Engineering Implementation of the Acosta Fermentation Method to Obtain Cuban Schnapps with Reduced Concentrations of Higher Alcohols
Abstract
:1. Introduction
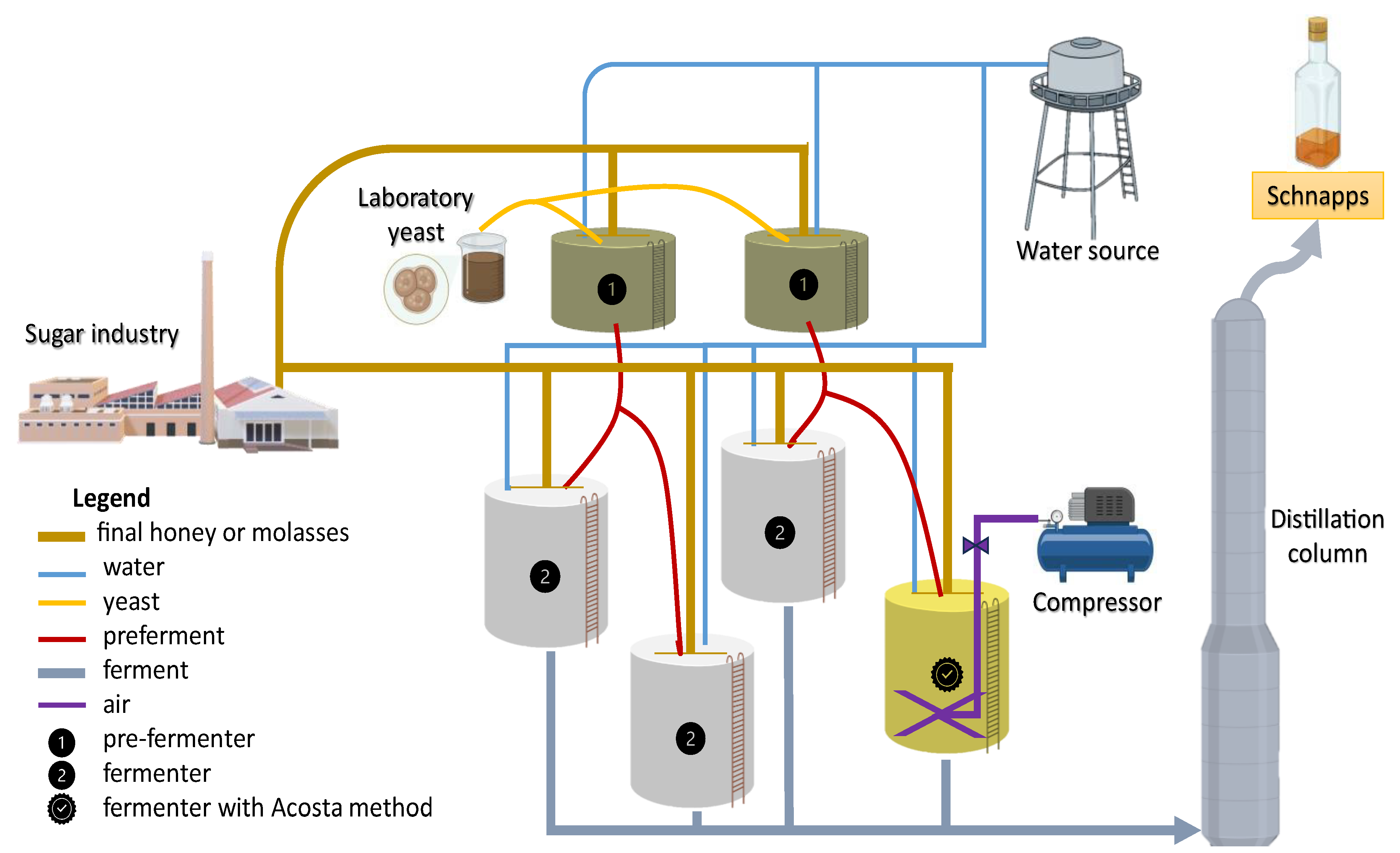
2. Materials and Methods
2.1. Reagents
2.2. Guided Study
2.3. Raw Material Characterization
2.3.1. Obtainment of Molasses and Molasses Dilution
2.3.2. Determination of Soluble Solids
2.3.3. Density Determination
2.3.4. Determination of Sludge Content
- S → Sludge in molasses sample.
- Pv → Precipitate volume in mL.
- m → Mass of molasses in 10 mL of solution (g).
- ρ → Specific density of molasses.
- 2 → Dilution factor.
2.3.5. pH Determination
2.3.6. Determination of Total Reducing Sugars
2.3.7. Determination of Free Reducing Sugars
2.3.8. Determination of Sucrose
- Suc. → Sucrosa (%).
- TRS → Total reducing sugars (%).
- FRS → Free reducing sugars (%).
2.3.9. Determination of Total Sugars
- TS → Total sugars (%).
- Suc. → Sucrosa (%).
- FRS → Free reducing sugars (%).
2.3.10. Determination of Infermentable Reducing Sugars
- IRS → Infermentable reducing sugars (%).
- V1 → Average volume of inverted sugar reference solution at 2 mg/mL consumed in the 2 blank titrations (mL).
- V2 → Average volume of inverted sugar reference solution at 2 mg/mL consumed in the 2 sample titrations (mL).
- a → Mass of sample used in the preparation of the test solution (g).
- b → Volume of test solution pipetted (mL).
- 0.002 → Factor corresponding to the concentration of the inverted sugar solution.
- 250 → Dilution factor.
- 100 → Percentage expression.
2.3.11. Determination of Fermentable Reducing Sugars
2.4. Pre-Fermentation and Fermentation States
2.4.1. Analytical Measurements
2.4.2. S. cerevisiae Cell Count
2.4.3. S. cerevisiae Viability
2.4.4. S. cerevisiae Budding
2.4.5. Microbial Contamination
2.4.6. Alcohol Degree Determination
2.4.7. Determination of Higher Alcohols
- M → Content of higher alcohols (mg/L).
- C → Concentration value obtained by substituting absorbance on the graph or in the equation of the line of best fit (g/100 L).
- f → Dilution factor of the test sample.
- 103 → Conversion factor to express the result in mg/L of alcohol at 100 °GL.
- Ø → Alcoholic strength of the test sample.
2.5. Cuban Schnapps Product
2.5.1. The Alcohol Content and Higher Alcohol Content
2.5.2. Total Acidity Determination
- TAc → Total acidity (mg/L).
- V → Total volume of 0.025 N sodium hydroxide solution consumed in the titration (mL).
- Vb → Volume of sodium hydroxide consumed in the neutralization of water (mL).
- F → General and alcoholic strength correction factor.
- 1500 → Factor to express the concentration in mg/L.
- Vm → Volume of test sample of brandy in mL.
2.5.3. Total Ester Determination
- B → Total ester content.
- V1 → Volume of the 0.1 N sodium hydroxide solution (mL).
- N1 → Exact normality of the sodium hydroxide solution.
- V2 → Volume of the 0.1 N hydrochloric acid solution consumed in the titration (mL).
- N2 → Exact normality of the hydrochloric acid solution used in the titration.
- e → Volume of the test sample in mL.
- Ø → Alcoholic strength of the test sample.
- 88 → Equivalent gram of ethyl acetate.
- 105 → Conversion factor to express the result in mg/L of alcohol at 100 °GL.
3. Results
3.1. Characterization of the Raw Material
3.2. Study of Pre-Fermentation
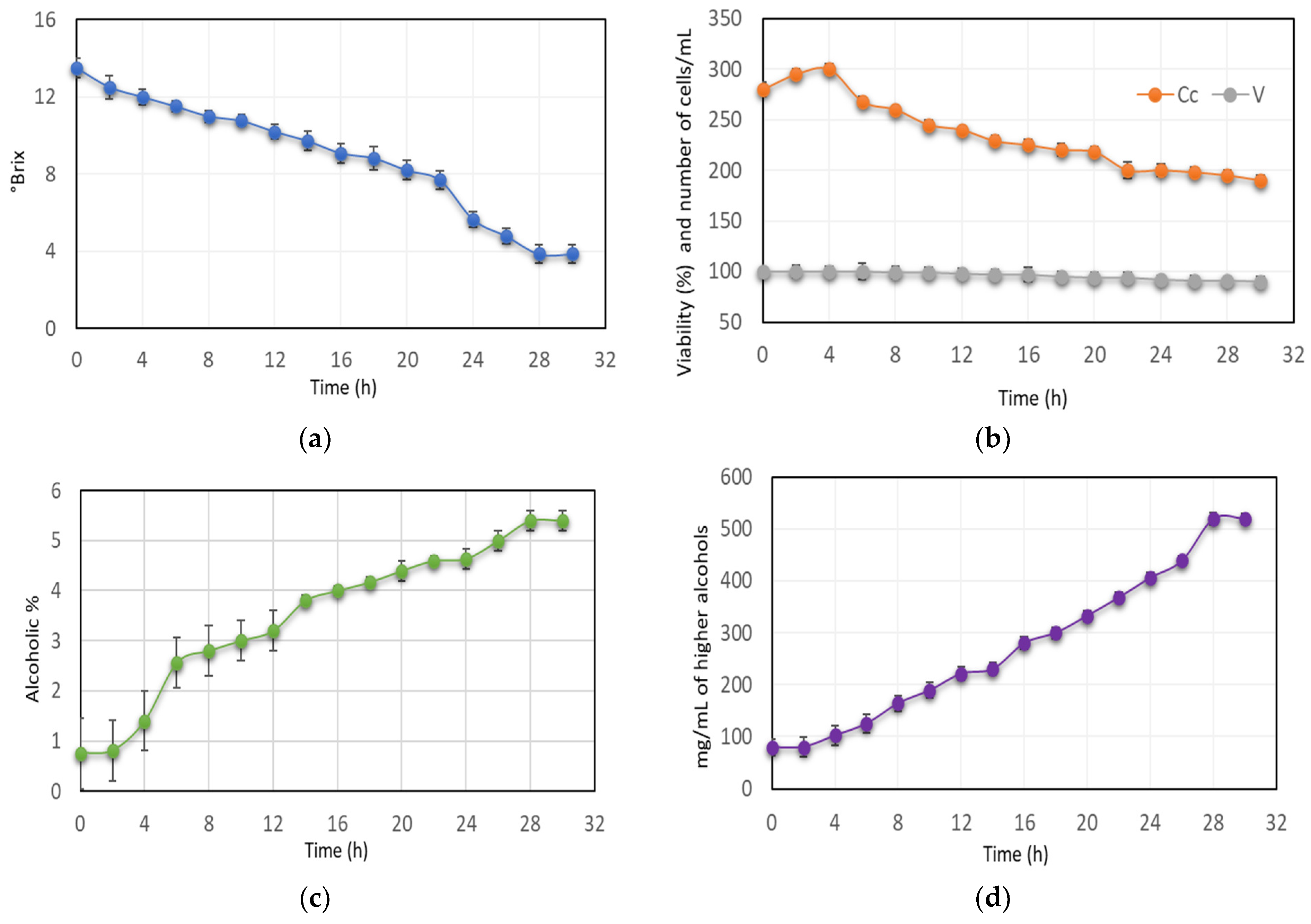
3.3. Study of Fermentation
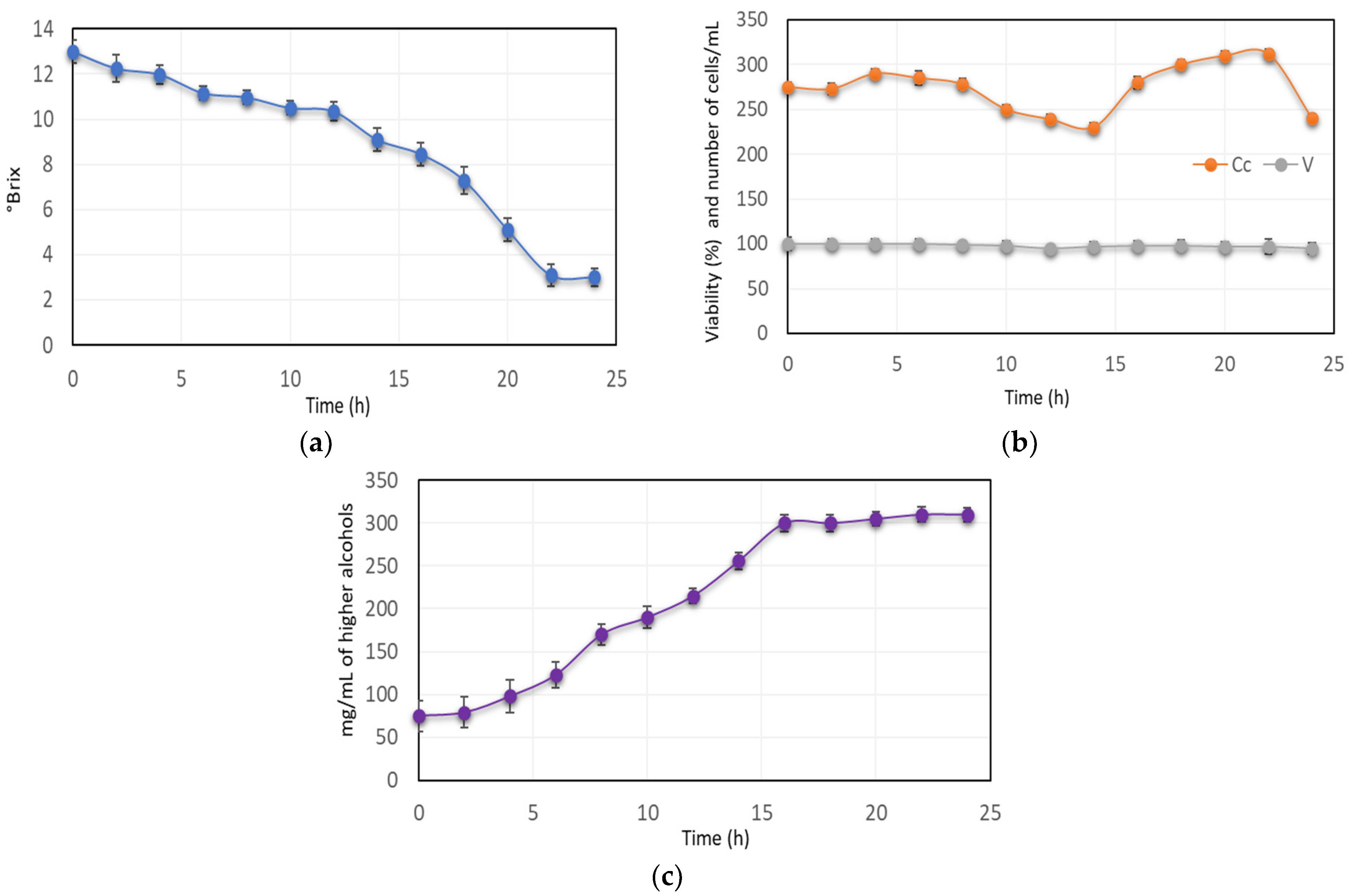
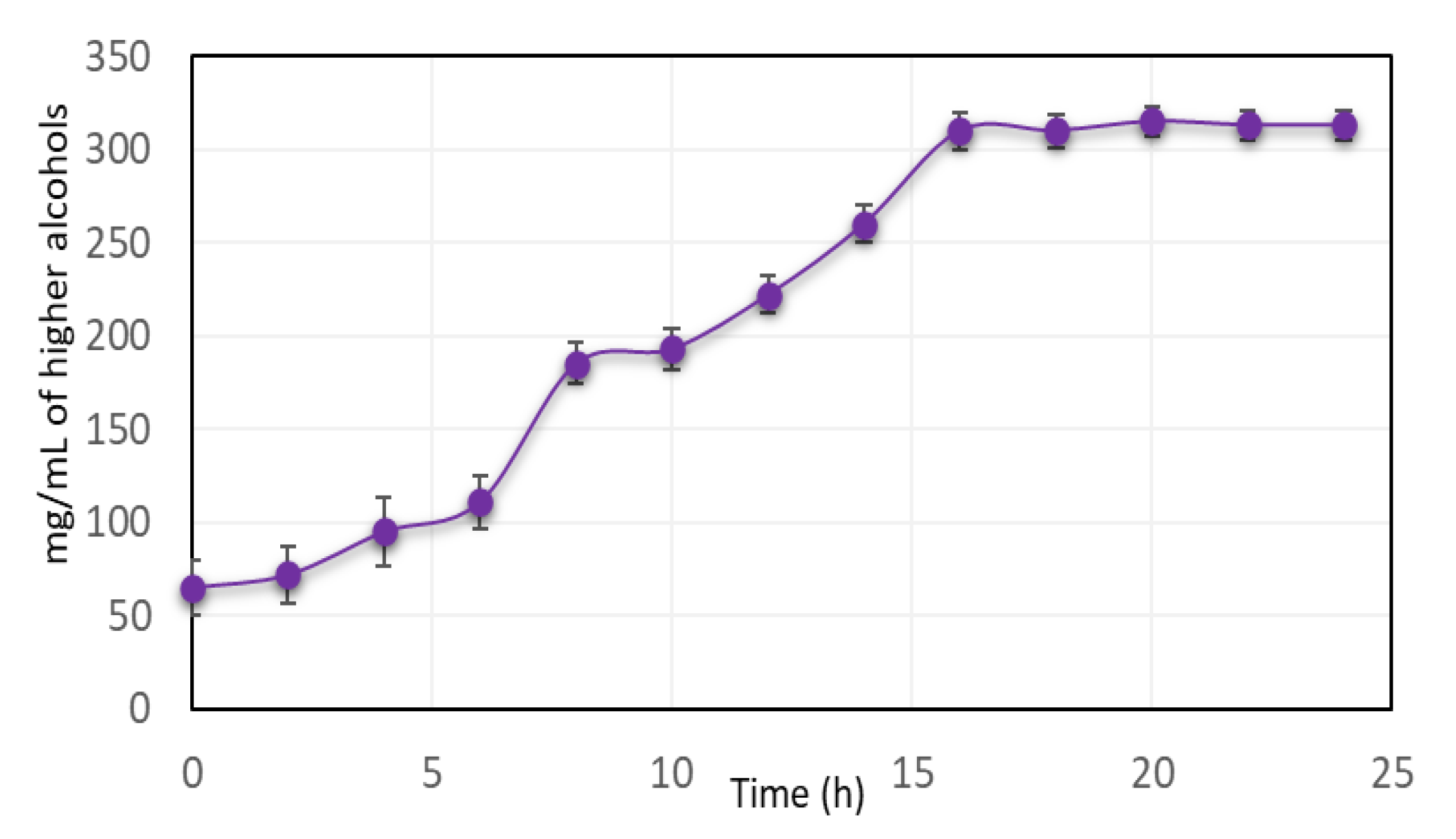
3.4. Follow-Up of the Fermentation Proposal at the Laboratory Level, Acosta Fermentation Method
3.5. Follow-Up of the Fermentation Proposal at the Laboratory Level, Acosta Fermentation Method
3.6. Characterization of the Schnapps Obtained
4. Discussion
Contribution to the Food Industry and Sustainability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mosquera, J.T. Aspectos Toxicológicos, Psicológicos y Sociales. Relacionados con el Consumo de Bebidas Alcohólicas, 1st ed.; Editorial Universidad Nacional de Colombia: Bogotá, Colombia, 2012. [Google Scholar]
- Nevárez-Loor, G.; Intriago-Flor, F.; Plúa-Barcia, J.L. Evaluación de las condiciones higiénica sanitarias en la elaboración de alcohol artesanal en Manabí. Técnica Rev. Agrocienc. 2021, 11, 28–33. [Google Scholar]
- Díaz, O.C.; de Armas Martínez, A.C.; Carvajal, Y.A.; Aguilar, I.G. Proposal for technological modification in a cuban distillery based on a consumption indexes study. Rev. Centro Azúcar 2021, 48, 117–126. [Google Scholar]
- Borroto-Mato, D.; Lorenzo-Izquierdo, M.; García-Gutiérrez, R.; Herrera-Marrero, N. Determinación de alcoholes superiores por cromatografía de gases en destilados. ICIDCA Sobre Deriv. Caña Azúcar 2021, 55, 63–69. [Google Scholar]
- Quiñones, I. Resolución No. 12/19; Gaceta Oficial de la República de Cuba; Ministerio de la Industria Alimentaria: Havana, Cuba, 2019. [Google Scholar]
- Mulet-Hing, M. Automatización de la destilación de alcohol de la UEB destilería de la ronera Santiago de Cuba. Tecnol. Química 2013, 33, 1–9. [Google Scholar]
- Hua, Y. Research progress of higher alcohols as alternative fuels for compression ignition engines. Fuel 2024, 357, 129749. [Google Scholar] [CrossRef]
- NC 535:2007; Bebidas Alcohólicas. Determinación de Alcoholes Superiores. Método Espectrofotométrico. Oficina Nacional de Normalización: La Habana, Cuba, 2007.
- Burini, J.A.; Eizaguirre, J.I.; Loviso, C.; Libkind, D. Levaduras no convencionales como herramientas de innovación y diferenciación en la producción de cerveza. Rev. Argent. Microbiol. 2021, 53, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, P.; Zhang, P.; Lu, J.; Chen, Y.; Xiao, D.; Guo, X. Unraveling the aroma profiling of Baijiu: Sensory characteristics of aroma compounds, analytical approaches, key odor-active compounds in different Baijiu, and their synthesis mechanisms. Trends Food Sci. Technol. 2024, 146, 104376. [Google Scholar] [CrossRef]
- Morales-Cusme, E.J.; Palacios-Macías, M.G. Evaluación en la Implementación de Buenas Prácticas de Manufactura para el Aguardiente de Caña en la Fábrica “Alcívar”. Master’s Thesis, ESPAM MFL, Calceta, Ecuador, 2021. [Google Scholar]
- Muñoz, F.M. Impacto Orgenoléptico Causado por la Variación de Constituyentes Nutricionales, en la Fermentación de un Vino Cabernet Sauvignon. Bachelor’s Thesis, Facultad de Enología y Agroindustrias, Universidad Juan Agustín Maza, Mendoza, Argentina, 2021. [Google Scholar]
- Loviso, C.L.; Libkind, D. Síntesis y regulación de los compuestos del aroma y sabor derivados de la levadura en la cerveza: Alcoholes superiores. Rev. Argent. Microbiol. 2019, 51, 386–397. [Google Scholar] [CrossRef]
- Ferremi Leali, N.; Salvetti, E.; Luzzini, G.; Salini, A.; Slaghenaufi, D.; Fusco, S.; Ugliano, M.; Torriani, S.; Binati, R.L. Differences in the Volatile Profile of Apple Cider Fermented with Schizosaccharomyces pombe and Schizosaccharomyces japonicus. Fermentation 2024, 10, 128. [Google Scholar] [CrossRef]
- Romero-Rodríguez, R.; Durán-Guerrero, E.; Castro, R.; Díaz, A.B.; Lasanta, C. Evaluation of the influence of the microorganisms involved in the production of beers on their sensory characteristics. Food Bioprod. Process. 2022, 135, 33–47. [Google Scholar] [CrossRef]
- Palacios-Bereche, M.C.; Palacios-Bereche, R.; Ensinas, A.V.; Gallego, A.G.; Modesto, M.; Nebra, S.A. Brazilian sugar cane industry—A survey on future improvements in the process energy management. Energy 2022, 259, 124903. [Google Scholar] [CrossRef]
- Santos, F.; Eichler, P.; Machado, G.; De Mattia, J.; De Souza, G. By-products of the sugarcane industry. In Sugarcane Biorefinery, Technology and Perspectives; Academic Press: Cambridge, MA, USA, 2020; pp. 21–48. [Google Scholar]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fagioli, L.; Fusaro, I.; Biagi, G.; Formigoni, A.; Mammi, L. Characterization of molasses chemical composition. J. Dairy Sci. 2020, 103, 6244–6249. [Google Scholar] [CrossRef]
- Ghorai, S.; Banik, S.P.; Verna, D.; Chowdhury, S.; Mukherjee, S.; Khowala, S. Fungal biotechnology in food and feed processing. Food Res. Int. 2009, 42, 577–587. [Google Scholar] [CrossRef]
- Torres, D. Estudio de la Etapa de Fermentación Alcohólica Utilizando Mezclas de Diferentes Sustratos; Depertamento de Ingeniería Química, Facultad de Química y Farmacia, Universidad Central Marta Abreu de Las Villas: Santa Clara, Cuba, 2005. [Google Scholar]
- Sherman, F. Getting started with yeast. Methods Enzymol 2002, 350, 3–41. [Google Scholar]
- García, R. Cultivo Mixto en el Desarrollo de la Fermentación Para la Producción de Alcohol Orgánico. Ph.D. Thesis, Depertamento de Licenciatura en Química, Facultad de Química y Farmacia, Universidad Central Marta Abreu de Las Villas, Santa Clara, Cuba, 2006. [Google Scholar]
- Arencibia, Y. Impacto de la Recirculación de Vinazas a la Etapa Fermentativa en el Proceso de Producción de Etanol en la Destilería Heriberto Duquesne. Ph.D. Thesis, Universidad Central Marta Abreu de Las Villas, Santa Clara, Cuba, 2014. [Google Scholar]
- Cirilo, V.S.; Santillán, P.S.; Salado, N.T.; Pérez, J.H. Composición de la pared celular de vástago de coco tratado por fermentación solida usando Pleurotus ostreatus como inóculo. Lat. Am. Arch. Anim. Prod. 2023, 31, 175–179. [Google Scholar]
- Rodríguez-Páez, D.; Zumalacárregui-de Cárdenas, L.; Pérez-Ones, O.; Martínez-Guía, Y. Recuperación de los fondajes de la fermentación alcohólica para su uso como alimento animal. ICIDCA Sobre Deriv. Caña Azúcar 2022, 56, 23–30. [Google Scholar]
- Vera Cajilema, J.L. Plan de Negocio Para la Creación de una Planta Destiladora de Alcohol de Caña de Azúcar, Cantón Guaranda, Provincia del Bolívar. Master’s Thesis, Universidad Laica Vicente Rocafuerte de Guayaquil, Bolívar, Ecuador, 2021. [Google Scholar]
- Campués, J.K.; Tarupí, J.C. Obtención de Alcohol a Partir de Jugo de Caña, Cachaza y Melaza, Mediante la Incorporación de dos Niveles de Fermento (Saccharomyces cerevisiae). Master’s Thesis, Universidad Técnica del Norte, Facultad de Ingeniería en Ciencias Agropecuarias y Ambientales, Ibarra, Ecuador, 2011. [Google Scholar]
- Acosta, D.R.; Jover, J.A. Método de Fermentación “Acosta” Para Obtener Concentraciones de Alcoholes Superiores en Aguardientes Menores de 350 mg/L; Universidad Central “Marta Abreu” de las Villas, Facultad de Química, Departamento de Licenciatura en Química: Santa Clara, Cuba, 2009. [Google Scholar]
- Austin, G.T.; Espinoza, M.E.; Sánchez, J.; Viesca, R. Manual de Procesos Químicos en la Industria; McGraw-Hill: New York, NY, USA, 1992; Volume 1. [Google Scholar]
- Mangwanda, T.W.; Mani, J.S.; Johnson, J.B.; Jackson, S.; McKeown, T.; Naiker, M. Physicochemical and nutritional analysis of molasses for rum fermentation. Biol. Life Sci. Forum 2023, 26, 105. [Google Scholar] [CrossRef]
- Ribeiro, N.N.; Cazadore, V.C.; Madaleno, L.L. Use of Distillery Effluents in Dilution of Molasses for Ethanol Production. Sugar Tech. 2023, 25, 366–372. [Google Scholar] [CrossRef]
- Hawaz, E.; Tafesse, M.; Tesfaye, A.; Kiros, S.; Beyene, D.; Kebede, G.; Boekhout, T.; Groenwald, M.; Theelen, B.; Degefe, A.; et al. Bioethanol production from sugarcane molasses by co-fermentation of Saccharomyces cerevisiae isolate TA2 and Wickerhamomyces anomalus isolate HCJ2F-19. Ann. Microbiol. 2024, 74, 13. [Google Scholar] [CrossRef]
- NC 81-06:1988; Industria Azucarera. Miel Final. Determinación del Contenido de Sólidos Solubles. Oficina Nacional de Normalización: La Habana, Cuba, 1988.
- Vázquez, M. Manual de Técnicas Analíticas para Destilerías; Editorial ICIDCA: La Habana, Cuba, 2013; pp. 7–55. ISBN 978-959-71-65-35-4. [Google Scholar]
- NC 81-40:1988; Industria Azucarera. Miel Final. Determinación del Contenido de Lodos. Oficina Nacional de Normalización: La Habana, Cuba, 1988.
- NC 81-41:1988; Industria Azucarera. Miel Final. Determinación de pH. Oficina Nacional de Normalización: La Habana, Cuba, 1988.
- NC 81-42:1988; Industria Azucarera. Miel Final. Determinación de Azúcares Reductores Totales. Oficina Nacional de Normalización: La Habana, Cuba, 1988.
- NC 81-43:1988; Industria Azucarera. Miel Final. Determinación de Azúcares Fermentables. Método Indirecto. Oficina Nacional de Normalización: La Habana, Cuba, 1988.
- NC 83-26:1987; Industria de Fermentación. Alcohol Etílico. Determinación del Grado Alcohólico. Oficina Nacional de Normalización: La Habana, Cuba, 1987.
- NC 534:2007; Bebidas alcohólicas. Determinación de Ésteres Totales. Método de Saponificación. Oficina Nacional de Normalización: La Habana, Cuba, 2007.
- NC 715:2009; Miel Final (Melaza). Especificaciones. Oficina Nacional de Normalización: La Habana, Cuba, 2009.
- Pérez-Bermúdez, I.; Ribas-García, M.; Ibañez-Fuentes, M.L.; Saura-Laria, G.; GarridoCarralero, N. Análisis de la influencia de la calidad de la miel final y el tiempo perdido sobre la eficiencia industrial en la producción de etanol. ICIDCA Sobre Deriv. Caña Azúcar 2015, 49, 58–63. [Google Scholar]
- Mesa, L.; González, E.; González, M.; Agüero, G. La producción de etanol. Alternativas de materias primas. Rev. Cubana Química 2005, 17, 129–137. [Google Scholar]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Principios de Bioquímica; Ediciones Omega: Barcelona, Spain, 2005. [Google Scholar]


| Accessory | Amount |
|---|---|
| Pipeline ½ | 12 m |
| Hose ½ | 1 m |
| Cubit ½ | 4 units |
| T connection ½ | 1 unit |
| Valve ½ | 1 unit |
| Parameter | Results |
|---|---|
| °Brix | 84.8 ± 1.71 |
| Temperature (°C) | 29 ± 0.9 |
| Density (g/mL) | 1.44402 ± 0.018 |
| pH | 5.7 ± 0.14 |
| Sludge content (%) | 6.17 ± 0.92 |
| Total reducing sugars (%) | 56.05 ± 1.87 |
| Free reducing sugars (%) | 16.67 ± 2.01 |
| Sucrose (%) | 37.41 |
| Total sugars (%) | 54.08 |
| Infermentable reducing sugars (%) | 4.25 ± 0.39 |
| Parameter | Results |
|---|---|
| °Brix | 7.79 ± 0.61 |
| Temperature (°C) | 31 ± 0.8 |
| Cell count (Mcel/mL) | 410 ± 31.5 |
| Viability (%) | 100 ± 0.0 |
| Budding (%) | 56.8 ± 4.89 |
| Bacterial contamination | No |
| Higher alcohols (mg/L) | 0.70 ± 0.32 |
| Parameter | Results |
|---|---|
| Alcoholic degree (°GL) | 75 ± 1.2 |
| Acidity (mg/L) | 15 ± 1.8 |
| Esters (mg/L) | 40 ± 3.84 |
| Higher alcohols (mg/L) | 132.5 ± 3.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergel-Alfonso, A.A.; Acosta-Martínez, D.R.; Arencibia-Sánchez, J.A.; Rodríguez-Félix, F.; Reyes-Delgado, Y.; González-Morales, R.V.; Benítez-Sánchez, R.; Gonzalez-Bravo, A.L.; Tapia-Hernández, J.A. Engineering Implementation of the Acosta Fermentation Method to Obtain Cuban Schnapps with Reduced Concentrations of Higher Alcohols. Processes 2024, 12, 1064. https://doi.org/10.3390/pr12061064
Vergel-Alfonso AA, Acosta-Martínez DR, Arencibia-Sánchez JA, Rodríguez-Félix F, Reyes-Delgado Y, González-Morales RV, Benítez-Sánchez R, Gonzalez-Bravo AL, Tapia-Hernández JA. Engineering Implementation of the Acosta Fermentation Method to Obtain Cuban Schnapps with Reduced Concentrations of Higher Alcohols. Processes. 2024; 12(6):1064. https://doi.org/10.3390/pr12061064
Chicago/Turabian StyleVergel-Alfonso, Ariel Alain, Delvis Rafael Acosta-Martínez, José Ariel Arencibia-Sánchez, Francisco Rodríguez-Félix, Yosviel Reyes-Delgado, Rosa Virginia González-Morales, Rosbel Benítez-Sánchez, Ana Liz Gonzalez-Bravo, and José Agustín Tapia-Hernández. 2024. "Engineering Implementation of the Acosta Fermentation Method to Obtain Cuban Schnapps with Reduced Concentrations of Higher Alcohols" Processes 12, no. 6: 1064. https://doi.org/10.3390/pr12061064
APA StyleVergel-Alfonso, A. A., Acosta-Martínez, D. R., Arencibia-Sánchez, J. A., Rodríguez-Félix, F., Reyes-Delgado, Y., González-Morales, R. V., Benítez-Sánchez, R., Gonzalez-Bravo, A. L., & Tapia-Hernández, J. A. (2024). Engineering Implementation of the Acosta Fermentation Method to Obtain Cuban Schnapps with Reduced Concentrations of Higher Alcohols. Processes, 12(6), 1064. https://doi.org/10.3390/pr12061064









