Strength and Contaminant Toxicity Leaching Characteristics of MgO-Solidified Silt
Abstract
1. Introduction
2. Materials and Methods
2.1. Dredged Silt
2.2. Test Methods
3. Test Results
3.1. Unconfined Compressive Strength
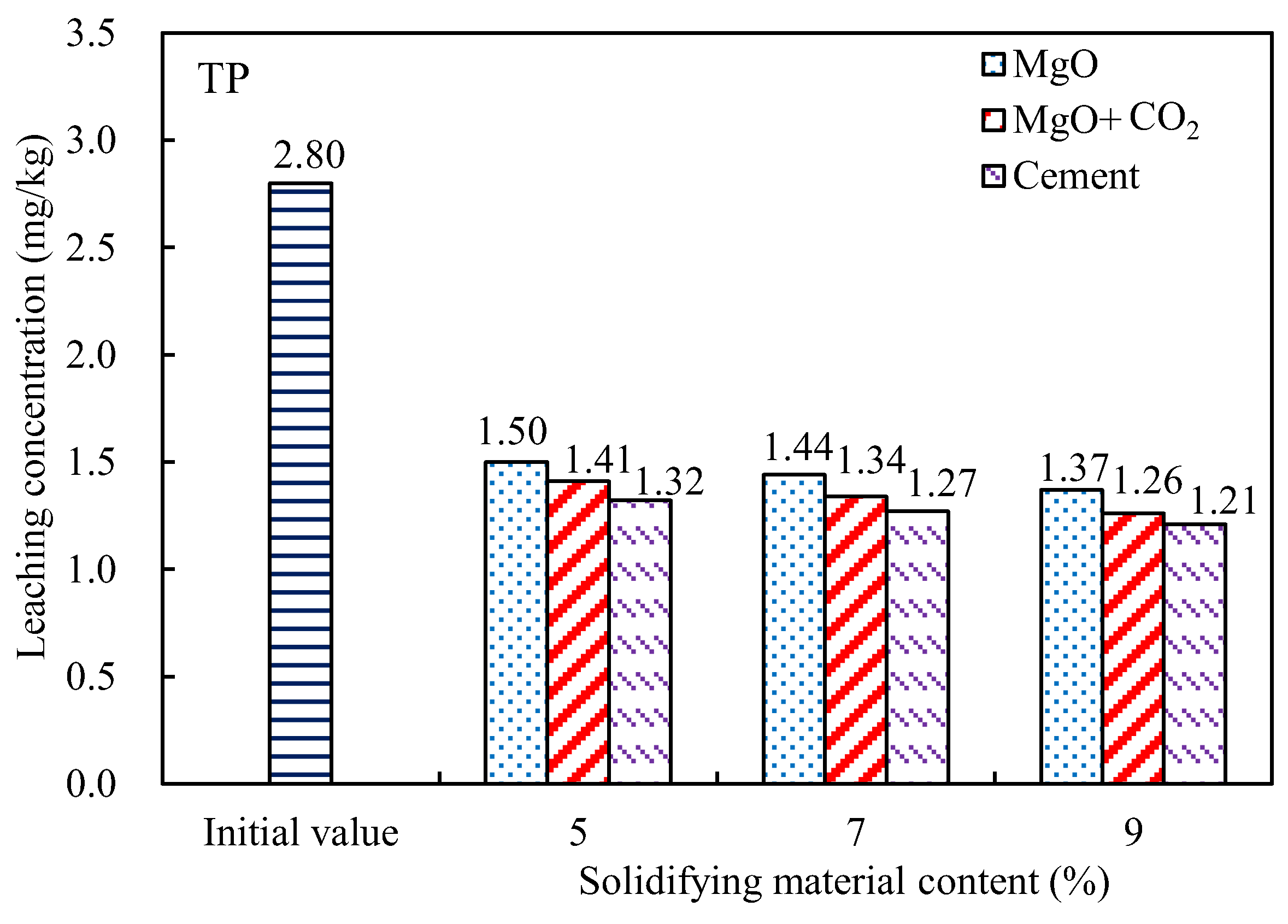
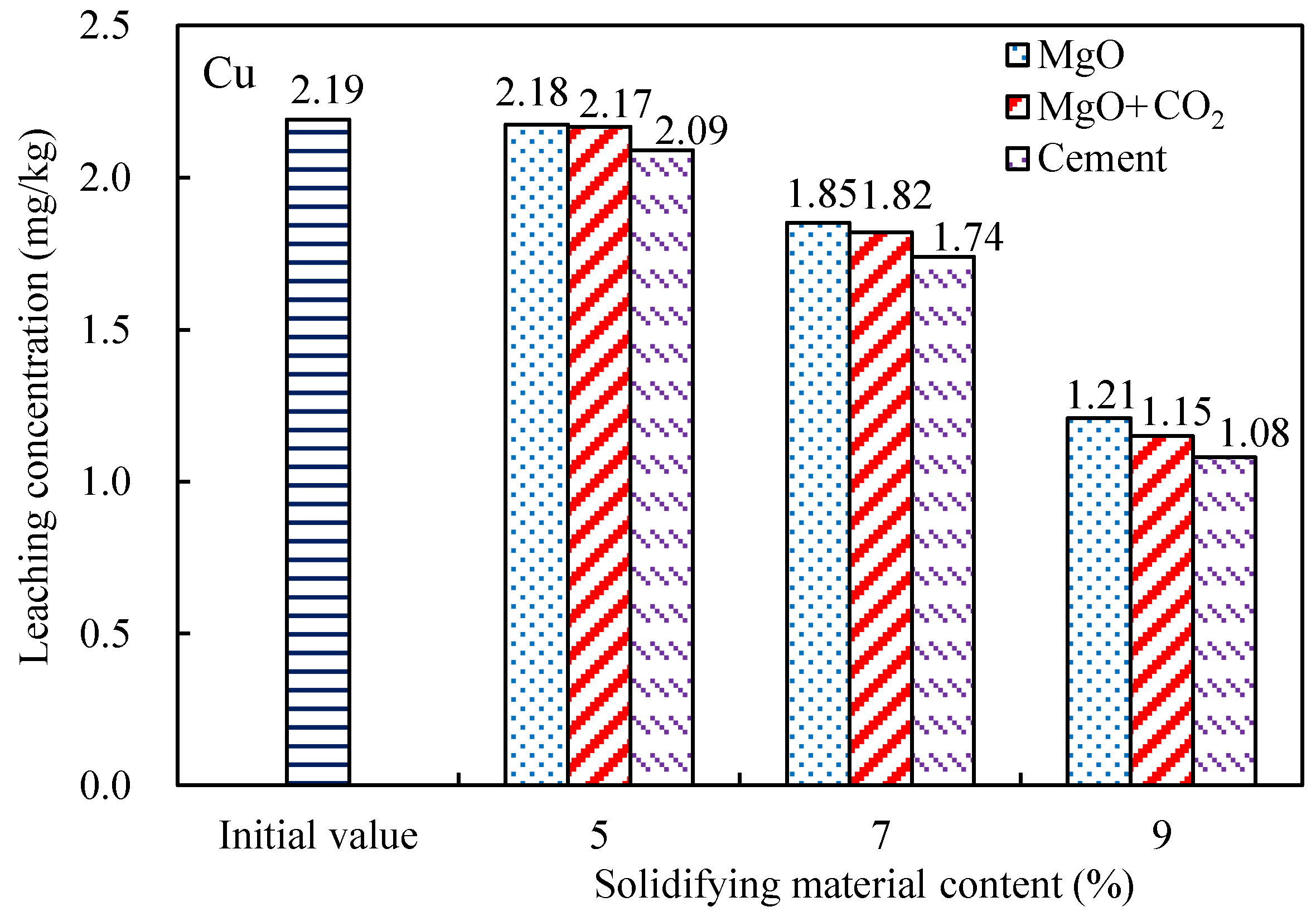
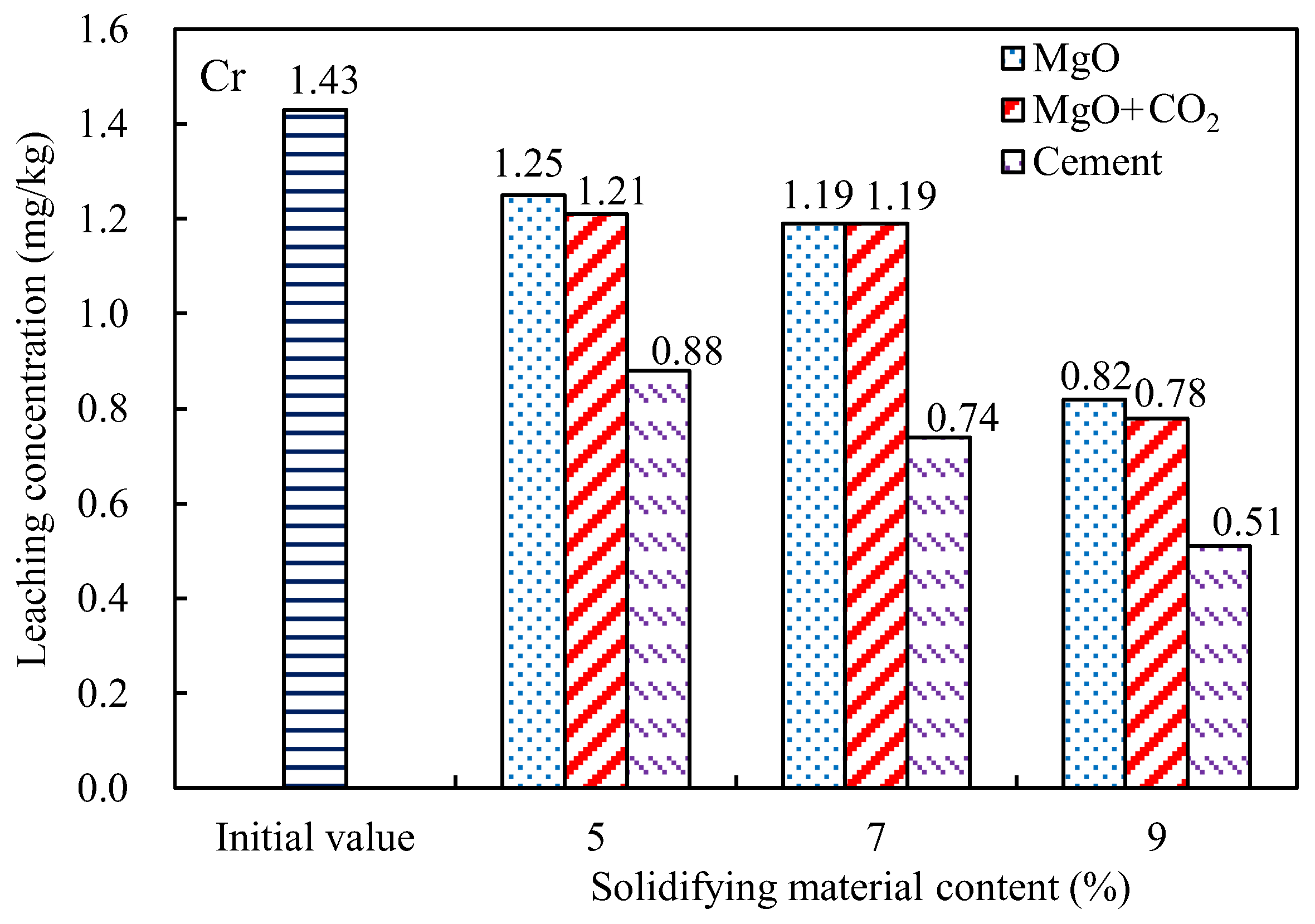
3.2. Contaminant Leaching
4. Discussion
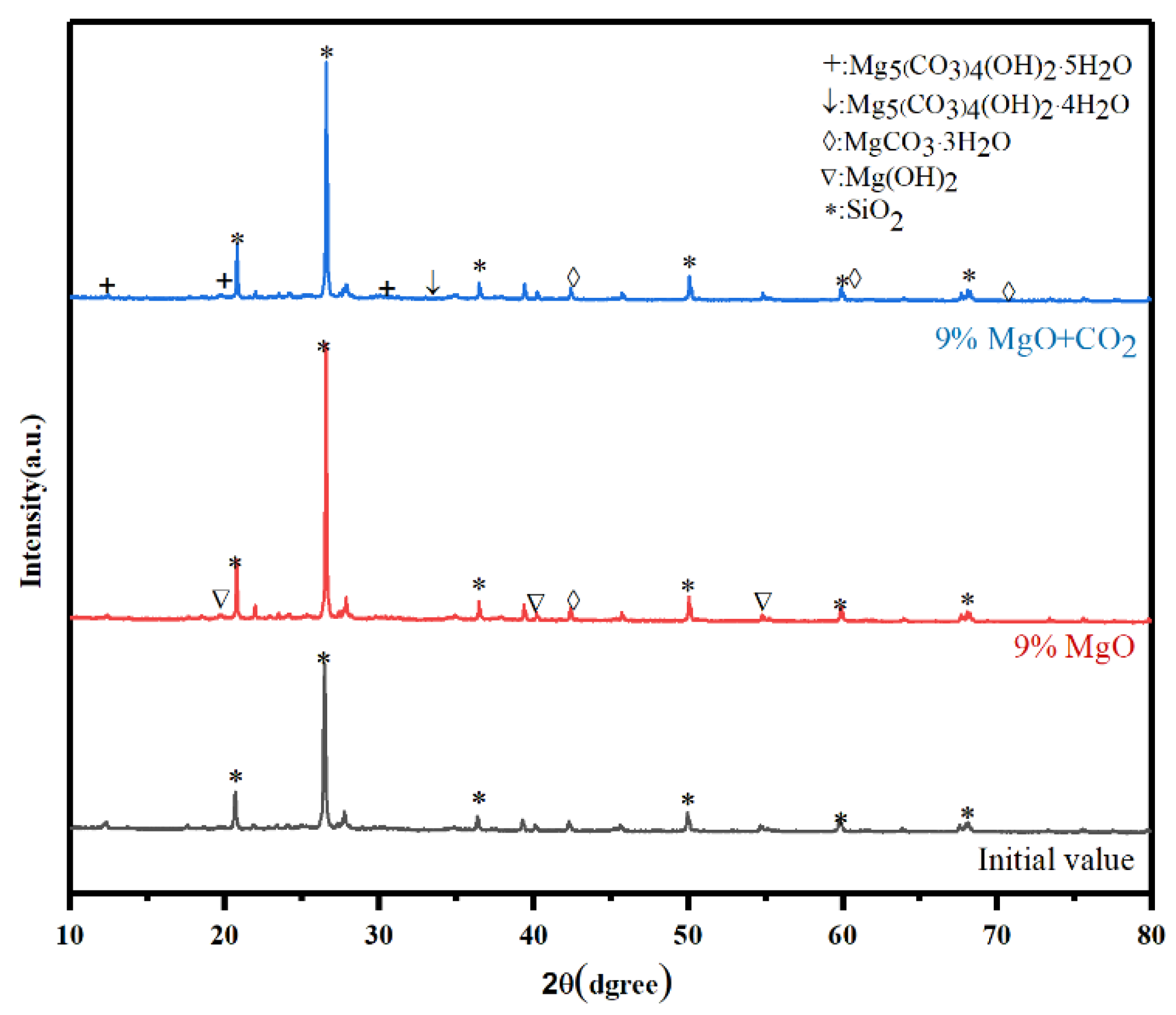
Mechanism of MgO Solidification and CO2 Carbonization
5. Conclusions
- The UCS of MgO-solidified silt increased with the MgO content and curing time. The UCS of the solidified silt with a MgO content of 9% and curing time of 14 d reached 111 kPa. A longer curing time resulted in a more significant effect of the MgO content, and a higher MgO content resulted in a more notable effect of curing time on the UCS.
- The CO2-injection treatment could further increase the UCS of the MgO-solidified silt by approximately 25%. The UCS values of the MgO-solidified silt without and with the CO2 injection were approximately 60% and 80%, respectively, of those of the cement-solidified silt.
- The leaching concentrations of nutrient salts (TN and TP) and heavy metal pollutants (Cu, Cr, and Ni) in the dredged silt decreased with increasing MgO content. The concentrations of TN and TP in the leached MgO-solidified silt with the CO2 injection were only approximately 5% higher than those of the cement-solidified silt. The effect of MgO solidification with carbonization was most significant for Ni, the leaching concentration of which was reduced by more than 65%. However, the concentrations of Cu in the leached MgO-solidified silts were closest to those of the cement-solidified silts.
- SEM and XRD results showed that the addition of MgO to the silt formed flocculated magnesium hydroxide colloidal material that filled the silt pores. As the MgO content increased, the pore size significantly reduced and the silt pores were gradually filled. Flake-like material appeared following carbonization, which was identified as hydromagnesite and dypingite. Compared with the addition of MgO alone, its combination with carbonation enhanced the overall compactness of the silt, thus greatly improving its strength.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhong, J.; Chen, C.; Yu, J.; Shen, Q.; Liu, C.; Fan, C. Effect of dredging and capping with clean soil on the mitigation of algae-induced black blooms in Lake Taihu, China: A simulation study. J. Environ. Manag. 2022, 302, 114106. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gong, H.; Shi, W.; Fang, N.; Tan, Y.; Zhou, W.; Huang, J.; Dai, L.; Dai, X.; Guo, Y. Insights into multisource sludge distributed in the Yangtze River basin, China: Characteristics, correlation, treatment and disposal. J. Environ. Sci. 2023, 126, 321–332. [Google Scholar] [CrossRef]
- Shu, S.; Li, L.; Cao, M.; Pan, Z.; Li, R.; Xu, G.; Tang, Y. Coupling effect of solidification and consolidation on characteristics of the dredged silt. Environ. Sci. Pollut. Res. 2024, 31, 10887–10895. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, D.N. Characterization of sediments for sustainable development: State of the art. Mar. Georesour. Geotechnol. 2015, 33, 447–465. [Google Scholar] [CrossRef]
- Tang, C.S.; Cheng, Q.; Wang, P.; Wang, H.S.; Wang, Y.; Inyang, H.I. Hydro-mechanical behavior of fiber reinforced dredged sludge. Eng. Geol. 2020, 276, 105779. [Google Scholar] [CrossRef]
- Niu, Y.; Jiang, X.; Wang, K.; Xia, J.D.; Jiao, W.; Niu, Y.; Yu, H. Meta analysis of heavy metal pollution and sources in surface sediments of Lake Taihu, China. Sci. Total Environ. 2020, 700, 134509. [Google Scholar] [CrossRef] [PubMed]
- Andreottola, G.; Bonomo, L.; De Gioannis, G.; Ferrarese, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Saponaro, S. Lab-scale feasibility tests for sediment treatment using different physico-chemical techniques. J. Soils Sediments 2010, 10, 142–150. [Google Scholar] [CrossRef]
- Yuan, H.; Song, S.; An, S.; Liu, E. Ecological risk assessment of potentially toxic elements (PTEs) in the soil-plant system after reclamation of dredged sediment. Environ. Sci. Pollut. Res. 2018, 25, 29181–29191. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zeng, J.; Bai, H.; Hao, Y.; Yan, X.; Wang, S. Revitalizing urban lake cleanup: Optimizing flocculation and dewatering of dredged sludge using cation polyacrylamide. Environ. Sci. Pollut. Res. 2023, 30, 119462–119472. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, C.L.; Chiu, A.C. Soil–water transfer mechanism for solidified dredged materials. J. Geotech. Geoenviron. Eng. 2007, 133, 588–598. [Google Scholar] [CrossRef]
- Smith, B.T.; Howard, I.L.; Vahedifard, F. Lightly cemented dredged sediments for sustainable reuse. Environ. Geotech. 2018, 5, 324–335. [Google Scholar] [CrossRef]
- Shu, S.; Xue, X.; Wang, Q.; Li, Y.; Hadkhale, P. Long-term environmental impact of an artificial island of solidified dredged marine silt. Environ. Geotech. 2023, 40, 1–10. [Google Scholar] [CrossRef]
- Ige, O.E.; Olanrewaju, O.A.; Duffy, K.J.; Obiora, C. A review of the effectiveness of Life Cycle Assessment for gauging environmental impacts from cement production. J. Clean. Prod. 2021, 324, 129213. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Z.; Liang, Y.; Wang, X.; Liu, M. Experimental study on solidified dredged sediment with MgO and industrial waste residue. Constr. Build. Mater. 2023, 366, 130105. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, C.; Zhang, Y.; Liu, H. A data-driven approach for the measurement and improvement of regional industrial ecological efficiency for carbon peaking and carbon neutralization. Environ. Sci. Pollut. Res. 2023, 30, 7655–7670. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Liu, S. Compaction and mechanical characteristics and stabilization mechanism of carbonated reactive MgO-stabilized silt. KSCE J. Civ. Eng. 2017, 21, 2641–2654. [Google Scholar] [CrossRef]
- Liu, S.Y.; Cao, J.J.; Cai, G.H. Microscopic mechanism of carbonization and solidification of silty clay by activated magnesium oxide. Rock. Soil. Mech. 2018, 39, 1543–1552. (In Chinese) [Google Scholar]
- Wang, D.X.; Xiao, J.; Gao, X. Strength gain and microstructure of carbonated reactive MgO-fly ash solidified sludge from East Lake, China. Eng. Geol. 2019, 251, 37–47. [Google Scholar] [CrossRef]
- CJ/T 221-2005; Determination Method for Municipal Sludge in Wastewater Treatment Plant. Ministry of Construction of the People’s Republic of China. China Architecture & Building Press: Beijing, China, 2005. (In Chinese)
- HJ/T 200-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Acetic Acid Buffer Solution Method. State Environmental Protection Administration of the People’s Republic of China. China Environmental Science Press: Beijing, China, 2007. (In Chinese)
- GB/T 50123-2019; Standard for Geotechnical Test Method. Ministry of Housing and Urban-Rural Development of the People’s Republic of China. China Architecture & Building Press: Beijing, China, 2019. (In Chinese)







| Parameter | Value |
|---|---|
| Moisture content (%) | 60.74 |
| Liquid limit (%) | 61.50 |
| Plastic limit (%) | 25.80 |
| Density (g/cm3) | 1.72 |
| Organic content (%) | 3.30 |
| Sand fraction content (%) | 2 |
| Silt fraction content (%) | 78 |
| Clay fraction content (%) | 20 |
| Indicators | Total Concentration | Leaching Concentration |
|---|---|---|
| TN | 1372.05 | 58.86 |
| TP | 545.90 | 2.80 |
| Cr | 117.20 | 1.43 |
| Ni | 91.13 | 0.52 |
| Cu | 21.58 | 2.19 |
| Zn | 1.95 | Not detected |
| Pb | 1.67 | Not detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, S.; Zhou, X.; Gong, Y.; Wang, H.; Tang, Y.; Chen, J. Strength and Contaminant Toxicity Leaching Characteristics of MgO-Solidified Silt. Processes 2024, 12, 1086. https://doi.org/10.3390/pr12061086
Shu S, Zhou X, Gong Y, Wang H, Tang Y, Chen J. Strength and Contaminant Toxicity Leaching Characteristics of MgO-Solidified Silt. Processes. 2024; 12(6):1086. https://doi.org/10.3390/pr12061086
Chicago/Turabian StyleShu, Shi, Xiaohuan Zhou, Yujie Gong, Haohui Wang, Yan Tang, and Junhao Chen. 2024. "Strength and Contaminant Toxicity Leaching Characteristics of MgO-Solidified Silt" Processes 12, no. 6: 1086. https://doi.org/10.3390/pr12061086
APA StyleShu, S., Zhou, X., Gong, Y., Wang, H., Tang, Y., & Chen, J. (2024). Strength and Contaminant Toxicity Leaching Characteristics of MgO-Solidified Silt. Processes, 12(6), 1086. https://doi.org/10.3390/pr12061086







