Chitosan-Based Grafted Cationic Magnetic Material to Remove Emulsified Oil from Wastewater: Performance and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Emulsified, Oily Wastewater
2.3. Synthesis of FS@CTS-P(AM-DMC)
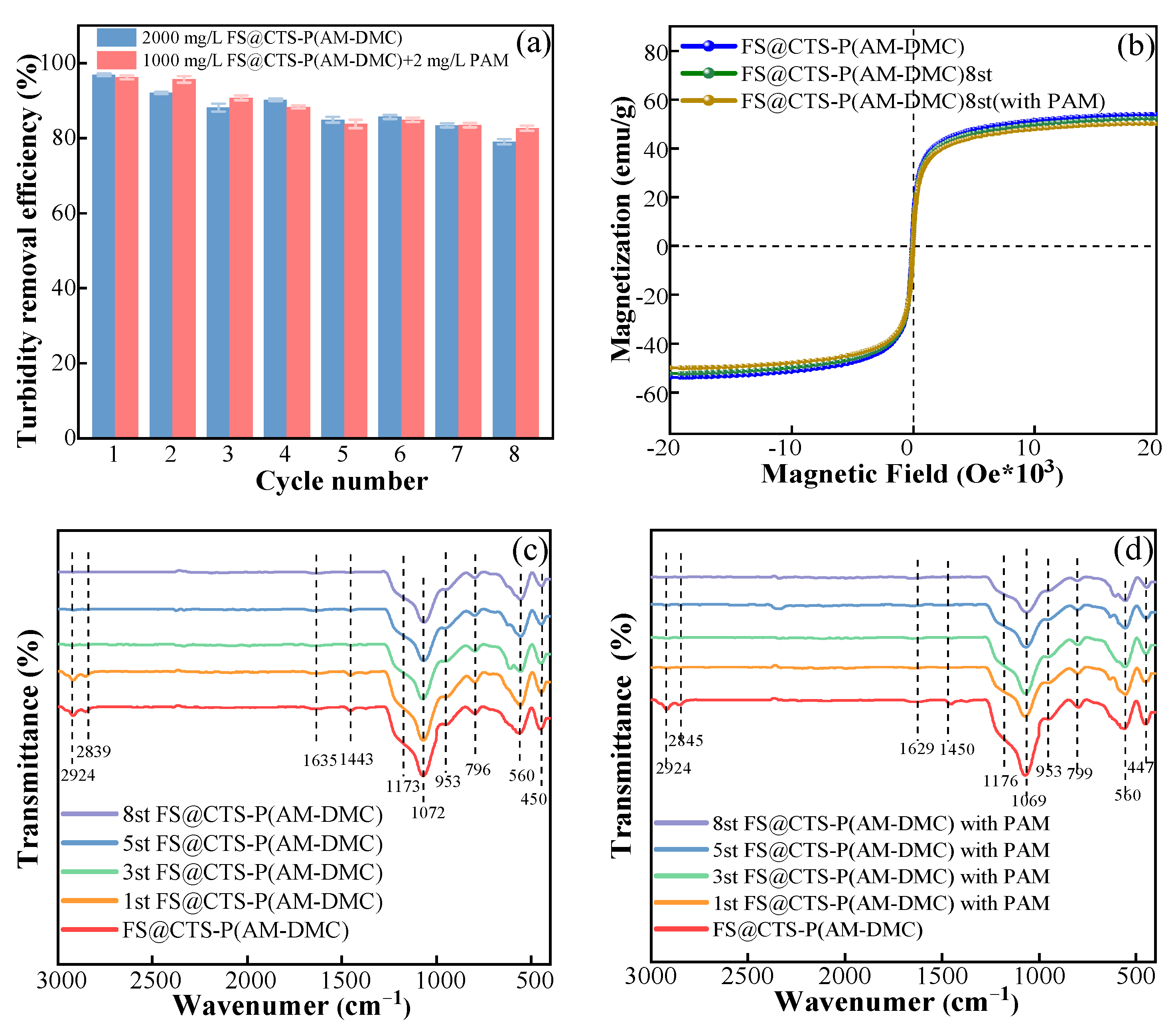
2.4. Characterization and Analysis
2.5. Flocculation Operation
2.6. Distribution of Floc Size
2.7. Recycling Experiment
2.8. Microscopic Image Analysis
2.9. PIV Measurement of Magnetophoretic Flow Field
2.10. Interaction Energy Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Flocculation Performance
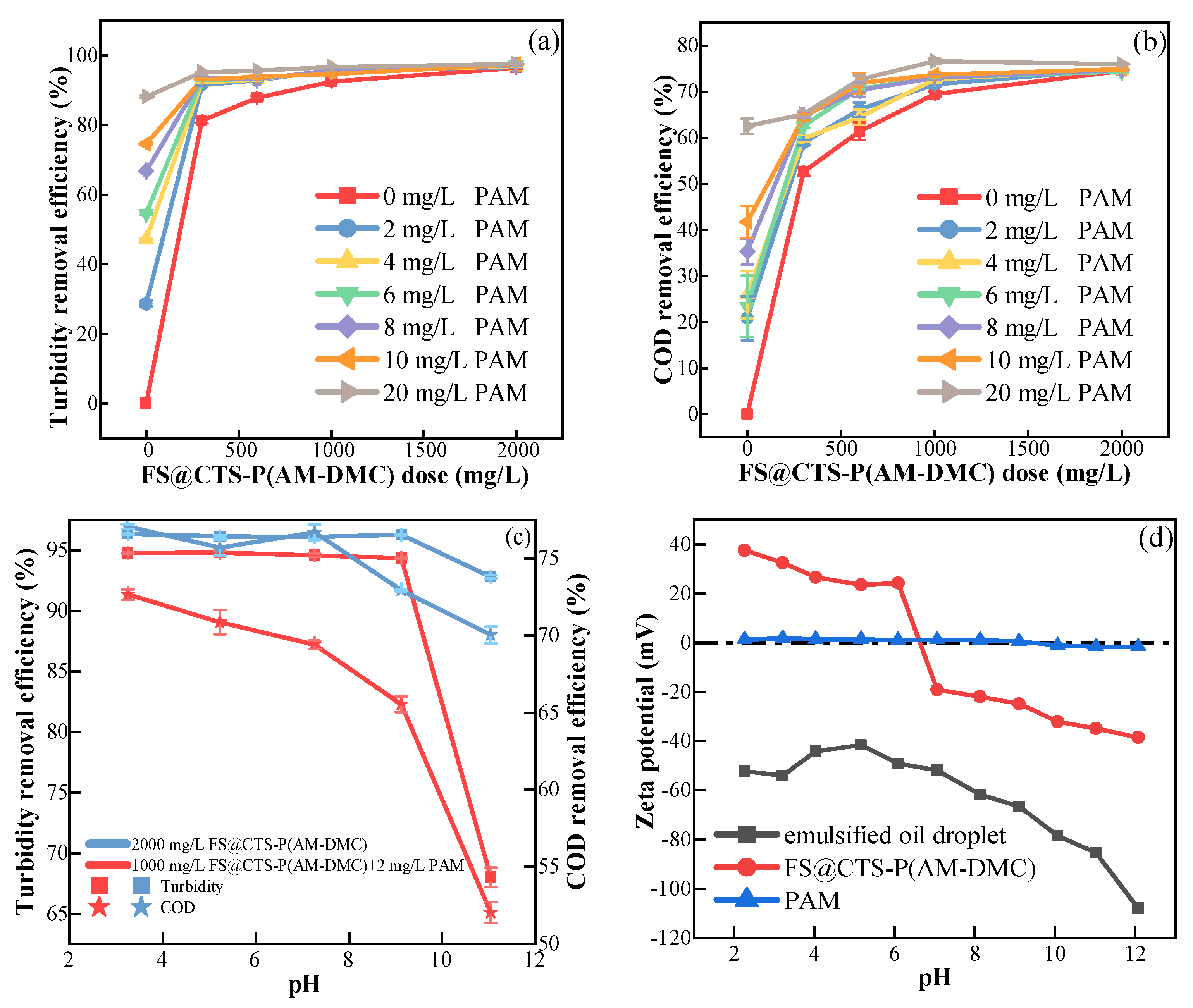
3.1.1. Effects of Flocculant Dose
3.1.2. Effects of pH Values
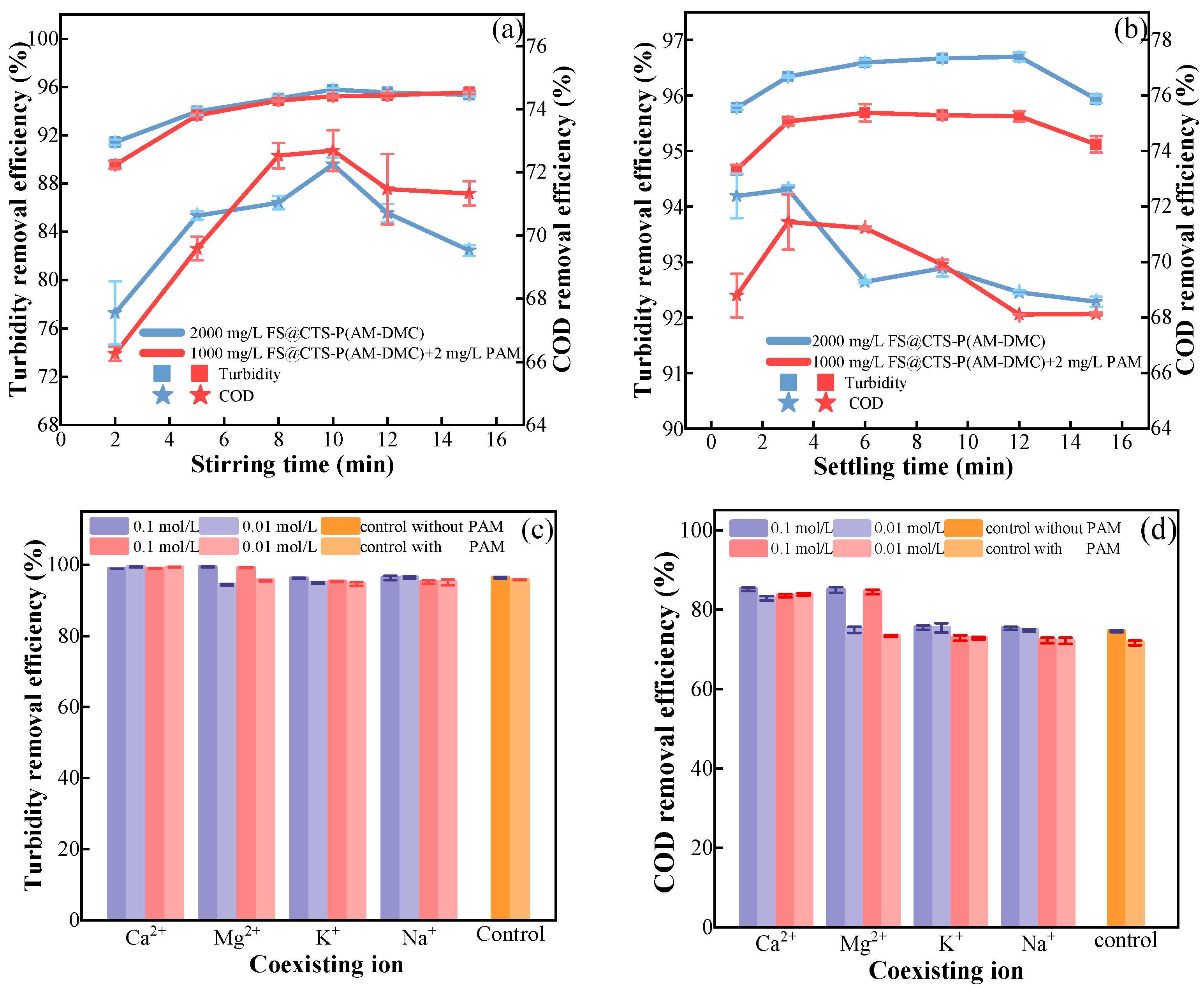
3.1.3. Effects of Stirring and Settling Time
3.1.4. Effects of Coexisting Ions
3.1.5. Effects of Magnetic Field Strength
3.2. Recovery and Reuse
3.3. Magnetic Flocculation Mechanism
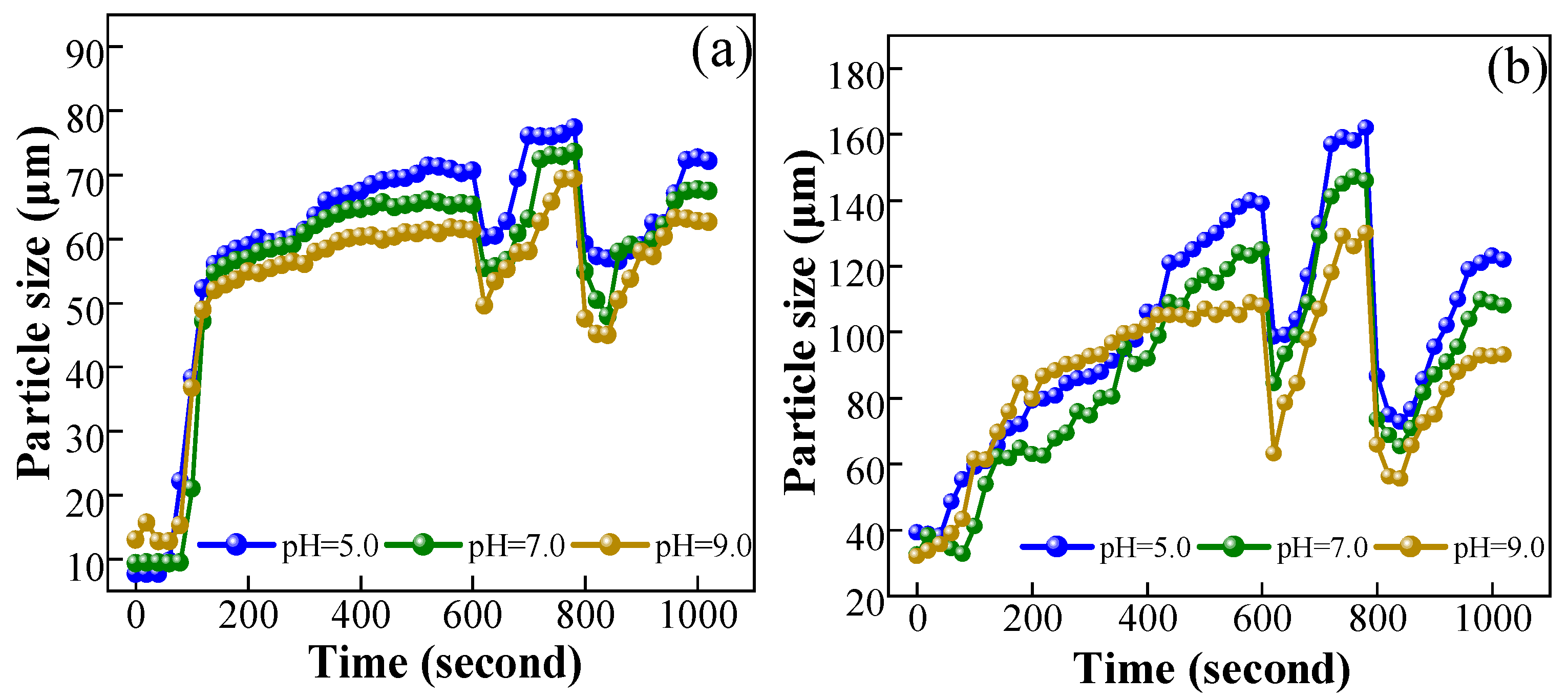
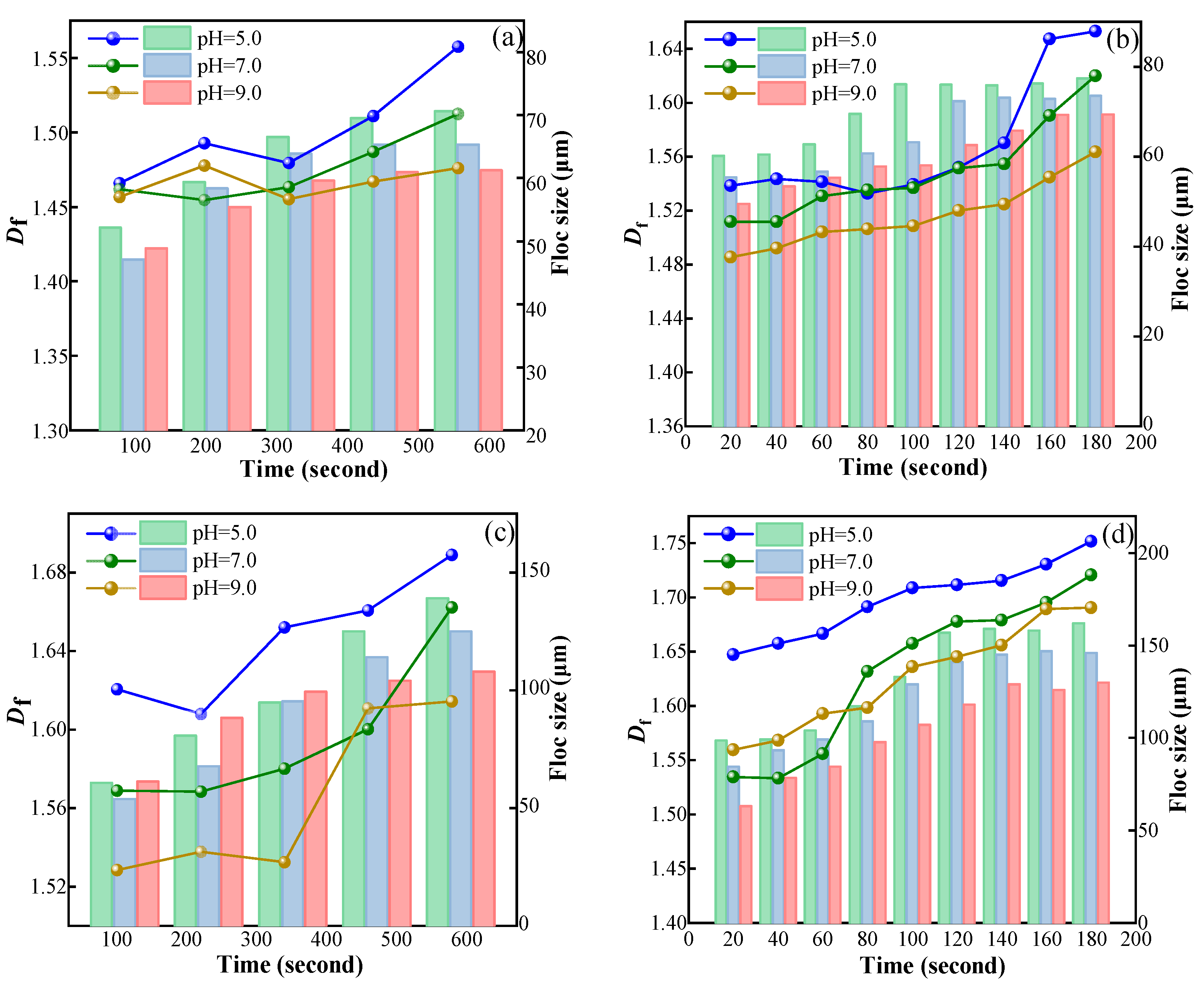
3.3.1. Floc Size Distribution
3.3.2. Fractal Dimension of Magnetic Flocs
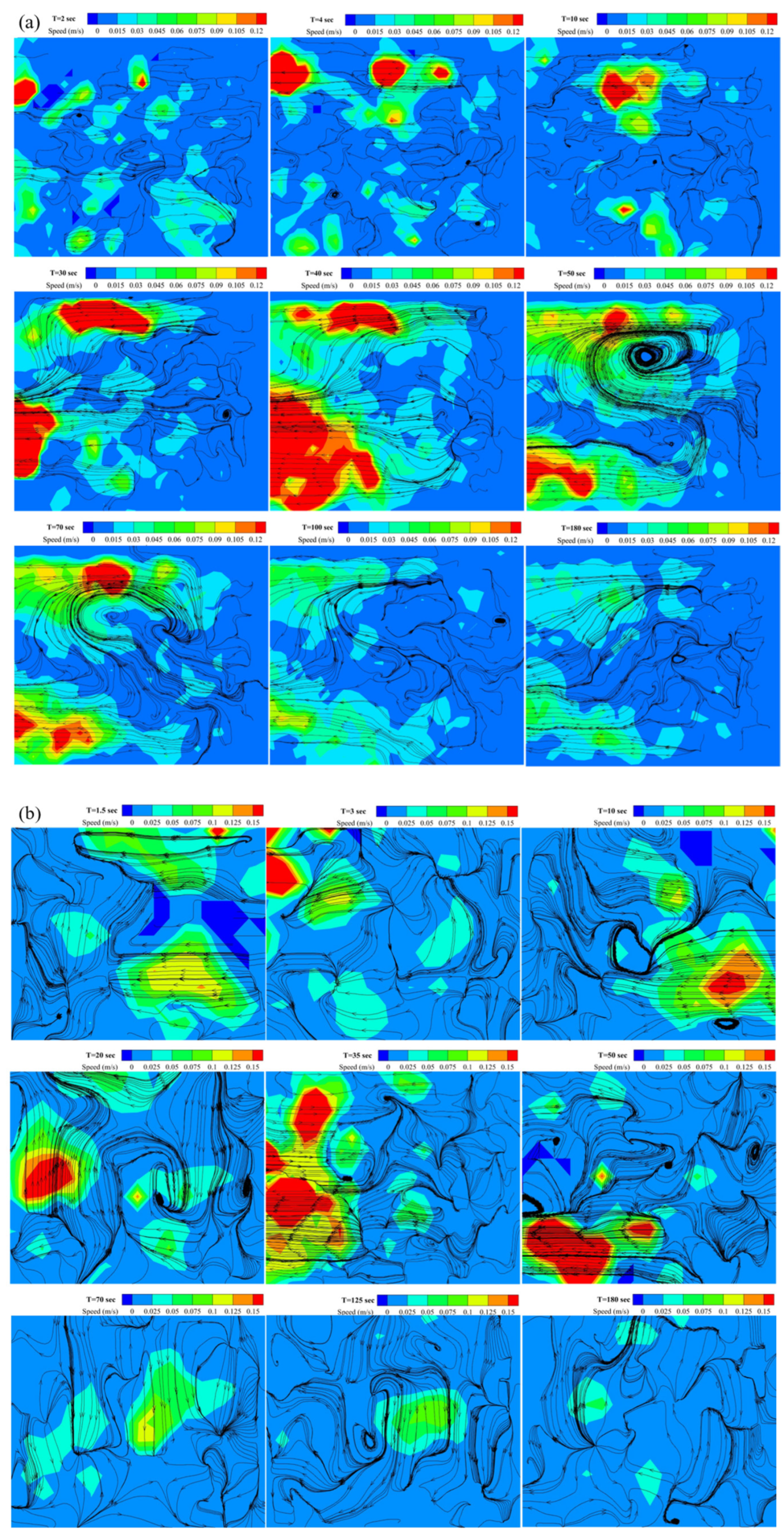
3.3.3. PIV Analysis of Velocity Field
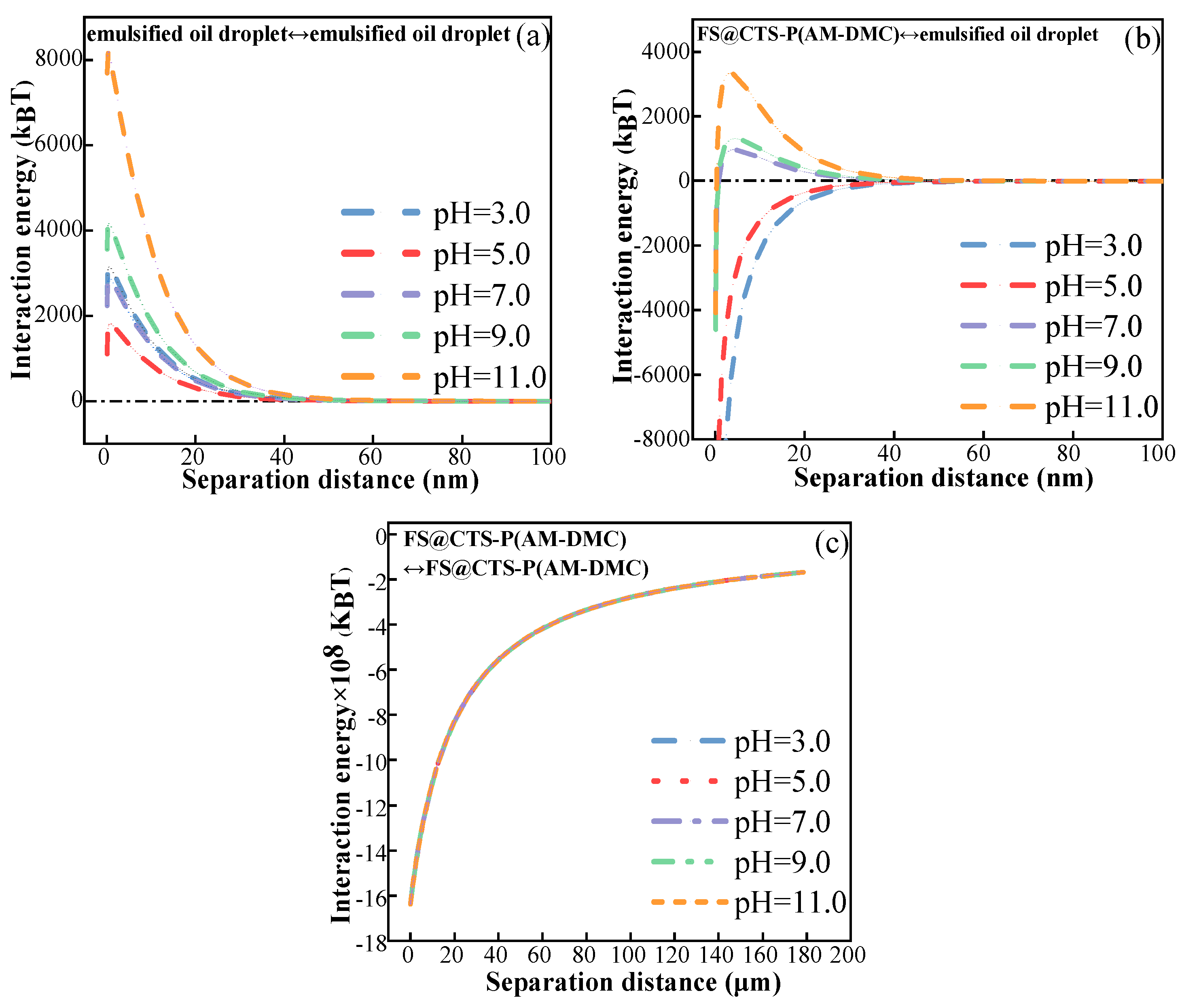
3.3.4. Interaction Energy Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, D.; Zheng, D.; Li, Z.; Wang, C.; Li, J.; Zhang, M. Research Advances in Wood Composites in Applications of Industrial Wastewater Purification and Solar-Driven Seawater Desalination. Polymers 2023, 15, 4712. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Wibowo, A.; Karim, Z.; Posoknistakul, P.; Matsagar, B.M.; Wu, K.C.-W.; Sakdaronnarong, C. Wastewater Treatment Using Membrane Bioreactor Technologies: Removal of Phenolic Contaminants from Oil and Coal Refineries and Pharmaceutical Industries. Polymers 2024, 16, 443. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, W.; Zhu, Z.; Xu, B. In Situ Fenton Triggered PDA Coating Copper Mesh with Underwater Superoleophobic Property for Oily Wastewater Pretreatment. Processes 2021, 9, 1665. [Google Scholar] [CrossRef]
- Lü, T.; Qi, D.; Zhang, D.; Fu, K.; Li, Y.; Zhao, H. Fabrication of recyclable multi-responsive magnetic nanoparticles for emulsified oil-water separation. J. Clean. Prod. 2020, 255, 120293. [Google Scholar] [CrossRef]
- He, J.; Zhao, H.; Li, X.; Su, D.; Zhang, F.; Ji, H.; Liu, R. Superelastic and superhydrophobic bacterial cellulose/silica aerogels with hierarchical cellular structure for oil absorption and recovery. J. Hazard. Mater. 2018, 346, 199–207. [Google Scholar] [CrossRef]
- Medeiros, A.D.L.M.d.; Silva Junior, C.J.G.d.; Amorim, J.D.P.d.; Durval, I.J.B.; Costa, A.F.d.S.; Sarubbo, L.A. Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes 2022, 10, 743. [Google Scholar] [CrossRef]
- Azevedo, A.; Etchepare, R.; Calgaroto, S.; Rubio, J. Aqueous dispersions of nanobubbles: Generation, properties and features. Miner. Eng. 2016, 94, 29–37. [Google Scholar] [CrossRef]
- Bastos, P.; Santos, M.; Carvalho, P.; Velizarov, S.; Crespo, J. Pilot scale reverse osmosis refinery wastewater treatment—A techno-economical and sustainability assessment. Environ. Sci. Water Res. Technol. 2021, 7, 549–561. [Google Scholar] [CrossRef]
- Abdurahman, N.H.; Rosli, Y.M.; Azhari, N.H.; Hayder, G.; Norasyikin, I. A Hybrid Ultrasonic Membrane Anaerobic System (UMAS) Development for Palm Oil Mill Effluent (POME) Treatment. Processes 2023, 11, 2477. [Google Scholar] [CrossRef]
- Wu, M.; Zhai, M.; Li, X. Adsorptive removal of oil drops from ASP flooding-produced water by polyether polysiloxane-grafted ZIF-8. Powder Technol. 2021, 378, 76–84. [Google Scholar] [CrossRef]
- Ma, J.; Shi, J.; Ding, H.; Zhu, G.; Fu, K.; Fu, X. Synthesis of cationic polyacrylamide by low-pressure UV initiation for turbidity water flocculation. Chem. Eng. J. 2017, 312, 20–29. [Google Scholar] [CrossRef]
- Jia, H.; Liu, W.; Wang, J.; Ngo, H.H.; Guo, W.; Zhang, H. Optimization of sensing performance in an integrated dual sensors system combining microbial fuel cells and upflow anaerobic sludge bed reactor. Chemosphere 2018, 210, 931–940. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Zhang, L.; Zhang, S.; Sun, Y.; Shah, K. Treatment of Oil-Contaminated Water by Modified Polysilicate Aluminum Ferric Sulfate. Processes 2018, 6, 95. [Google Scholar] [CrossRef]
- Parrino, F.; Corsino, S.F.; Bellardita, M.; Loddo, V.; Palmisano, L.; Torregrossa, M.; Viviani, G. Sequential biological and photocatalysis based treatments for shipboard slop purification: A pilot plant investigation. Process Saf. Environ. Prot. 2019, 125, 288–296. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Zheng, H.; Wang, Y.; Sun, Y.; Zhao, C.; Zhang, S. Rapid and efficient removal of heavy metal and cationic dye by carboxylate-rich magnetic chitosan flocculants: Role of ionic groups. Carbohydr. Polym. 2018, 181, 327–336. [Google Scholar] [CrossRef]
- Du, C.; Hu, Y.; Han, H.; Sun, W.; Hou, P.; Liu, R.; Wang, L.; Yang, Y.; Liu, R.; Sun, L.; et al. Magnetic separation of phosphate contaminants from starch wastewater using magnetic seeding. Sci. Total Env. 2019, 695, 133723. [Google Scholar] [CrossRef]
- Zhang, L.; Verstraete, W.; de Lourdes Mendoza, M.; Lu, Z.; Liu, Y.; Huang, G.; Cai, L. Decrease of dissolved sulfide in sewage by powdered natural magnetite and hematite. Sci. Total Env. 2016, 573, 1070–1078. [Google Scholar] [CrossRef]
- Lü, T.; Chen, Y.; Qi, D.; Cao, Z.; Zhang, D.; Zhao, H. Treatment of emulsified oil wastewaters by using chitosan grafted magnetic nanoparticles. J. Alloys Compd. 2017, 696, 1205–1212. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, T.; Zhang, F. Evaluation of oilfield-produced water treated with a prepared magnetic inorganic polymer: Poly(silicate aluminum)/magnetite. J. Appl. Polym. Sci. 2017, 135, 4. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Jia, H.; Wen, H.; Li, J.; Liu, W.; Li, J. The investigation on Fe3O4 magnetic flocculation for high efficiency treatment of oily micro-polluted water. J. Env. Manag. 2019, 244, 399–407. [Google Scholar] [CrossRef]
- Al-Rubaie, M.; Dixon, M.; Abbas, T. Use of flocculated magnetic separation technology to treat Iraqi oilfield co-produced water for injection purpose. Desalination Water Treat. 2013, 53, 2086–2091. [Google Scholar] [CrossRef]
- Ma, J.; Fu, X.; Jiang, L.; Zhu, G.; Shi, J. Magnetic flocculants synthesized by Fe3O4 coated with cationic polyacrylamide for high turbid water flocculation. Env. Sci. Pollut. Res. Int. 2018, 25, 25955–25966. [Google Scholar] [CrossRef] [PubMed]
- Bakhteeva, I.A.; Medvedeva, I.V.; Filinkova, M.S.; Byzov, I.V.; Zhakov, S.V.; Uimin, M.A.; Yermakov, A.E. Magnetic sedimentation of nonmagnetic TiO2 nanoparticles in water by heteroaggregation with Fe-based nanoparticles. Sep. Purif. Technol. 2019, 218, 156–163. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Qin, L.; Li, H.; Liang, W. Magnetic coagulation and flocculation of a kaolin suspension using Fe3O4 coated with SiO2. J. Environ. Chem. Eng. 2021, 9, 105980. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Qin, L.; Liang, W. Self-assembly of Fe3O4 with natural tannin as composites for microalgal harvesting. Fuel 2022, 321, 124038. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Ma, J.; Xingang, L.; Al-Shiaani, N.; He, L. Fast demulsification of oil-water emulsions at room temperature by functionalized magnetic nanoparticles. Sep. Purif. Technol. 2021, 274, 118967. [Google Scholar] [CrossRef]
- Lü, T.; Qi, D.; Zhang, D.; Lin, S.; Mao, Y.; Zhao, H. Facile synthesis of N-(aminoethyl)-aminopropyl functionalized core-shell magnetic nanoparticles for emulsified oil-water separation. J. Alloys Compd. 2018, 769, 858–865. [Google Scholar] [CrossRef]
- Shao, S.; Li, Y.; Lü, T.; Qi, D.; Zhang, D.; Zhao, H. Removal of Emulsified Oil from Aqueous Environment by Using Polyvinylpyrrolidone-Coated Magnetic Nanoparticles. Water 2019, 11, 1993. [Google Scholar] [CrossRef]
- Ma, J.; Wu, G.; Zhang, R.; Xia, W.; Nie, Y.; Kong, Y.; Jia, B.; Li, S. Emulsified oil removal from steel rolling oily wastewater by using magnetic chitosan-based flocculants: Flocculation performance, mechanism, and the effect of hydrophobic monomer ratio. Sep. Purif. Technol. 2023, 304, 122329. [Google Scholar] [CrossRef]
- Ma, J.; Fu, X.; Xia, W.; Zhang, R.; Fu, K.; Wu, G.; Jia, B.; Li, S.; Li, J. Removal of emulsified oil from water by using recyclable chitosan based covalently bonded composite magnetic flocculant: Performance and mechanism. J. Hazard. Mater. 2021, 419, 126529. [Google Scholar] [CrossRef]
- Wang, R.; Cai, Y.; Su, Z.; Ma, X.; Wu, W. High positively charged Fe3O4 nanocomposites for efficient and recyclable demulsification of hexadecane-water micro-emulsion. Chemosphere 2022, 291, 133050. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Fang, Y.; San, K.N.E.; Fan, Y. Smart and recyclable admicelle-coated Fe3O4 nanoparticles for treating oily wastewater. J. Environ. Chem. Eng. 2022, 10, 107445. [Google Scholar] [CrossRef]
- You, Z.; Xu, H.; Sun, Y.; Zhang, S.; Zhang, L. Effective treatment of emulsified oil wastewater by the coagulation-flotation process. RSC Adv. 2018, 8, 40639–40646. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Du, S.; Liang, W. Synthesis of chitosan-based grafting magnetic flocculants for flocculation of kaolin suspensions. J. Environ. Sci. 2024, 139, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Du, S.; Cheng, P.; Liang, W. Magnetic coagulation and flocculation of kaolin suspension using Fe3O4 with plant polyphenol self-assembled flocculants. Int. J. Biol. Macromol. 2023, 253, 126578. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhu, Q.; Bian, J. Preparation of a recyclable demulsifier for the treatment of emulsified oil wastewater by chitosan modification and sodium oleate grafting Fe3O4. J. Environ. Chem. Eng. 2021, 9, 105663. [Google Scholar] [CrossRef]
- Hamedi, H.; Rezaei, N.; Zendehboudi, S. Investigation of Emulsified Oil Adsorption onto Functionalized Magnetic Nanoparticles—Kinetic and Isotherm Models. Energies 2023, 16, 8073. [Google Scholar] [CrossRef]
- Lu, J.-w.; Yuan, Z.-t.; Guo, X.-f.; Tong, Z.-y.; Li, L.-x. Magnetic separation of pentlandite from serpentine by selective magnetic coating. Int. J. Miner. Metall. Mater. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Tang, H.; Wang, L.; Sun, W.; Hu, Y.; Han, H.; Zhai, J. Electric arc furnace dust as magnetic carrier particles for removal of micro-fine particles from suspensions. Sep. Purif. Technol. 2017, 176, 220–230. [Google Scholar] [CrossRef]
- Paula, F.L.O.; Castro, L.L.; Cassiano, T.S.A.; dos Santos, S.G.; Gomide, G.; Depeyrot, J.; Campos, A.F.C. pH-dependent phase transitions in ferrofluids: A Monte Carlo simulation study using an extended DLVO model. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130578. [Google Scholar] [CrossRef]
- Shan, J.; Zhou, X. Starting Conditions of Particle Migration in Tight Sandstone Reservoir Development. Processes 2020, 8, 1491. [Google Scholar] [CrossRef]
- Zhang, C.; Dostál, J.; Heidinger, S.; Günther, S.; Odenbach, S. Experimental Investigation of a Pulsation Reactor via Optical Methods. Processes 2024, 12, 385. [Google Scholar] [CrossRef]
- Fleischhauer, E.; Azimi, F.; Tkacik, P.; Keanini, R.; Mullany, B. Application of particle image velocimetry (PIV) to vibrational finishing. J. Mater. Process. Technol. 2016, 229, 322–328. [Google Scholar] [CrossRef]
- Patalano, A.; García, C.M.; Rodríguez, A. Rectification of Image Velocity Results (RIVeR): A simple and user-friendly toolbox for large scale water surface Particle Image Velocimetry (PIV) and Particle Tracking Velocimetry (PTV). Comput. Geosci. 2017, 109, 323–330. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, Y.; Wang, H.; Gao, M. The Influence of a Manifold Structure on the Measurement Results of a PIV Flowmeter. Processes 2024, 12, 144. [Google Scholar] [CrossRef]
- Sarno, L.; Tai, Y.-C.; Carravetta, A.; Martino, R.; Nicolina Papa, M.; Kuo, C.-Y. Challenges and improvements in applying a particle image velocimetry (PIV) approach to granular flows. J. Phys. Conf. Ser. 2019, 1249, 012011. [Google Scholar] [CrossRef]
- Liang, J.; Du, N.; Song, S.; Hou, W. Magnetic demulsification of diluted crude oil-in-water nanoemulsions using oleic acid-coated magnetite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 197–202. [Google Scholar] [CrossRef]
- Harford, A.J.; Hogan, A.C.; Jones, D.R.; van Dam, R.A. Ecotoxicological assessment of a polyelectrolyte flocculant. Water Res. 2011, 45, 6393–6402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, C.-l.; Zhou, Y.-c.; Shen, Y.; Wang, Y.-w.; Ge, S.-f. Study on the Effect of Coagulant Ratio on Dehydration Performance of Acrylic Sludge. IOP Conf. Ser. Earth Environ. Sci. 2018, 146, 012076. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Mamba, B.B.; Verliefde, A.R.; Mhlanga, S.D. Adsorption of phenolic compounds by polyacrylonitrile nanofibre membranes: A pretreatment for the removal of hydrophobic bearing compounds from water. J. Environ. Chem. Eng. 2019, 7, 103254. [Google Scholar] [CrossRef]
- Wu, L.J.; Gao, Y.; Xu, X.; Deng, J.; Liu, H. Excellent coagulation performance of polysilicate aluminum ferric for treating oily wastewater from Daqing gasfield: Responses to polymer properties and coagulation mechanism. J. Environ. Manag. 2024, 356, 120642. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, Y.V.; Litmanovich, A.D.; Platé, N.A. On the Kinetics of Polyacrylamide Alkaline Hydrolysis. Macromolecules 1998, 31, 4642–4644. [Google Scholar] [CrossRef]
- Nie, C.; Han, G.; Ni, J.; Guan, S.; Du, H.; Zhang, Y.; Wang, H. Stability Dynamic Characteristic of Oil-in-Water Emulsion from Alkali-Surfactant-Polymer Flooding. ACS Omega 2021, 6, 19058–19066. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Y.; Ming, X.; Chen, B.; Li, Z. Enhancing anti-washout behavior of cement paste by polyacrylamide gelation: From floc properties to mechanism. Cem. Concr. Compos. 2023, 136, 104887. [Google Scholar] [CrossRef]
- Tang, C.C.; Zhang, X.Y.; Wang, R.; Wang, T.Y.; He, Z.W.; Wang, X.C. Calcium ions-effect on performance, growth and extracellular nature of microalgal-bacterial symbiosis system treating wastewater. Env. Res. 2022, 207, 112228. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Guo, L.; Chen, Y.; She, Z.; Gao, M.; Zhao, Y.; Shao, M. Effect of Magnet Powder (Fe3O4) on Aerobic Granular Sludge (AGS) Formation and Microbial Community Structure Characteristics. ACS Sustain. Chem. Eng. 2018, 6, 9707–9715. [Google Scholar] [CrossRef]
- Hezave, A.Z.; Dorostkar, S.; Ayatollahi, S.; Nabipour, M.; Hemmateenejad, B. Investigating the effect of ionic liquid (1-dodecyl-3-methylimidazolium chloride ([C12mim] [Cl])) on the water/oil interfacial tension as a novel surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 63–71. [Google Scholar] [CrossRef]
- Pu, L.; Zeng, Y.J.; Xu, P.; Li, F.Z.; Zong, M.H.; Yang, J.G.; Lou, W.Y. Using a novel polysaccharide BM2 produced by Bacillus megaterium strain PL8 as an efficient bioflocculant for wastewater treatment. Int. J. Biol. Macromol. 2020, 162, 374–384. [Google Scholar] [CrossRef]
- Liu, B.; Lu, H.; Wu, S.; Wang, Z.; Feng, L.; Zheng, H. Octopus tentacle-like molecular chains in magnetic flocculant enhances the removal of Cu(II) and malachite green in water. Sep. Purif. Technol. 2022, 282, 120139. [Google Scholar] [CrossRef]
- Nwodo, U.; Agunbiade, M.; Green, E.; Mabinya, L.; Okoh, A. A Freshwater Streptomyces, Isolated from Tyume River, Produces a Predominantly Extracellular Glycoprotein Bioflocculant. Int. J. Mol. Sci. 2012, 13, 8679–8695. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Kuttuva Rajarao, G. Effective water content reduction in sewage wastewater sludge using magnetic nanoparticles. Bioresour. Technol. 2014, 153, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Kim, S.B. DLVO and XDLVO calculations for bacteriophage MS2 adhesion to iron oxide particles. J. Contam. Hydrol. 2015, 181, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, W.; Jia, H.; Zhang, H. Effects of recycling flocculation membrane filtration on drinking water treatment. Aqua 2013, 62, 433. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, C.; Zheng, H.; Wang, Y.; Zhao, C.; Zhao, C.; Zhang, S. Polymer-grafted magnetic microspheres for enhanced removal of methylene blue from aqueous solutions. RSC Adv. 2017, 7, 47029–47037. [Google Scholar] [CrossRef]
- Hadizade, G.; Binaeian, E.; Emami, M.R.S. Preparation and characterization of hexagonal mesoporous silica/polyacrylamide nanocomposite capsule (PAM-HMS) for dye removal from aqueous solutioxns. J. Mol. Liq. 2017, 238, 499–507. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Li, A.; Yang, H. Evaluation of structural effects on the flocculation performance of a co-graft starch-based flocculant. Water Res. 2017, 118, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Sun, W.; Sun, Y.; Chen, L.; Xu, Y.; Tang, M. Synthesis, Characterization, and Sludge Dewaterability Evaluation of the Chitosan-Based Flocculant CCPAD. Polymers 2019, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, T.; Yuan, b.; Li, R.; Yang, H.; Li, A. Flocculation of Both Kaolin and Hematite Suspensions Using the Starch-Based Flocculants and Their Floc Properties. Ind. Eng. Chem. Res. 2015, 54, 59–67. [Google Scholar] [CrossRef]
- Machado, C.A.; Esteves, A.F.; Pires, J.C.M. Optimization of Microalgal Harvesting with Inorganic and Organic Flocculants Using Factorial Design of Experiments. Processes 2022, 10, 1124. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, G.; Huang, J.; Xu, H.; Feng, J.; Song, P.; Li, M.; Wang, L. Assessing the effect of flow fields on flocculation of kaolin suspension using microbial flocculant GA1. RSC Adv. 2014, 4, 40464–40473. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q. Hydrodynamics of froth flotation and its effects on fine and ultrafine mineral particle flotation: A literature review. Miner. Eng. 2021, 173, 107220. [Google Scholar] [CrossRef]
- Yan, M.; Guo, K.; Gao, Y.; Yue, Q.; Gao, B. Insights into the control mechanism of different coagulation pretreatment on ultrafiltration membrane fouling for oily wastewater treatment. Sep. Purif. Technol. 2023, 327, 124907. [Google Scholar] [CrossRef]
- Geng, Y.; Nie, Y.; Du, H.; Ma, T.; Li, L.; Zhao, C.; Xue, N.; Shen, Q. Coagulation performance and floc characteristics of Fe–Ti–V ternary inorganic coagulant for organic wastewater treatment. J. Water Process Eng. 2023, 56, 104344. [Google Scholar] [CrossRef]
- Xu, W.; Gao, B. Effect of shear conditions on floc properties and membrane fouling in coagulation/ultrafiltration hybrid process—The significance of Alb species. J. Membr. Sci. 2012, 415–416, 153–160. [Google Scholar] [CrossRef]
- Lipus, L.C.; Krope, J.; Crepinsek, L. Dispersion Destabilization in Magnetic Water Treatment. J. Colloid. Interface Sci. 2001, 236, 60–66. [Google Scholar] [CrossRef]
- de Oliveira Anício, S.; dos Santos Lopes, V.; de Oliveira, A.L. PSD and Fractal Dimension for flocculation with different parameters and ferric chloride, aluminium polychloride and aluminium sulfate as coagulants. J. Water Process Eng. 2021, 43, 102180. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y. Influence of single- and dual-flocculant conditioning on the geometric morphology and internal structure of activated sludge. Powder Technol. 2015, 270, 1–9. [Google Scholar] [CrossRef]
- Ban, Y.; Liu, L.; Du, J.; Ma, C. Investigation of the treatment efficiency and mechanism of microporous flocculation magnetic fluidized bed (MFMFB) reactor for Pb(II)-containing wastewater. Sep. Purif. Technol. 2024, 334, 125963. [Google Scholar] [CrossRef]
- Salim, S.; Shi, Z.; Vermue, M.H.; Wijffels, R.H. Effect of growth phase on harvesting characteristics, autoflocculation and lipid content of Ettlia texensis for microalgal biodiesel production. Bioresour. Technol. 2013, 138, 214–221. [Google Scholar] [CrossRef]
- Nabweteme, R.; Yoo, M.; Kwon, H.-S.; Kim, Y.J.; Hwang, G.; Lee, C.-H.; Ahn, I.-S. Application of the extended DLVO approach to mechanistically study the algal flocculation. J. Ind. Eng. Chem. 2015, 30, 289–294. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Xie, S.; Duan, W.; Chen, W. Roles and Influences of Kerosene on Chalcopyrite Flotation in MgCl2 Solution: EDLVO and DFT Approaches. Minerals 2022, 12, 48. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Chew, J.W. Assessment of oil fouling by oil-membrane interaction energy analysis. J. Membr. Sci. 2018, 560, 21–29. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Syngouna, V.I. Attachment of bacteriophages MS2 and PhiX174 onto kaolinite and montmorillonite: Extended-DLVO interactions. Colloids Surf. B Biointerfaces 2012, 92, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, H.; Sun, L.; Shi, J.; Cao, X.; Yuan, S. Molecular Dynamics Study on Aggregating Behavior of Asphaltene and Resin in Emulsified Heavy Oil Droplets with Sodium Dodecyl Sulfate. Energy Fuels 2018, 32, 12383–12393. [Google Scholar] [CrossRef]
- Xu, J.; Yu, H.Q.; Li, X.Y. Probing the contribution of extracellular polymeric substance fractions to activated-sludge bioflocculation using particle image velocimetry in combination with extended DLVO analysis. Chem. Eng. J. 2016, 303, 627–635. [Google Scholar] [CrossRef]
- Mohamed Noor, M.H.; Ngadi, N.; Mohammed Inuwa, I.; Opotu, L.A.; Mohd Nawawi, M.G. Synthesis and application of polyacrylamide grafted magnetic cellulose flocculant for palm oil wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 104014. [Google Scholar] [CrossRef]
- Hu, L.; Gao, S.; Ding, X.; Wang, D.; Jiang, J.; Jin, J.; Jiang, L. Photothermo-Responsive Single-Walled Carbon Nanotube-Based Ultrathin Membranes for On/Off Switchable Separation of Oil-in-Water Nanoemulsions. ACS Nano 2015, 9, 4835–4842. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Xu, Z.; Zhang, Y.; Fang, S.; Song, X.; Xiong, Y. Core-shell composite nanoparticles with magnetic and temperature dual stimuli-responsive properties for removing emulsified oil. Adv. Powder Technol. 2017, 28, 1291–1297. [Google Scholar] [CrossRef]
- Mirshahghassemi, S.; Lead, J.R. Oil Recovery from Water under Environmentally Relevant Conditions Using Magnetic Nanoparticles. Environ. Sci. Technol. 2015, 49, 11729–11736. [Google Scholar] [CrossRef]
- Xu, H.; Jia, W.; Ren, S.; Wang, J. Novel and recyclable demulsifier of expanded perlite grafted by magnetic nanoparticles for oil separation from emulsified oil wastewaters. Chem. Eng. J. 2018, 337, 10–18. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, S.; Qi, D.; Zhang, D.; Zhao, H. Enhanced demulsification from aqueous media by using magnetic chitosan-based flocculant. J. Colloid Interface Sci. 2018, 518, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, H.; Yan, J.; Hou, W. Demulsification of Oleic-Acid-Coated Magnetite Nanoparticles for Cyclohexane-in-Water Nanoemulsions. Energy Fuels 2014, 28, 6172–6178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Liu, C.; Cheng, P.; Liang, W. Chitosan-Based Grafted Cationic Magnetic Material to Remove Emulsified Oil from Wastewater: Performance and Mechanism. Processes 2024, 12, 797. https://doi.org/10.3390/pr12040797
Du S, Liu C, Cheng P, Liang W. Chitosan-Based Grafted Cationic Magnetic Material to Remove Emulsified Oil from Wastewater: Performance and Mechanism. Processes. 2024; 12(4):797. https://doi.org/10.3390/pr12040797
Chicago/Turabian StyleDu, Sicong, Chuang Liu, Peng Cheng, and Wenyan Liang. 2024. "Chitosan-Based Grafted Cationic Magnetic Material to Remove Emulsified Oil from Wastewater: Performance and Mechanism" Processes 12, no. 4: 797. https://doi.org/10.3390/pr12040797




