Effect of Y2O3 Content on Microstructure and Wear Resistance of Laser Cladding Layer of Stellite-6 Alloy
Abstract
:1. Introduction
2. Experiment
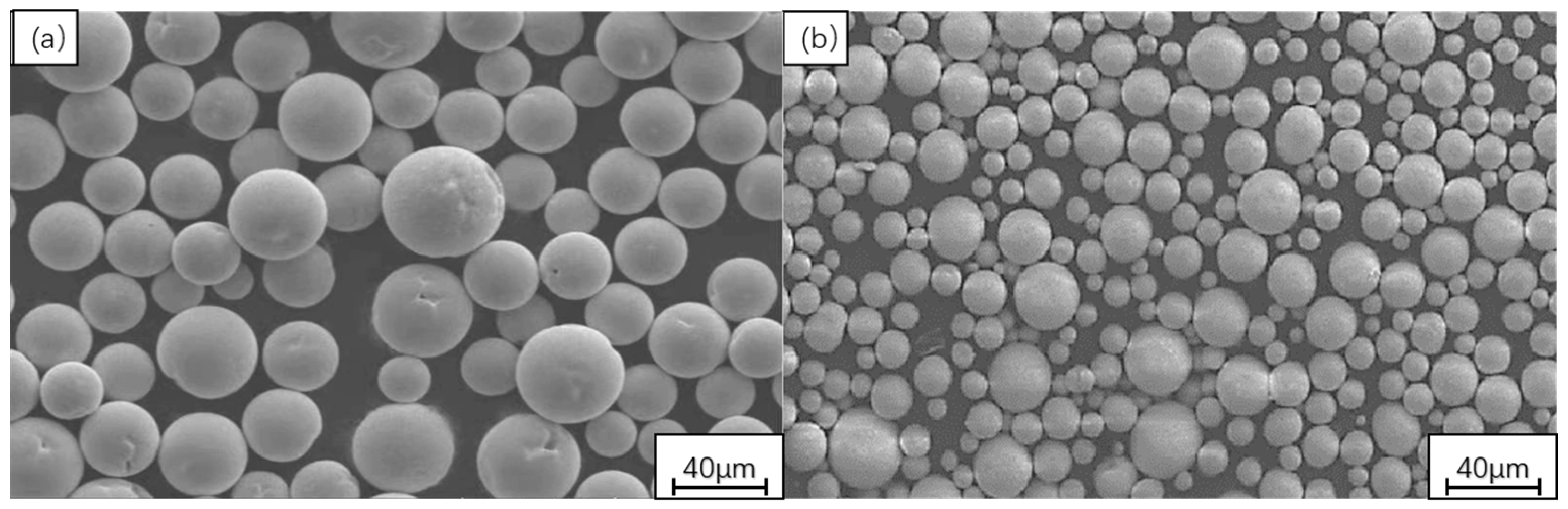
2.1. Experimental Materials
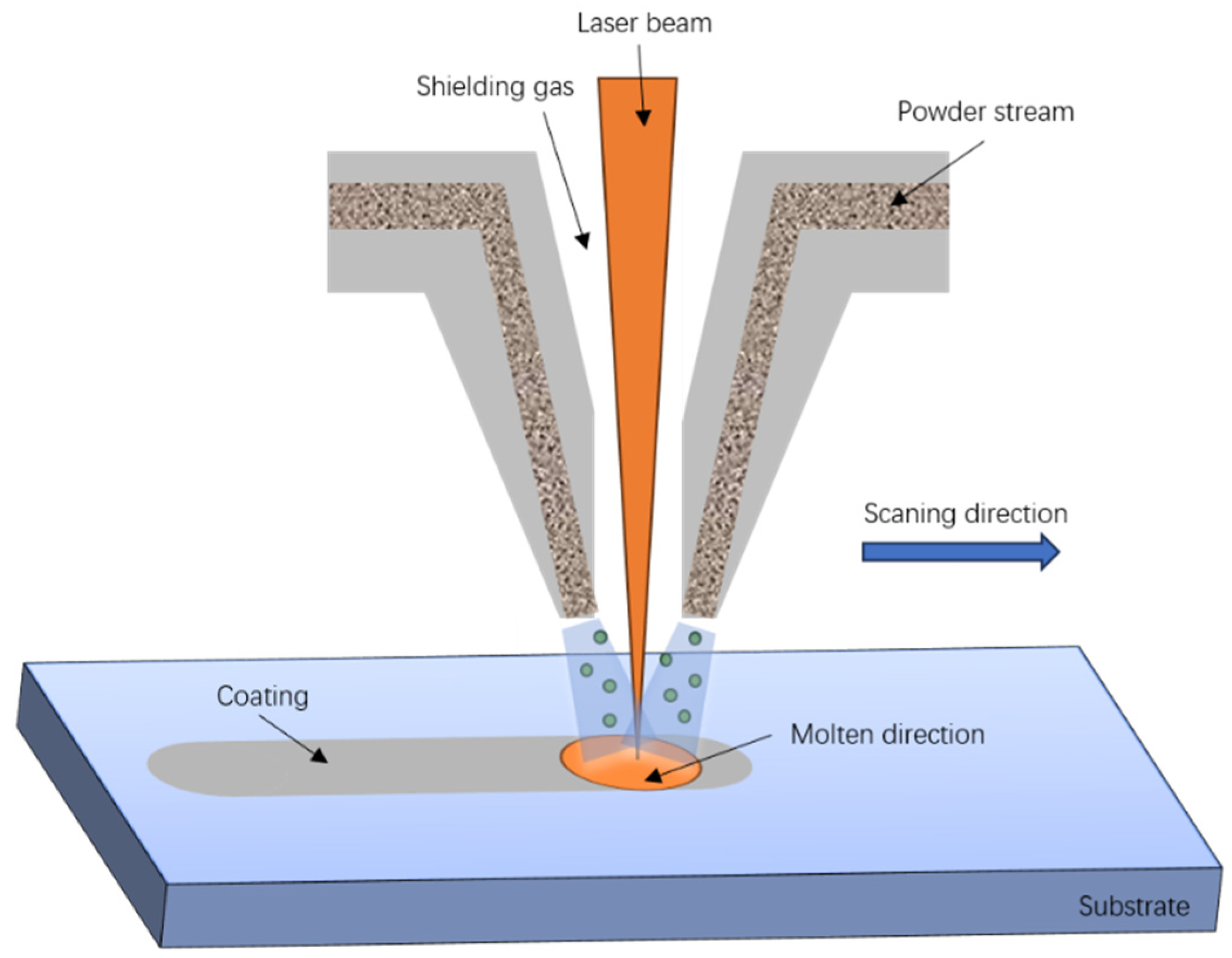
2.2. Experimental Methods
3. Results and Discussion
3.1. Macroscopic Morphology
3.2. Phase Composition
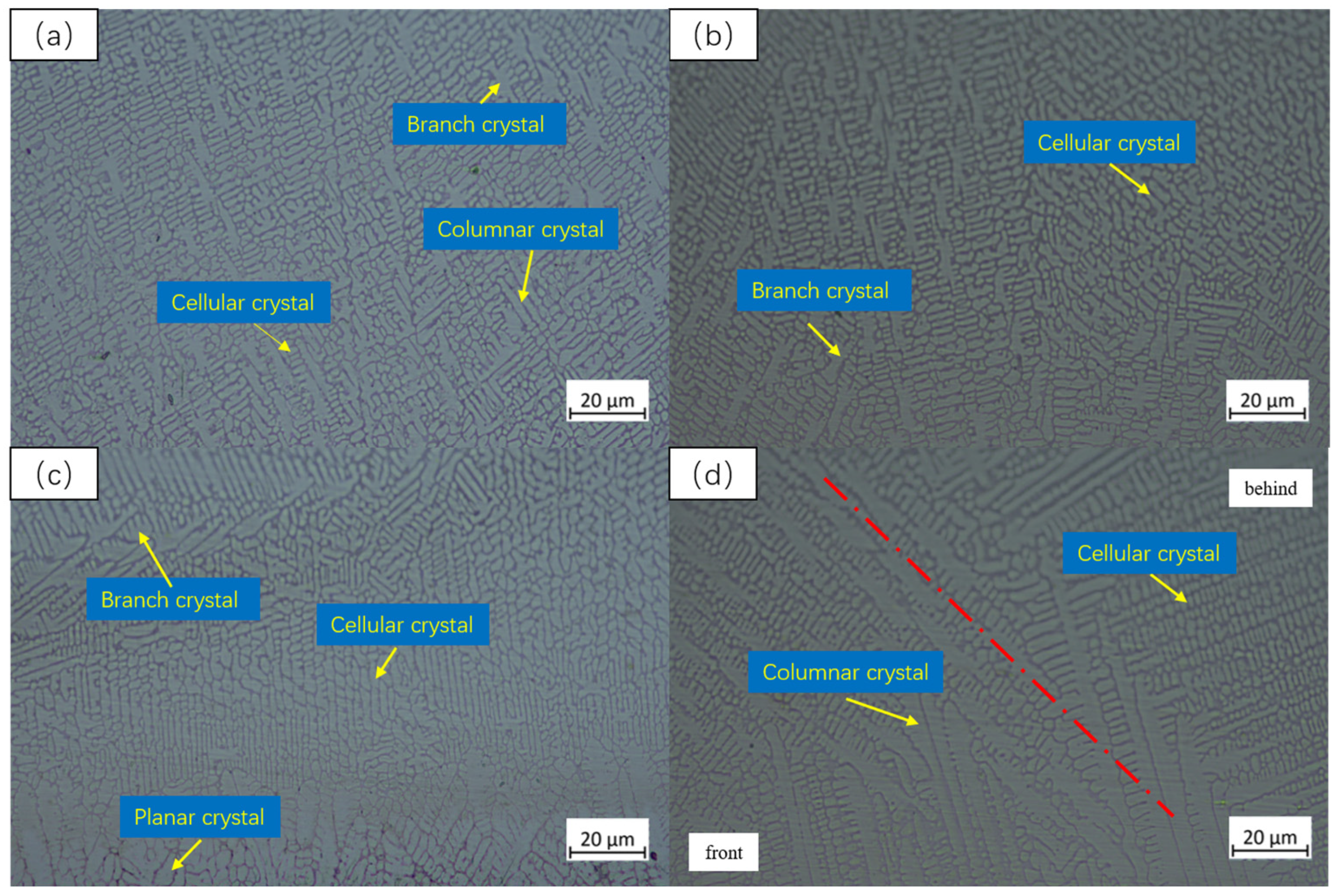
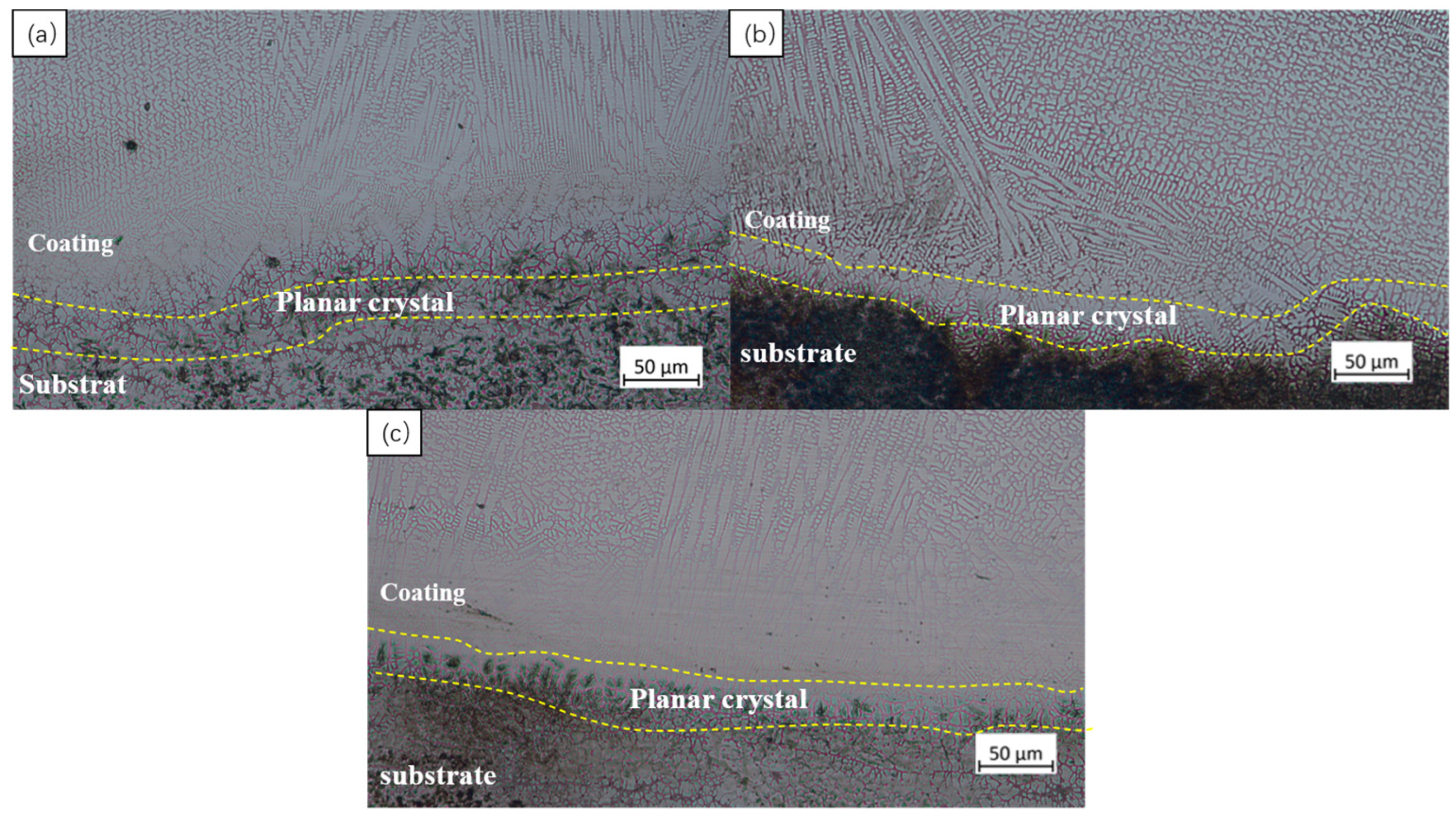
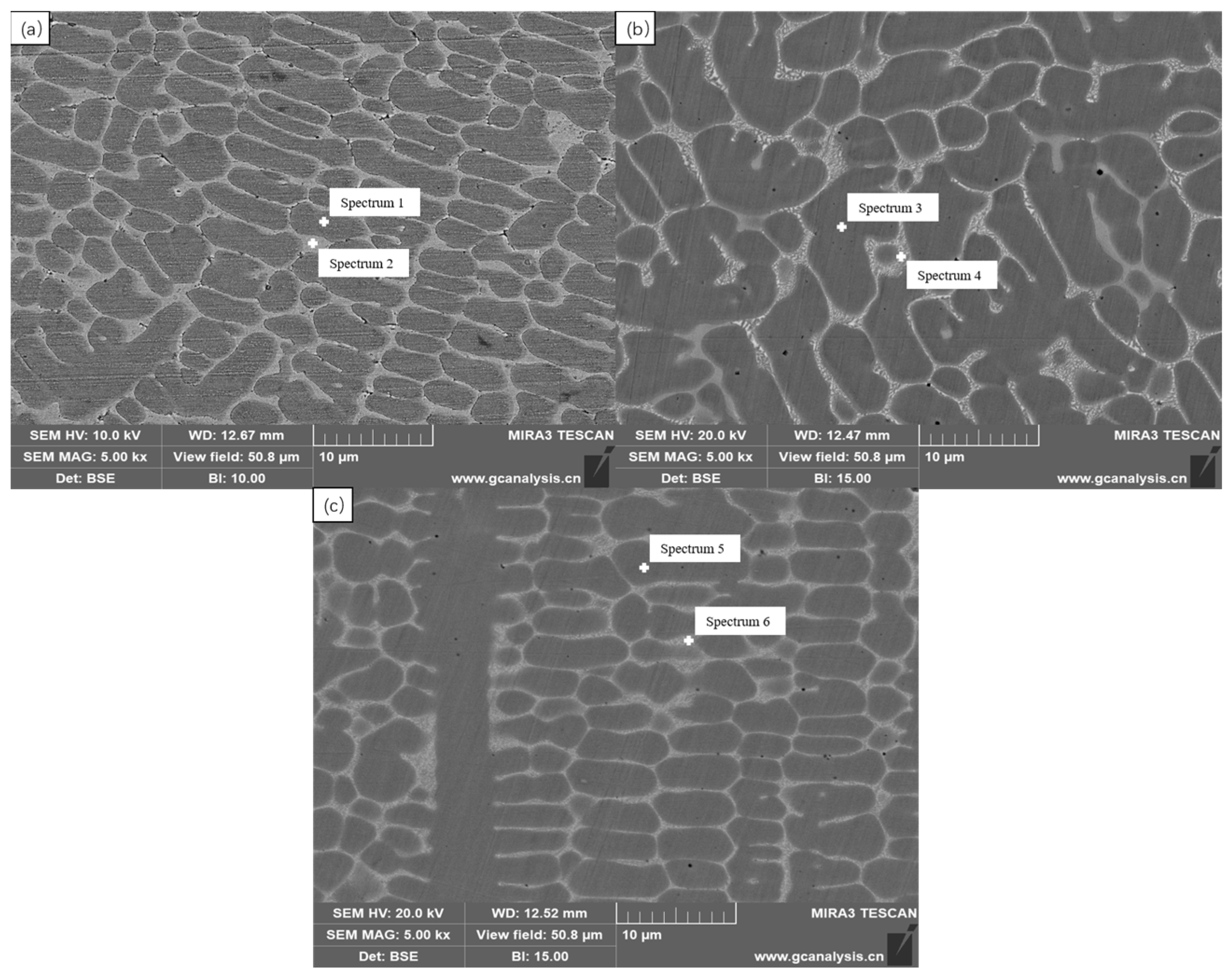
3.3. Microstructure
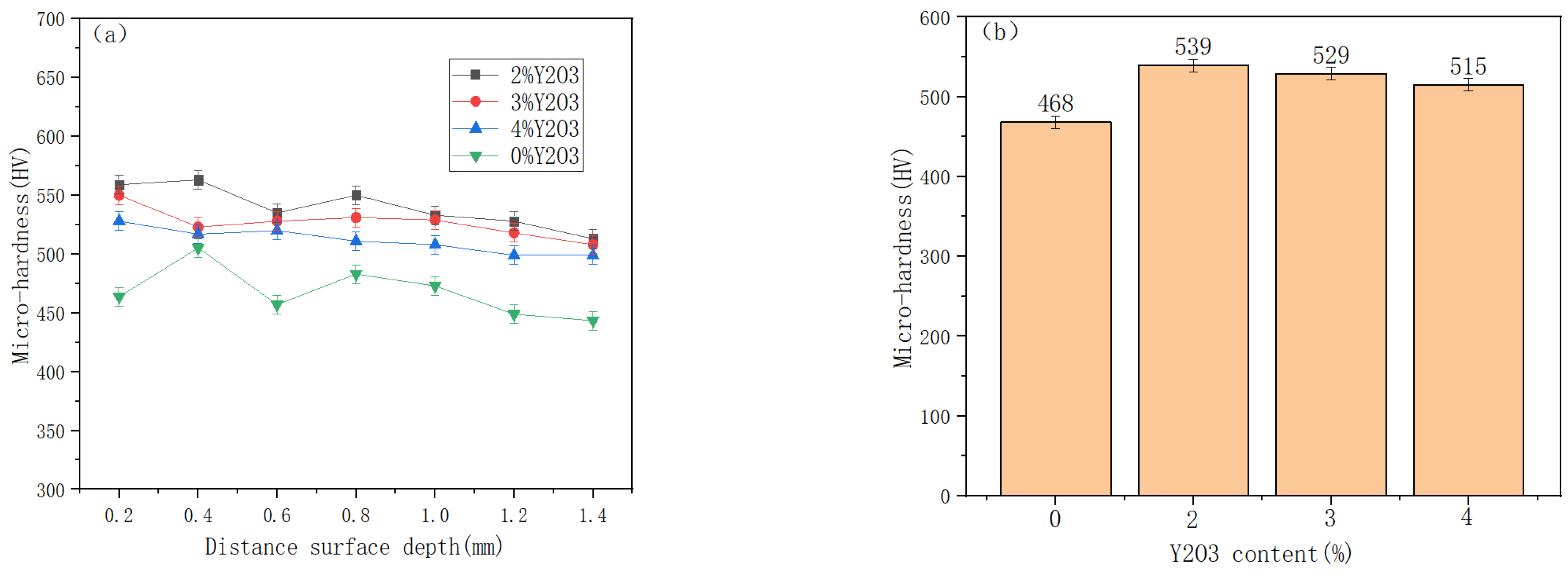
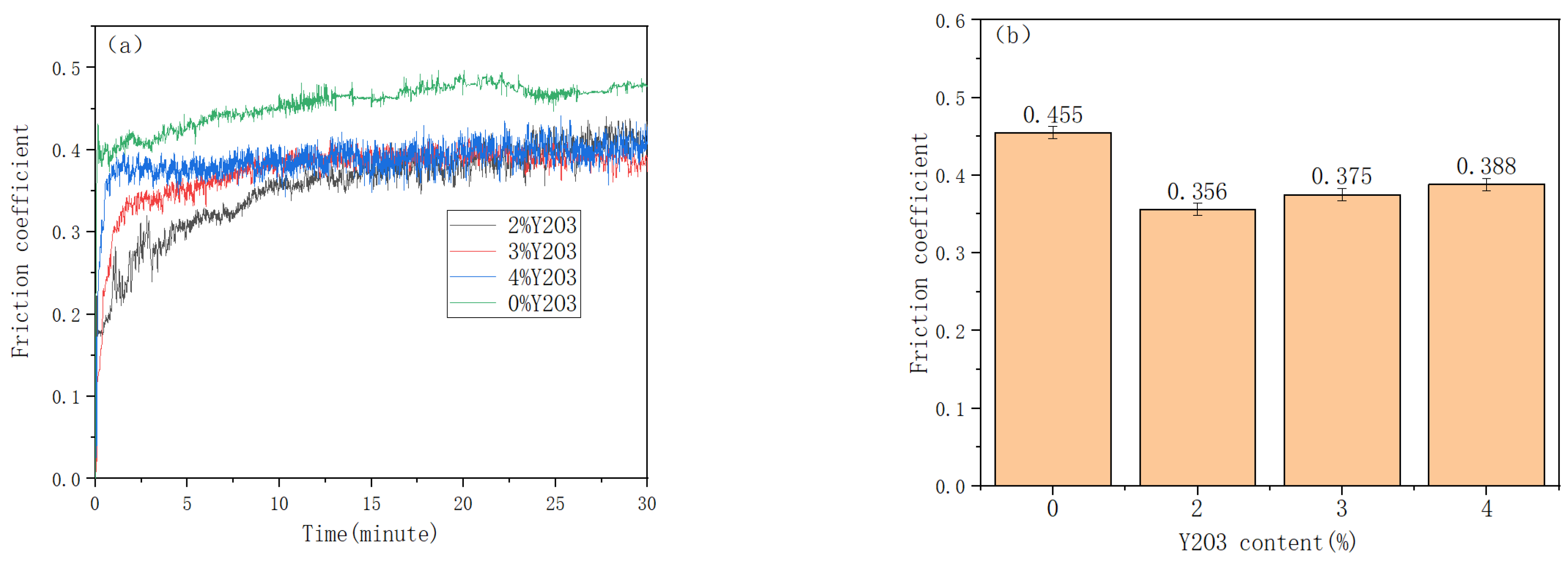
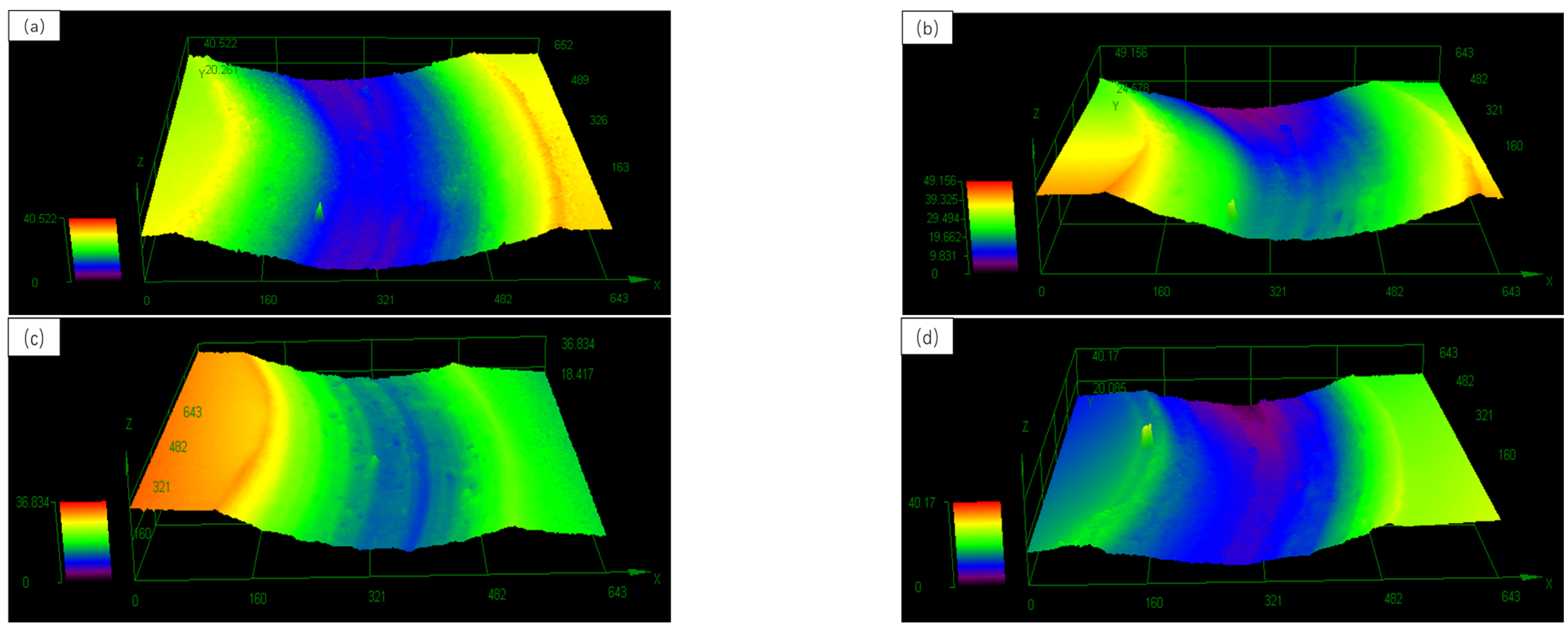
3.4. Microhardness and Friction Wear of Cladding Layer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Liu, H. Technology and Application of Laser Rapid Manufacturing; Chemical Industry Press: Beijing, China, 2009; p. 149. [Google Scholar]
- Wang, H.P.; Zhu, P.H.; Shu, F.Y.; He, P. Simulation of temperature field and performance of laser cladding on 65Mn steel surface. J. Mech. Eng. 2023, 59, 216–224. [Google Scholar] [CrossRef]
- Bozzi, A.C.; Ramos, F.D.; Vargas, D.B.O. Microabrasive wear behavior of different stellites obtained by laser cladding and casting processes. Wear 2023, 524–525, 204857. [Google Scholar] [CrossRef]
- Chen, K.; Chen, Y.; Kong, L.; Cao, J.; Liu, Z.; Liang, X. Research progress and perspective in the annular laser cladding technology. Surf. Technol. 2022, 51, 1–10+41. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Ou, W.; Yuan, X.; Zhang, L.; Xu, J.; Yang, J.; Jiao, Y.; Zhang, H.; Chen, Z.; et al. Cracking mechanisms and prevention of Stellite 6# surface layer built by laser cladding during thermal cyclic process. Int. J. Fatig. 2023, 168, 107439. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, B.; Cui, H.; Lu, F.; Xu, P. Microstructure, wear, and oxidation resistance of nanostructured carbide-strengthened cobalt-based composite coatings on Invar alloys by laser cladding. J. Surf. Coat. Technol. 2020, 381, 125188. [Google Scholar] [CrossRef]
- Qin, R.; Chen, B.; Zhang, G.; Liu, W.; Li, N.; Huang, S.; Gao, C. Effect of Post-heat Treatment on Microstructure and Mechanical Properties of Laser Cladded High Co-Ni Secondary Hardening Steel Coating. J. Phys. Conf. Ser. 2024, 2706, 012023. [Google Scholar] [CrossRef]
- Fan, L.; Chen, H.; Zhu, G.; Cao, Q.; Dong, L. Tribological Properties Laser-Cladded Spherical WB-Reinforced Co-Based Coatings under Low-Temperature Friction. Materials 2023, 16, 6444. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Shi, Y.; Jiang, G.; Tang, S.; He, X. Influence of carrier gas flow rate on microstructure and corrosion resistance of laser clad Ni-based coatings with Y2O3. Heat Treat. Met. 2024, 49, 281–290. [Google Scholar] [CrossRef]
- Guo, X. Preparation Technology and Tribological Properties of Ni-Based Self-Lubricating Coating on H13 Steel Surface; University of Jinan: Jinan, China, 2023. [Google Scholar] [CrossRef]
- Yang, F.; Chen, F.; Chen, J.; Xu, Z.; Zhao, Z.; Wang, Z.; He, Z. Microstructures and properties of La2O3 cladding layer on 316L steel. J. Univ. Sci. Technol. 2020, 43, 6. [Google Scholar] [CrossRef]
- Guo, J.T.; Huai, K.W.; Gao, Q.; Ren, W.L.; Li, G.S. Effects of rare earth elements on the microstructure and mechanical properties of NiAl-based eutectic alloy. Intermetallics 2007, 15, 727–733. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, C.; Liu, J.; Xu, H. Effect of rare earth La2O3 content on laser cladding 304L coating. Appl. Laser 2021, 41, 968–973. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, X.; Jiang, J.; Lian, G.; Chen, C. Study on the Effect of Rare Earth Oxide Addition on the Microstructure and Properties of Ni60/WC-Ni Coatings Prepared by Laser Cladding. Materials 2023, 16, 7263. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wang, L.; Gao, Z.; Yang, X.; Liu, T.; Zhan, X. Microstructure and element distribution characteristics of Y2O3 modulated WC reinforced coating on Invar alloys by laser cladding. Opt. Laser Technol. 2022, 153, 108205. [Google Scholar] [CrossRef]
- Kang, R.Q.; Liu, W.J.; Ma, M.X.; Zhong, M.L.; Zhang, W.M.; Zhang, H.J. The effect of rear earth oxides on particles distribution in laser cladding particles reinforced Ni-base composite coatings. Appl. Laser 2009, 29, 374–378. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Hao, H.; Gao, Q.; Cao, Y.B.; Han, R.H.; Qi, H.B. Strengthening mechanism and high-temperature properties of H13 + WC/Y2O3 laser-cladding coatings. Surf. Coat. Technol. 2021, 405, 126544. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Xu, X.; Luo, K.; Lu, J. The effect of Y2O3 nanoparticle addition on the microstructure and high-temperature corrosion resistance of IN718 deposited by extreme high-speed laser cladding. Surf. Coat. Technol. 2024, 476, 130235. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Liu, S.; Qu, K. Microstructure and high-temperature properties of Fe-Ti-Cr-Mo-BC-Y2O3 laser cladding coating. J. Rare Earths 2020, 38, 683–688. [Google Scholar] [CrossRef]
- Voropaev, A.A.; Protsenko, V.G.; Anufriyev, D.A.; Kuznetsov, M.V.; Mukhin, A.A.; Sviridenko, M.N.; Kuryntsev, S.V. Influence of Laser Beam Wobbling Parameters on Microstructure and Properties of 316L Stainless Steel Multi Passed Repaired Parts. Materials 2022, 15, 722. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, C.; Zhang, M. Effect of heat treatments on the microstructural evolution and tribological behaviors of a NiCrSiB/Y2O3 alloy fabricated by laser additive manufacturing. Mater. Charact. 2020, 165, 110401. [Google Scholar] [CrossRef]
- Zhu, R.; Li, Z.; Li, X.; Sun, Q. Microstructure and properties of the low-power-laser clad coatings on magnesium alloy with different amount of rare earth addition. Appl. Surf. Sci. 2015, 353, 405–413. [Google Scholar] [CrossRef]
- Li, M.; He, Y.; Yuan, X. Effect of nano-Y2O3 on microstructure of laser cladding cobalt-based alloy coatings. Appl. Surf. Sci. 2005, 252, 2882–2887. [Google Scholar] [CrossRef]
- Quazi, M.M.; Fazal, M.A.; Haseeb, A.S.M.A.; Yusof, F.; Masjuki, H.H.; Arslan, A. Effect of rare earth elements and their oxides on tribo-mechanical performance of laser claddings: A review. J. Rare Earths 2016, 34, 549–564. [Google Scholar] [CrossRef]






| Element | W | Mo | Cr | V | C | Mn | Si | Cu | Ni | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 6.19 | 4.94 | 4.10 | 1.87 | 0.882 | 0.34 | 0.40 | 0.10 | 0.12 | Bal. |
| Element | C | Cr | Si | W | Fe | Mo | Ni | Mn | Co |
|---|---|---|---|---|---|---|---|---|---|
| Content | 1.15 | 29.00 | 1.10 | 4.00 | 3.00 | 1.00 | 3.00 | 0.50 | Bal. |
| Process Parameters | Operation Range |
|---|---|
| Laser power (W) | 1600 |
| Scanning speed (mm/s) | 15 |
| Powder feeding rate (r/min) | 15 |
| Laser spot diameter (mm) | 3 |
| Overlap rate (%) | 30 |
| Protecting gas (Ar L/min) | 5 |
| Spectrum Label | Spectrum1 | Spectrum2 | Spectrum3 | Spectrum4 | Spectrum5 | Spectrum6 |
|---|---|---|---|---|---|---|
| C | 2.25 | 4.01 | 2.60 | 4.88 | 1.72 | 3.84 |
| Si | 0.19 | - | 0.20 | - | 0.34 | 0.21 |
| V | 0.50 | 1.54 | 0.43 | 1.93 | 0.45 | 1.49 |
| Cr | 13.71 | 21.73 | 13.76 | 27.53 | 15.77 | 29.26 |
| Mn | - | 0.46 | - | - | - | - |
| Fe | 44.98 | 33.96 | 46.42 | 33.68 | 38.33 | 30.36 |
| Co | 34.71 | 24.10 | 34.11 | 22.81 | 41.39 | 28.13 |
| Cu | 1.60 | 7.82 | 0.40 | - | - | - |
| Mo | 0.51 | 2.14 | 0.52 | 2.98 | 0.52 | 2.00 |
| W | 1.54 | 4.25 | 1.55 | 6.21 | 1.48 | 4.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, K.; Feng, A.; Ye, Z. Effect of Y2O3 Content on Microstructure and Wear Resistance of Laser Cladding Layer of Stellite-6 Alloy. Processes 2024, 12, 1119. https://doi.org/10.3390/pr12061119
Xia K, Feng A, Ye Z. Effect of Y2O3 Content on Microstructure and Wear Resistance of Laser Cladding Layer of Stellite-6 Alloy. Processes. 2024; 12(6):1119. https://doi.org/10.3390/pr12061119
Chicago/Turabian StyleXia, Kun, Aixin Feng, and Zhuolun Ye. 2024. "Effect of Y2O3 Content on Microstructure and Wear Resistance of Laser Cladding Layer of Stellite-6 Alloy" Processes 12, no. 6: 1119. https://doi.org/10.3390/pr12061119





