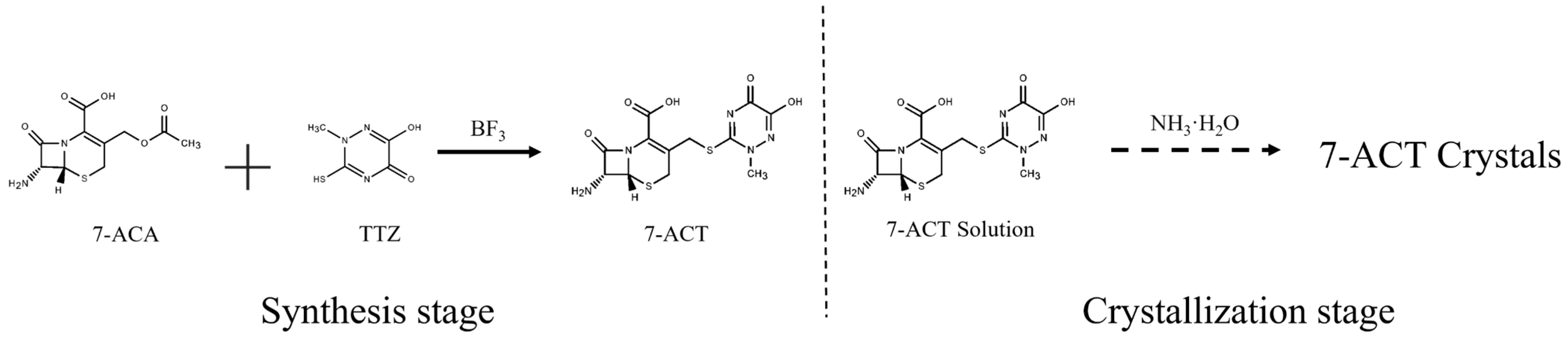
2.2. Synthesis and Crystallization of 7-ACT
- (1)
- 7-ACT synthesis operation
- (2)
- 7-ACT crystallization operation
2.3. HPLC Analysis
2.4. Online Monitoring with Raman Analyzer
3. Results
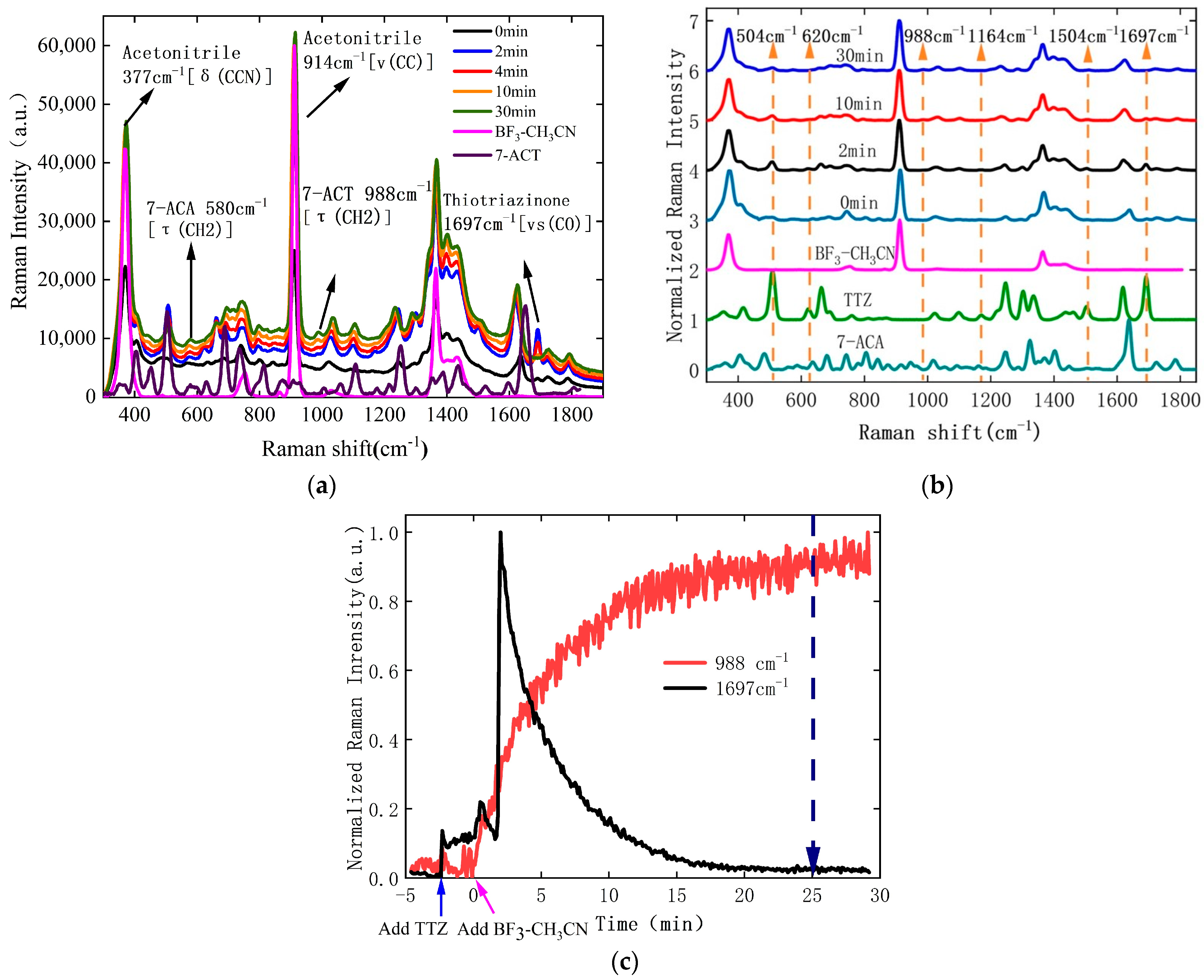
3.1. Monitoring of 7-ACT Synthesis Reaction
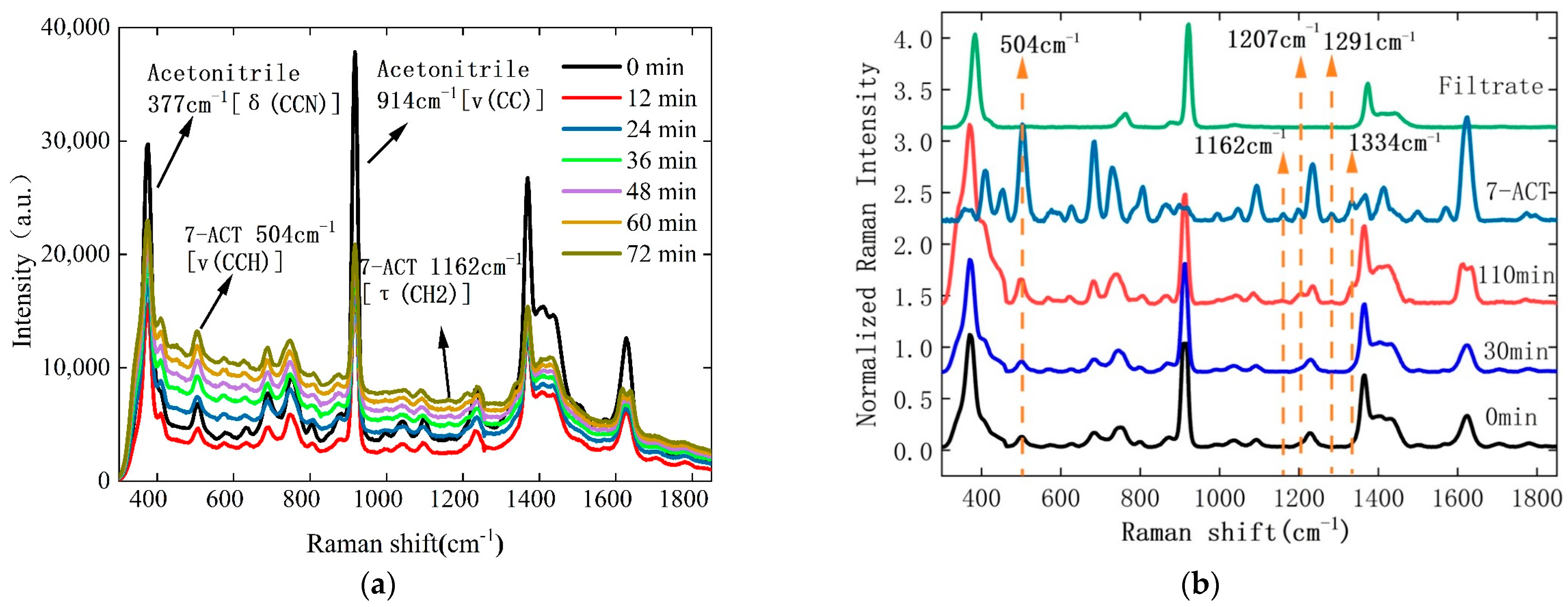
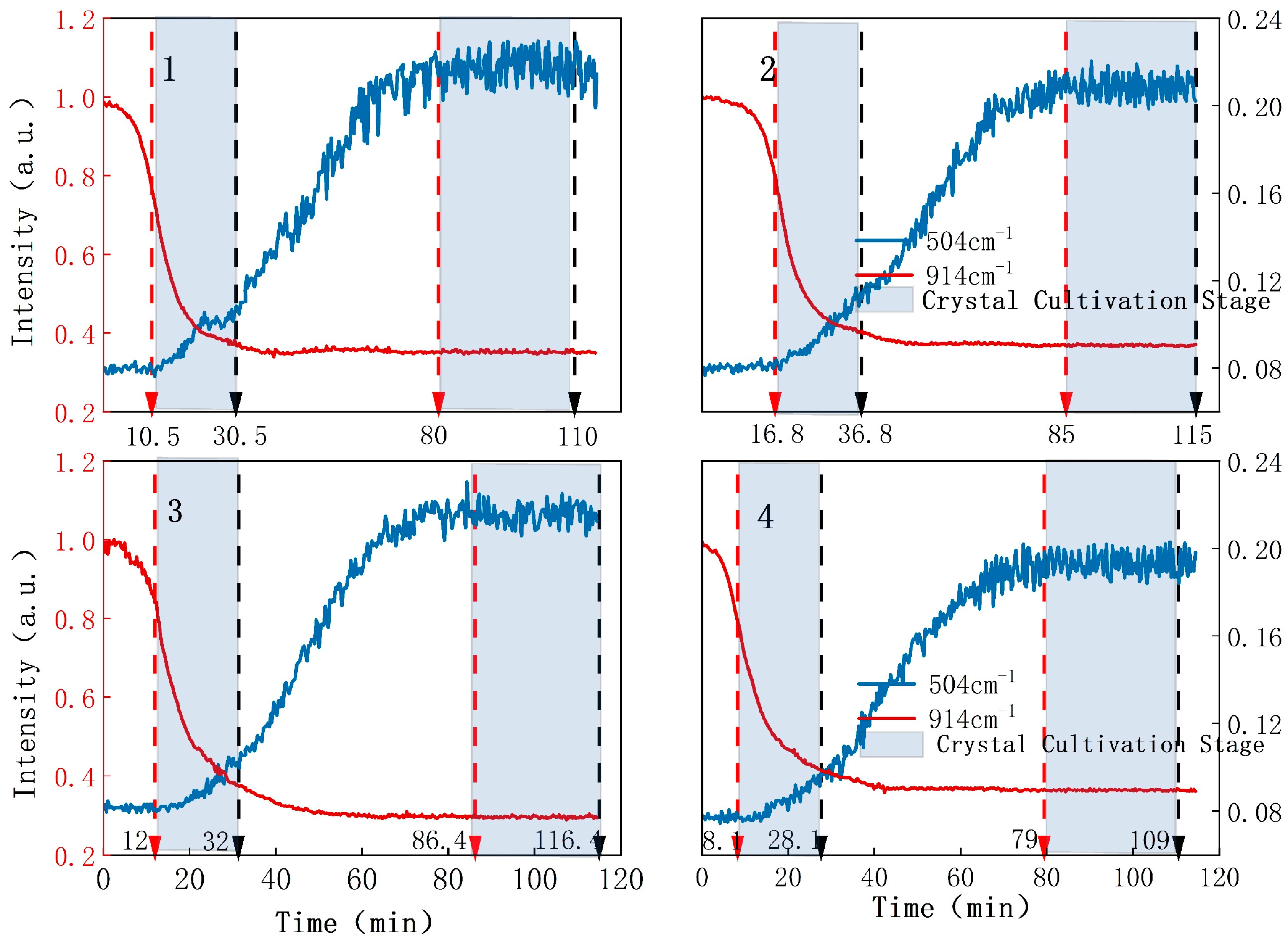
3.2. Monitoring of 7-ACT Crystallization Process
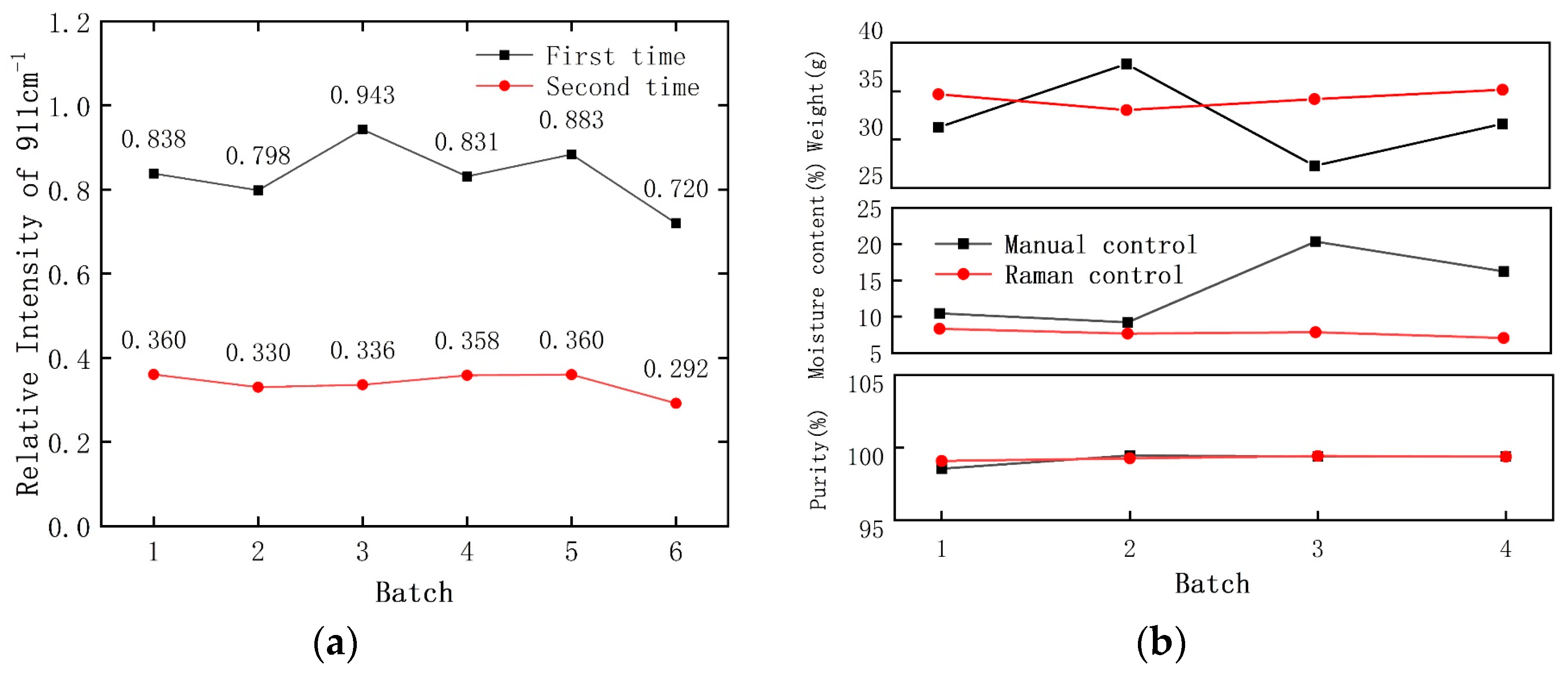
3.3. Control of the Crystallization Process Based on Raman Monitoring
4. Conclusions and Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ν | Stretching |
| νs | Symmetric stretching |
| νas | Asymmetric stretching |
| ω | Wagging |
| δ | Scissoring |
| τ | Twisting |
References
- Sangroniz, L.; Cavallo, D.; Müller, A.J. Self-Nucleation Effects on Polymer Crystallization. Macromolecules 2020, 53, 4581–4604. [Google Scholar] [CrossRef]
- Mcginty, J.; Yazdanpanah, N.; Price, C.; ter Horst, J.H.; Sefcik, J. Nucleation and Crystal Growth in Continuous Crystallization; Yazdanpanah, N., Nagy, Z.K., Eds.; The Royal Society of Chemistry: London, UK, 2020; ISBN 978-1-78801-214-0. [Google Scholar]
- Paus, R.; Ji, Y. Modeling and predicting the influence of variable factors on dissolution of crystalline pharmaceuticals. Chem. Eng. Sci. 2016, 145, 10–20. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Zhang, M.; Guo, M.; Xu, S.; Yin, Q. Determination of metastable zone and induction time of analgin for cooling crystallization. Chin. J. Chem. Eng. 2017, 25, 313–318. [Google Scholar] [CrossRef]
- Kelton, K.F. Analysis of crystallization kinetics. Mater. Sci. Eng. A 1997, 226–228, 142–150. [Google Scholar] [CrossRef]
- Orehek, J.; Teslić, D.; Likozar, B. Continuous Crystallization Processes in Pharmaceutical Manufacturing: A Review. Org. Process Res. Dev. 2021, 25, 16–42. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; York, P. Crystallization processes in pharmaceutical technology and drug delivery design. J. Cryst. Growth 2000, 211, 122–136. [Google Scholar] [CrossRef]
- Vengrenovich, R.D.; Gudyma, Y.V.; Yarema, S.V. Ostwald ripening under dislocation diffusion. Scr. Mater. 2002, 46, 363–367. [Google Scholar] [CrossRef]
- Ohyama, M.; Kudo, S.; Amari, S.; Takiyama, H. Production of crystalline particles with high homogeneity in reaction crystallization by using pH-solubility-profile. J. Ind. Eng. Chem. 2019, 75, 38–43. [Google Scholar] [CrossRef]
- Lahiq, A.A.; Alshahrani, S.M. State-of-the-art review on various mathematical approaches towards solving population balanced equations in pharmaceutical crystallization process. Arab. J. Chem. 2023, 16, 104929. [Google Scholar] [CrossRef]
- Vedantam, S.; Ranade, V.V. Crystallization: Key thermodynamic, kinetic and hydrodynamic aspects. Sadhana 2013, 38, 1287–1337. [Google Scholar] [CrossRef]
- Ito, F.; Suzuki, Y.; Fujimori, J.; Sagawa, T.; Hara, M.; Seki, T.; Yasukuni, R.; Chapelle, M.L.D.L. Direct Visualization of the Two-step Nucleation Model by Fluorescence Color Changes during Evaporative Crystallization from Solution. Sci. Rep. 2016, 6, 22918. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, H.; Jia, C.; Tang, Y.; Yang, H.; Chen, C.; Gou, K.; Zhou, Y.; Zhang, D.; Liu, S. Advances in Crystallization Regulation and Defect Suppression Strategies for All-inorganic CsPbX3 Perovskite Solar Sells. Prog. Mater. Sci. 2024, 141, 101223. [Google Scholar] [CrossRef]
- Abdollahi, J.; Dubljevic, S. Crystal radius and temperature regulation in Czochralski crystallization process. In Proceedings of the 2013 American Control Conference, Washington, DC, USA, 17–19 June 2013. [Google Scholar]
- Gao, Z.; Rohani, S.; Gong, J.; Wang, J. Recent Developments in the Crystallization Process: Toward the Pharmaceutical Industry. Engineering 2017, 3, 343–353. [Google Scholar] [CrossRef]
- Tavare, N.S. Crystallization Techniques and Phenomena. In Industrial Crystallization: Process Simulation Analysis and Design; Tavare, N.S., Ed.; Springer: Boston, MA, USA, 1995; pp. 465–499. ISBN 978-1-4899-0233-7. [Google Scholar]
- Liang, M.; Jin, F.; Liu, R.; Yu, Y.; Su, R.; Wang, L.; Qi, W.; He, Z. Shape evolution and thermal stability of lysozyme crystals: Effect of pH and temperature. Bioprocess. Biosyst. Eng. 2013, 36, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, P.; Suryanarayanan, R. The Effect of Crystallizing and Non-crystallizing Cosolutes on Succinate Buffer Crystallization and the Consequent pH Shift in Frozen Solutions. Pharm. Res. 2011, 28, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Eren, A.; Civati, F.; Ma, W.; Gamekkanda, J.C.; Myerson, A.S. Continuous crystallization and its potential use in drug substance Manufacture: A review. J. Cryst. Growth 2023, 601, 126958. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. The kinetics of crystallization of poly (ethylene terephthalate) measured by FTIR spectroscopy. Eur. Polym. J. 2013, 49, 1722–1730. [Google Scholar] [CrossRef]
- Lewiner, F.; Klein, J.P.; Puel, F.; Févotte, G. On-line ATR FTIR measurement of supersaturation during solution crystallization processes. Calibration and applications on three solute/solvent systems. Chem. Eng. Sci. 2001, 56, 2069–2084. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly(ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]
- Zhang, F.; Du, K.; Guo, L.; Huo, Y.; He, K.; Shan, B. Progress, problems, and potential of technology for measuring solution concentration in crystallization processes. Measurement 2022, 187, 110328. [Google Scholar] [CrossRef]
- Verstraeten, M.; Van Hauwermeiren, D.; Hellings, M.; Hermans, E.; Geens, J.; Vervaet, C.; Nopens, I.; De Beer, T. Model-based NIR spectroscopy implementation for in-line assay monitoring during a pharmaceutical suspension manufacturing process. Int. J. Pharm. 2018, 546, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, T.; Ma, Y.; Xue, F.; Gao, Z.; Hou, B.; Gong, J. Application of PAT-based feedback control approaches in pharmaceutical crystallization. Crystals 2021, 11, 221. [Google Scholar] [CrossRef]
- Acevedo, D.; Wu, W.; Yang, X.; Pavurala, N.; Mohammad, A.; O’Connor, T.F. Evaluation of focused beam reflectance measurement (FBRM) for monitoring and predicting the crystal size of carbamazepine in crystallization processes. Crystengcomm 2021, 23, 972–985. [Google Scholar] [CrossRef]
- Leite, L.C.; Bernardo, A. A New Alternative for Monitoring the Crystallization Process through Artificial Neural Networks and FBRM Signal. 2023. Available online: https://www.researchsquare.com/article/rs-3492230/v1 (accessed on 1 October 2023).
- Li, X.; Xu, D.; Yang, J.; Yan, Z.; Luo, T.; Li, X.; Zhang, Z.; Wang, X. Utilization of FBRM and PVM to analyze the effects of different additives on the crystallization of ammonium dihydrogen phosphate. J. Cryst. Growth 2021, 576, 126378. [Google Scholar] [CrossRef]
- Salami, H.; Mcdonald, M.A.; Bommarius, A.S.; Rousseau, R.W.; Grover, M.A. In Situ Imaging Combined with Deep Learning for Crystallization Process Monitoring: Application to Cephalexin Production. Org. Process Res. Dev. 2021, 25, 1670–1679. [Google Scholar] [CrossRef]
- De Castro, M.L.; Priego-Capote, F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, L.; Xu, S.; Du, S.; Dong, W.; Gong, J. Optimization of cooling strategy and seeding by FBRM analysis of batch crystallization. J. Cryst. Growth 2018, 486, 1–9. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, K.M.; Poornachary, S.K.; Dalvi, S.V. Elucidating the Polymorphic Behavior of Curcumin during Antisolvent Crystallization: Insights from Raman Spectroscopy and Molecular Modeling. Cryst. Growth Des. 2020, 20, 6008–6023. [Google Scholar] [CrossRef]
- Garbacz, P.; Paukszta, D.; Sikorski, A.; Wesolowski, M. Structural Characterization of Co-Crystals of Chlordiazepoxide with p-Aminobenzoic Acid and Lorazepam with Nicotinamide by DSC, X-ray Diffraction, FTIR and Raman Spectroscopy. Pharmaceutics 2020, 12, 648. [Google Scholar] [CrossRef]
- Pataki, H.; Markovits, I.; Vajna, B.; Nagy, Z.K.; Marosi, G. In-Line Monitoring of Carvedilol Crystallization Using Raman Spectroscopy. Cryst. Growth Des. 2012, 12, 5621–5628. [Google Scholar] [CrossRef]
- Celik Onar, H.; Ergin, M.F.; Yasa, H. Investigating the Role of Citric Acid as a Natural Acid on the Crystallization of Amoxicillin Trihydrate. Acs Omega 2023, 8, 36344–36354. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, L.; Chu, Z.; Tong, Z. Seeded Epitaxial Growth of Crystallizable Polymers Governed by Crystallization Temperatures. Macromolecules 2023, 56, 5984–5992. [Google Scholar] [CrossRef]
- Wang, Z.; Sohn, I. A review of in situ observations of crystallization and growth in high temperature oxide melts. Jom 2018, 70, 1210–1219. [Google Scholar] [CrossRef]
- Da Trindade, M.T.; Salgado, H.R.N. A critical review of analytical methods for determination of ceftriaxone sodium. Crit. Rev. Anal. Chem. 2018, 48, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Owens, H.M.; Dash, A.K. Ceftriaxone sodium: Comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 2003, 30, 21–57. [Google Scholar] [CrossRef]
- Gilles, F.; Nesrine, G. On Multiple Nucleation Bursts During Solution Crystallization in Pure and Impure Solvent. Cryst. Growth Des. 2012, 12, 3407–3417. [Google Scholar] [CrossRef]
- Wu, D.-X. Technological optimization of preparing 7-ACT, Intermediate of Ceftriaxone sodium. Strait Pharm. J. 2011, 23. [Google Scholar] [CrossRef]
- Uckert, K.; Bhartia, R.; Michel, J. A semi-autonomous method to detect cosmic rays in Raman hyperspectral data sets. Appl. Spectrosc. 2019, 73, 1019–1027. [Google Scholar] [CrossRef]
- Sinfield, J.V.; Monwuba, C.K. Assessment and correction of turbidity effects on Raman observations of chemicals in aqueous solutions. Appl. Spectrosc. 2014, 68, 1381–1392. [Google Scholar] [CrossRef]
- Laven, P. MiePlot (a Computer Program for Scattering of Light from a Sphere Using Mie Theory & the Debye Series). 2011. Available online: http://www.philiplaven.com/mieplot.htm (accessed on 10 September 2023).
- Wriedt, T. Mie Theory: A Review. In The Mie Theory: Basics and Applications; Hergert, W., Wriedt, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 53–71. ISBN 978-3-642-28738-1. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Chang, B.; Li, Z.; Chen, B.; Ji, K.; Chen, Y.; Ou, S.; Zhang, F.; Wei, J.; Ruan, Y. Automatic Control of Nucleation and Crystal Growth Using Online Raman Analyzer. Processes 2024, 12, 774. https://doi.org/10.3390/pr12040774
Li A, Chang B, Li Z, Chen B, Ji K, Chen Y, Ou S, Zhang F, Wei J, Ruan Y. Automatic Control of Nucleation and Crystal Growth Using Online Raman Analyzer. Processes. 2024; 12(4):774. https://doi.org/10.3390/pr12040774
Chicago/Turabian StyleLi, Aofei, Boxue Chang, Zhen Li, Biao Chen, Kaidi Ji, Yangshun Chen, Shiqiang Ou, Fengming Zhang, Jiaoning Wei, and Yinlan Ruan. 2024. "Automatic Control of Nucleation and Crystal Growth Using Online Raman Analyzer" Processes 12, no. 4: 774. https://doi.org/10.3390/pr12040774




