Study on Pyrolysis Behavior of Avermectin Mycelial Residues and Characterization of Obtained Gas, Liquid, and Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Methods
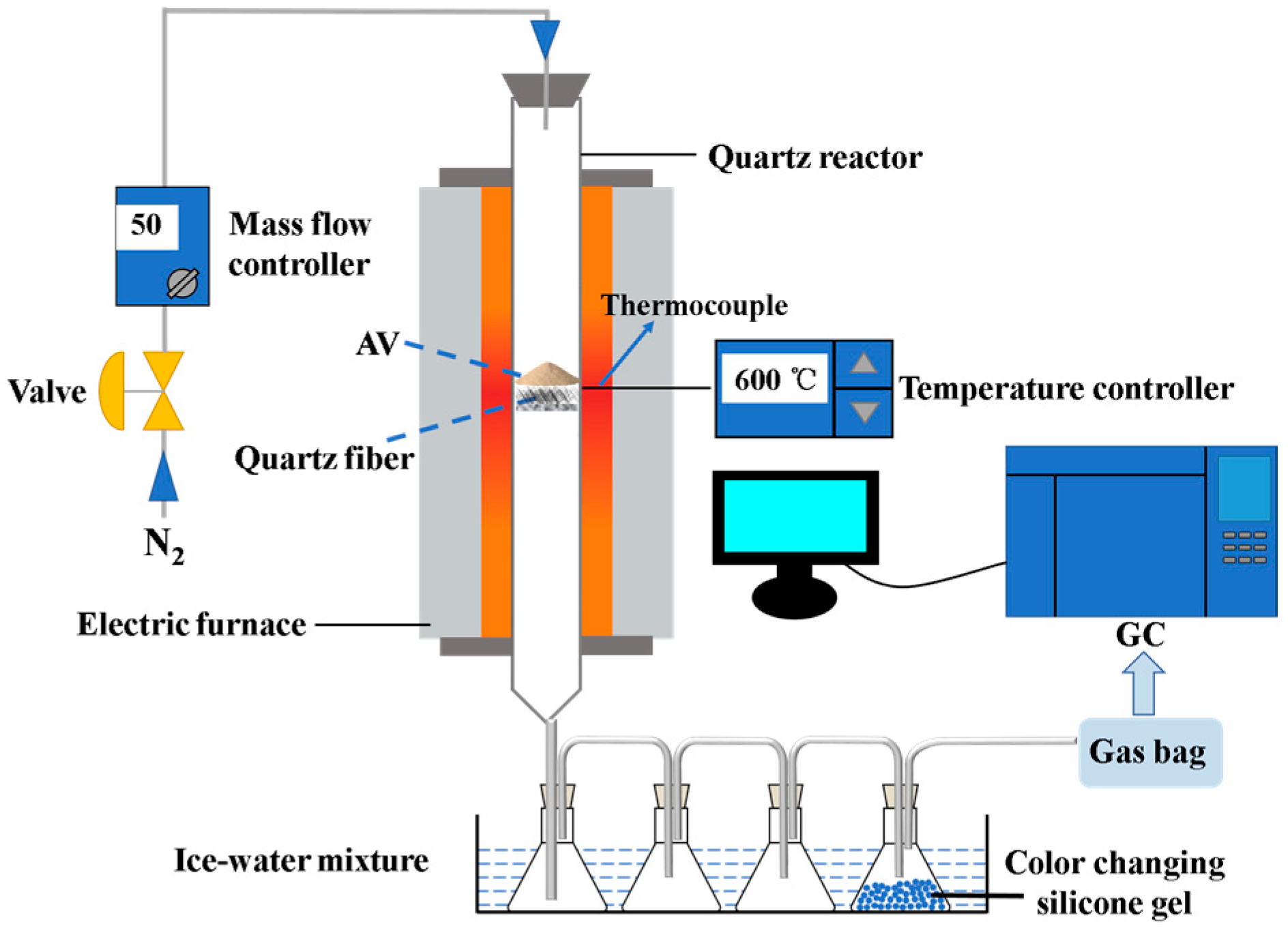
2.2. Experimental Apparatus and Procedure
2.3. Feedstock and Pyrolysis Product Characterization
3. Results and Discussion
3.1. TG Analysis of AV Material
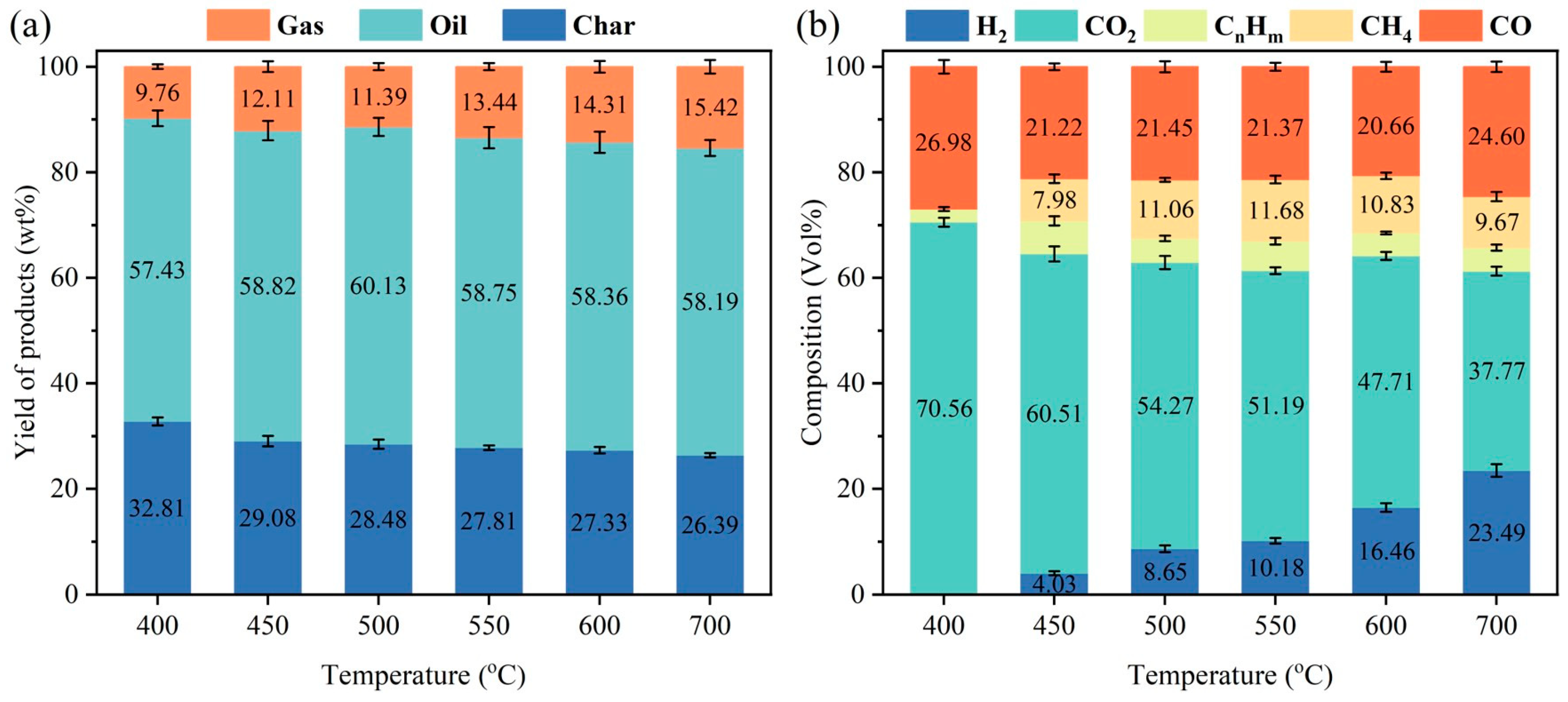
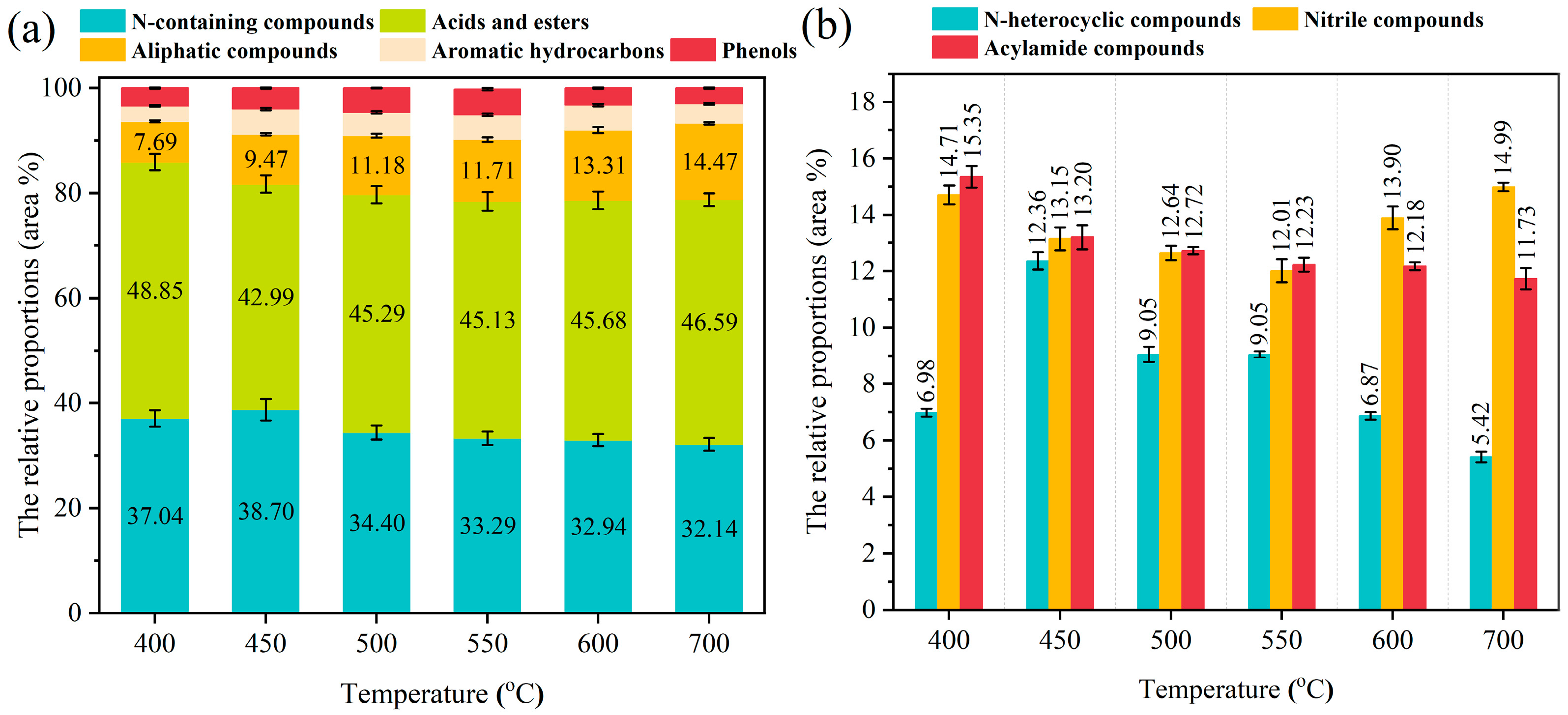
3.2. Product Yields and Composition Analysis of Pyrolysis Gas and Bio-Oil
3.3. Characterization of Pyrolysis Biochar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ying, G.G.; He, L.Y.; Ying, A.J.; Zhang, Q.Q.; Liu, Y.S.; Zhao, J.L. China Must Reduce Its Antibiotic Use. Environ. Sci. Technol. 2017, 51, 1072–1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuan, R.; Chu, L. The Occurrence, Distribution and Degradation of Antibiotics by Ionizing Radiation: An Overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhuan, R.; Chu, L.; Xiang, X.; Sun, H.; Wang, J. Inactivation of Antibiotic Resistance Genes in Antibiotic Fermentation Residues by Ionizing Radiation: Exploring the Development of Recycling Economy in Antibiotic Pharmaceutical Factory. Waste Manag. 2019, 84, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, C.; Tian, Y.; Xi, B.; Guo, W.; Tan, W. Fate of Antibiotic Resistance Genes in Industrial-Scale Rapid Composting of Pharmaceutical Fermentation Residue: The Role Implications of Microbial Community Structure and Mobile Genetic Elements. Environ. Pollut. 2021, 291, 118155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, Z.; Wen, Q.; Ma, J.; He, Z. Assessment of Maturity during Co-Composting of Penicillin Mycelial Dreg via Fluorescence Excitation-Emission Matrix Spectra: Characteristics of Chemical and Fluorescent Parameters of Water-Extractable Organic Matter. Chemosphere 2016, 155, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, G.; Li, X.; Zou, S.; Li, P.; Hu, Z.; Li, J. Occurrence and Elimination of Antibiotics at Four Sewage Treatment Plants in the Pearl River Delta (PRD), South China. Water Res. 2007, 41, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Geng, Y.; Hong, J.; Kua, H.W.; Xu, C.; Yu, N. Life Cycle Assessment of Antibiotic Mycelial Residues Management in China. Renew. Sustain. Energy Rev. 2017, 79, 830–838. [Google Scholar] [CrossRef]
- Chen, G.; Liu, H.; Li, J.; Yan, B.; Dong, L. Treatment of antibiotic mycelial fermentation residue: The critical review. Environ. Chem. 2021, 40, 459–473. [Google Scholar]
- Wang, G.; Liu, H.; Wang, J.; Gong, P.; Cai, C.; Dai, X.; Wang, P. Pretreatment of Spiramycin Fermentation Residue by Thermally Activated Peroxydisulfate for Improving Biodegradability: Insights into Matrix Disintegration and Antibiotics Degradation. Chem. Eng. J. 2022, 427, 130973. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, M.; Han, T.; Bai, X.; Tang, X.; Duan, E.; Kang, A.; Zheng, Z.; Cheng, F. Application Potential of Antibiotic Fermentation Residue for Co-Combustion with Coal: Thermal Behavior, Gaseous Products, and Kinetics. Fuel 2023, 335, 126953. [Google Scholar] [CrossRef]
- Wei, X.; Huang, S.; Wu, Y.; Wu, S.; Yang, J. A Comprehensive Study on Torrefaction of Penicillin Mycelial Residues: Analysis of Product Characteristics and Conversion Mechanisms of N. Fuel 2022, 330, 125703. [Google Scholar] [CrossRef]
- Wei, X.; Huang, S.; Wu, Y.; Wu, S. Effects of Demineralization and Devolatilization on Fast Pyrolysis Behaviors and Product Characteristics of Penicillin Mycelial Residues. J. Hazard. Mater. 2022, 430, 128359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Z.; Xu, G.; Li, G. Magnetic Porous Biochar with Nanostructure Surface Derived from Penicillin Fermentation Dregs Pyrolysis with K2FeO4 Activation: Characterization and Application in Penicillin Adsorption. Bioresour. Technol. 2021, 327, 124818. [Google Scholar] [CrossRef] [PubMed]
- Halil, D. Comprehensive Assessment of Thermochemical Processes for Sustainable Waste Management and Resource Recovery. Processes 2023, 11, 2092. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Wei, X.; Zhang, S.; Chen, J.; Ren, Z.J. CO2 Activation Promotes Available Carbonate and Phosphorus of Antibiotic Mycelial Fermentation Residue-Derived Biochar Support for Increased Lead Immobilization. Chem. Eng. J. 2018, 334, 1101–1107. [Google Scholar] [CrossRef]
- Gao, T.; Shi, W.; Zhao, M.; Huang, Z.; Liu, X.; Ruan, W. Preparation of Spiramycin Fermentation Residue Derived Biochar for Effective Adsorption of Spiramycin from Wastewater. Chemosphere 2022, 296, 133902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Z.; Xu, G.; Li, G. Pyrolysis of Penicillin Fermentation Residue and Sludge to Produce Biochar: Antibiotic Resistance Genes Destruction and Biochar Application in the Adsorption of Penicillin in Water. J. Hazard. Mater. 2021, 413, 125385. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, Y.; Ma, C.; Zhu, G.; Wang, Y.; Li, C. Pyrolysis of Antibiotic Mycelial Residue for Biochar: Kinetic Deconvolution, Biochar Properties, and Heavy Metal Immobilization. J. Environ. Manag. 2023, 328, 116956. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, C.; Xing, Y.; Li, Z.; Li, Y.; Yang, J.; Feng, L.; Hu, J.; Sun, H. Thermal Characteristics and Product Formation Mechanism during Pyrolysis of Penicillin Fermentation Residue. Bioresour. Technol. 2019, 277, 46–54. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, L.; Wang, Q.; Wang, J.; Wang, X.; Yang, J.; Tang, J. Nitrogen-Doped Porous Graphitized Carbon from Antibiotic Bacteria Residues Induced by Sodium Carbonate and Application in Li-Ion Battery. J. Electroanal. Chem. 2021, 889, 115179. [Google Scholar] [CrossRef]
- Hao, R.; Du, L.; Gu, X.; Li, S. Facile Synthesis of N-Rich Carbon Nanosheets Derived from Antibiotic Mycelial Dregs as Efficient Catalysts for Peroxymonosulfate Activation. Sep. Purif. Technol. 2023, 306, 122571. [Google Scholar] [CrossRef]
- Hao, X.; Chen, J.; Tan, R.; Ma, L.; Pan, J. Molecular Characterization and Functional Analysis of Glutathione S-Transferase Genes of Pine Wood Nematode (Bursaphelenchus Xylophilus) for Avermectin. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lu, H.; Xin, H.; Shi, Q.; Li, D.; Liu, L.; Sun, Z. Improve Avermectin Fermentation Residue Decomposition Efficiency, Bacterial Community and Mature Compost Quality by Inoculating Mature Compost. Waste Biomass Valorization 2024. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Chen, L.; Sun, L.; Zhao, B.; Si, H.; Xie, X.; Meng, F. Pyrolysis of Sawdust with Various Fe-Based Catalysts: Influence of Support Characteristics on Hydrogen-Rich Gas Production. J. Anal. Appl. Pyrolysis 2019, 137, 29–36. [Google Scholar] [CrossRef]
- Zou, L.; He, X.; Yang, W.; Shao, H.; Wang, Y.; Zhao, Q. Co-Pyrolysis of Peanut Shell with Municipal Sludge: Reaction Mechanism, Product Distribution, and Synergy. Environ. Sci. Pollut. Res. 2023, 30, 94081–94096. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Zhang, J.; Xu, F.; Yang, D. Determination of main component content in soybean by thermogravimetric analysis. Cereal Feed Ind. 2016, 11, 56–61. [Google Scholar]
- Xiao, R.; Sun, X.; Wang, J.; Feng, J.; Li, R.; Zhang, Z.; Wang, J.J.; Amjad, A. Characteristics and Phytotoxicity Assay of Biochars Derived from a Zn-Rich Antibiotic Residue. J. Anal. Appl. Pyrolysis 2015, 113, 575–583. [Google Scholar] [CrossRef]
- Wei, X.; Huang, S.; Yang, J.; Liu, P.; Li, X.; Wu, Y.; Wu, S. Adsorption of Phenol from Aqueous Solution on Activated Carbons Prepared from Antibiotic Mycelial Residues and Traditional Biomass. Fuel Process. Technol. 2023, 242, 107663. [Google Scholar] [CrossRef]
- Wei, X.; Huang, S.; Wu, Y.; Wu, S. Effects of Washing Pretreatment on Properties and Pyrolysis Biochars of Penicillin Mycelial Residues. Biomass Bioenergy 2022, 161, 106477. [Google Scholar] [CrossRef]
- Feng, L.; Li, Z.; Hong, C.; Xing, Y.; Qin, Y.; Lü, Y.; Zhao, X.; Lü, J. Characteristic Analysis of Bio-Oil from Penicillin Fermentation Residue by Catalytic Pyrolysis. Environ. Technol. 2023, 44, 2481–2489. [Google Scholar] [CrossRef]
- Chen, Y.; Du, L.; Li, S.; Song, W.; Jensen, P.A.; Lin, W. Pyrolysis of Antibiotic Mycelial Dreg and Characterization of Obtained Gas, Liquid and Biochar. J. Hazard. Mater. 2021, 402, 123826. [Google Scholar] [CrossRef] [PubMed]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical Processing of Sewage Sludge to Energy and Fuel: Fundamentals, Challenges and Considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Du, J.; Li, C.; Zhou, X. Pyrolysis behavior of antibiotic residues and the mechanism of nitrogen evolution. J. Fuel Chem. Technol. 2023, 51, 949–958. [Google Scholar] [CrossRef]
- Li, Y.; Hong, C.; Li, Z.; Xing, Y.; Chang, X.; Zheng, Z.; Zhao, X. Study on the Nitrogen Migration Mechanism during Penicillin Fermentation Residue Fast Pyrolysis Based on the Substance Transformation and Canonical Variational Theory. Sci. Total Environ. 2020, 737, 139739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hong, C.; Wang, Y.; Xing, Y.; Chang, X.; Zheng, Z.; Li, Z.; Zhao, X. Nitrogen Migration Mechanism during Pyrolysis of Penicillin Fermentation Residue Based on Product Characteristics and Quantum Chemical Analysis. ACS Sustain. Chem. Eng. 2020, 8, 7721–7740. [Google Scholar] [CrossRef]
- Santos, R.M.; Santos, A.O.; Sussuchi, E.M.; Nascimento, J.S.; Lima, Á.S.; Freitas, L.S. Pyrolysis of Mangaba Seed: Production and Characterization of Bio-Oil. Bioresour. Technol. 2015, 196, 43–48. [Google Scholar] [CrossRef]
- Paula, S.; Cindy, A.B.; Melisa, B.; Richard, P.; Marisa, F.; Ulises, S. Characterization of Pyrolytic Tars Derived from Different Biomasse. Processes 2024, 12, 817. [Google Scholar] [CrossRef]
- Yuan, H.; Li, C.; Shan, R.; Zhang, J.; Chen, Y. Nitrogen-containing Species Evolution during Co-Pyrolysis of Gentamicin Residue and Biomass. J. Anal. Appl. Pyrolysis 2023, 169, 105812. [Google Scholar] [CrossRef]
- Li, J.; Xiong, Z.; Zeng, K.; Zhong, D.; Zhang, X.; Chen, W.; Nzihou, A.; Flamant, G.; Yang, H.; Chen, H. Characteristics and Evolution of Nitrogen in the Heavy Components of Algae Pyrolysis Bio-Oil. Environ. Sci. Technol. 2021, 55, 6373–6385. [Google Scholar] [CrossRef]
- Hu, J.; Hong, C.; Zhao, C.; Si, Y.; Xing, Y.; Ling, W.; Zhang, B.; Li, Z.; Wang, Y.; Feng, L.; et al. Nitrogen Self-Doped Hierarchical Porous Carbon via Penicillin Fermentation Residue (PR) Hydrothermal Carbonization (HTC) and Activation for Supercapacitance. J. Alloys Compd. 2022, 918, 165452. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, X.; Wang, J.; Jin, X.; Liu, Y.; Qian, F.; Zhang, S.; Chen, J. Combustion of Hazardous Biological Waste Derived from the Fermentation of Antibiotics Using TG–FTIR and Py–GC/MS Techniques. Bioresour. Technol. 2015, 193, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Monroe, A.P.R.; Silva, A.V.S.; Melo, M.S.; Da Silva, J.B.S.; Peña Garcia, R.R.; Rios, M.A.D.S.; Bizzo, W.A.; Cruz, G. Evaluation of the Bioenergy Potential of Blends (Green Coconut Shells and Fish Scales) as a Feedstock in Thermochemical Processes for Clean Energy Production. Processes 2024, 12, 710. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Cen, K.; Luo, M.; Li, H.; Lu, B. Pyrolysis Polygeneration of Poplar Wood: Effect of Heating Rate and Pyrolysis Temperature. Bioresour. Technol. 2016, 218, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Zhu, X.; Luo, J.; Zhou, S.; Zhang, C.; Fan, J.; Clark, J.H.; Zhang, S. Phosphorus and Nitrogen Transformation in Antibiotic Mycelial Residue Derived Hydrochar and Activated Pyrolyzed Samples: Effect on Pb (II) Immobilization. J. Hazard. Mater. 2020, 393, 122446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; Zhao, X.; Wang, S.; Xing, G. Comparisons of Biochar Properties from Wood Material and Crop Residues at Different Temperatures and Residence Times. Energy Fuels 2013, 27, 5890–5899. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, G.; Zhao, P.; Areeprasert, C.; Shen, Y.; Yoshikawa, K.; Xu, G. Hydrothermal Treatment of Antibiotic Mycelial Dreg: More Understanding from Fuel Characteristics. Chem. Eng. J. 2015, 273, 147–155. [Google Scholar] [CrossRef]
- Feng, L.; Xing, Y.; Yang, P. Characteristics of pyrolysis and gaseous pollutant emissions of antibiotic bacterial residue. Saf. Environ. Eng. 2018, 25, 89–96. [Google Scholar] [CrossRef]



| Proximate Analysis (wt%) a | Ultimate Analysis (wt%) | H/C Ratio | O/C Ratio | HHV (MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatiles | Fixed Carbon | C | H | O b | N | S | |||
| 4.41 | 8.70 | 75.77 | 11.12 | 45.32 | 6.26 | 31.95 | 6.76 | 0.61 | 1.66 | 0.56 | 19.46 |
| Samples | Elements (wt%) | |||
|---|---|---|---|---|
| C | O | P | Ca | |
| AV | 28.43 ± 0.23 | 62.93 ± 0.06 | 1.85 ± 0.17 | 6.78 ± 0.13 |
| 400 °C | 31.14 ± 0.13 | 47.79 ± 0.29 | 5.80 ± 0.11 | 15.27 ± 0.10 |
| 500 °C | 22.52 ± 0.19 | 36.28 ± 0.24 | 7.74 ± 0.20 | 33.28 ± 0.15 |
| 600 °C | 17.60 ± 0.16 | 39.64 ± 0.11 | 9.43 ± 0.19 | 33.33 ± 0.08 |
| 700 °C | 21.87 ± 0.26 | 37.40 ± 0.14 | 9.03 ± 0.09 | 31.71 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Hou, J.; Chen, L.; Yang, F.; Li, T.; Sun, L.; Hua, D. Study on Pyrolysis Behavior of Avermectin Mycelial Residues and Characterization of Obtained Gas, Liquid, and Biochar. Processes 2024, 12, 1118. https://doi.org/10.3390/pr12061118
Yang S, Hou J, Chen L, Yang F, Li T, Sun L, Hua D. Study on Pyrolysis Behavior of Avermectin Mycelial Residues and Characterization of Obtained Gas, Liquid, and Biochar. Processes. 2024; 12(6):1118. https://doi.org/10.3390/pr12061118
Chicago/Turabian StyleYang, Shuangxia, Jianjun Hou, Lei Chen, Feixia Yang, Tianjin Li, Laizhi Sun, and Dongliang Hua. 2024. "Study on Pyrolysis Behavior of Avermectin Mycelial Residues and Characterization of Obtained Gas, Liquid, and Biochar" Processes 12, no. 6: 1118. https://doi.org/10.3390/pr12061118
APA StyleYang, S., Hou, J., Chen, L., Yang, F., Li, T., Sun, L., & Hua, D. (2024). Study on Pyrolysis Behavior of Avermectin Mycelial Residues and Characterization of Obtained Gas, Liquid, and Biochar. Processes, 12(6), 1118. https://doi.org/10.3390/pr12061118






