Three-Dimensional Heterogeneous Salt Cavern Underground Gas Storage Water Solution Cavity Model Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mathematical Modelling of Cavity Expansion
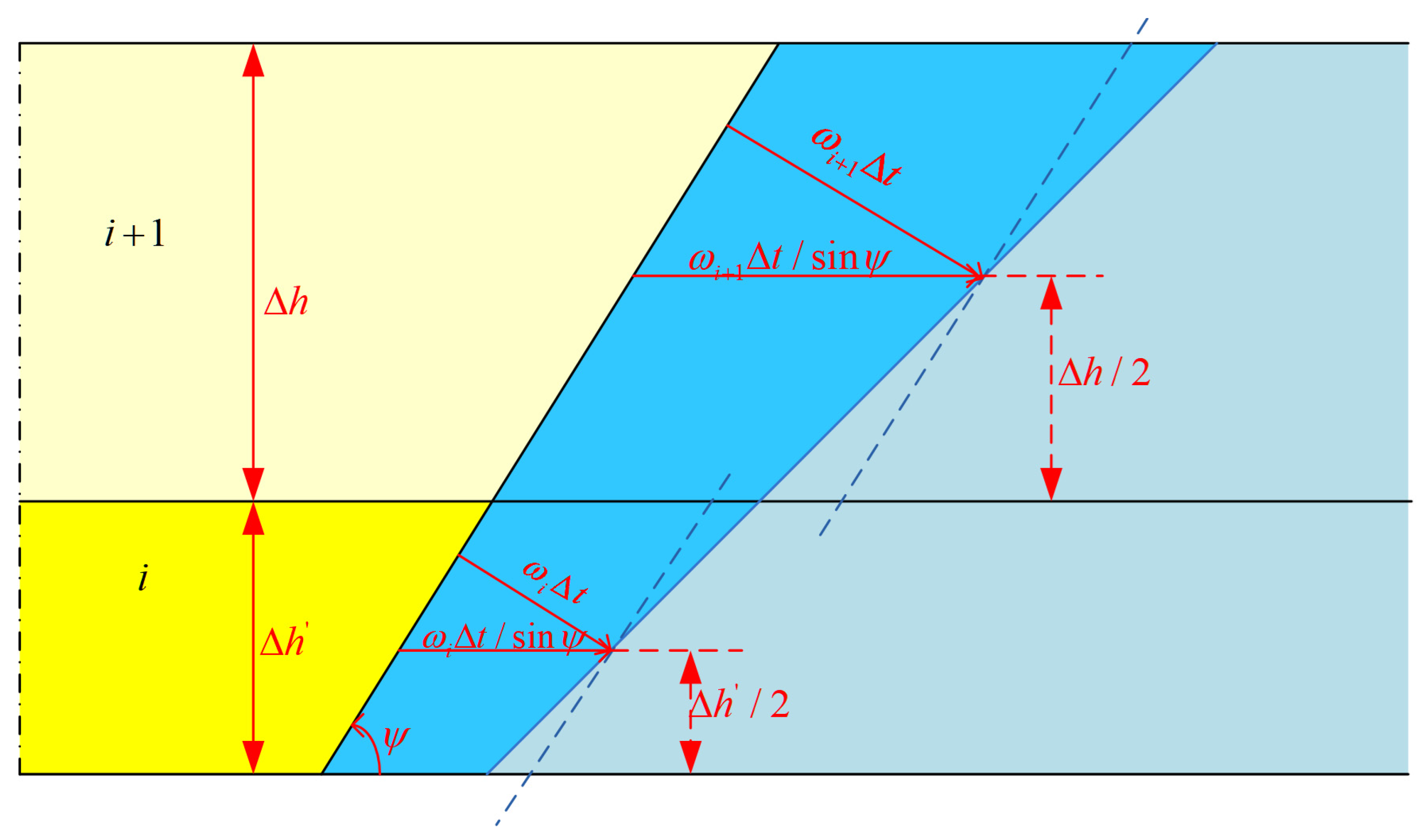
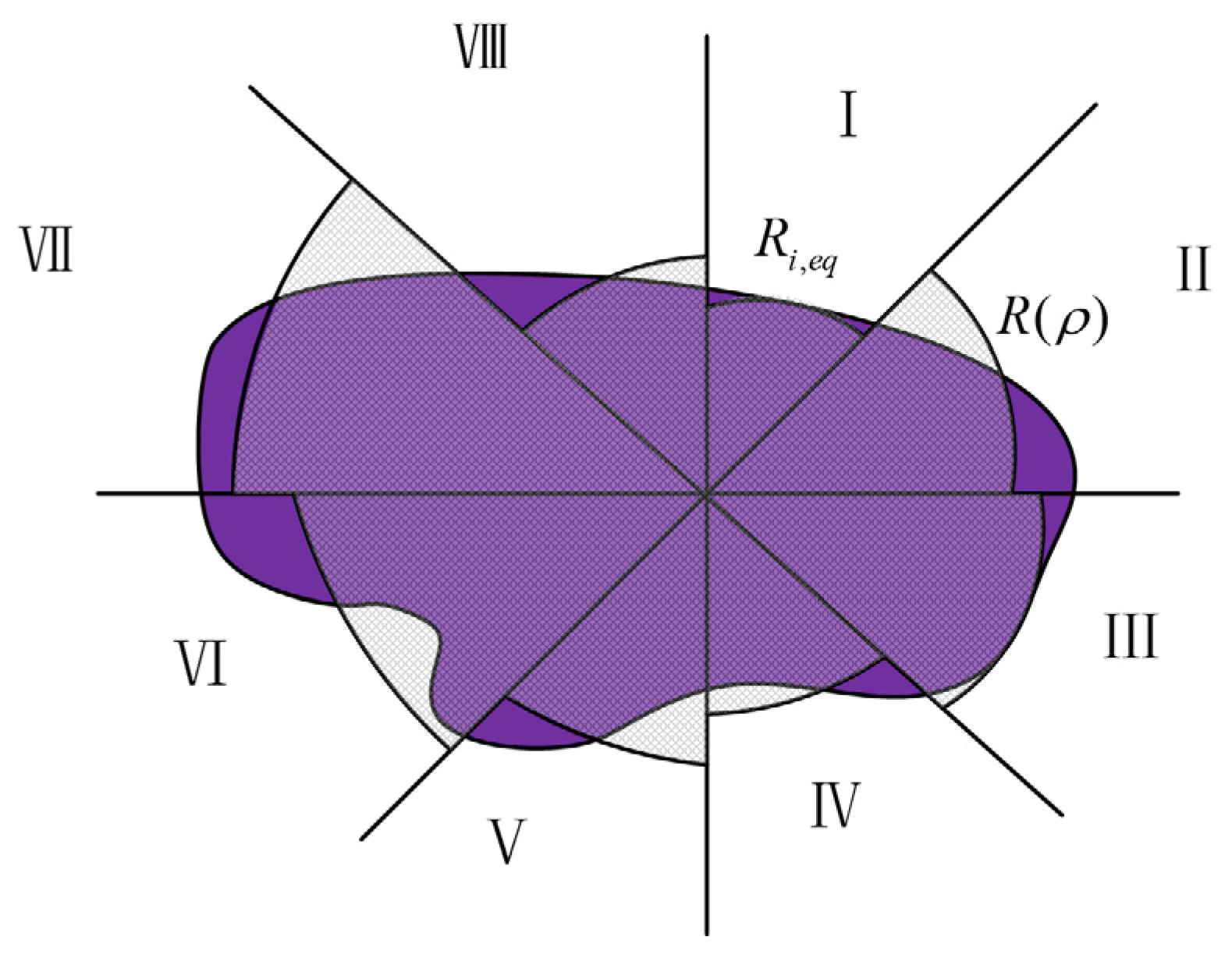
2.2. Cavity Morphology Post-Processing Model
2.3. Mathematical Modeling of Brine Concentration in the Cavity
3. Results
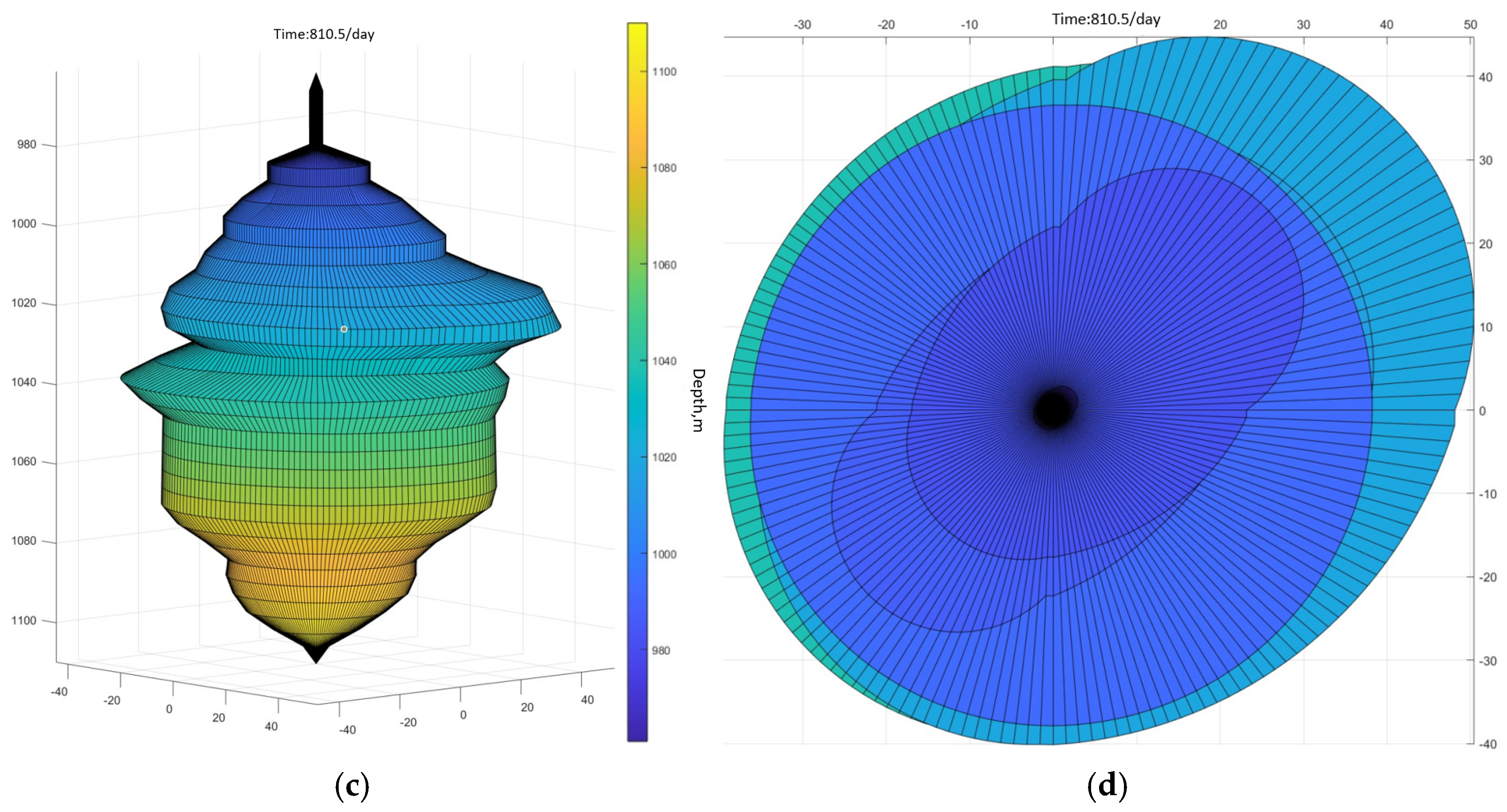
3.1. Cavity Simulation Demonstration
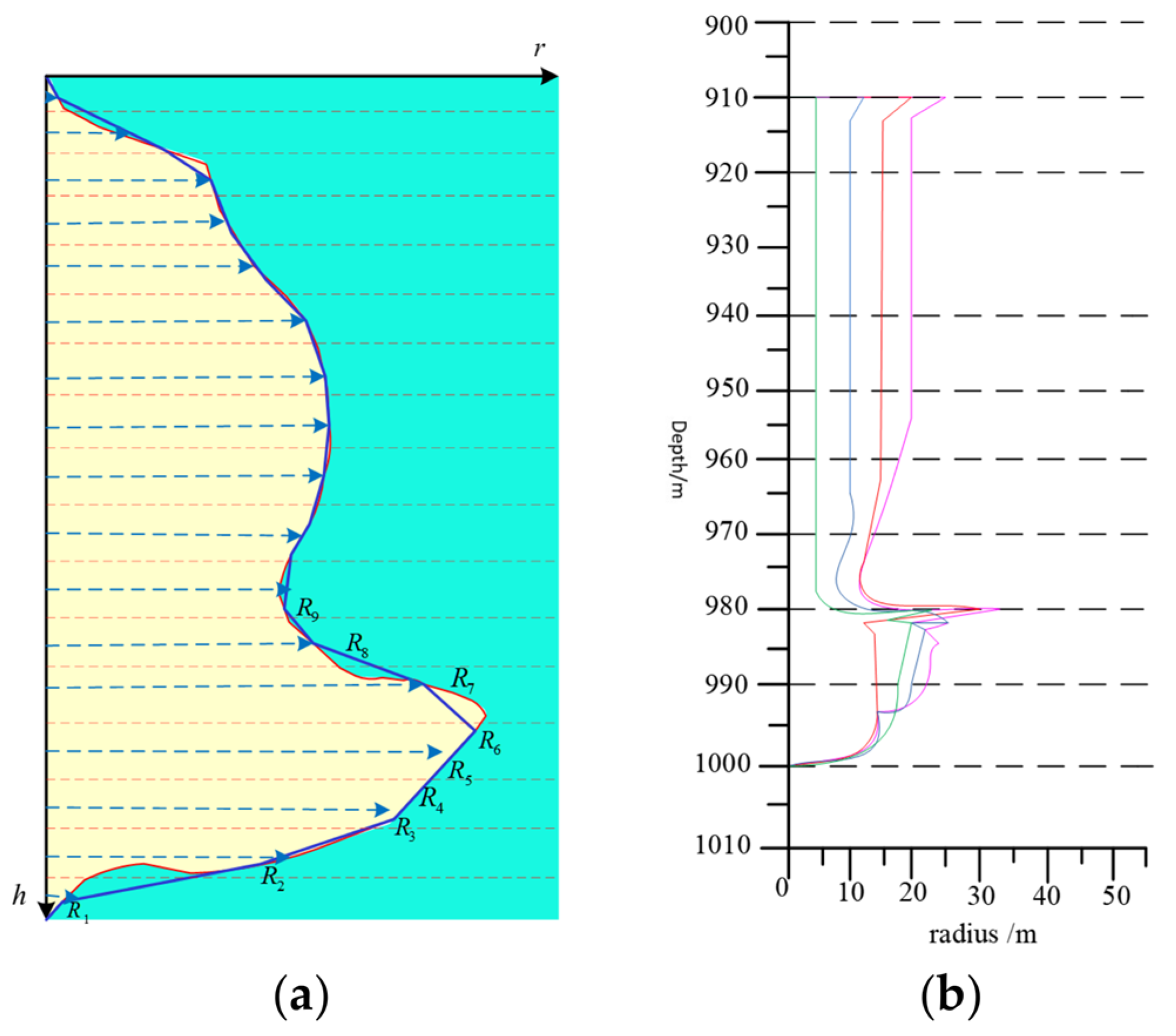
3.2. Mathematical Model Validation
3.3. Analysis of Results
4. Discussion
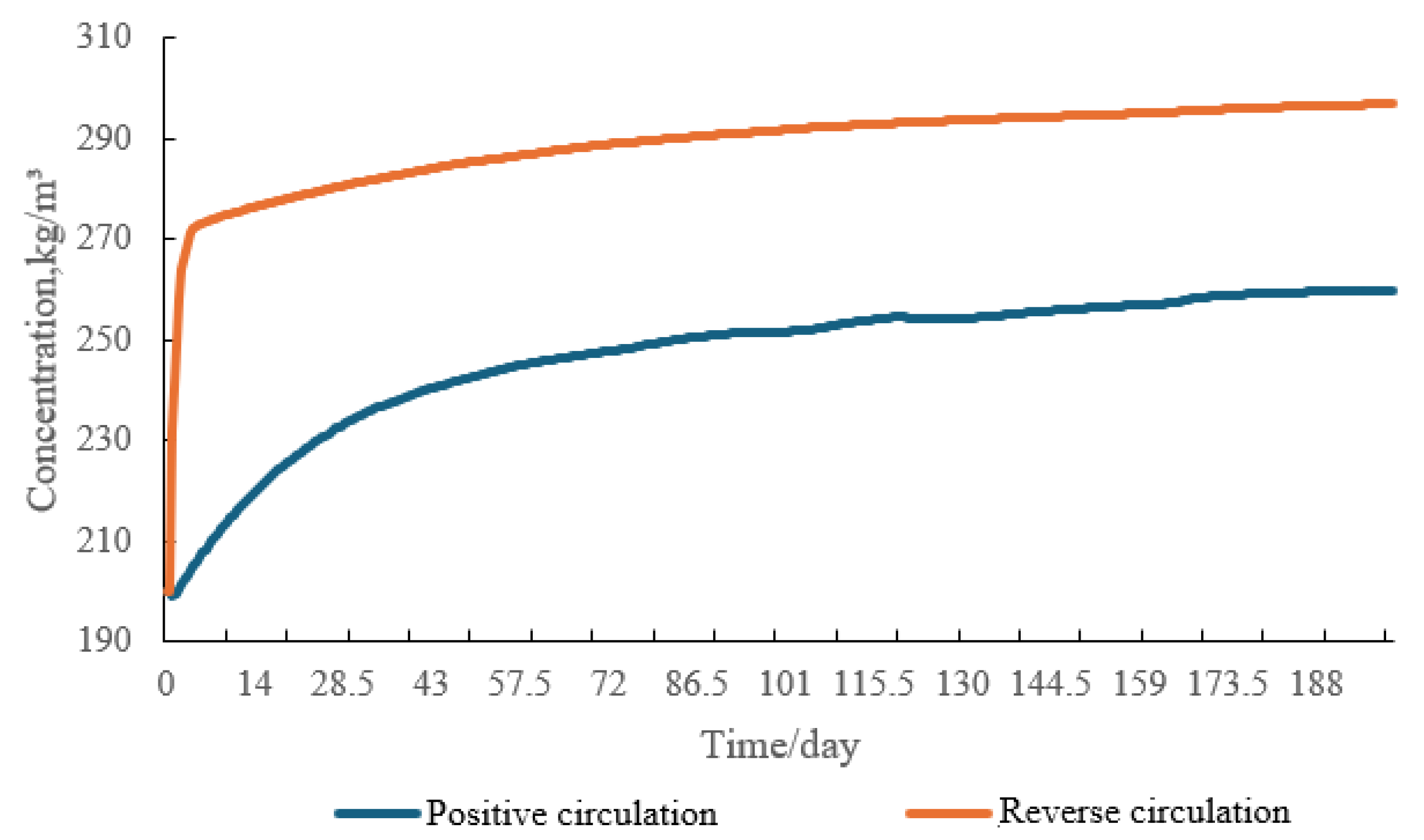
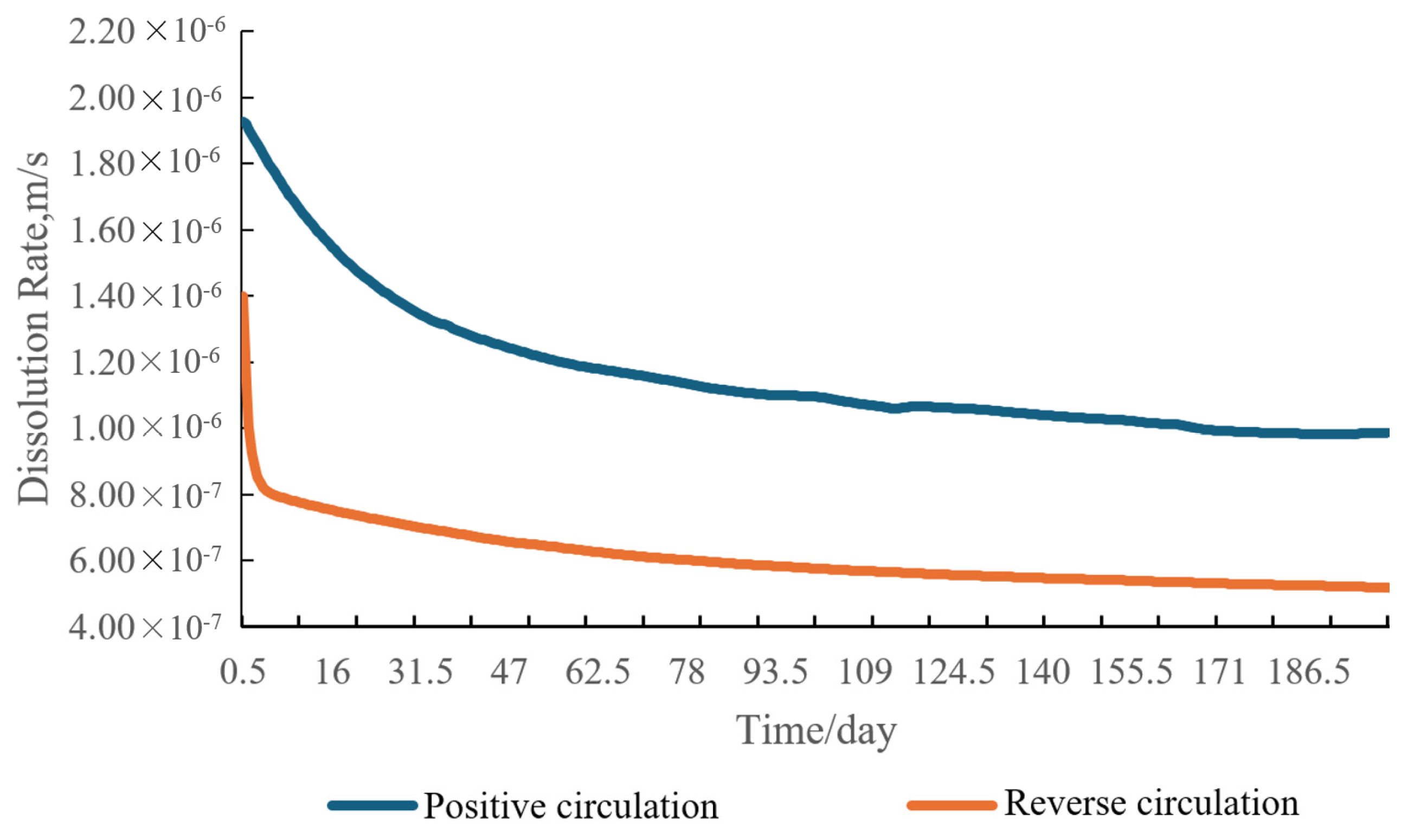
4.1. Circulation Mode Analysis
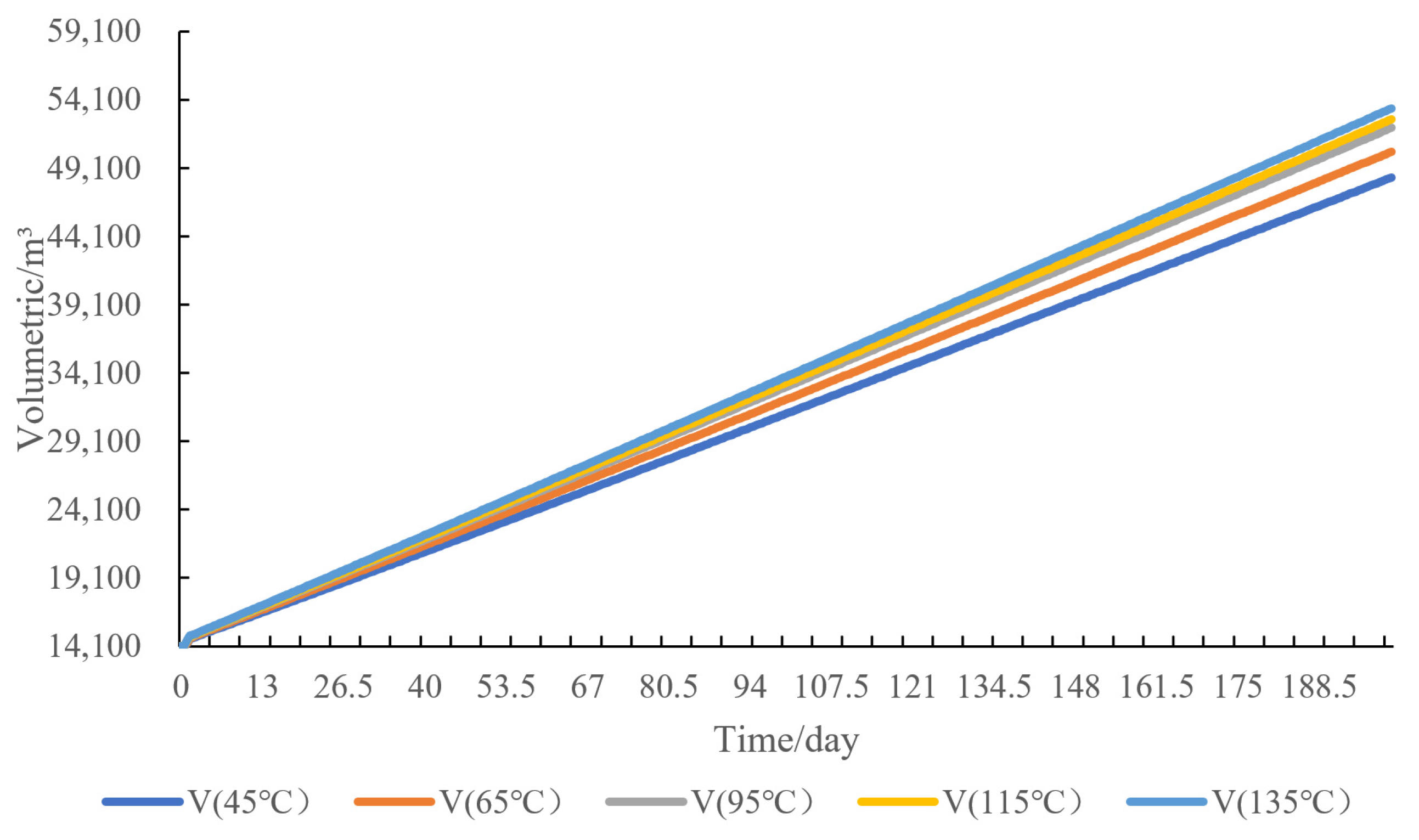
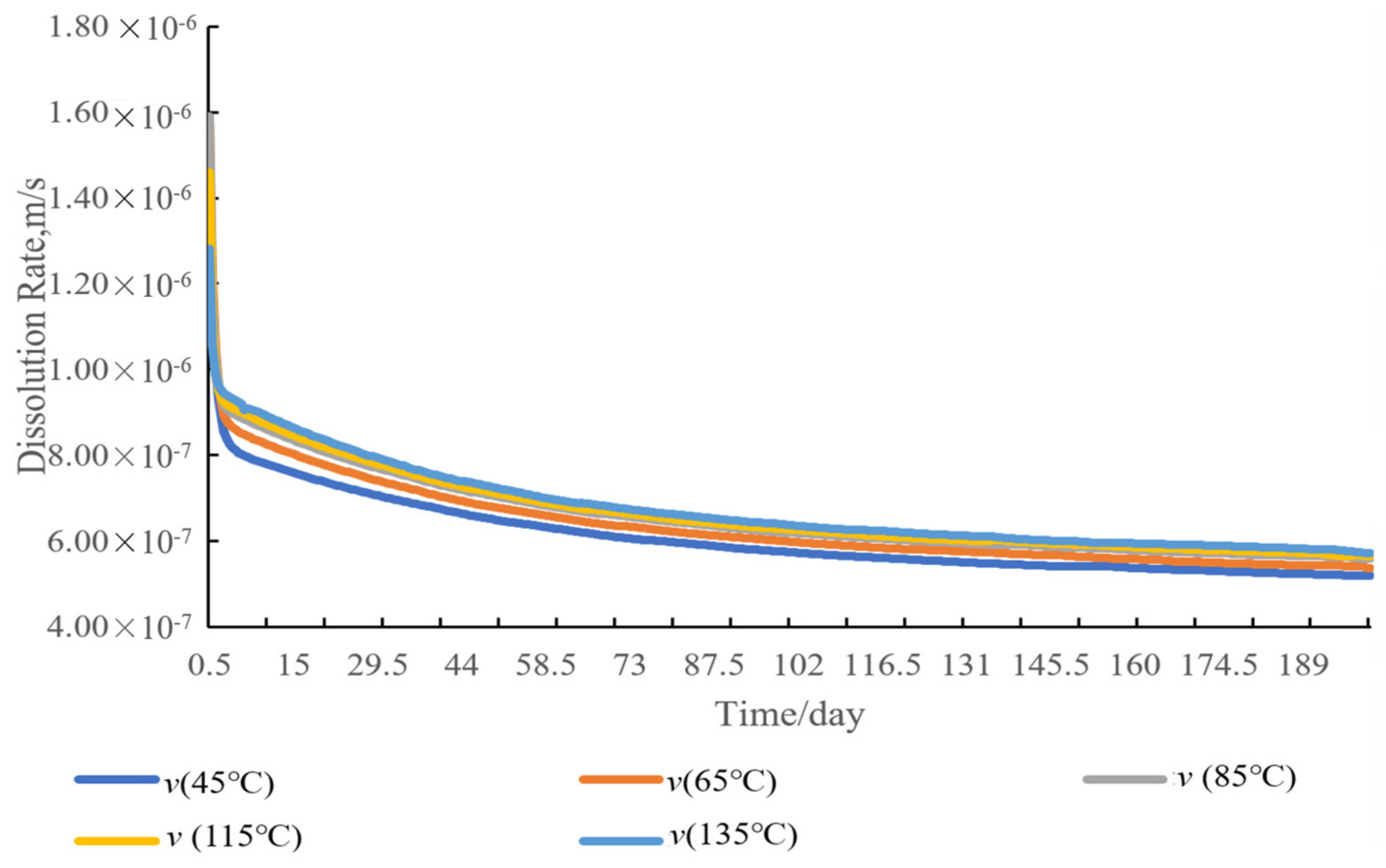
4.2. Temperature Sensitivity Analysis
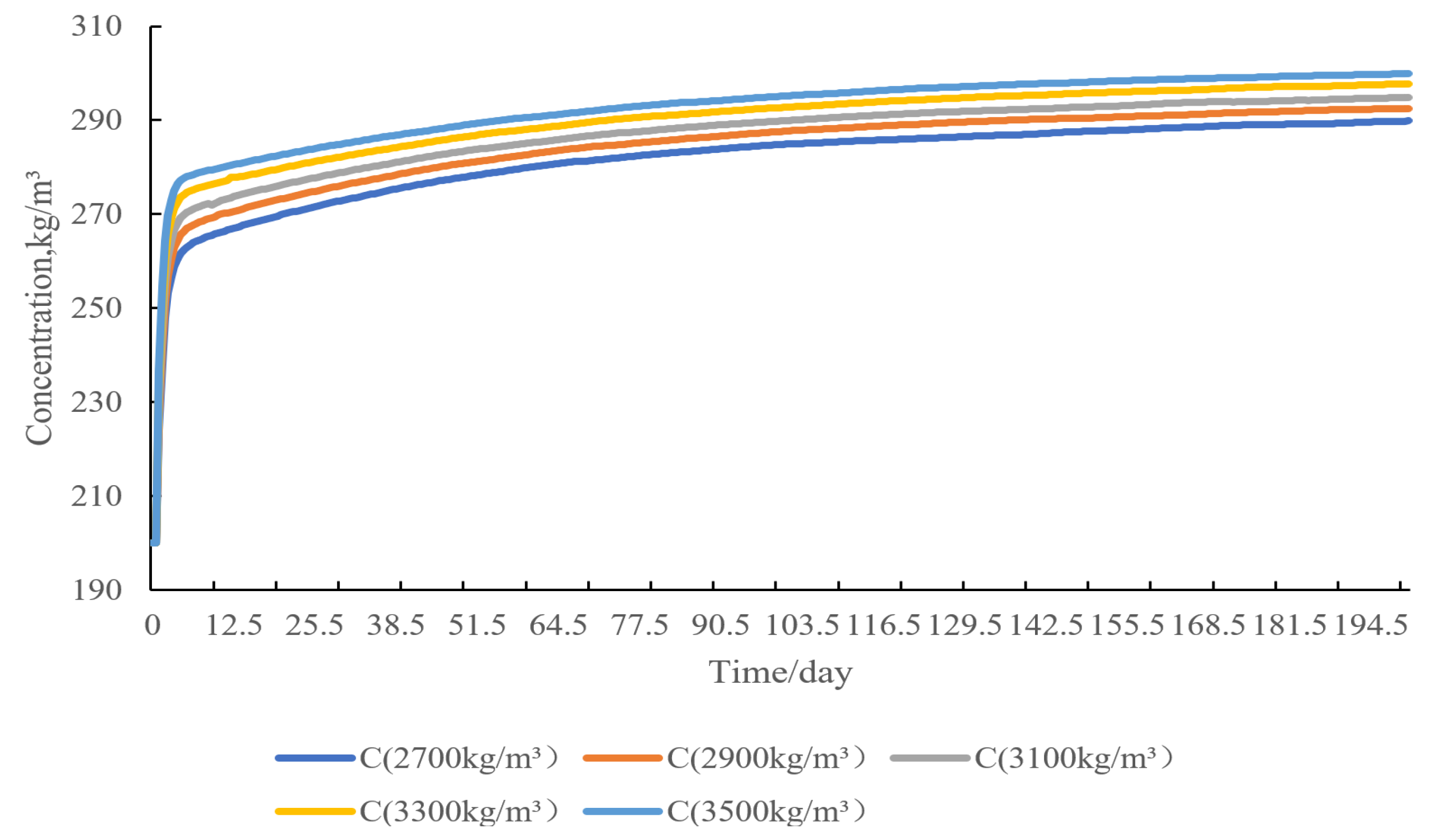
4.3. Saltstone Density Sensitivity Analysis
4.4. Sensitivity Analysis of Insoluble Matter Content
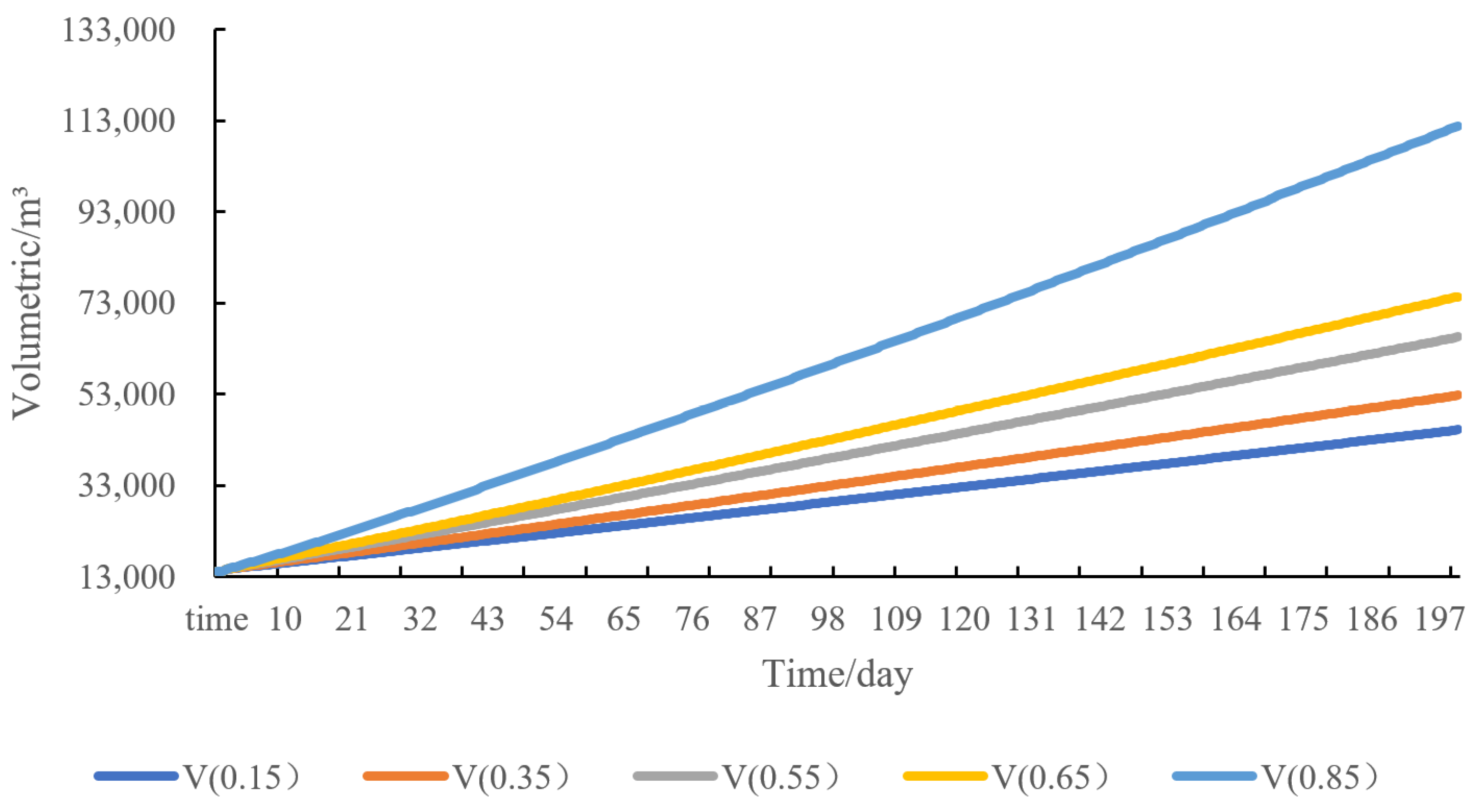
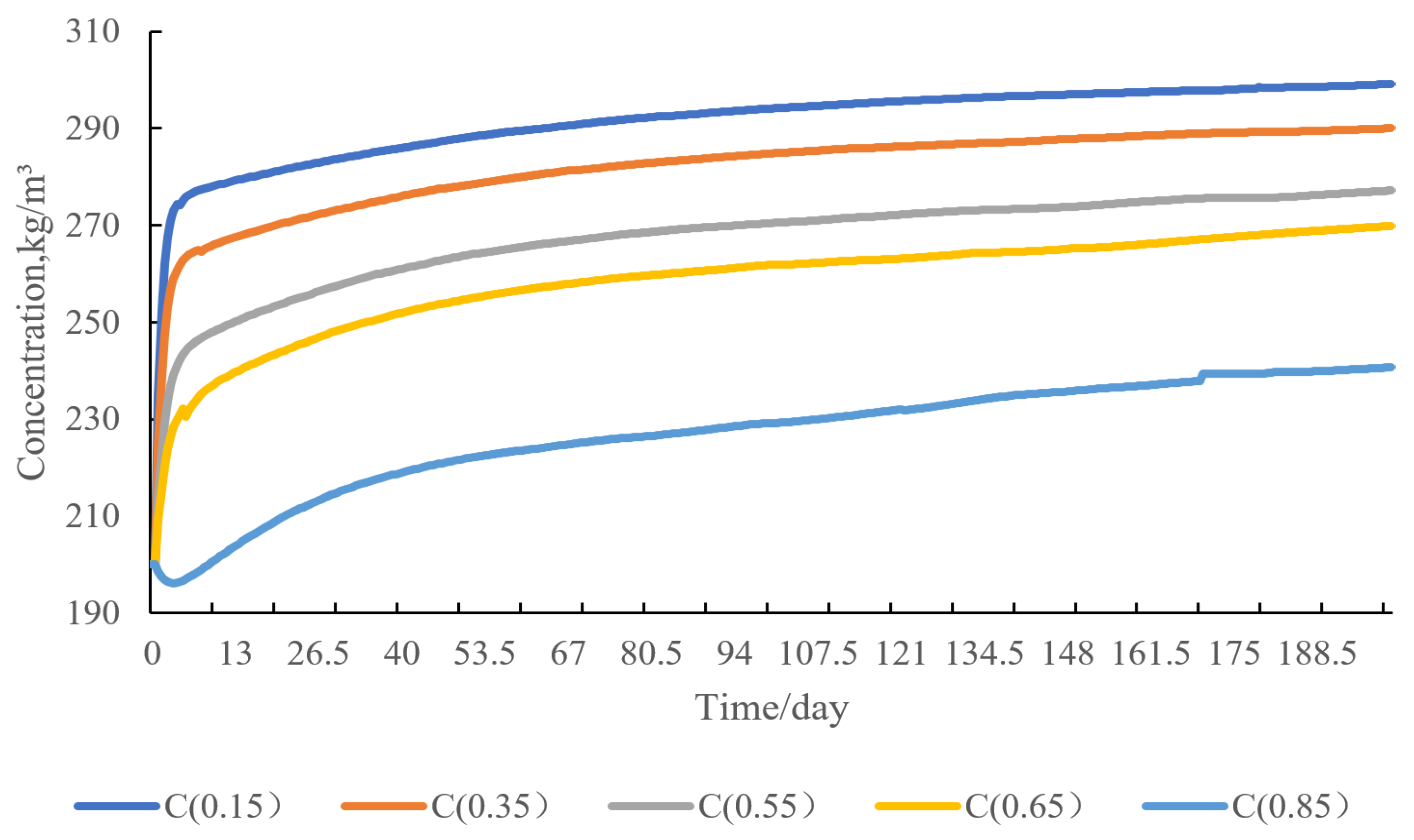
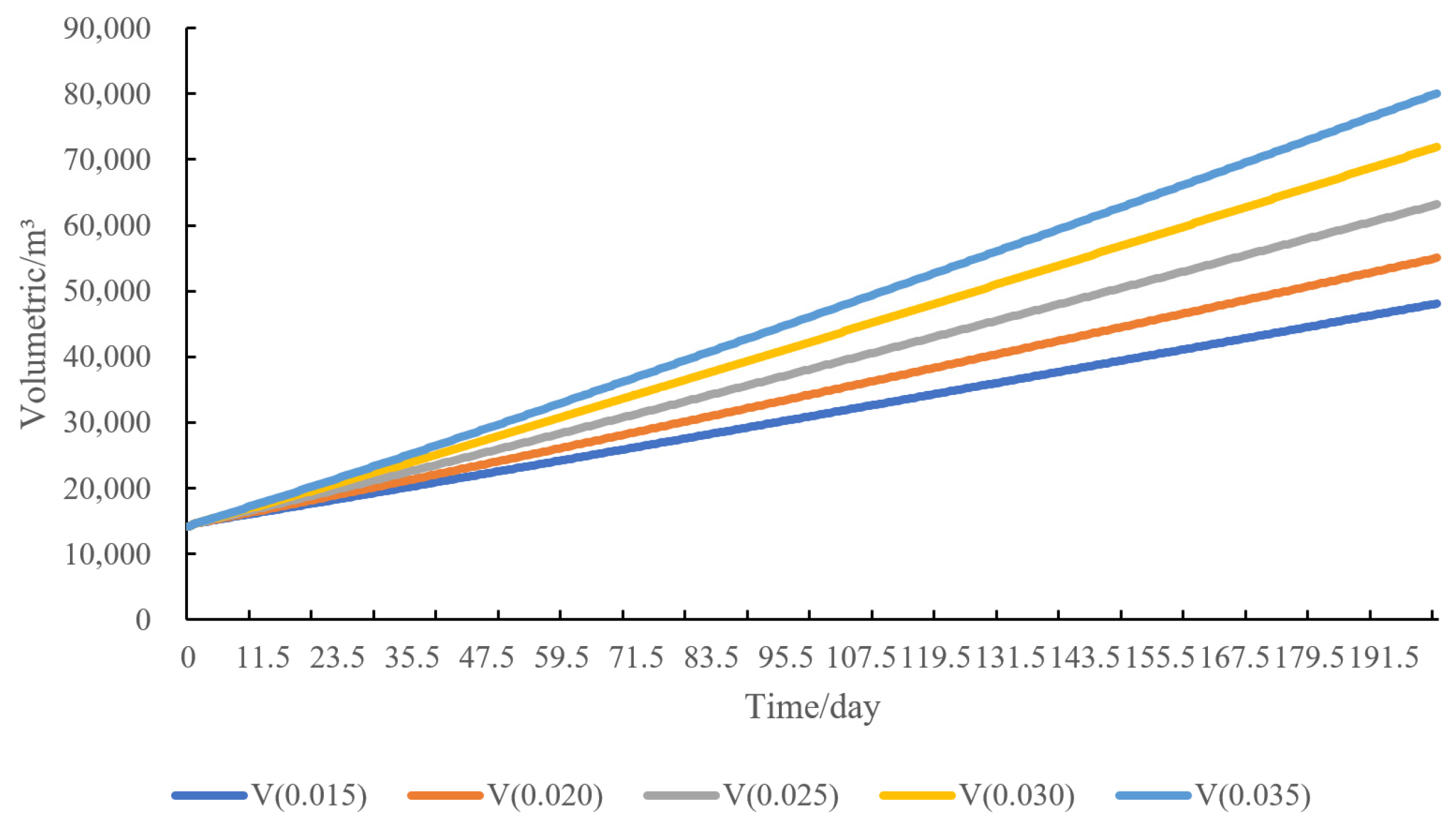
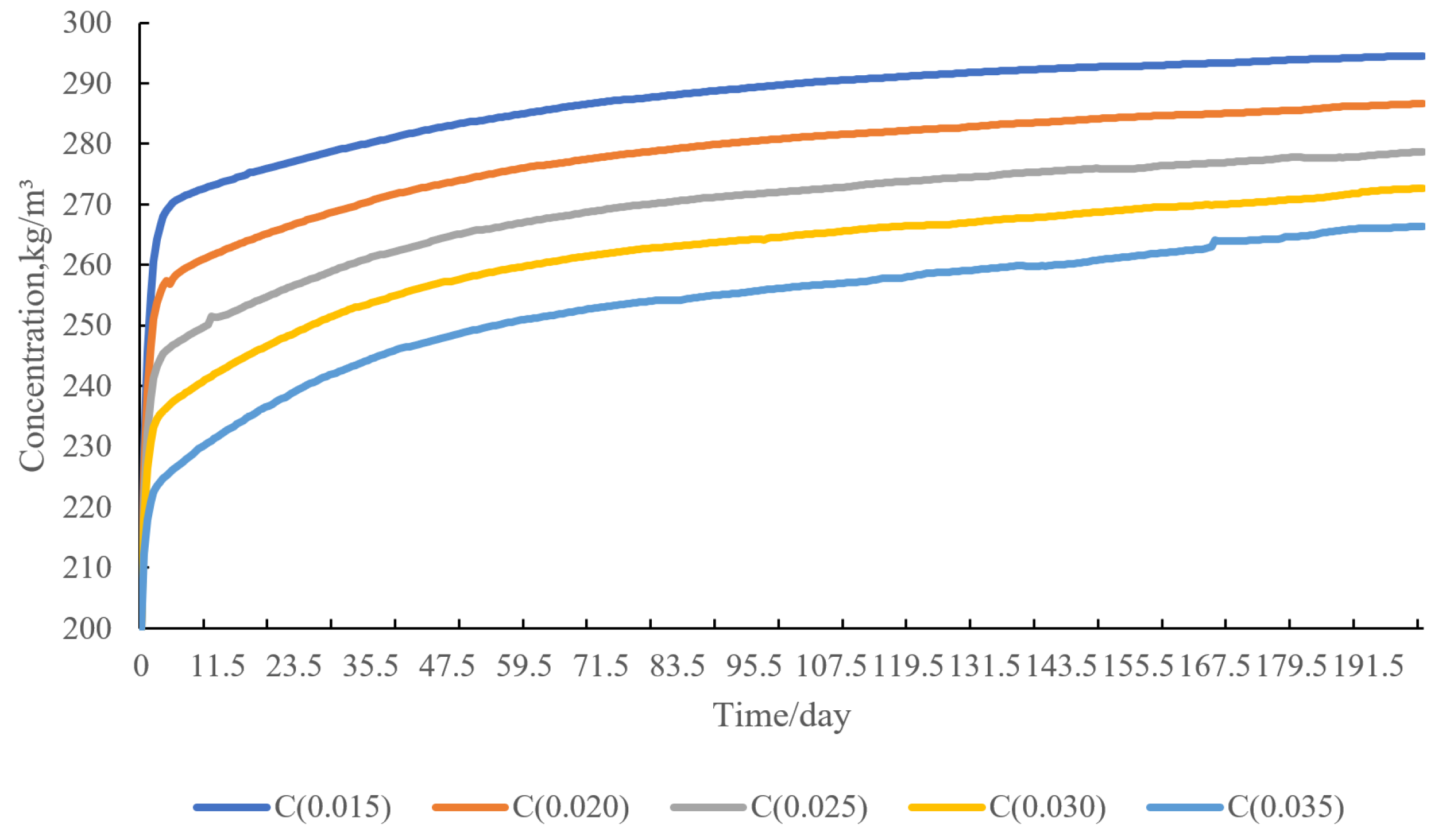
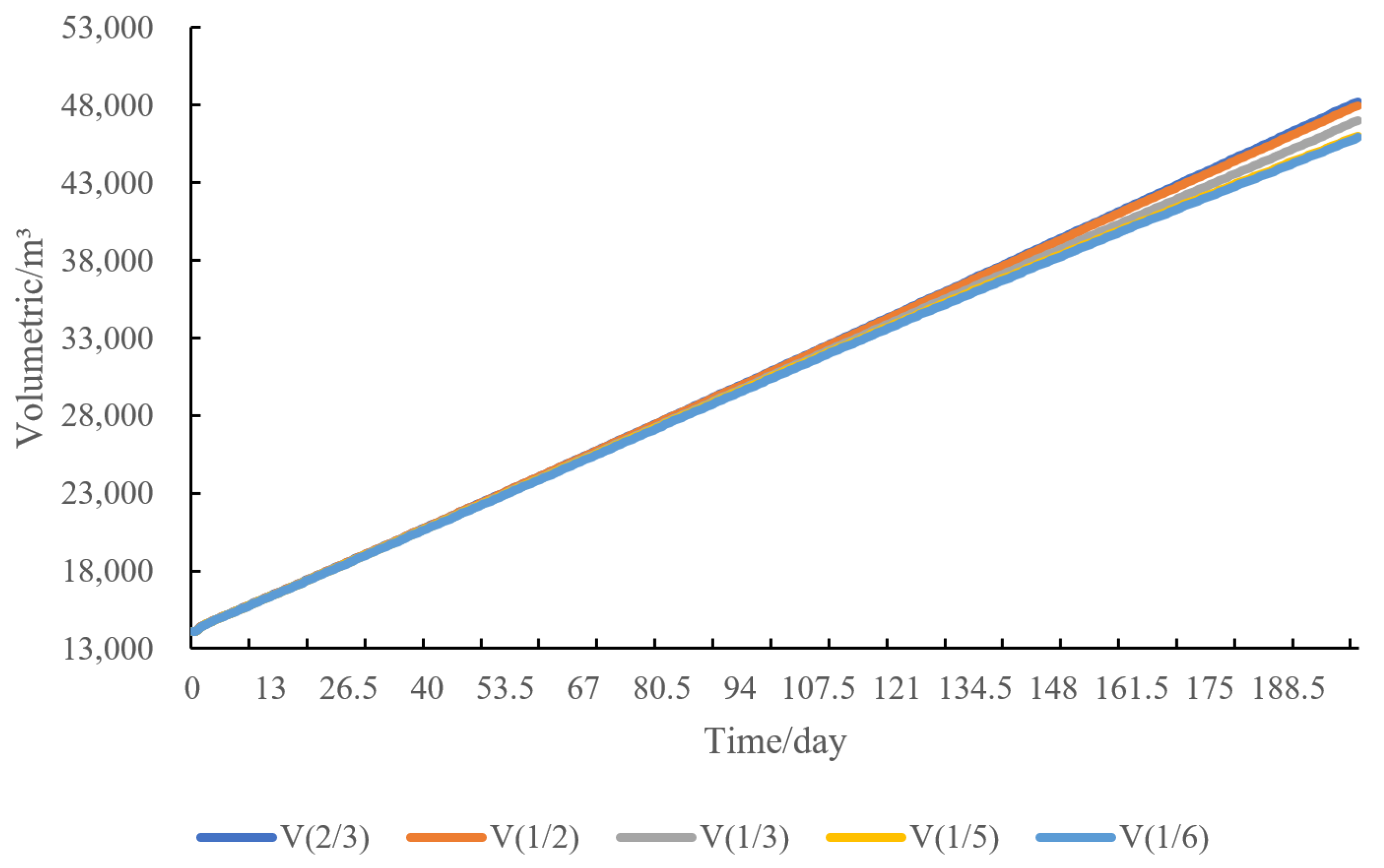
4.5. Sensitivity Analysis of Water Injection Volume
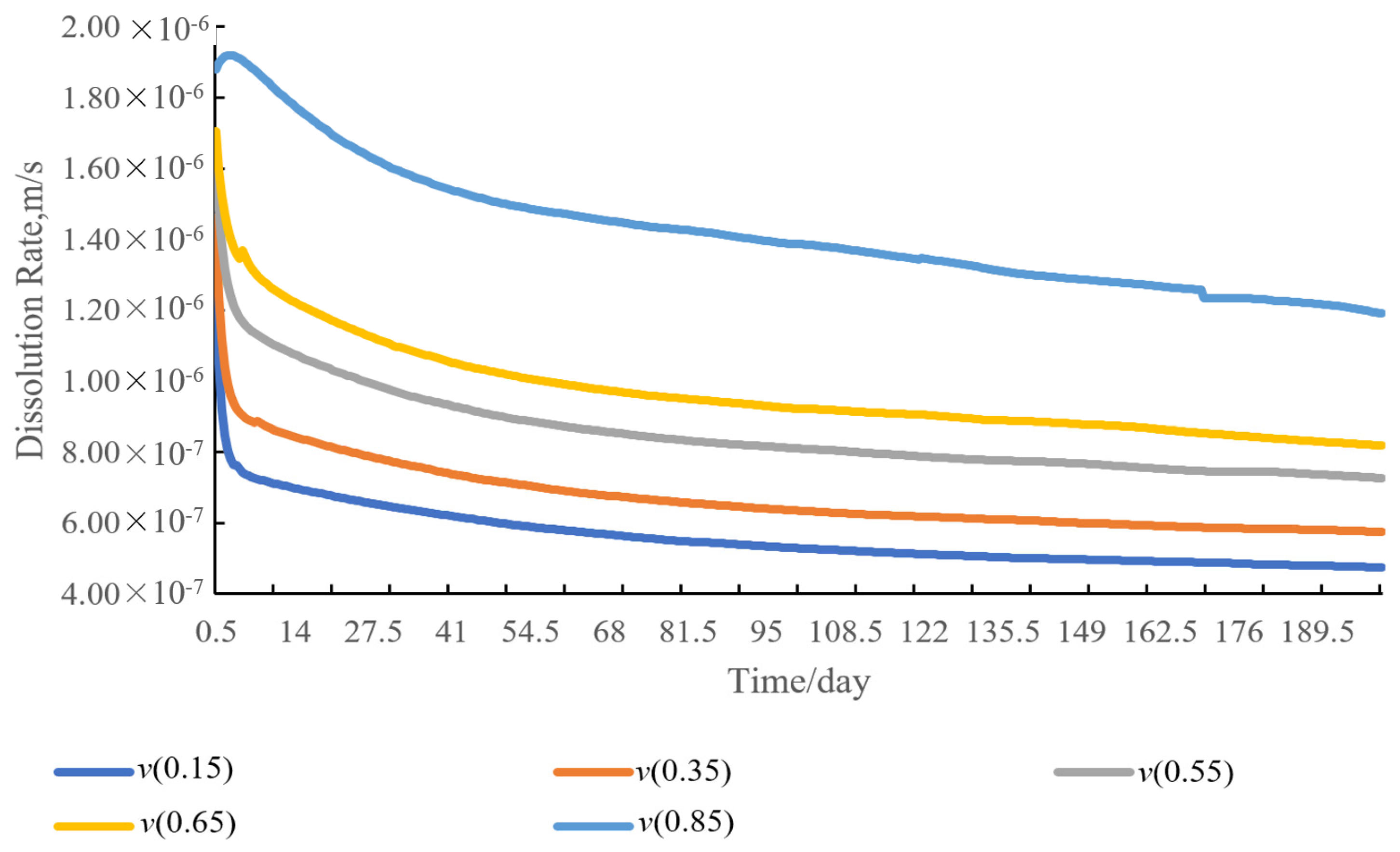
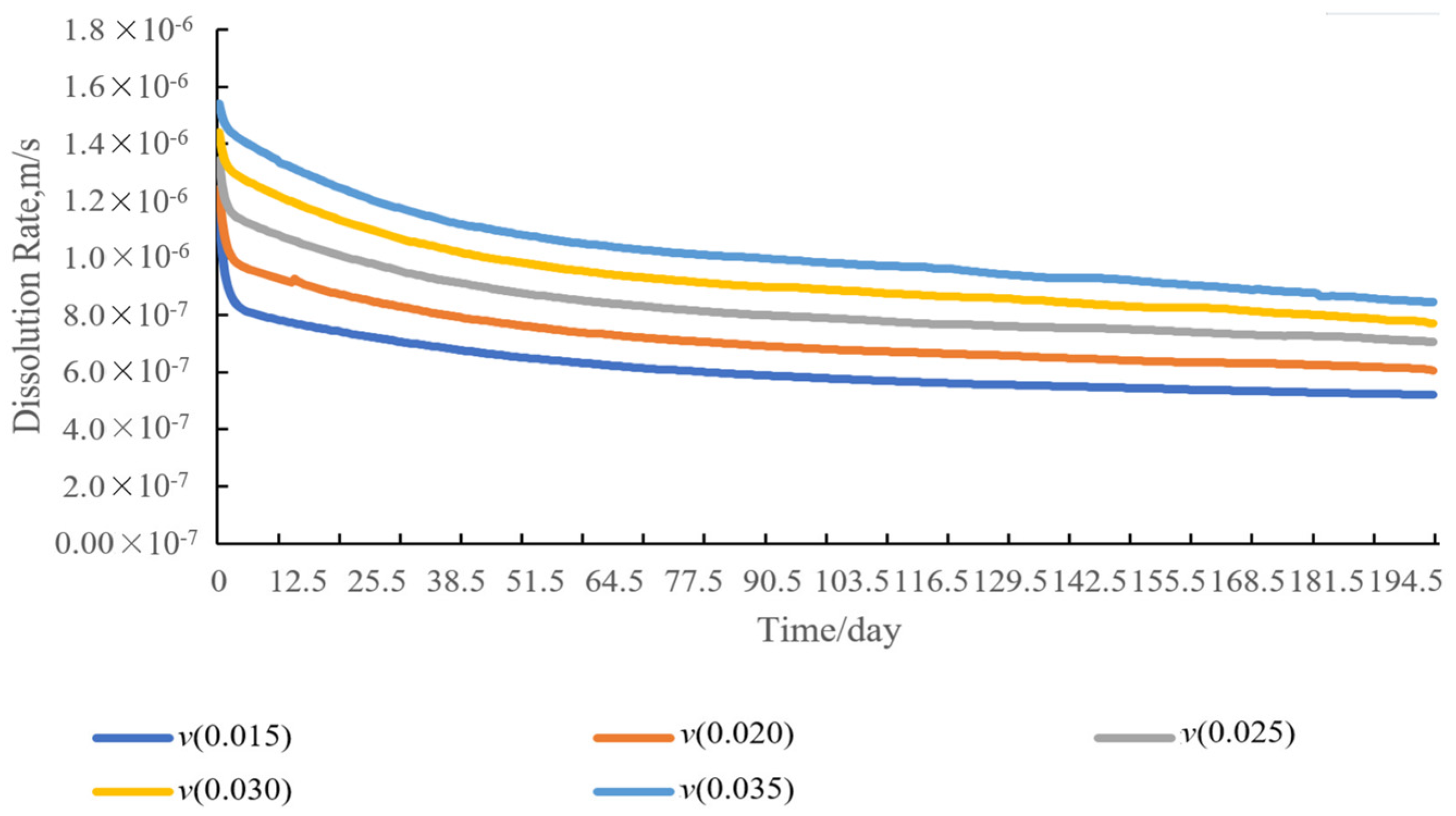
4.6. Sensitivity Analysis of Dissolution Rate of Entrained Salt Rocks
5. Conclusions
- (1)
- The equivalent line segment method is used to describe the cavity contour, and compared with the equivalent tangent point method, which moves the contour points in the horizontal direction by fixing the vertical height, the contour points can be moved in both the horizontal direction and in the vertical direction using this method to avoid the drawbacks of a jagged cavity contour appearing, brought about by the equivalent tangent point method.
- (2)
- A three-dimensional non-homogeneous salt cavern reservoir water solution cavity model was established, and the cavity was divided into several sectors in the horizontal direction and several layers in the vertical direction. The geological parameters, such as the density of the salt rock and the insoluble content in each sector, satisfied the Gaussian distribution, and the temperature varied linearly according to the number of layers in the vertical direction. The numerical simulation software for the water solution cavity construction of a three-dimensional non-homogeneous salt cavern reservoir was prepared using MATLAB R2023b software.
- (3)
- Using the experimental data of the salt system distribution in the Fusi section of the Funing Group and the parameters of the cavity expansion results of the salt cavity of the Jintan reservoir’s early well No. 52, the numerical simulation results of the cavity produced by this software, CALES, and winUbro were compared. The results show that the cavity shapes simulated by this software maintain a high degree of consistency with the actual cavity shapes, and at the same time, they demonstrate a high degree of accuracy in terms of maximum radius and volume error. This excellence is mainly attributed to the 3D non-homogeneous model introduced in the software, which successfully captures and accurately reflects the effect of non-homogeneous geology on the cavity shape.
- (4)
- The time standard for completing the cavity plays a decisive role in the cavity expansion process. In particular, in practical engineering applications, the time of cavity expansion not only affects the overall project schedule but also directly relates to the economic efficiency and safety management of the dissolution process. Under the requirement of rapid completion of cavity construction, the model proposed in this study can predict the cavity expansion time more accurately and ensure the completion of cavity construction within the optimal time, thus reducing the project cost and improving the efficiency of resource utilization. Therefore, the reasonable setting and accurate prediction of time standards are crucial to the success of cavity construction.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; He, Y.; Zhou, Y.; Ji, S.; Luo, Y. Comparison and Selection of Natural Gas Peaking Methods. Nat. Gas Oil 2009, 27, 5–9+58+63. [Google Scholar]
- Zhou, X. Effective natural gas peaking and storage technology—Underground gas storage. Nat. Gas Ind. 2013, 33, 95–99. [Google Scholar]
- Ding, G.; Jing, L.; Ren, Y.; Zhao, X.; Ran, L. Suggestions for building China’s natural gas peaking reserve and emergency response system. Nat. Gas Ind. 2009, 29, 98–100+144. [Google Scholar] [CrossRef]
- Wang, F.; Wang, D. Peaking technology of underground gas storage for gas pipeline. Pet. Plan. Des. 2011, 22, 28–30. [Google Scholar]
- Korzeniowski, W.; Skrzypkowski, K.; Poborska-Młynarska, K. The Idea of the Recovery of Municipal Solid Waste Incineration (MSWI) Residues in Kłodawa Salt Mine S.A. by Filling the Excavations with Self-Solidifying Mixtures. Arch. Min. Sci. 2023, 63, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Nolen, J.S.; Von Hantlemann, O.; Meisters, S.; Kleinitz, W.; Heiblinger, J. Numerical simulation of the solution mining process. In Proceedings of the SPE European Spring Meeting, Amsterdam, The Netherlands, 29 May 1974; Society of Petroleum Engineers: Amsterdam, The Netherlands, 1974. [Google Scholar]
- Reda, D.C.; Russo, A.J. Experimental studies of saltcavity leaching by freshwater injection. SPE Prod. Eng. 1986, 1, 82–86. [Google Scholar] [CrossRef]
- Kunstman, A.S.; Urbanczyk, K.M. UBRO-a computer model for designing salt cavern leaching process developed at CHEMKOP. In SMRI Meeting Paper; Solution Mining Research Institute: Paris, France, 1990; pp. 167–172. [Google Scholar]
- Ban, F.; Gao, S.; Shan, W.; Xiong, W. Modeling of rock salt erosion in water-soluble gas storage reservoirs. J. Liaoning Univ. Eng. Technol. 2005, 24 (Suppl. S2), 102–104. [Google Scholar]
- Grüschow, N.; Rembe, M. Numerical modeling of potash solution mining caverns. In Proceedings of the Solution Mining Research Institute Fall 2012 Technical Conference, Bremen, Germany, 1–2 October 2012. [Google Scholar]
- Wang, T.; Yang, C.; Li, J.; Li, J.; Shi, X.; Ma, H. Failure Analysis of Overhanging Blocks in the Walls of a Gas Storage Salt Cavern: A Case Study. Rock Mech. Rock Eng. 2016, 50, 125–137. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Yang, C.; Li, Y.; Wang, T.; Ma, H.; Shi, H.; Li, J.; Liu, J. Repair of irregularly shaped salt caven gas storage by re-leaching under gas blanket. J. Nat. Gas Sci. Eng. 2017, 45, 848–859. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Shi, X.; Xu, W.; Yang, C. Modeling the construction of energy storage salt caverns in bedded salt. Appl. Energy 2019, 255, 113866. [Google Scholar] [CrossRef]
- Katarzyna, C. Refinements to Cavity Morphology Predictions Under Various Operational Conditions. J. Nat. Gas Sci. Eng. 2021, 78, 103413. [Google Scholar]
- Cyran, K.; Kowalski, M. Shape Modelling and Volume Optimisation of Salt Caverns for Energy Storage. Appl. Sci. 2021, 11, 423. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Zhang, S.; Shi, X.; Xu, W.; Zhuang, D.; Liu, J.; Li, Q.; Chen, Y. Machine-learning-based capacity prediction and construction parameter optimization for energy storage salt caverns. Energy 2022, 254, 124238. [Google Scholar] [CrossRef]
- Wang, T.; Ding, Z.; He, T.; Xie, D.; Liao, Y.; Chen, J.; Zhu, K. Stability of the horizontal salt cavern used for different energy storage under varying geological conditions. J. Energy Storage 2024, 84, 110817. [Google Scholar] [CrossRef]
- Saberian, A. Study on Mixing in Water-Brine Systems; Solution Mining Research Institute: Paris, France, 1971. [Google Scholar]
- Saberian, A.; Von Schonfeldt, H.A. Convective mixing of water with brine around the periphery of vertical tube. In Proceedings of the 1973 Fourth Symposium on Salt, Houston, TX, USA, 8–12 April 1973. [Google Scholar]
- Saberian, A. Cavity Development in a Three Layer Bedded Salt Model; Solution Mining Research Institute: Paris, France, 1977. [Google Scholar]
- Saberian, A. SALGAS User’s Manual, Volume 1—Theories, Formulas & Program Description; SMRI Research Project Report No. 84-003-SMRI; Solution Mining Research Institute: Paris, France, 1984. [Google Scholar]
- Podio, A.L.; Saberian, A. Optirnization of solution mining operations. In Proceedings of the 5th International Symposium on Salt-Northem Ohio Geological Society, Hamburg, Germany, 29 June 1979; pp. 87–103. [Google Scholar]
- Ehgartner, B.L.; Sobolik, S.R. Analysis of Cavern Shapes for the Strategic Petroleum Reserve; Technical Report; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2006. [CrossRef]
- Wang, T.; Yang, C.; Shi, X.; Ma, H.; Li, Y.; Yang, Y.; Daemen, J.J.K. Failure analysis of thick interlayer from leaching of bedded salt caverns. Int. J. Rock Mech. Min. Sci. 2015, 73, 175–183. [Google Scholar] [CrossRef]
- Edler, D. Numerical simulation of cavern shape development during product withdrawal with fresh water theory and case study. In Proceedings of the 9th International Symposium on Salt, Beijing, China, 3 September 2009. [Google Scholar]
- Saberian, A. Numerical Simulation of Development of Solution-Mined Storage Cavities; Texas University: Austin, TX, USA, 1974. [Google Scholar]
- Kunstman, A.; Urbanczyk, K.; Mlynarska, K.P. Solution Mining in Salt Deposits-Outline of Recent Development Trends; AGH University of Science and Technology: Krakow, Poland, 2007; pp. 106–118. [Google Scholar]
- Ma, H.; Chen, F.; Yang, C.; Shi, X.; Zhang, Z. Experimental study on dissolution rate of deep salt rocks. Min. Ind. Res. Dev. 2010, 30, 9–13. [Google Scholar] [CrossRef]

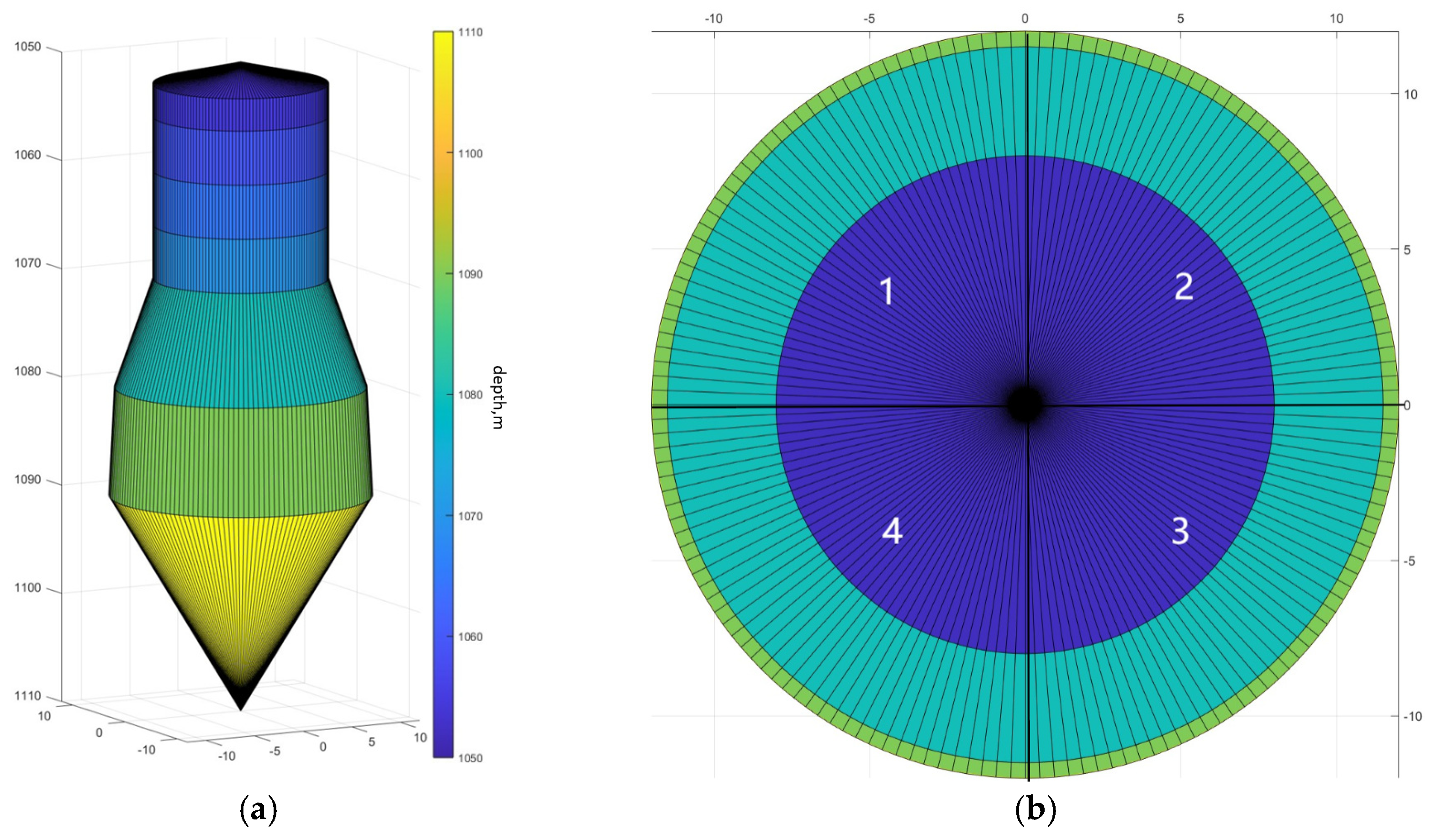








| Sector Number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Insoluble content range | 0.5–0.6 | 0.2–0.3 | 0.15–0.2 | 0.8–0.9 |
| Saltstone density range, kg/m3 | 2400–2500 | 2300–3200 | 3600–3800 | 3000–3200 |
| Lumen Formation Stage | Circulation Mode | Time/Day | Engine Capacity /(m3/d) | Blanket Depth/m | Inner Pipe Depth/m | Outer Pipe Depth/m |
|---|---|---|---|---|---|---|
| 1 | Positive circulation | 150 | 100 | 1049 | 1106 | 1071 |
| 2 | Positive circulation | 150 | 100 | 1035 | 1079 | 1056 |
| 3 | Positive circulation | 150 | 100 | 1020 | 1075 | 1041 |
| 4 | Reverse circulation | 180 | 100 | 1000 | 1046 | 1070 |
| 5 | Reverse circulation | 150 | 100 | 981 | 1030 | 1062 |
| 6 | Reverse circulation | 30 | 100 | 961 | 1030 | 1062 |
| Floor Number | Well Section (m) | Layer Thickness (m) | Natural Gamma (API) | Compensating for Sound Waves (us/m) | Compensation Density (g/cm3) | Photoelectric Absorption Index (b/ev) | Mud Content (%) | Explanation of Conclusions | Note |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 794.8~809.0 | 14.2 | 9.94 | 226.31 | 2.06 | 4.06 | 3.66 | saline rock formation | |
| 2 | 809.0~812.8 | 3.8 | 15.90 | 235.98 | 2.24 | 3.69 | 18.50 | saline rock formation | clay |
| 3 | 812.8~814.9 | 2.1 | 79.00 | 296.38 | 2.33 | 3.23 | 99.90 | mudstone layer | |
| 4 | 814.9~828.4 | 13.5 | 11.28 | 232.19 | 2.09 | 3.87 | 4.26 | saline rock formation | |
| 5 | 828.4~832.2 | 3.8 | 16.77 | 232.64 | 2.32 | 3.35 | 42.02 | saline rock formation | clay |
| 6 | 832.2~852.6 | 20.4 | 10.87 | 219.9 | 2.08 | 3.51 | 4.08 | saline rock formation | |
| 7 | 852.6~856.0 | 3.4 | 32.87 | 231.51 | 2.16 | 3.56 | 19.95 | saline rock formation | clay |
| 8 | 856.0~858.8 | 2.8 | 15.37 | 226.07 | 2.07 | 3.76 | 6.30 | saline rock formation | |
| 9 | 858.8~861.5 | 2.7 | 20.70 | 233.04 | 2.25 | 3.56 | 36.69 | saline rock formation | clay |
| 10 | 861.5~871.6 | 10.1 | 12.29 | 224.39 | 2.06 | 3.71 | 4.74 | saline rock formation | |
| 11 | 871.6~873.3 | 1.7 | 56.51 | 237.53 | 2.36 | 3.21 | 57.92 | mudstone layer | saline |
| 12 | 873.3~877.5 | 4.2 | 14.72 | 227.65 | 2.09 | 3.66 | 5.96 | saline rock formation | |
| 13 | 877.5~878.6 | 1.1 | 33.53 | 234.46 | 2.28 | 3.47 | 20.14 | saline rock formation | saline |
| 14 | 878.6~879.8 | 1.2 | 13.94 | 221.18 | 2.08 | 3.70 | 5.56 | saline rock formation | |
| 15 | 879.8~881.0 | 1.2 | 23.50 | 242.25 | 2.25 | 3.48 | 28.45 | saline rock formation | clay |
| 16 | 881.0~884.7 | 3.7 | 11.92 | 228.50 | 2.08 | 3.86 | 4.57 | saline rock formation | |
| 17 | 884.7~885.9 | 1.2 | 30.65 | 234.35 | 2.26 | 3.44 | 17.98 | saline rock formation | clay |
| 18 | 885.9~905.9 | 20.0 | 12.06 | 223.22 | 2.06 | 3.80 | 4.63 | saline rock formation | |
| 19 | 905.9~924.0 | 18.1 | 105.37 | 279.34 | 2.56 | 3.05 | 99.9 | mudstone layer |
| Cavitation Parameters | Circulation Mode | Blanket Depth/m | Outer Pipe Depth/m | Inner Pipe Depth/m | Water Injection Displacement m3/h | Cumulative Luminalization Time/Day |
|---|---|---|---|---|---|---|
| 1 | Positive circulation | 882 | 890 | 906 | 30 | 46 |
| 2 | Positive circulation | 882 | 890 | 906 | 60 | 214 |
| 3 | Reverse circulation | 872 | 880 | 900 | 80 | 306 |
| 4 | Reverse circulation | 862 | 880 | 900 | 80 | 405 |
| 5 | Reverse circulation | 852 | 880 | 900 | 80 | 440 |
| 6 | Reverse circulation | 842 | 860 | 880 | 80 | 532 |
| 7 | Reverse circulation | 832 | 860 | 880 | 80 | 642 |
| 8 | Reverse circulation | 820 | 860 | 880 | 80 | 654 |
| Parameter Name | First Stage |
|---|---|
| Injection time, day | 200 |
| Inner pipe depth, m | 1095 |
| Outer pipe depth, m | 1065 |
| Blanket depth, m | 1050 |
| Circulation mode | Positive circulation |
| Injection flow, m3/s | 0.016 |
| Saltstone density, kg/m3 | 3000–3200 |
| Insoluble content | 0.2–0.3 |
| Insoluble expansion coefficient | 1.7 |
| Stratigraphical temperature, °C | 41 |
| Intercalated saline rock (geology) Dissolution rate, m/s | Lateral solution: 0.00000024472 Paracentesis: 0.00000038444 |
| Sandwich section | 1050–1060 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen, X.; Meng, X.; Ren, Z.; Cao, J. Three-Dimensional Heterogeneous Salt Cavern Underground Gas Storage Water Solution Cavity Model Study. Processes 2024, 12, 1124. https://doi.org/10.3390/pr12061124
Cen X, Meng X, Ren Z, Cao J. Three-Dimensional Heterogeneous Salt Cavern Underground Gas Storage Water Solution Cavity Model Study. Processes. 2024; 12(6):1124. https://doi.org/10.3390/pr12061124
Chicago/Turabian StyleCen, Xueqi, Xinggang Meng, Zongxiao Ren, and Jiajun Cao. 2024. "Three-Dimensional Heterogeneous Salt Cavern Underground Gas Storage Water Solution Cavity Model Study" Processes 12, no. 6: 1124. https://doi.org/10.3390/pr12061124





