Towards Reliable Prediction of Performance for Polymer Electrolyte Membrane Fuel Cells via Machine Learning-Integrated Hybrid Numerical Simulations
Abstract
1. Introduction
2. Applications, Potentials, and Hurdles of PEMFC System Integration
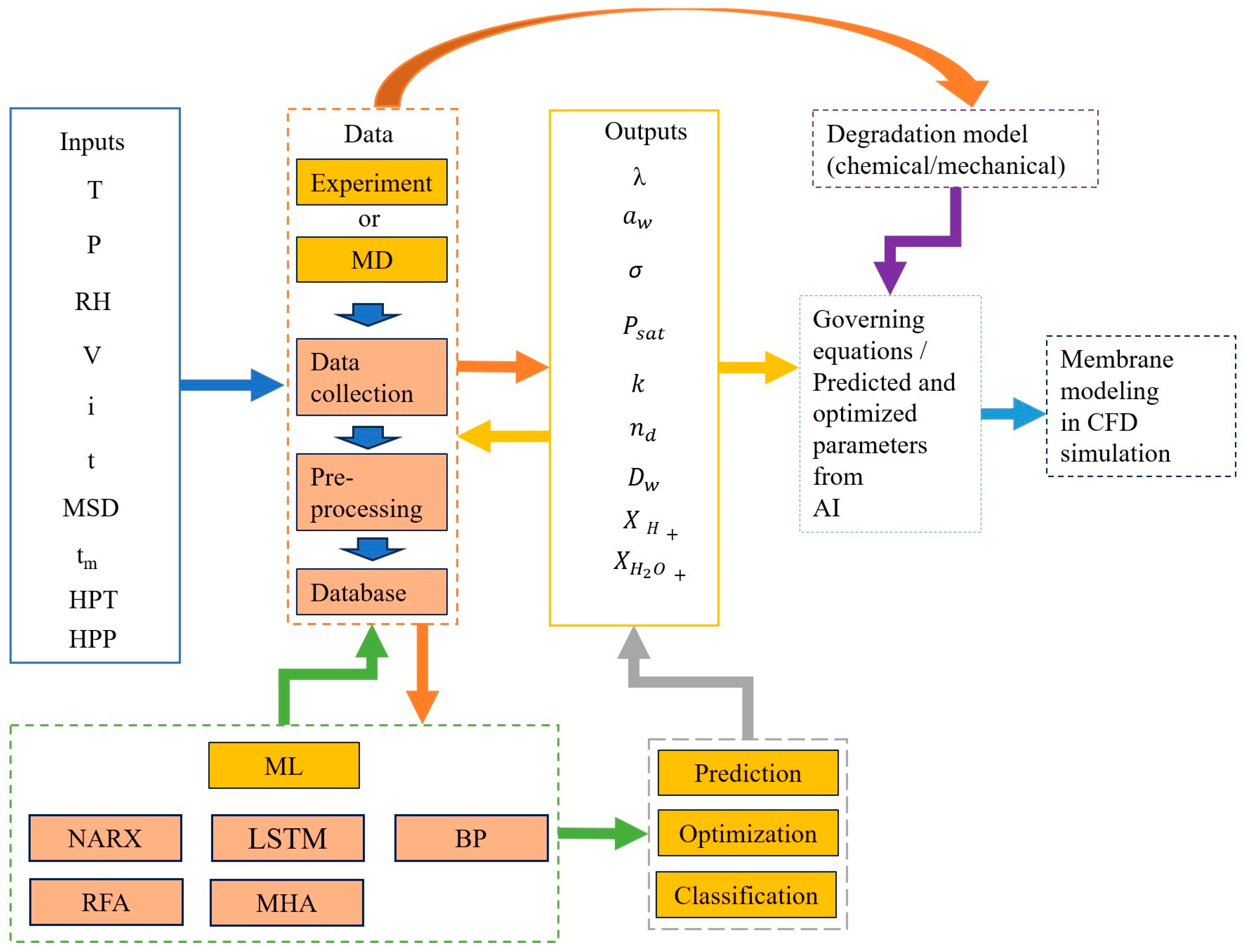
3. PEMFC Modeling Approaches
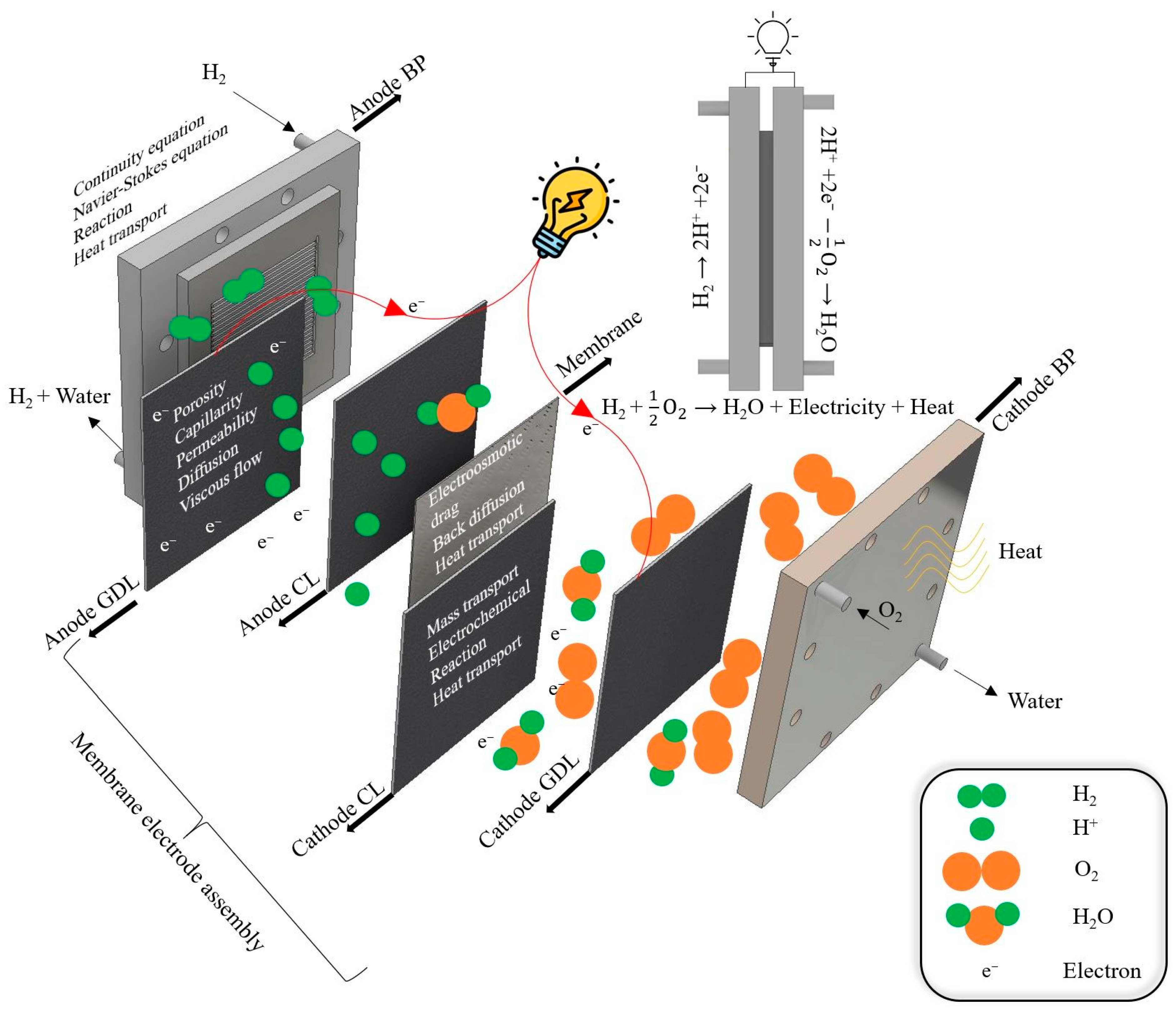
4. PEMFC Fundamentals and Modeling Consideration of Components with ML
4.1. Modeling of Polymer Electrolyte Membrane
4.1.1. Microscopic
4.1.2. Macroscopic
Sorption Model
Transport Models
Diffusive Model
Chemical Potential Model
- Chemical potential of proton, Φ2
- Transport coefficient of water, αw
- Chemical potential of water, μw
Hydraulic Model
Combination Model
4.1.3. Combined Model (Microscopic and Macroscopic)
4.1.4. Issues Related to State-of-Art Membrane Modeling
4.1.5. ML in the Field of Membrane
4.1.6. Integration of ML in Membrane Modeling
4.2. Modeling of Catalyst Layer (CL)
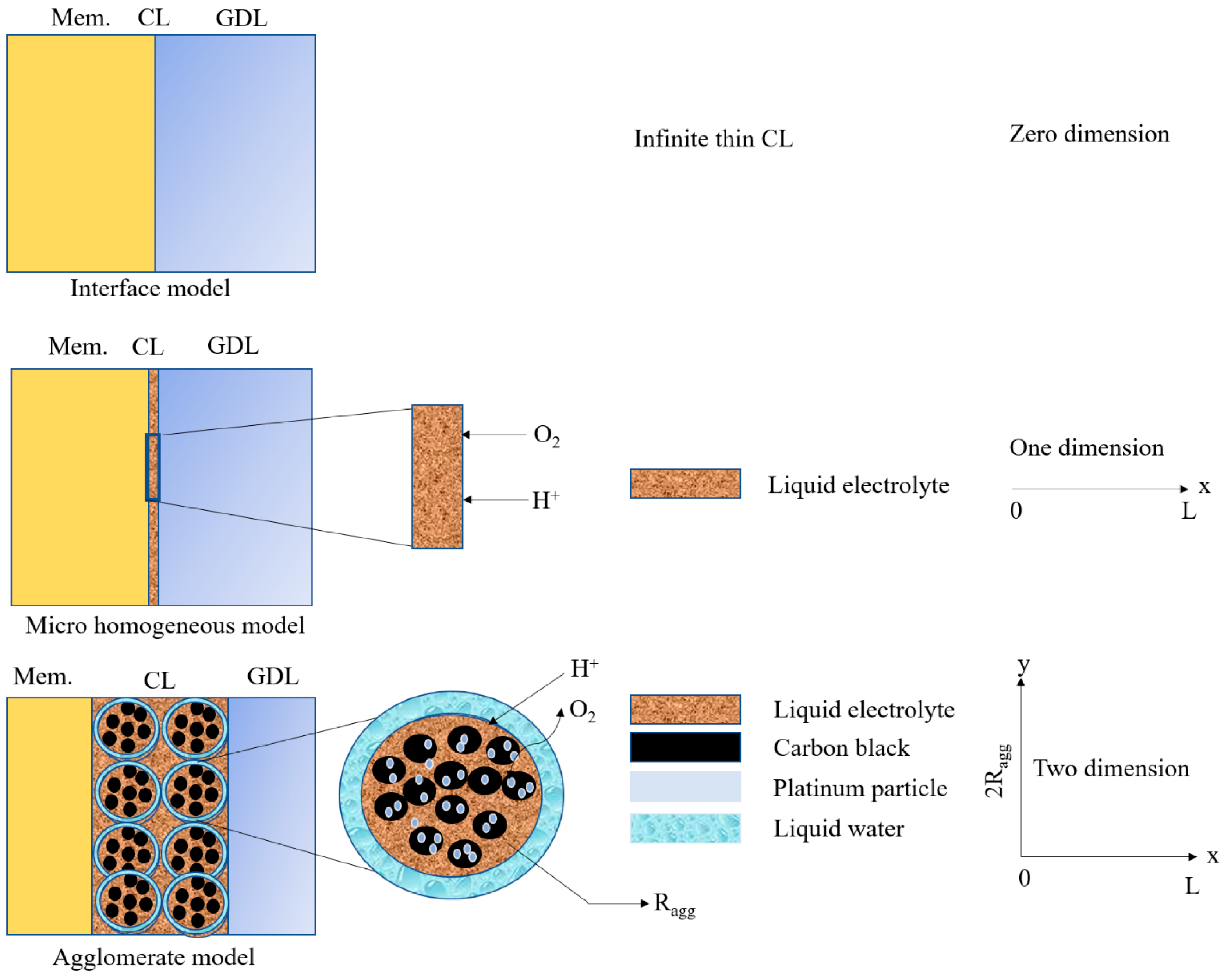
4.2.1. Interface Model
4.2.2. Microscopic and Single Pore Models
4.2.3. Simple Macrohomogeneous Model
Porous Electrode Model
Agglomerate Model
Electrochemical Kinetics Equation of CL
4.2.4. Heat Transfer in the CL
4.2.5. Issues Related to State-of-Art CL Modeling
4.2.6. ML in the Field of CL
4.2.7. Integration of ML in CL Modeling
4.3. Modeling of Gas Diffusion Layer (GDL)
4.3.1. Modeling Porous Structure
Porosity
Wettability, Permeability, and Capillary Effect
Electric Conductivity
4.3.2. Transport Properties in GDL
Transport of Gas Phase
Transport of Liquid Phase
4.3.3. Lattice Boltzmann Method (LBM)
4.3.4. Issues Related to State-of-Art GDL Modeling
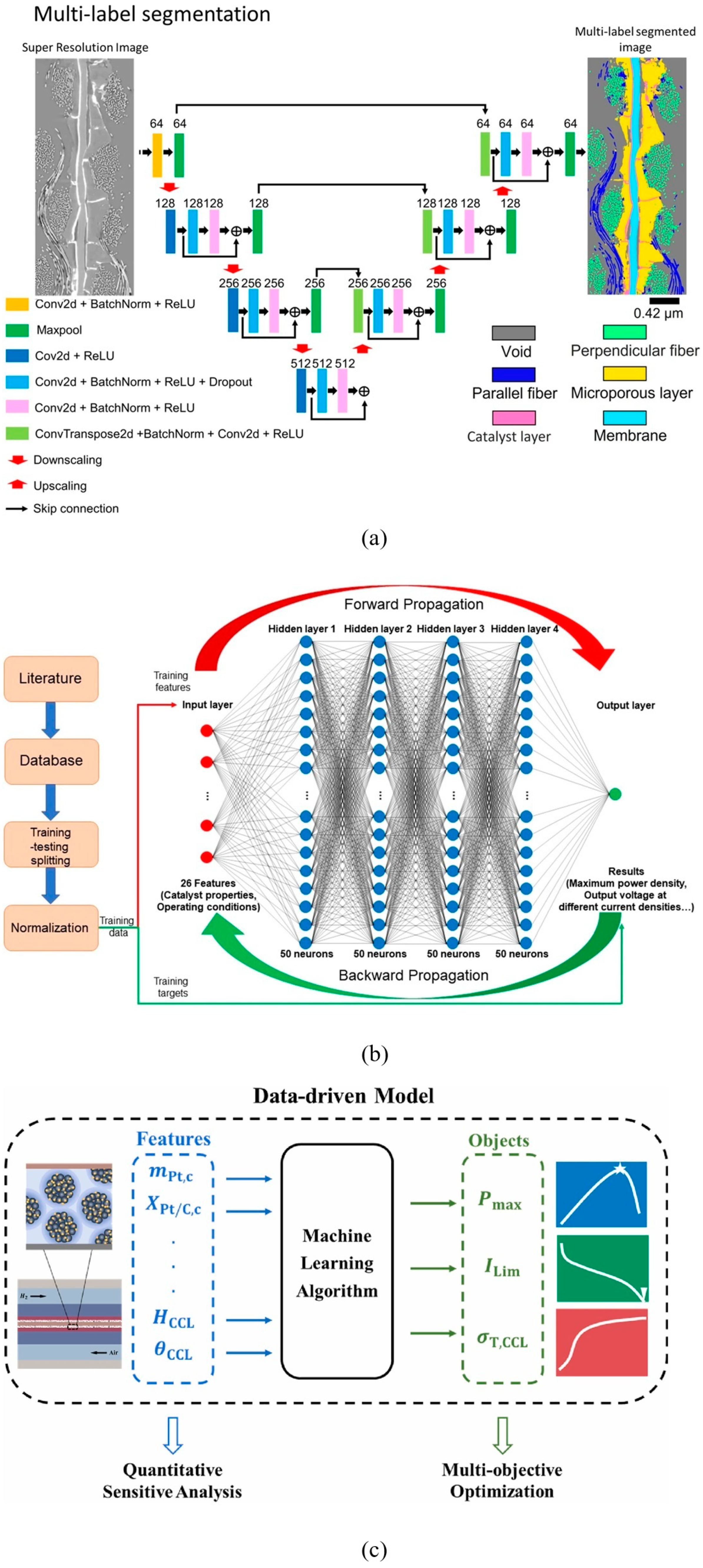
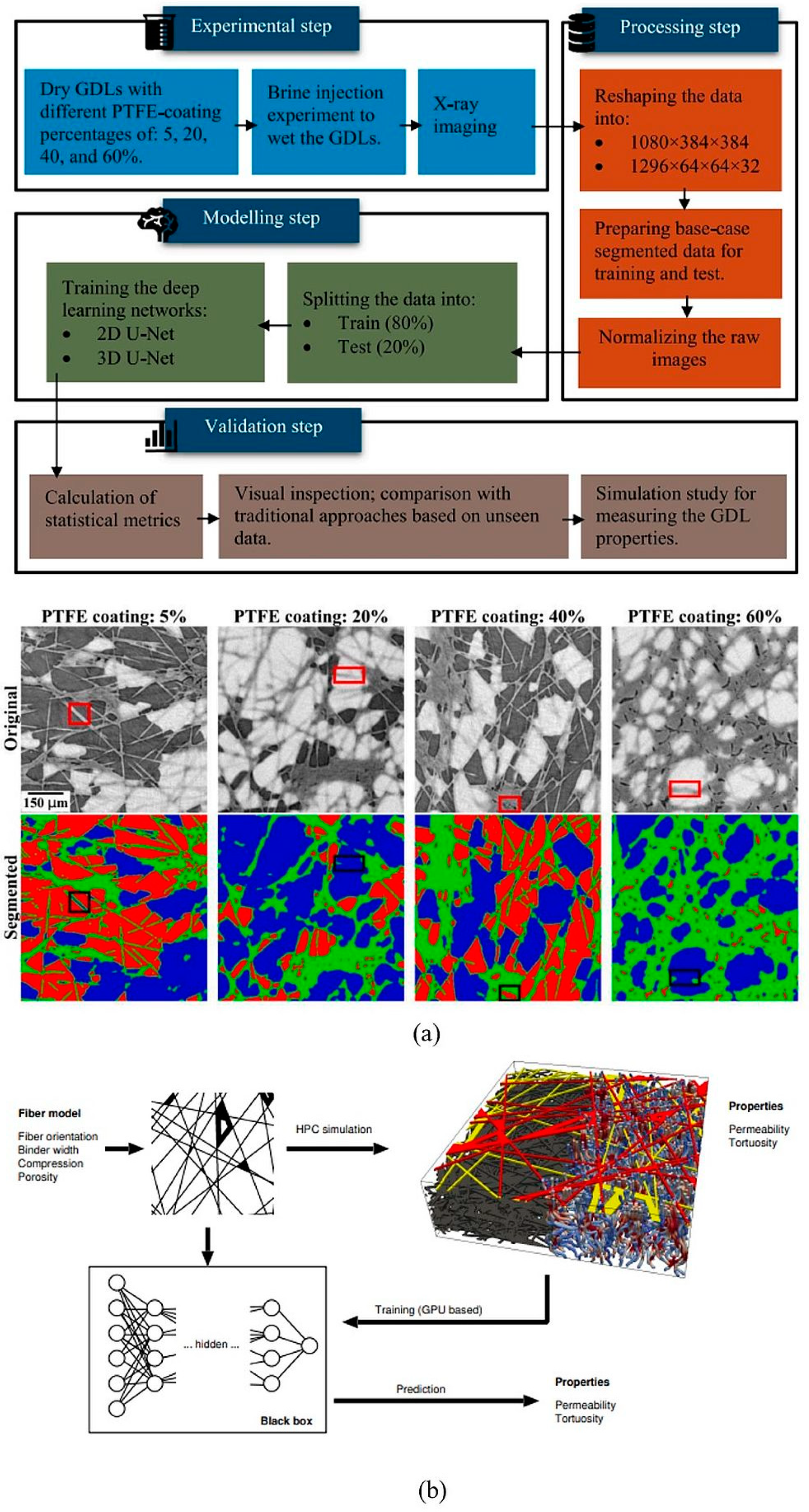
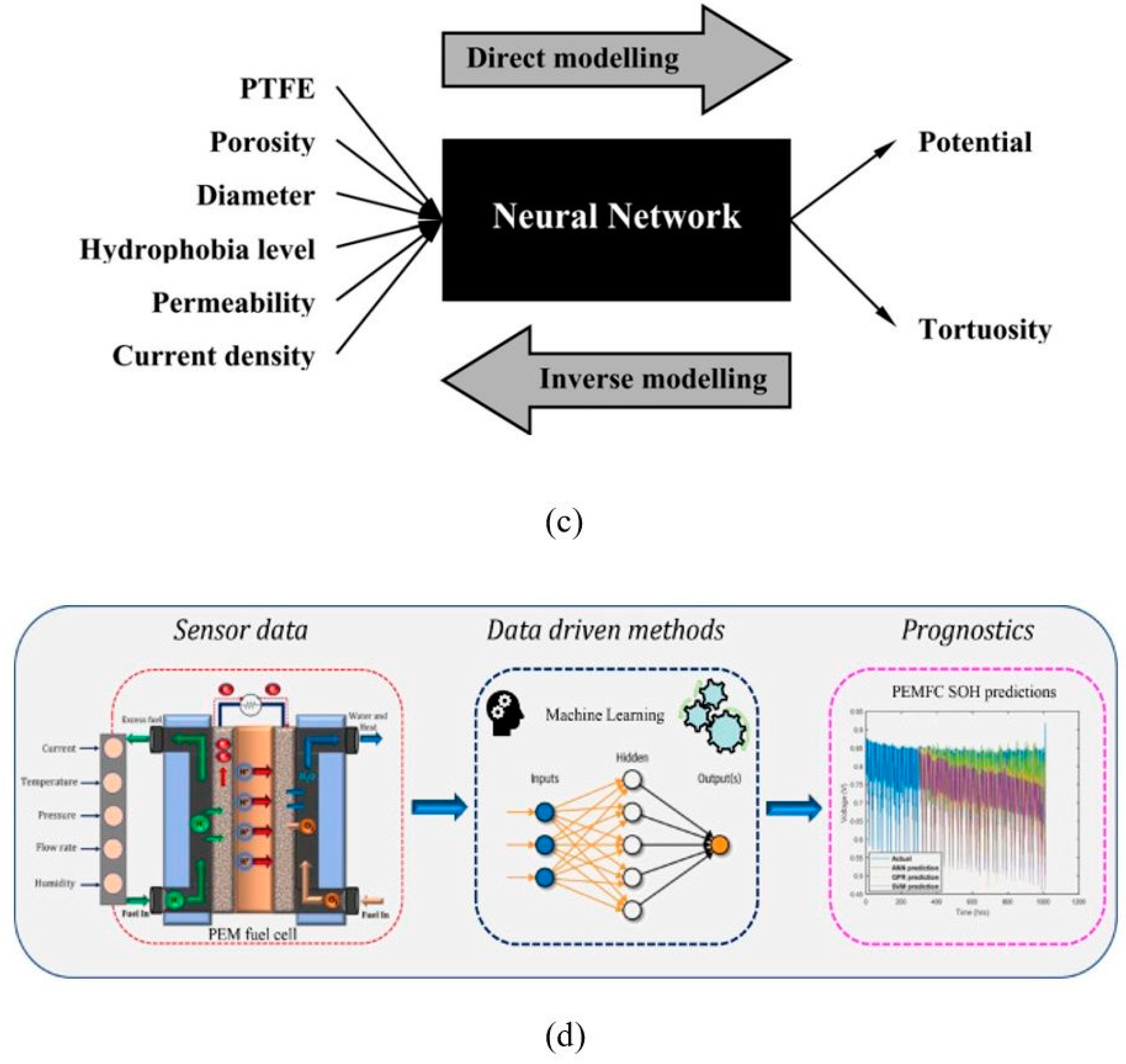
4.3.5. ML in the Field of GDL
4.3.6. Integration of ML in GDL Modeling
4.4. Modeling of Bi-Polar Plate (BP)
4.4.1. Flow Inside the Channel
4.4.2. Modeling of Pressure Drop
4.4.3. Issues Related to State-of-Art BP Modeling
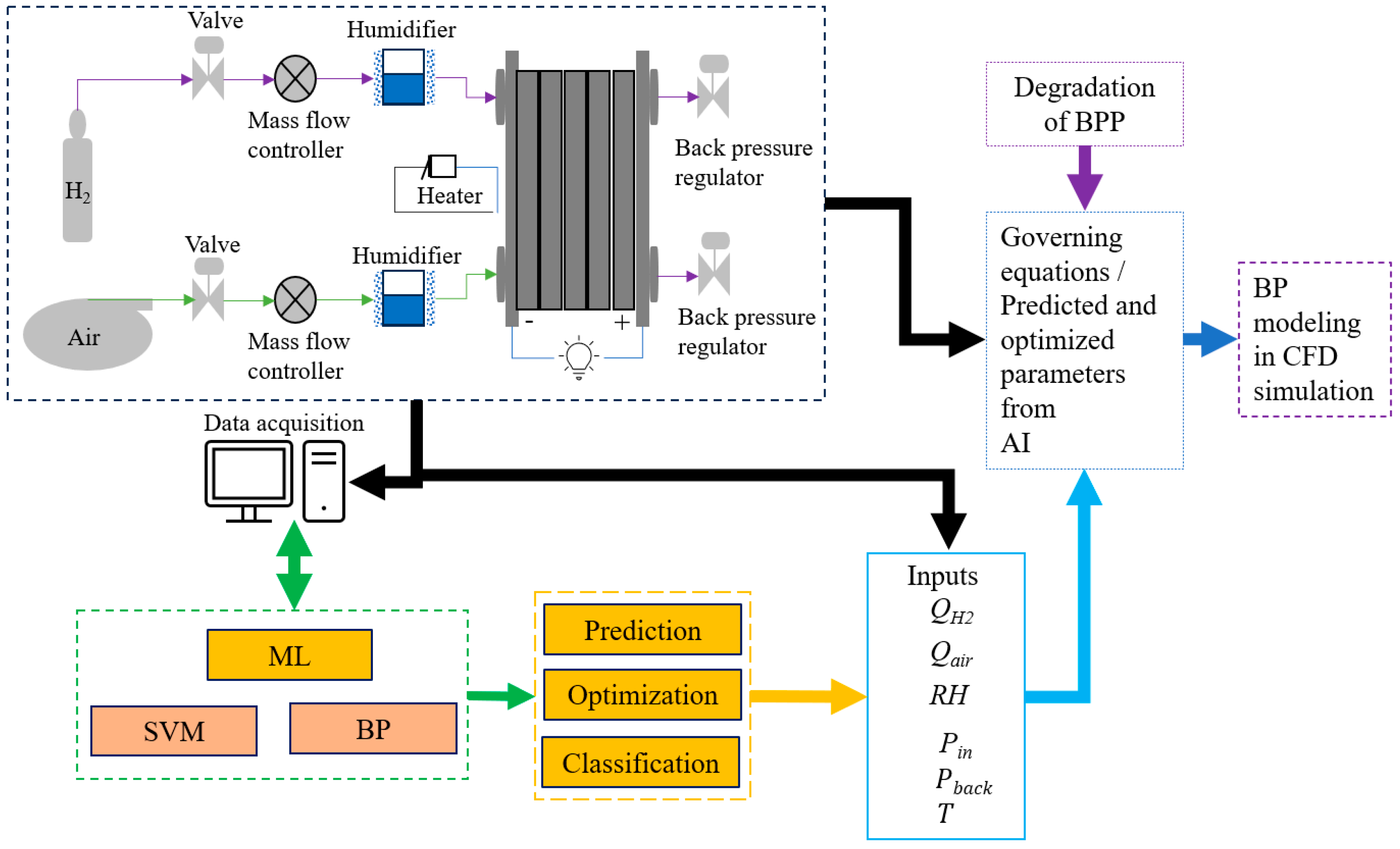
4.4.4. ML in the Field of BP
4.4.5. Integration of ML in BP Modeling
5. Final Overview
6. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFD | computational fluid dynamics |
| CL | catalyst layer |
| GDL | gas diffusion layer |
| HOR | hydrogen oxidation reaction |
| HPT | hot press time |
| HPP | hot press pressure |
| ORR | oxygen reduction reaction |
| MD | molecular dynamics |
| MEA | membrane electrode assembly |
| MSD | mean square displacement |
| MHA | Meta-heuristic algorithms |
| PFSA | perfluorosulfonic acid |
| Pt | platinum |
| PTFE | polytetrafluorethylene |
| LE | liquid equilibrated |
| SPNB | sulfonated polynorbornene |
| PEMFC | proton electrolyte membrane fuel cell |
| VE | vapor equilibrated |
| Symbols | |
| a | water activity (1/m) |
| A | specific external surface area, cm2 |
| interfacial surface area between phases k and p per unit volume, 1/cm | |
| c | molar concentration, kmol/m3 |
| solubility of oxygen, mol/cm3 | |
| interstitial concentration of species i in phase k, mol/cm3 | |
| total solution concentration or molar density, mol/cm3 | |
| heat capacity of phase k, J/(g·K) | |
| D | mass diffusivity (m2/s) |
| hydraulic diameter, cm | |
| Knudsen diffusion coefficient of species i, cm2/s | |
| diffusion coefficient of i in j, cm2/s | |
| effectiveness factor | |
| E | cell potential, V |
| interaction force between phases, N/cm3 | |
| f | friction factor |
| F | Faraday’s constant |
| flux density of species i in phase k relative to the mass-averaged velocity of phase k, mol/(cm2·s) | |
| oxygen flux per unit volume, mol/(cm2·s) | |
| heat transfer coefficient, J/(cm2·s·K) | |
| latent heat of evaporation, J/kg | |
| partial molar enthalpy of species i in phase k, J/mol | |
| heat or enthalpy of evaporation, J/mol | |
| normal interfacial current transferred per unit interfacial area across the interface between the electronically conducting phase and phase k due to electron-transfer reaction h, A/cm | |
| transfer current density of reaction h per unit interfacial area between phases k and p, A/cm | |
| exchange current density for reaction h, A/cm2 | |
| length, cm | |
| k | permeability, m2 |
| ORR rate constant | |
| m | mass |
| M | molecular weight, g/mol |
| electroosmotic drag coefficient | |
| superficial flux density of species i in phase k, mol/(cm2·s) | |
| p | partial pressure, atm |
| geometric flow rate, cm3/s | |
| rate of reaction per unit of interfacial area between phases k and p, mol/(cm2·s) | |
| total ohmic resistance, Ω/cm2 | |
| R | ideal gas constant, 8.3143 J/(mol·K) |
| rate of homogeneous reaction g in phase k, mol/(cm3·s) | |
| rate of evaporation, mol/(cm3·s) | |
| s | water volume fraction |
| gas volume fraction | |
| S | source term |
| stoichiometric coefficient of species i residing in phase k and participating in electron transfer reaction h | |
| stoichiometric coefficient of species i residing in phase k and participating in electron transfer reaction g | |
| T | temperature, K |
| u | mobility, (m2·kmol)/(J·s) |
| velocity vector, cm/s | |
| potential intercept for a polarization equation, V | |
| reversible cell potential of reaction h, V | |
| enthalpy potential, V | |
| V | volume, m3 |
| molar volume, cm3/mol | |
| mole fraction of species i | |
| z | charge number of valence |
| Greek letters | |
| transfer coefficient, water transport coefficient kmol2/(J·m·s) | |
| roughness factor | |
| electrolyte thickness, cm | |
| porosity | |
| overpotential, V | |
| contact angle | |
| ionic conductivity of the membrane, S/cm | |
| λ | water content |
| dynamic viscosity, kg/(m·s) | |
| μw | membrane water chemical potential |
| velocity, cm/s | |
| fluid density average velocity, cm/s | |
| ρ | density, g/cm3 |
| σ | standard conductivity in the electronically conducting phase, S/cm |
| liquid–water surface tension, N/m | |
| tortuosity | |
| chemical potential | |
| Thiele modulus | |
| thermal conductivity, W/(m·K) | |
| Subscripts and superscripts | |
| agg | agglomerate |
| act | activation |
| an | anode |
| cat | cathode |
| chan | channel |
| eq | equilibrium |
| ext | external to the control volume |
| f | sulfonic acid group |
| G | gas phase |
| H2 | hydrogen |
| H2O | water |
| i, j | ith and jth components |
| ion | ionic |
| lim | limiting |
| L, l | liquid phase |
| k | phase |
| O2 | oxygen |
| ref | reference |
| sat | saturated |
| sol | solvent (ionomer) |
| non-frozen membrane water to vapor | |
| Naf | Nafion |
| w | water phase |
| vap | vapor |
| water to liquid (and vice versa) | |
References
- Caetano, N.S.; Mata, T.M.; Martins, A.A.; Felgueiras, M.C. New Trends in Energy Production and Utilization. Energy Procedia 2017, 107, 7–14. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Sugiawan, Y.; Managi, S. New evidence of energy-growth nexus from inclusive wealth. Renew. Sustain. Energy Rev. 2019, 103, 40–48. [Google Scholar] [CrossRef]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen fuel and fuel cell technology for cleaner future: A review. Environ. Sci. Pollut. Res. 2021, 28, 15607–15626. [Google Scholar]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Felseghi, R.-A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen fuel cell technology for the sustainable future of stationary applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Dincer, I. Hydrogen and fuel cell technologies for sustainable future. Jordan J. Mech. Ind. Eng. 2008, 2, 1–14. [Google Scholar]
- Xing, H.; Stuart, C.; Spence, S.; Chen, H. Fuel cell power systems for maritime applications: Progress and perspectives. Sustainability 2021, 13, 1213. [Google Scholar] [CrossRef]
- Mallouppas, G.; Yfantis, E.A. Decarbonization in shipping industry: A review of research, technology development, and innovation proposals. J. Mar. Sci. Eng. 2021, 9, 415. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Sulaiman, N.; Hannan, M.A.; Mohamed, A.; Ker, P.J.; Majlan, E.H.; Daud, W.R.W. Optimization of energy management system for fuel-cell hybrid electric vehicles: Issues and recommendations. Appl. Energy 2018, 228, 2061–2079. [Google Scholar] [CrossRef]
- Dall’Armi, C.; Pivetta, D.; Taccani, R. Health-Conscious Optimization of Long-Term Operation for Hybrid PEMFC Ship Propulsion Systems. Energies 2021, 14, 3813. [Google Scholar] [CrossRef]
- Lee, H.; Ryu, B.; Anh, D.P.; Roh, G.; Lee, S.; Kang, H. Thermodynamic analysis and assessment of novel ORC-DEC integrated PEMFC system for liquid hydrogen fueled ship application. Int. J. Hydrogen Energy 2023, 48, 3135–3153. [Google Scholar] [CrossRef]
- Wu, D.; Peng, C.; Yin, C.; Tang, H. Review of system integration and control of proton exchange membrane fuel cells. Electrochem. Energy Rev. 2020, 3, 466–505. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, G.; Jiang, Y.; Wang, R.; Sheng, X.; Xi, F.; Zhao, Z.; Chen, W.; Zhu, Y.; Wang, Y.; et al. “3D+ 1D” modeling approach toward large-scale PEM fuel cell simulation and partitioned optimization study on flow field. eTransportation 2020, 6, 100090. [Google Scholar] [CrossRef]
- He, C.; Desai, S.; Brown, G.; Bolleplli, S. PEM fuel cell catalysts: Cost, performance, and durability. Electrochem. Soc. Interface 2005, 14, 41. [Google Scholar] [CrossRef]
- Raj, A.; Shamim, T. Investigation of the effect of multidimensionality in PEM fuel cells. Energy Convers. Manag. 2014, 86, 443–452. [Google Scholar] [CrossRef]
- Ji, M.; Wei, Z. A review of water management in polymer electrolyte membrane fuel cells. Energies 2009, 2, 1057–1106. [Google Scholar] [CrossRef]
- Goebel, S.G. Evaporative Cooled Fuel Cell. US Patent 6960404 B2; General Motors Corporation, 1 November 2005. [Google Scholar]
- Kandlikar, S.G.; Lu, Z. Thermal management issues in a PEMFC stack–A brief review of current status. Appl. Therm. Eng. 2009, 29, 1276–1280. [Google Scholar] [CrossRef]
- Moreno, N.G.; Molina, M.C.; Gervasio, D.; Robles, J.F.P. Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renew. Sustain. Energy Rev. 2015, 52, 897–906. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.; Karpenko-Jereb, L.; Ashton, S. Advanced CFD Analysis of an Air-cooled PEM Fuel Cell Stack Predicting the Loss of Performance with Time. Fuel Cells 2016, 16, 490–503. [Google Scholar] [CrossRef]
- Wang, L.; Quan, Z.; Zhao, Y.; Yang, M.; Zhang, J. Experimental investigation on thermal management of proton exchange membrane fuel cell stack using micro heat pipe array. Appl. Therm. Eng. 2022, 214, 118831. [Google Scholar] [CrossRef]
- Huang, Z.; Jian, Q.; Zhao, J. Thermal management of open-cathode proton exchange membrane fuel cell stack with thin vapor chambers. J. Power Sources 2021, 485, 229314. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Lu, Z.; Domigan, W.E.; White, A.D.; Benedict, M.W. Measurement of flow maldistribution in parallel channels and its application to ex-situ and in-situ experiments in PEMFC water management studies. Int. J. Heat Mass Transf. 2009, 52, 1741–1752. [Google Scholar] [CrossRef]
- Werner, C.; Busemeyer, L.; Kallo, J. The impact of operating parameters and system architecture on the water management of a multifunctional PEMFC system. Int. J. Hydrogen Energy 2015, 40, 11595–11603. [Google Scholar] [CrossRef]
- Li, W.; Jing, S.; Wang, S.; Wang, C.; Xie, X. Experimental investigation of expanded graphite/phenolic resin composite bipolar plate. Int. J. Hydrogen Energy 2016, 41, 16240–16246. [Google Scholar] [CrossRef]
- Kahveci, E.E.; Taymaz, I. Experimental study on performance evaluation of PEM fuel cell by coating bipolar plate with materials having different contact angle. Fuel 2019, 253, 1274–1281. [Google Scholar] [CrossRef]
- Haraldsson, K.; Wipke, K. Evaluating PEM fuel cell system models. J. Power Sources 2004, 126, 88–97. [Google Scholar] [CrossRef]
- Arvay, A.; French, J.; Wang, J.-C.; Peng, X.-H.; Kannan, A. Nature inspired flow field designs for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2013, 38, 3717–3726. [Google Scholar] [CrossRef]
- Hamdollahi, S.; Jun, L. A Review on Modeling of Proton Exchange Membrane Fuel Cell. Chem. Ind. Chem. Eng. Q. 2023, 29, 61–74. [Google Scholar] [CrossRef]
- Arif, M.; Cheung, S.C.; Andrews, J. Different approaches used for modeling and simulation of polymer electrolyte membrane fuel cells: A review. Energy Fuels 2020, 34, 11897–11915. [Google Scholar] [CrossRef]
- D’adamo, A.; Haslinger, M.; Corda, G.; Höflinger, J.; Fontanesi, S.; Lauer, T. Modelling Methods and Validation Techniques for CFD Simulations of PEM Fuel Cells. Process 2021, 9, 688. [Google Scholar] [CrossRef]
- Legala, A.; Zhao, J.; Li, X. Machine learning modeling for proton exchange membrane fuel cell performance. Energy AI 2022, 10, 100183. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, F.; Quan, H.-B.; Yin, R.-J.; Tao, W.-Q. PEMFC Output Voltage Prediction Based on Different Machine Learning Regression Models. In Proceedings of the 2022 5th International Conference on Energy, Electrical and Power Engineering (CEEPE), Chongqing, China, 22–24 April 2022. [Google Scholar]
- Kheirandish, A.; Shafiabady, N.; Dahari, M.; Kazemi, M.S.; Isa, D. Modeling of commercial proton exchange membrane fuel cell using support vector machine. Int. J. Hydrogen Energy 2016, 41, 11351–11358. [Google Scholar] [CrossRef]
- Ghosh, S.; Routh, A.; Rahaman, M.; Ghosh, A. Modeling and control of a PEM fuel cell performance using Artificial Neural Networks to maximize the real time efficiency. In Proceedings of the 2019 International Conference on Energy Management for Green Environment (UEMGREEN), Kolkata, India, 25–27 September 2019. [Google Scholar]
- Seyhan, M.; Akansu, Y.E.; Murat, M.; Korkmaz, Y.; Akansu, S.O. Performance prediction of PEM fuel cell with wavy serpentine flow channel by using artificial neural network. Int. J. Hydrogen Energy 2017, 42, 25619–25629. [Google Scholar] [CrossRef]
- Jemeï, S.; Hissel, D.; Péra, M.; Kauffmann, J. On-board fuel cell power supply modeling on the basis of neural network methodology. J. Power Sources 2003, 124, 479–486. [Google Scholar] [CrossRef]
- Jemeïjemei, S.; Hissel, D.; Pérapera, M.-C.; Kauffmann, J.M. A new modeling approach of embedded fuel-cell power generators based on artificial neural network. IEEE Trans. Ind. Electron. 2008, 55, 437–447. [Google Scholar] [CrossRef]
- Cho, Y.; Hwang, G.; Gbadago, D.Q.; Hwang, S. Artificial neural network-based model predictive control for optimal operating conditions in proton exchange membrane fuel cells. J. Clean. Prod. 2022, 380, 135049. [Google Scholar] [CrossRef]
- Nanadegani, F.S.; Lay, E.N.; Iranzo, A.; Salva, J.A.; Sunden, B. On neural network modeling to maximize the power output of PEMFCs. Electrochim. Acta 2020, 348, 136345. [Google Scholar] [CrossRef]
- Salva, J.A.; Iranzo, A.; Rosa, F.; Tapia, E.; Lopez, E.; Isorna, F. Optimization of a PEM fuel cell operating conditions: Obtaining the maximum performance polarization curve. Int. J. Hydrogen Energy 2016, 41, 19713–19723. [Google Scholar] [CrossRef]
- Mawardi, A.; Yang, F.; Pitchumani, R. Optimization of the operating parameters of a proton exchange membrane fuel cell for maximum power density. J. Fuel Cell Sci. Technol. 2005, 2, 121–135. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, H.; Chen, G.; Wang, H.; Lu, L.; Liu, J.; Xing, L. Integration of multi-physics and machine learning-based surrogate modelling approaches for multi-objective optimization of deformed GDL of PEM fuel cells. Energy AI 2023, 14, 100261. [Google Scholar] [CrossRef]
- Wan, W.; Yang, Y.; Li, Y.; Xie, C.; Song, J.; Deng, Z.; Tan, J.; Zhang, R. Operating Conditions Combination Analysis Method of Optimal Water Management State for PEM Fuel Cell. Green Energy Intell. Transp. 2023, 2, 100105. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, M.; Quan, S. Model predictive control of water management in PEMFC. J. Power Sources 2008, 180, 322–329. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, K.; Zhou, S.; Shan, J.; Bao, D. Features selection and substitution in PEM fuel cell water management failures diagnosis. Fuel Cells 2021, 21, 512–522. [Google Scholar] [CrossRef]
- Laribi, S.; Mammar, K.; Sahli, Y.; Koussa, K. Analysis and diagnosis of PEM fuel cell failure modes (flooding & drying) across the physical parameters of electrochemical impedance model: Using neural networks method. Sustain. Energy Technol. Assess. 2019, 34, 35–42. [Google Scholar]
- Laribi, S.; Mammar, K.; Sahli, Y.; Koussa, K. Fault diagnosis of thermal management system in a polymer electrolyte membrane fuel cell. Energy 2021, 214, 119062. [Google Scholar]
- Pourrahmani, H.; Siavashi, M.; Moghimi, M. Design optimization and thermal management of the PEMFC using artificial neural networks. Energy 2019, 182, 443–459. [Google Scholar] [CrossRef]
- Maleki, E.; Maleki, N. Artificial neural network modeling of Pt/C cathode degradation in PEM fuel cells. J. Electron. Mater. 2016, 45, 3822–3834. [Google Scholar] [CrossRef]
- Kui, C.; Laghrouche, S.; Djerdir, A. Proton exchange membrane fuel cell degradation and remaining useful life prediction based on artificial neural network. In Proceedings of the 2018 7th International Conference on Renewable Energy Research and Applications (ICRERA), Paris, France, 14–17 October 2018. [Google Scholar]
- Matsuura, T.; Chen, J.; Siegel, J.B.; Stefanopoulou, A.G. Degradation phenomena in PEM fuel cell with dead-ended anode. Int. J. Hydrogen Energy 2013, 38, 11346–11356. [Google Scholar] [CrossRef]
- Han, I.-S.; Chung, C.-B. A hybrid model combining a support vector machine with an empirical equation for predicting polarization curves of PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 7023–7028. [Google Scholar] [CrossRef]
- Khajeh-Hosseini-Dalasm, N.; Ahadian, S.; Fushinobu, K.; Okazaki, K.; Kawazoe, Y. Prediction and analysis of the cathode catalyst layer performance of proton exchange membrane fuel cells using artificial neural network and statistical methods. J. Power Sources 2011, 196, 3750–3756. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells–a review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Advances in stationary and portable fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef]
- Du, B.; Guo, Q.; Pollard, R.; Rodriguez, D.; Smith, C.; Elter, J. PEM fuel cells: Status and challenges for commercial stationary power applications. JOM 2006, 58, 45–49. [Google Scholar] [CrossRef]
- Mancino, A.N.; Menale, C.; Vellucci, F.; Pasquali, M.; Bubbico, R. PEM Fuel Cell Applications in Road Transport. Energies 2023, 16, 6129. [Google Scholar] [CrossRef]
- Sürer, M.G.; Arat, H.T. Advancements and current technologies on hydrogen fuel cell applications for marine vehicles. Int. J. Hydrogen Energy 2022, 47, 19865–19875. [Google Scholar] [CrossRef]
- Dyantyi, N.; Parsons, A.; Sita, C.; Pasupathi, S. PEMFC for aeronautic applications: A review on the durability aspects. Open Eng. 2017, 7, 287–302. [Google Scholar] [CrossRef]
- Karlsson, V.; Ahlmark, D. An Environmental Perspective on the Feasibility of Using Existing PEMFC Technology in General Aviation. Bachelor Thesis, Royal Institute of Technology, Stockholm, Sweden, 26 May 2013. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-141020 (accessed on 22 May 2024).
- Jahnke, T.; Futter, G.; Latz, A.; Malkow, T.; Papakonstantinou, G.; Tsotridis, G.; Schott, P.; Gérard, M.; Quinaud, M.; Quiroga, M.; et al. Performance and degradation of Proton Exchange Membrane Fuel Cells: State of the art in modeling from atomistic to system scale. J. Power Sources 2016, 304, 207–233. [Google Scholar] [CrossRef]
- Petrone, R.; Zheng, Z.; Hissel, D.; Péra, M.; Pianese, C.; Sorrentino, M.; Becherif, M.; Yousfi-Steiner, N. A review on model-based diagnosis methodologies for PEMFCs. Int. J. Hydrogen Energy 2013, 38, 7077–7091. [Google Scholar] [CrossRef]
- Kandidayeni, M.; Macias, A.; Amamou, A.; Boulon, L.; Kelouwani, S.; Chaoui, H. Overview and benchmark analysis of fuel cell parameters estimation for energy management purposes. J. Power Sources 2018, 380, 92–104. [Google Scholar] [CrossRef]
- Costa, M.A.; Wullt, B.; Norrlöf, M.; Gunnarsson, S. Failure detection in robotic arms using statistical modeling, machine learning and hybrid gradient boosting. Measurement 2019, 146, 425–436. [Google Scholar] [CrossRef]
- Ashraf, H.; Abdellatif, S.O.; Elkholy, M.M.; El-Fergany, A.A. Computational techniques based on artificial intelligence for extracting optimal parameters of PEMFCs: Survey and insights. Arch. Comput. Methods Eng. 2022, 29, 3943–3972. [Google Scholar] [CrossRef]
- Shah, A.; Luo, K.; Ralph, T.; Walsh, F. Recent trends and developments in polymer electrolyte membrane fuel cell modelling. Electrochim. Acta 2011, 56, 3731–3757. [Google Scholar] [CrossRef]
- Shah, A.; Luo, K.; Ralph, T.; Walsh, F. CFD assisted modeling for control system design: A case study. Simul. Model. Pract. Theory 2009, 17, 730–742. [Google Scholar]
- Al-Baghdadi, M.A.S. Modelling of proton exchange membrane fuel cell performance based on semi-empirical equations. Renew. Energy 2005, 30, 1587–1599. [Google Scholar] [CrossRef]
- Pisani, L.; Murgia, G.; Valentini, M.; D’aguanno, B. A new semi-empirical approach to performance curves of polymer electrolyte fuel cells. J. Power Sources 2002, 108, 192–203. [Google Scholar] [CrossRef]
- Menesklou, P.; Sinn, T.; Nirschl, H.; Gleiss, M. Grey box modelling of decanter centrifuges by coupling a numerical process model with a neural network. Minerals 2021, 11, 755. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Peng, L.; Zhang, J.; Shao, Z.; Huang, J.; Sun, C.; Ouyang, M.; He, X. Recent progress on the key materials and components for proton exchange membrane fuel cells in vehicle applications. Energies 2016, 9, 603. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, B.; Ling, Z.; Liu, Q.; Fu, X.; Zhang, Y.; Zhang, R.; Hu, S.; Zhao, F.; Li, X.; et al. Advances in ionogels for proton-exchange membranes. Sci. Total Environ. 2024, 921, 171099. [Google Scholar] [CrossRef] [PubMed]
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Beuscher, U.; Cleghorn, S.J.; Johnson, W.B. Challenges for PEM fuel cell membranes. Int. J. Energy Res. 2005, 29, 1103–1112. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Valenzuela, E.; Gamboa, S.; Sebastian, P.; Moreira, J.; Pantoja, J.; Ibañez, G.; Reyes, A.; Campillo, B.; Serna, S. Proton charge transport in nafion nanochannels. J. Nano Res. 2009, 5, 31–36. [Google Scholar] [CrossRef]
- Chen, Q.; Schmidt-Rohr, K. Backbone Dynamics of the Nafion Ionomer Studied by 19F-13C Solid-State NMR. Macromol. Chem. Phys. 2007, 208, 2189–2203. [Google Scholar] [CrossRef]
- Rao, Z.; Zheng, C.; Geng, F. Proton conduction of fuel cell polymer membranes: Molecular dynamics simulation. Comput. Mater. Sci. 2018, 142, 122–128. [Google Scholar] [CrossRef]
- Harvey, D.A.; Bellemare-Davis, A.; Karan, K.; Jayansankar, B.; Pharoah, J.G.; Colbow, V.; Young, A.; Wessel, S. Statistical simulation of the performance and degradation of a PEMFC membrane electrode assembly. ECS Trans. 2013, 50, 147. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kang, H.; Sohn, Y.-J.; Lee, J.; Shim, S.; Lee, S.G. Molecular dynamics simulation study on the effect of perfluorosulfonic acid side chains on oxygen permeation in hydrated ionomers of PEMFCs. Sci. Rep. 2021, 11, 8702. [Google Scholar] [CrossRef]
- Srinophakun, T.; Martkumchan, S. Ionic conductivity in a chitosan membrane for a PEM fuel cell using molecular dynamics simulation. Carbohydr. Polym. 2012, 88, 194–200. [Google Scholar] [CrossRef]
- Chen, L.; He, Y.-L.; Tao, W.-Q. The temperature effect on the diffusion processes of water and proton in the proton exchange membrane using molecular dynamics simulation. Numer. Heat Transf. Part A Appl. 2014, 65, 216–228. [Google Scholar] [CrossRef]
- Zheng, C.; Geng, F.; Rao, Z. Proton mobility and thermal conductivities of fuel cell polymer membranes: Molecular dynamics simulation. Comput. Mater. Sci. 2017, 132, 55–61. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, T.-H.; Kim, D.J.; Nam, S.Y. Molecular dynamics simulation of the functional group effect in hydrocarbon anionic exchange membranes. Int. J. Hydrogen Energy 2017, 42, 20895–20903. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, S.Y.; Kim, H.-J.; Jang, S.S.; Lee, S.G. Distribution characteristics of phosphoric acid and PTFE binder on Pt/C surfaces in high-temperature polymer electrolyte membrane fuel cells: Molecular dynamics simulation approach. Int. J. Hydrogen Energy 2021, 46, 17295–17305. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.J.; Smith, G. Modelling the proton-conductive membrane in practical polymer electrolyte membrane fuel cell (PEMFC) simulation: A review. Membranes 2020, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- von Schröder, P. Über Erstarrungs-und Quellugserscheinungen von Gelatine. Z. Für Phys. Chem. 1903, 45, 75–117. [Google Scholar] [CrossRef]
- Onishi, L.M.; Prausnitz, J.M.; Newman, J. Water− Nafion equilibria. Absence of schroeder’s paradox. J. Phys. Chem. B 2007, 111, 10166–10173. [Google Scholar] [CrossRef]
- Jeck, S.; Scharfer, P.; Kind, M. Absence of Schroeder’s paradox: Experimental evidence for water-swollen Nafion® membranes. J. Membr. Sci. 2011, 373, 74–79. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334. [Google Scholar] [CrossRef]
- Newman, J.; Balsara, N.P. Electrochemical Systems; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Ye, Q.; Van Nguyen, T. Three-dimensional simulation of liquid water distribution in a PEMFC with experimentally measured capillary functions. J. Electrochem. Soc. 2007, 154, B1242. [Google Scholar] [CrossRef]
- Kulikovsky, A. Quasi-3D modeling of water transport in polymer electrolyte fuel cells. J. Electrochem. Soc. 2003, 150, A1432. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Transport in polymer-electrolyte membranes: I. Physical model. J. Electrochem. Soc. 2003, 150, A1008. [Google Scholar] [CrossRef]
- Pasaogullari, U.; Wang, C.-Y.; Chen, K.S. Two-phase transport in polymer electrolyte fuel cells with bilayer cathode gas diffusion media. J. Electrochem. Soc. 2005, 152, A1574. [Google Scholar] [CrossRef]
- Einstein, A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen [AdP 17, 549 (1905)]. Ann. Der Phys. 2005, 14 (Suppl. S1), 182–193. [Google Scholar] [CrossRef]
- Nernst, W. Zur kinetik der in lösung befindlichen körper. Z. Für Phys. Chem. 1888, 2, 613–637. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley & Sons: New York, NY, USA, 1960; Volume 413, p. 362. [Google Scholar]
- Bennion, D.N. Mass Transport of Binary Electrolyte Solutions in Membranes; Department of Engineering, University of California: Berkeley, CA, USA, 1966. [Google Scholar]
- Pintauro, P.N.; Bennion, D.N. Mass transport of electrolytes in membranes. 1. Development of mathematical transport model. Ind. Eng. Chem. Fundam. 1984, 23, 230–234. [Google Scholar] [CrossRef]
- Koter, S.; Hamann, C. Characteristics of ion-exchange membranes for electrodialysis on the basis of irreversible thermodynamics. J. Non-Equilibrium Thermodyn. 1990, 15, 315–334. [Google Scholar] [CrossRef]
- Auclair, B.; Nikonenko, V.; Larchet, C.; Métayer, M.; Dammak, L. Correlation between transport parameters of ion-exchange membranes. J. Membr. Sci. 2002, 195, 89–102. [Google Scholar] [CrossRef]
- Baschuk, J.; Li, X. A general formulation for a mathematical PEM fuel cell model. J. Power Sources 2005, 142, 134–153. [Google Scholar] [CrossRef]
- Baschuk, J.; Li, X. A comprehensive, consistent and systematic mathematical model of PEM fuel cells. Appl. Energy 2009, 86, 181–193. [Google Scholar] [CrossRef]
- Fimrite, J.; Struchtrup, H.; Djilali, N. Transport phenomena in polymer electrolyte membranes: I. modeling framework. J. Electrochem. Soc. 2005, 152, A1804. [Google Scholar] [CrossRef]
- Wöhr, M.; Bolwin, K.; Schnurnberger, W.; Fischer, M.; Neubrand, W.; Eigenberger, G. Dynamic modelling and simulation of a polymer membrane fuel cell including mass transport limitation. Int. J. Hydrogen Energy 1998, 23, 213–218. [Google Scholar] [CrossRef]
- Berg, P.; Promislow, K.; Pierre, J.S.; Stumper, J.; Wetton, B. Water management in PEM fuel cells. J. Electrochem. Soc. 2004, 151, A341. [Google Scholar] [CrossRef]
- Thampan, T.; Malhotra, S.; Tang, H.; Datta, R. Modeling of conductive transport in proton-exchange membranes for fuel cells. J. Electrochem. Soc. 2000, 147, 3242. [Google Scholar] [CrossRef]
- Mason, E.A.; Malinauskas, A.P. Gas Transportin Porous Media: The Dusty-Gas Model; Elsevier: New York, NY, USA, 1983. [Google Scholar]
- Weber, A.Z.; Newman, J. Modeling transport in polymer-electrolyte fuel cells. Chem. Rev. 2004, 104, 4679–4726. [Google Scholar] [CrossRef]
- Bernardi, D.M.; Verbrugge, M.W. Mathematical model of a gas diffusion electrode bonded to a polymer electrolyte. AIChE J. 1991, 37, 1151–1163. [Google Scholar] [CrossRef]
- Bernardi, D.M.; Verbrugge, M.W. A mathematical model of the solid-polymer-electrolyte fuel cell. J. Electrochem. Soc. 1992, 139, 2477. [Google Scholar] [CrossRef]
- Verbrugge, M.W.; Hill, R.F. Ion and Solvent Transport in Ion-Exchange Membranes: I. A Macrohomogeneous Mathematical Model. J. Electrochem. Soc. 1990, 137, 886. [Google Scholar] [CrossRef]
- Verbrugge, M.W.; Hill, R.F. Analysis of Promising Perfluorosulfonic Acid Membranes for Fuel-Cell Electrolytes. J. Electrochem. Soc. 1990, 137, 3770. [Google Scholar] [CrossRef]
- Verbrugge, M.W.; Hill, R.F. Transport phenomena in perfluorosulfonic acid membranes during the passage of current. J. Electrochem. Soc. 1990, 137, 1131. [Google Scholar] [CrossRef]
- Schlögl, R. Zur Theorie der anomalen Osmose. Z. Für Phys. Chem. 1955, 3, 73–102. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Transport in polymer-electrolyte membranes: II. Mathematical model. J. Electrochem. Soc. 2004, 151, A311. [Google Scholar] [CrossRef]
- Costamagna, P. Transport phenomena in polymeric membrane fuel cells. Chem. Eng. Sci. 2001, 56, 323–332. [Google Scholar] [CrossRef]
- Jung, S.Y.; Nguyen, T.V. An along-the-channel model for proton exchange membrane fuel cells. J. Electrochem. Soc. 1998, 145, 1149. [Google Scholar]
- You, L.; Liu, H. A two-phase flow and transport model for the cathode of PEM fuel cells. Int. J. Heat Mass Transf. 2002, 45, 2277–2287. [Google Scholar] [CrossRef]
- Hertwig, K.; Martens, L.; Karwoth, R. Mathematical Modelling and Simulation of Polymer Electrolyte Membrane Fuel Cells. Part I: Model Structures and Solving an Isothermal One-Cell Model. Fuel Cells 2002, 2, 61–77. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Derouin, C.; Radzinski, S.; Sherman, R.J.; Smith, V.T.; Springer, T.E.; Gottesfeld, S. Water uptake by and transport through Nafion® 117 membranes. J. Electrochem. Soc. 1993, 140, 1041. [Google Scholar] [CrossRef]
- Zawodzinski, T.A., Jr.; Springer, T.E.; Uribe, F.; Gottesfeld, S., Jr. Characterization of polymer electrolytes for fuel cell applications. Solid State Ion. 1993, 60, 199–211. [Google Scholar] [CrossRef]
- van Bussel, H.P.; Koene, F.G.; Mallant, R.K. Dynamic model of solid polymer fuel cell water management. J. Power Sources 1998, 71, 218–222. [Google Scholar] [CrossRef]
- Berning, T. On the Nature of Electro-Osmotic Drag. Energies 2020, 13, 4726. [Google Scholar] [CrossRef]
- Berning, T. On water transport in polymer electrolyte membranes during the passage of current. Int. J. Hydrogen Energy 2011, 36, 9341–9344. [Google Scholar] [CrossRef]
- Fuller, T.F. Solid-Polymer-Electrolyte Fuel Cells; University of California: Berkeley, CA, USA, 1992. [Google Scholar]
- Carcadea, E.; Ingham, D.B.; Stefanescu, I.; Ionete, R.; Ene, H. The influence of permeability changes for a 7-serpentine channel pem fuel cell performance. Int. J. Hydrogen Energy 2011, 36, 10376–10383. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Davey, J.; Valerio, J.; Gottesfeld, S. The water content dependence of electro-osmotic drag in proton-conducting polymer electrolytes. Electrochim. Acta 1995, 40, 297–302. [Google Scholar] [CrossRef]
- Seddiq, M.; Khaleghi, H.; Mirzaei, M. Numerical analysis of gas cross-over through the membrane in a proton exchange membrane fuel cell. J. Power Sources 2006, 161, 371–379. [Google Scholar] [CrossRef]
- de Bruijn, F.A.; Makkus, R.C.; Mallant, R.K.A.; Janssen, G.J.M. Materials for state-of-the-art PEM fuel cells, and their suitability for operation above 100 C. Adv. Fuel Cells 2007, 1, 235–336. [Google Scholar]
- Eikerling, M.; Kharkats, Y.I.; Kornyshev, A.A.; Volfkovich, Y.M. Phenomenological theory of electro-osmotic effect and water management in polymer electrolyte proton-conducting membranes. J. Electrochem. Soc. 1998, 145, 2684. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.; Stimming, U. Electrophysical properties of polymer electrolyte membranes: A random network model. J. Phys. Chem. B 1997, 101, 10807–10820. [Google Scholar] [CrossRef]
- Rui, Z.; Liu, J. Understanding of free radical scavengers used in highly durable proton exchange membranes. Prog. Nat. Sci. Mater. Int. 2020, 30, 732–742. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.; Shin, S.-H.; Kim, N.; Shin, D.; Bae, B. Increasing the durability of polymer electrolyte membranes using organic additives. ACS Omega 2018, 3, 11262–11269. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, V.; Arges, C.G.; Ramani, V. Investigation of polymer electrolyte membrane chemical degradation and degradation mitigation using in situ fluorescence spectroscopy. Proc. Natl. Acad. Sci. USA 2012, 109, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chen, W.; Lu, S.; Chen, X. Parameter study of high-temperature proton exchange membrane fuel cell using data-driven models. Int. J. Hydrogen Energy 2019, 44, 28958–28967. [Google Scholar] [CrossRef]
- Huo, W.; Li, W.; Zhang, Z.; Sun, C.; Zhou, F.; Gong, G. Performance prediction of proton-exchange membrane fuel cell based on convolutional neural network and random forest feature selection. Energy Convers. Manag. 2021, 243, 114367. [Google Scholar] [CrossRef]
- Briceno-Mena, L.A.; Venugopalan, G.; Romagnoli, J.A.; Arges, C.G. Machine learning for guiding high-temperature PEM fuel cells with greater power density. Patterns 2021, 2, 100187. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Wang, R.; Ding, Y.; Yin, W.; Liu, Y.; Li, J.; Liu, J. Designing AI-Aided Analysis and Prediction Models for Nonprecious Metal Electrocatalyst-Based Proton-Exchange Membrane Fuel Cells. Angew. Chem. 2020, 132, 19337–19345. [Google Scholar] [CrossRef]
- Amirinejad, M.; Tavajohi-Hasankiadeh, N.; Madaeni, S.S.; Navarra, M.A.; Rafiee, E.; Scrosati, B. Adaptive neuro-fuzzy inference system and artificial neural network modeling of proton exchange membrane fuel cells based on nanocomposite and recast Nafion membranes. Int. J. Energy Res. 2013, 37, 347–357. [Google Scholar] [CrossRef]
- Musharavati, F. Four dimensional bio-inspired optimization approach with artificial intelligence for proton exchange membrane fuel cell. Int. J. Energy Res. 2022, 46, 21424–21440. [Google Scholar] [CrossRef]
- Briceno-Mena, L.A.; Romagnoli, J.A.; Arges, C.G. PemNet: A Transfer Learning-Based Modeling Approach of High-Temperature Polymer Electrolyte Membrane Electrochemical Systems. Ind. Eng. Chem. Res. 2022, 61, 3350–3357. [Google Scholar] [CrossRef]
- Foughali, Y.; Mankour, M.; Sekour, M.; Azzeddine, H.A.; Larbaoui, A.; Chaouch, D.-E.; Berka, M. A RBF artificial neural network to predict a fuel cell maximum power point. Prz. Elektrotechniczny 2022, 1, 102–106. [Google Scholar] [CrossRef]
- Li, G.; Zhu, Y.; Guo, Y.; Mabuchi, T.; Li, D.; Huang, S.; Wang, S.; Sun, H.; Tokumasu, T. Deep Learning to Reveal the Distribution and Diffusion of Water Molecules in Fuel Cell Catalyst Layers. ACS Appl. Mater. Interfaces 2023, 15, 5099–5108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Liu, W.; Mao, L. Diagnosing Improper Membrane Water Content in Proton Exchange Membrane Fuel Cell Using Two-Dimensional Convolutional Neural Network. Energies 2022, 15, 4247. [Google Scholar] [CrossRef]
- Huo, H.; Chen, J.; Wang, K.; Wang, F.; Jin, G.; Chen, F. State Estimation of Membrane Water Content of PEMFC Based on GA-BP Neural Network. Sustainability 2023, 15, 9094. [Google Scholar] [CrossRef]
- Gu, X.; Hou, Z.; Cai, J. Data-based flooding fault diagnosis of proton exchange membrane fuel cell systems using LSTM networks. Energy AI 2021, 4, 100056. [Google Scholar] [CrossRef]
- Nourizadeh, A. Machine Learning of PEM Fuel Cell Degradation: Artificial Neural Network and Long Short-Term Memory Recurrent Neural Network; University of California: Irvine, CA, USA, 2023. [Google Scholar]
- Nguyen, H.-L.; Lee, S.-M.; Yu, S. A Comprehensive Review of Degradation Prediction Methods for an Automotive Proton Exchange Membrane Fuel Cell. Energies 2023, 16, 4772. [Google Scholar] [CrossRef]
- Gatto, I.; Saccà, A.; Baglio, V.; Aricò, A.S.; Oldani, C.; Merlo, L.; Carbone, A. Evaluation of hot pressing parameters on the electrochemical performance of MEAs based on Aquivion® PFSA membranes. J. Energy Chem. 2019, 35, 168–173. [Google Scholar] [CrossRef]
- Spiegel, C. PEM Fuel Cell Modeling and Simulation Using MATLAB; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Sui, P.-C.; Zhu, X.; Djilali, N. Modeling of PEM fuel cell catalyst layers: Status and outlook. Electrochem. Energy Rev. 2019, 2, 428–466. [Google Scholar] [CrossRef]
- Wilson, M.S.; Gottesfeld, S. Thin-film catalyst layers for polymer electrolyte fuel cell electrodes. J. Appl. Electrochem. 1992, 22, 1–7. [Google Scholar] [CrossRef]
- Janssen, G.; Overvelde, M. Water transport in the proton-exchange-membrane fuel cell: Measurements of the effective drag coefficient. J. Power Sources 2001, 101, 117–125. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Transport in polymer-electrolyte membranes: III. model validation in a simple fuel-cell model. J. Electrochem. Soc. 2004, 151, A326. [Google Scholar] [CrossRef]
- Bradean, R.; Promislow, K.; Wetton, B. Transport phenomena in the porous cathode of a proton exchange membrane fuel cell. Numer. Heat Transf. Part A Appl. 2002, 42, 121–138. [Google Scholar] [CrossRef]
- Weber, A.Z.; Darling, R.M.; Newman, J. Modeling two-phase behavior in PEFCs. J. Electrochem. Soc. 2004, 151, A1715. [Google Scholar] [CrossRef]
- Nam, J.H.; Kaviany, M. Effective diffusivity and water-saturation distribution in single-and two-layer PEMFC diffusion medium. Int. J. Heat Mass Transf. 2003, 46, 4595–4611. [Google Scholar] [CrossRef]
- Newman, J. Optimization of potential and hydrogen utilization in an acid fuel cell. Electrochim. Acta 1979, 24, 223–229. [Google Scholar] [CrossRef]
- Mann, R.F.; Amphlett, J.C.; Hooper, M.A.I.; Jensen, H.M.; Peppley, B.A.; Roberge, P.R. Development and application of a generalised steady-state electrochemical model for a PEM fuel cell. J. Power Sources 2000, 86, 173–180. [Google Scholar] [CrossRef]
- West, A.; Fuller, T. Influence of rib spacing in proton-exchange membrane electrode assemblies. J. Appl. Electrochem. 1996, 26, 557–565. [Google Scholar] [CrossRef]
- Yi, J.S.; Van Nguyen, T. Multicomponent transport in porous electrodes of proton exchange membrane fuel cells using the interdigitated gas distributors. J. Electrochem. Soc. 1999, 146, 38. [Google Scholar] [CrossRef]
- Berning, T.; Lu, D.M.; Djilali, N. Three-dimensional computational analysis of transport phenomena in a PEM fuel cell. J. Power Sources 2002, 106, 284–294. [Google Scholar] [CrossRef]
- Kazim, A.; Liu, H.; Forges, P. Modelling of performance of PEM fuel cells with conventional and interdigitated flow fields. J. Appl. Electrochem. 1999, 29, 1409–1416. [Google Scholar] [CrossRef]
- Singh, D.; Lu, D.; Djilali, N. A two-dimensional analysis of mass transport in proton exchange membrane fuel cells. Int. J. Eng. Sci. 1999, 37, 431–452. [Google Scholar] [CrossRef]
- Giner, J.; Hunter, C. The Mechanism of Operation of the Teflon-Bonded Gas Diffusion Electrode: A Mathematical Model. J. Electrochem. Soc. 1969, 116, 1124. [Google Scholar] [CrossRef]
- Grens, E.A.; Turner, R.M.; Katan, T. A model for analysis of porous gas electrodes. Adv. Energy Convers. 1964, 4, 109–119. [Google Scholar] [CrossRef]
- Chirkov, Y.G. Difference between hydrophobized and hydrophilic electrodes. III. Cylindrical gas pore model. Sov. Electrochem. 1975, 11, 36. [Google Scholar]
- Chirkov, Y.G. Mechanism of filling wetproofed electrodes with gas. Elektrokhimiya 1972, 8, 11. [Google Scholar]
- Cutlip, M. An approximate model for mass transfer with reaction inporous gas diffusion electrodes. Electrochim. Acta 1975, 20, 767–773. [Google Scholar] [CrossRef]
- Cutlip, M.; Yang, S.; Stonehart, P. Model development for porous gas-diffusion electrodes and application to the phosphoric-acid fuel-cell cathode. J. Electrochem. Soc. 1986, 86, 12. [Google Scholar]
- Iczkowski, R.P.; Cutlip, M.B. Voltage losses in fuel cell cathodes. J. Electrochem. Soc. 1980, 127, 1433. [Google Scholar] [CrossRef]
- Cutlip, M.; Yang, S.; Stonehart, P. Simulation and optimization of porous gas-diffusion electrodes used in hydrogen oxygen phosphoric acid fuel cells—II development of a detailed anode model. Electrochim. Acta 1991, 36, 547–553. [Google Scholar] [CrossRef]
- Vogel, W.; Lundquist, J.; Bradford, A. Reduction of oxygen on Teflon-backed platinum black electrodes. Electrochim. Acta 1972, 17, 1735–1744. [Google Scholar] [CrossRef]
- Björnbom, P. Modelling of a double-layered PTFE-bonded oxygen electrode. Electrochim. Acta 1987, 32, 115–119. [Google Scholar] [CrossRef]
- Antoine, O.; Bultel, Y.; Durand, R.; Ozil, P. Electrocatalysis, diffusion and ohmic drop in PEMFC: Particle size and spatial discrete distribution effects. Electrochim. Acta 1998, 43, 3681–3691. [Google Scholar] [CrossRef]
- Bultel, Y.; Ozil, P.; Durand, R. Modelling the mode of operation of PEMFC electrodes at the particle level: Influence of ohmic drop within the active layer on electrode performance. J. Appl. Electrochem. 1998, 28, 269–276. [Google Scholar] [CrossRef]
- Bultel, Y.; Ozil, P.; Durand, R. Modelling of mass transfer within the PEM fuel cell active layer: Limitations at the particle level. J. Appl. Electrochem. 1999, 29, 1025–1033. [Google Scholar] [CrossRef]
- Tiedemann, W.; Newman, J. Maximum effective capacity in an ohmically limited porous electrode. J. Electrochem. Soc. 1975, 122, 1482. [Google Scholar] [CrossRef]
- Kulikovsky, A.; Divisek, J.; Kornyshev, A. Modeling the cathode compartment of polymer electrolyte fuel cells: Dead and active reaction zones. J. Electrochem. Soc. 1999, 146, 3981. [Google Scholar] [CrossRef]
- Murgia, G.; Pisani, L.; Valentini, M.; D’aguanno, B. Electrochemistry and mass transport in polymer electrolyte membrane fuel cells I. Model. J. Electrochem. Soc. 2001, 149, A31. [Google Scholar] [CrossRef]
- Gurau, V.; Liu, H.; Kakac, S. Two-dimensional model for proton exchange membrane fuel cells. AIChE J. 1998, 44, 2410–2422. [Google Scholar] [CrossRef]
- Bevers, D.; WO¨HR, M.; Yasuda, K.; Oguro, A.K. Simulation of a polymer electrolyte fuel cell electrode. J. Appl. Electrochem. 1997, 27, 1254–1264. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A. Modelling the performance of the cathode catalyst layer of polymer electrolyte fuel cells. J. Electroanal. Chem. 1998, 453, 89–106. [Google Scholar] [CrossRef]
- Fuller, T.F.; Newman, J. Water and thermal management in solid-polymer-electrolyte fuel cells. J. Electrochem. Soc. 1993, 140, 1218. [Google Scholar] [CrossRef]
- Kornyshev, A.; Kulikovsky, A. Characteristic length of fuel and oxygen consumption in feed channels of polymer electrolyte fuel cells. Electrochim. Acta 2001, 46, 4389–4395. [Google Scholar] [CrossRef]
- Kulikovsky, A.A. Quasi Three–Dimensional Modelling of the PEM Fuel Cell: Comparison of the Catalyst Layers Performance. Fuel Cells 2001, 1, 162–169. [Google Scholar] [CrossRef]
- Wang, J.-T.; Savinell, R.F. Simulation studies on the fuel electrode of a H2O2 polymer electrolyte fuel cell. Electrochim. Acta 1992, 37, 2737–2745. [Google Scholar] [CrossRef]
- Perry, M.L.; Newman, J.; Cairns, E.J. Mass transport in gas-diffusion electrodes: A diagnostic tool for fuel-cell cathodes. J. Electrochem. Soc. 1998, 145, 5. [Google Scholar] [CrossRef]
- Um, S.; Wang, C.Y.; Chen, K. Computational fluid dynamics modeling of proton exchange membrane fuel cells. J. Electrochem. Soc. 2000, 147, 4485. [Google Scholar] [CrossRef]
- Marr, C.; Li, X. Composition and performance modelling of catalyst layer in a proton exchange membrane fuel cell. J. Power Sources 1999, 77, 17–27. [Google Scholar] [CrossRef]
- Gloaguen, F.; Convert, P.; Gamburzev, S.; Velev, O.; Srinivasan, S. An evaluation of the macro-homogeneous and agglomerate model for oxygen reduction in PEMFCs. Electrochim. Acta 1998, 43, 3767–3772. [Google Scholar] [CrossRef]
- Gloaguen, F.; Durand, R. Simulations of PEFC cathodes: An effectiveness factor approach. J. Appl. Electrochem. 1997, 27, 1029–1035. [Google Scholar] [CrossRef]
- Jaouen, F.; Lindbergh, G.; Sundholm, G. Investigation of Mass-Transport Limitations in the Solid Polymer Fuel Cell Cathode: I. Mathematical Model. J. Electrochem. Soc. 2002, 149, A437. [Google Scholar] [CrossRef]
- Siegel, N.P.; Ellis, M.W.; Nelson, D.J.; Von Spakovsky, M.R. Single Domain Pemfc Model Based on Agglomerate Catalyst Geometry. J. Power Sources 2003, 115, 81–89. [Google Scholar] [CrossRef]
- Broka, K.; Ekdunge, P. Modelling the PEM fuel cell cathode. J. Appl. Electrochem. 1997, 27, 281–289. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Antolini, E. Review in applied electrochemistry. Number 54 recent developments in polymer electrolyte fuel cell electrodes. J. Appl. Electrochem. 2004, 34, 563–576. [Google Scholar] [CrossRef]
- Mazumder, S.; Cole, J.V. Rigorous 3-D mathematical modeling of PEM fuel cells: I. Model predictions without liquid water transport. J. Electrochem. Soc. 2003, 150, A1503. [Google Scholar] [CrossRef]
- Mazumder, S.; Cole, J.V. Rigorous 3-D mathematical modeling of PEM fuel cells: II. Model predictions with liquid water transport. J. Electrochem. Soc. 2003, 150, A1510. [Google Scholar] [CrossRef]
- Rho, Y.W.; Srinivasan, S.; Kho, Y.T. Mass Transport Phenomena in Proton Exchange Membrane Fuel Cells Using O2/He, O2/Ar, and O2/N2 Mixtures: II. Theoretical Analysis. J. Electrochem. Soc. 1994, 141, 2089. [Google Scholar] [CrossRef]
- Maja, M.; Tosco, P.; Vanni, M. A One-Dimensional Model of Gas-Diffusion Electrodes for O2 Reduction. J. Electrochem. Soc. 2001, 148, A1368. [Google Scholar] [CrossRef]
- Pisani, L.; Murgia, G.; Valentini, M.; D’aguanno, B. A working model of polymer electrolyte fuel cells: Comparisons between theory and experiments. J. Electrochem. Soc. 2002, 149, A898. [Google Scholar] [CrossRef]
- Dannenberg, K.; Ekdunge, P.; Lindbergh, G. Mathematical model of the PEMFC. J. Appl. Electrochem. 2000, 30, 1377–1387. [Google Scholar] [CrossRef]
- Sui, P.-C.; Chen, L.-D.; Seaba, J.P.; Wariishi, Y. Modeling and Optimization of a PEMFC Catalyst Layer; SAE Transactions: Warrendale, PA, USA, 1999; pp. 729–737. [Google Scholar]
- Sivertsen, B.R.; Djilali, N. CFD-based modelling of proton exchange membrane fuel cells. J. Power Sources 2005, 141, 65–78. [Google Scholar] [CrossRef]
- d’Adamo, A.; Riccardi, M.; Borghi, M.; Fontanesi, S. CFD Modelling of a Hydrogen/Air PEM Fuel Cell with a Serpentine Gas Distributor. Processes 2021, 9, 564. [Google Scholar] [CrossRef]
- Wang, C.-Y. Fundamental Models for Fuel Cell Engineering. Chem. Rev. 2004, 104, 4727–4766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fan, L.; Sun, J.; Jiao, K. A 3D model of PEMFC considering detailed multiphase flow and anisotropic transport properties. Int. J. Heat Mass Transf. 2017, 115, 714–724. [Google Scholar] [CrossRef]
- Nitta, I.; Karvonen, S.; Himanen, O.; Mikkola, M. Modelling the effect of inhomogeneous compression of GDL on local transport phenomena in a PEM fuel cell. Fuel Cells 2008, 8, 410–421. [Google Scholar] [CrossRef]
- Zhang, G.; Jiao, K. Three-dimensional multi-phase simulation of PEMFC at high current density utilizing Eulerian-Eulerian model and two-fluid model. Energy Convers. Manag. 2018, 176, 409–421. [Google Scholar] [CrossRef]
- Wang, B.; Wu, K.; Xi, F.; Xuan, J.; Xie, X.; Wang, X.; Jiao, K. Numerical analysis of operating conditions effects on PEMFC with anode recirculation. Energy 2019, 173, 844–856. [Google Scholar] [CrossRef]
- Yang, X.-G.; Ye, Q.; Cheng, P. Matching of water and temperature fields in proton exchange membrane fuel cells with non-uniform distributions. Int. J. Hydrogen Energy 2011, 36, 12524–12537. [Google Scholar] [CrossRef]
- De Lile, J.R.; Zhou, S. Theoretical modeling of the PEMFC catalyst layer: A review of atomistic methods. Electrochim. Acta 2015, 177, 4–20. [Google Scholar] [CrossRef]
- Ju, H.; Wang, C.-Y. Experimental validation of a PEM fuel cell model by current distribution data. J. Electrochem. Soc. 2004, 151, A1954. [Google Scholar] [CrossRef]
- Kone, J.-P.; Zhang, X.; Yan, Y.; Hu, G.; Ahmadi, G. Three-dimensional multiphase flow computational fluid dynamics models for proton exchange membrane fuel cell: A theoretical development. J. Comput. Multiph. Flows 2017, 9, 3–25. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Falcão, D.; Oliveira, V.; Pinto, A. 1D+ 3D two-phase flow numerical model of a proton exchange membrane fuel cell. Appl. Energy 2017, 203, 474–495. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Jang, J.-H.; Yan, W.-M.; Li, H.-Y.; Liao, C.-C. A three-dimensional modeling of transport phenomena of proton exchange membrane fuel cells with various flow fields. Appl. Energy 2012, 96, 359–370. [Google Scholar] [CrossRef]
- Min, C.; He, Y.; Liu, X.; Yin, B.; Jiang, W.; Tao, W. Parameter sensitivity examination and discussion of PEM fuel cell simulation model validation: Part II: Results of sensitivity analysis and validation of the model. J. Power Sources 2006, 160, 374–385. [Google Scholar] [CrossRef]
- Tao, W.; Min, C.; Liu, X.; He, Y.; Yin, B.; Jiang, W. Parameter sensitivity examination and discussion of PEM fuel cell simulation model validation: Part I. Current status of modeling research and model development. J. Power Sources 2006, 160, 359–373. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Srinivasan, S.; Appleby, A.J.; Martin, C.R. Temperature dependence of the electrode kinetics of oxygen reduction at the platinum/Nafion® interface—A microelectrode investigation. J. Electrochem. Soc. 1992, 139, 2530. [Google Scholar] [CrossRef]
- Heidary, H.; Kermani, M.J.; Khajeh-Hosseini-Dalasm, N. Performance analysis of PEM fuel cells cathode catalyst layer at various operating conditions. Int. J. Hydrogen Energy 2016, 41, 22274–22284. [Google Scholar] [CrossRef]
- Harvey, D.; Pharoah, J.; Karan, K. A comparison of different approaches to modelling the PEMFC catalyst layer. J. Power Sources 2008, 179, 209–219. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Song, C.; Xia, Z.; Li, H.; Wang, H.; Zhang, J. PEM fuel cell relative humidity (RH) and its effect on performance at high temperatures. Electrochim. Acta 2008, 53, 5315–5321. [Google Scholar] [CrossRef]
- Dickinson, E.J.; Hinds, G. The Butler-Volmer equation for polymer electrolyte membrane fuel cell (PEMFC) electrode kinetics: A critical discussion. J. Electrochem. Soc. 2019, 166, F221. [Google Scholar] [CrossRef]
- Tomizawa, M.; Inoue, G.; Nagato, K.; Tanaka, A.; Park, K.; Nakao, M. Heterogeneous pore-scale model analysis of micro-patterned PEMFC cathodes. J. Power Sources 2023, 556, 232507. [Google Scholar] [CrossRef]
- So, M.; Park, K.; Ohnishi, T.; Ono, M.; Tsuge, Y.; Inoue, G. A discrete particle packing model for the formation of a catalyst layer in polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2019, 44, 32170–32183. [Google Scholar] [CrossRef]
- Inoue, G.; Park, K.; So, M.; Kimura, N.; Tsuge, Y. Microscale simulations of reaction and mass transport in cathode catalyst layer of polymer electrolyte fuel cell. Int. J. Hydrogen Energy 2022, 47, 12665–12683. [Google Scholar] [CrossRef]
- Olbrich, W.; Kadyk, T.; Sauter, U.; Eikerling, M. Wetting phenomena in catalyst layers of PEM fuel cells: Novel approaches for modeling and materials research. J. Electrochem. Soc. 2022, 169, 054521. [Google Scholar] [CrossRef]
- Xu, K.; Zhao, X.; Hu, X.; Guo, Z.; Ye, Q.; Li, L.; Song, J.; Song, P. The review of the degradation mechanism of the catalyst layer of membrane electrode assembly in the proton exchange membrane fuel cell. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Franco, A.A.; Schott, P.; Jallut, C.; Maschke, B. A dynamic mechanistic model of an electrochemical interface. J. Electrochem. Soc. 2006, 153, A1053. [Google Scholar] [CrossRef]
- Franco, A.A.; Tembely, M. Transient multiscale modeling of aging mechanisms in a PEFC cathode. J. Electrochem. Soc. 2007, 154, B712. [Google Scholar] [CrossRef]
- Franco, A.A.; Guinard, M.; Barthe, B.; Lemaire, O. Impact of carbon monoxide on PEFC catalyst carbon support degradation under current-cycled operating conditions. Electrochim. Acta 2009, 54, 5267–5279. [Google Scholar] [CrossRef]
- Malek, K.; Franco, A.A. Microstructure-based modeling of aging mechanisms in catalyst layers of polymer electrolyte fuel cells. J. Phys. Chem. B 2011, 115, 8088–8101. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.Z.; Borup, R.L.; Darling, R.M.; Das, P.K.; Dursch, T.J.; Gu, W.; Harvey, D.; Kusoglu, A.; Litster, S.; Mench, M.M.; et al. A critical review of modeling transport phenomena in polymer-electrolyte fuel cells. J. Electrochem. Soc. 2014, 161, F1254. [Google Scholar] [CrossRef]
- Da Wang, Y.; Meyer, Q.; Tang, K.; McClure, J.E.; White, R.T.; Kelly, S.T.; Crawford, M.M.; Iacoviello, F.; Brett, D.J.L.; Shearing, P.R.; et al. Large-scale physically accurate modelling of real proton exchange membrane fuel cell with deep learning. Nat. Commun. 2023, 14, 745. [Google Scholar] [CrossRef]
- Yao, J.; Yang, Y.; Hou, X.; Yang, Y.; Yang, F.; Wu, Z.; Zhang, Z. Fast design of catalyst layer with optimal electrical-thermal-water performance for proton exchange membrane fuel cells. J. Energy Chem. 2023, 81, 642–655. [Google Scholar] [CrossRef]
- Park, Y.; Hwang, C.-K.; Bang, K.; Hong, D.; Nam, H.; Kwon, S.; Yeo, B.C.; Go, D.; An, J.; Ju, B.-K.; et al. Machine learning filters out efficient electrocatalysts in the massive ternary alloy space for fuel cells. Appl. Catal. B Environ. 2023, 339, 123128. [Google Scholar] [CrossRef]
- Elçiçek, H.; Özdemir, O.K. Prediction of electrocatalyst performance of Pt/C using response surface optimization algorithm-based machine learning approaches. Int. J. Energy Res. 2022, 46, 21353–21372. [Google Scholar] [CrossRef]
- Zheng, L.; Hou, Y.; Zhang, T.; Pan, X. Performance prediction of fuel cells using long short-term memory recurrent neural network. Int. J. Energy Res. 2021, 45, 9141–9161. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, Y.; Ma, L.; Zhu, Y.; Li, Y.; Tao, J.; Tian, G. A hybrid prognostic method for proton-exchange-membrane fuel cell with decomposition forecasting framework based on AEKF and LSTM. Sensors 2022, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, K.; Zhao, H.; Li, J.; Sheng, X.; Yin, Y.; Du, Q.; Zu, B.; Han, L.; Jiao, K. Degradation prediction of proton exchange membrane fuel cell stack using semi-empirical and data-driven methods. Energy AI 2023, 11, 100205. [Google Scholar] [CrossRef]
- Sun, B.; Liu, X.; Wang, J.; Wei, X.; Yuan, H.; Dai, H. Short-term performance degradation prediction of a commercial vehicle fuel cell system based on CNN and LSTM hybrid neural network. Int. J. Hydrogen Energy 2023, 48, 8613–8628. [Google Scholar] [CrossRef]
- Sun, B.; Liu, X.; Wang, J.; Wei, X.; Yuan, H.; Dai, H. An improved neural network model for predicting the remaining useful life of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2023, 48, 25499–25511. [Google Scholar] [CrossRef]
- Lou, Y.; Hao, M.; Li, Y. Machine-learning-assisted insight into the cathode catalyst layer in proton exchange membrane fuel cells. J. Power Sources 2022, 543, 231827. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, B.; Sheng, X.; Wang, Y.; Ren, Q.; He, S.; Xuan, J.; Jiao, K. An artificial intelligence solution for predicting short-term degradation behaviors of proton exchange membrane fuel cell. Appl. Sci. 2021, 11, 6348. [Google Scholar] [CrossRef]
- Bi, W.; Gray, G.E.; Fuller, T.F. PEM fuel cell Pt/C dissolution and deposition in nafion electrolyte. Electrochem. Solid-State Lett. 2007, 10, B101. [Google Scholar] [CrossRef]
- Ma, R.; Yang, T.; Breaz, E.; Li, Z.; Briois, P.; Gao, F. Data-driven proton exchange membrane fuel cell degradation predication through deep learning method. Appl. Energy 2018, 231, 102–115. [Google Scholar] [CrossRef]
- Cindrella, L.; Kannan, A.; Lin, J.; Saminathan, K.; Ho, Y.; Lin, C.; Wertz, J. Gas diffusion layer for proton exchange membrane fuel cells—A review. J. Power Sources 2009, 194, 146–160. [Google Scholar] [CrossRef]
- Nabovati, A.; Hinebaugh, J.; Bazylak, A.; Amon, C.H. Effect of porosity heterogeneity on the permeability and tortuosity of gas diffusion layers in polymer electrolyte membrane fuel cells. J. Power Sources 2014, 248, 83–90. [Google Scholar] [CrossRef]
- Fadzillah, D.; Rosli, M.; Talib, M.; Kamarudin, S.; Daud, W. Review on microstructure modelling of a gas diffusion layer for proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2017, 77, 1001–1009. [Google Scholar] [CrossRef]
- Chu, H.-S.; Yeh, C.; Chen, F. Effects of porosity change of gas diffuser on performance of proton exchange membrane fuel cell. J. Power Sources 2003, 123, 1–9. [Google Scholar] [CrossRef]
- Xia, L.; Ni, M.; He, Q.; Xu, Q.; Cheng, C. Optimization of gas diffusion layer in high temperature PEMFC with the focuses on thickness and porosity. Appl. Energy 2021, 300, 117357. [Google Scholar] [CrossRef]
- Pasaogullari, U.; Wang, C.-Y. Two-phase transport and the role of micro-porous layer in polymer electrolyte fuel cells. Electrochim. Acta 2004, 49, 4359–4369. [Google Scholar] [CrossRef]
- Dullien, F.A. Porous Media: Fluid Transport and Pore Structure; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Udell, K.S. Heat transfer in porous media considering phase change and capillarity—The heat pipe effect. Int. J. Heat Mass Transf. 1985, 28, 485–495. [Google Scholar] [CrossRef]
- Ozden, A.; Alaefour, I.E.; Shahgaldi, S.; Li, X.; Colpan, C.O.; Hamdullahpur, F. Gas diffusion layers for PEM fuel cells: Ex-and in-situ characterization. In Exergetic, Energetic and Environmental Dimensions; Elsevier: Amsterdam, The Netherlands, 2018; pp. 695–727. [Google Scholar]
- Nam, J.; Chippar, P.; Kim, W.; Ju, H. Numerical analysis of gas crossover effects in polymer electrolyte fuel cells (PEFCs). Appl. Energy 2010, 87, 3699–3709. [Google Scholar] [CrossRef]
- Perng, S.-W.; Wu, H.-W. Non-isothermal transport phenomenon and cell performance of a cathodic PEM fuel cell with a baffle plate in a tapered channel. Appl. Energy 2011, 88, 52–67. [Google Scholar] [CrossRef]
- Ismail, M.S.; Hughes, K.J.; Ingham, D.B.; Ma, L.; Pourkashanian, M. Effects of anisotropic permeability and electrical conductivity of gas diffusion layers on the performance of proton exchange membrane fuel cells. Appl. Energy 2012, 95, 50–63. [Google Scholar] [CrossRef]
- Bruggeman, D. Dielectric constant and conductivity of mixtures of isotropic materials. Ann. Phys. 1935, 24, 636–679. [Google Scholar] [CrossRef]
- Li, G.; Pickup, P.G. Ionic conductivity of PEMFC electrodes: Effect of Nafion loading. J. Electrochem. Soc. 2003, 150, C745. [Google Scholar] [CrossRef]
- Rothfeld, L.B. Gaseous counterdiffusion in catalyst pellets. AIChE J. 1963, 9, 19–24. [Google Scholar] [CrossRef]
- Thampan, T.; Malhotra, S.; Zhang, J.; Datta, R. PEM fuel cell as a membrane reactor. Catal. Today 2001, 67, 15–32. [Google Scholar] [CrossRef]
- Nguyen, T.V.; White, R.E. A water and heat management model for proton-exchange-membrane fuel cells. J. Electrochem. Soc. 1993, 140, 2178. [Google Scholar] [CrossRef]
- Dohle, H.; Kornyshev, A.; Kulikovsky, A.; Mergel, J.; Stolten, D. The current voltage plot of PEM fuel cell with long feed channels. Electrochem. Commun. 2001, 3, 73–80. [Google Scholar] [CrossRef]
- Bear, J. Dynamics of Fluids in Porous Media Dover Publications; INC: New York, NY, USA, 1988. [Google Scholar]
- Leverett, M. Capillary behavior in porous solids. Trans. AIME 1941, 142, 152–169. [Google Scholar] [CrossRef]
- Kim, K.N.; Kang, J.H.; Lee, S.G.; Nam, J.H.; Kim, C.-J. Lattice Boltzmann simulation of liquid water transport in microporous and gas diffusion layers of polymer electrolyte membrane fuel cells. J. Power Sources 2015, 278, 703–717. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, Q.; Gu, S. Three-dimensional lattice-Boltzmann model for liquid water transport and oxygen diffusion in cathode of polymer electrolyte membrane fuel cell with electrochemical reaction. Electrochim. Acta 2018, 262, 282–296. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, Y.; Liu, J.; Xu, S.; Du, A. Lattice Boltzmann method modeling and experimental study on liquid water characteristics in the gas diffusion layer of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2022, 47, 10366–10380. [Google Scholar] [CrossRef]
- Salah, Y.B.; Tabe, Y.; Chikahisa, T. Gas channel optimisation for PEM fuel cell using the lattice Boltzmann method. Energy Procedia 2012, 28, 125–133. [Google Scholar] [CrossRef]
- Koorata, P.K. Deformation Mechanics of Fuel Cell Gas Diffusion Layer: Cyclic Response and Constitutive Model. J. Electrochem. Soc. 2022, 169, 104505. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, C.; Zhang, P.; Zhu, T.; Wang, S.; Zhu, Y.; Wang, X. Optimal design of a cathode flow field with a new arrangement of baffle plates for a high clean power generation of a polymer electrolyte membrane fuel cell. J. Clean. Prod. 2022, 375, 134187. [Google Scholar] [CrossRef]
- Poornesh, K.; Cho, C. Stability of polymer electrolyte membranes in fuel cells: Initial attempts to bridge physical and chemical degradation modes. Fuel Cells 2015, 15, 196–203. [Google Scholar] [CrossRef]
- Shinde, U.; Koorata, P.K.; Padavu, P. Electrical/flow heterogeneity of gas diffusion layer and inlet humidity induced performance variation in polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2023, 48, 12877–12892. [Google Scholar] [CrossRef]
- Mortazavi, M.; Tajiri, K. Effect of the PTFE content in the gas diffusion layer on water transport in polymer electrolyte fuel cells (PEFCs). J. Power Sources 2014, 245, 236–244. [Google Scholar] [CrossRef]
- Mathias, M.; Roth, J.; Fleming, J.; Lehnert, W.; Vielstich, W. Handbook of fuel cells—Fundamentals, technology and applications. Fuel Cell Technol. Appl. 2003, 3, 517–537. [Google Scholar]
- Bazylak, A.; Sinton, D.; Liu, Z.-S.; Djilali, N. Effect of compression on liquid water transport and microstructure of PEMFC gas diffusion layers. J. Power Sources 2007, 163, 784–792. [Google Scholar] [CrossRef]
- Radhakrishnan, V.; Haridoss, P. Effect of cyclic compression on structure and properties of a Gas Diffusion Layer used in PEM fuel cells. Int. J. Hydrogen Energy 2010, 35, 11107–11118. [Google Scholar] [CrossRef]
- Wu, J.; Martin, J.J.; Orfino, F.P.; Wang, H.; Legzdins, C.; Yuan, X.-Z.; Sun, C. In situ accelerated degradation of gas diffusion layer in proton exchange membrane fuel cell: Part I: Effect of elevated temperature and flow rate. J. Power Sources 2010, 195, 1888–1894. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, H.; Brandon, N.P. Gas diffusion layer degradation in proton exchange membrane fuel cells: Mechanisms, characterization techniques and modelling approaches. J. Power Sources 2021, 513, 230560. [Google Scholar] [CrossRef]
- El-Kharouf, A.; Mason, T.J.; Brett, D.J.; Pollet, B.G. Ex-situ characterisation of gas diffusion layers for proton exchange membrane fuel cells. J. Power Sources 2012, 218, 393–404. [Google Scholar] [CrossRef]
- Qiu, D.; Janssen, H.; Peng, L.; Irmscher, P.; Lai, X.; Lehnert, W. Electrical resistance and microstructure of typical gas diffusion layers for proton exchange membrane fuel cell under compression. Appl. Energy 2018, 231, 127–137. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Van Herle, J. The impacts of the gas diffusion layer contact angle on the water management of the proton exchange membrane fuel cells: Three-dimensional simulation and optimization. Int. J. Energy Res. 2022, 46, 16027–16040. [Google Scholar] [CrossRef]
- Bao, Y.; Gan, Y. Roughness effects of gas diffusion layers on droplet dynamics in PEMFC flow channels. Int. J. Hydrogen Energy 2020, 45, 17869–17881. [Google Scholar] [CrossRef]
- Ira, Y.; Bakhshan, Y.; Khorshidimalahmadi, J. Effect of wettability heterogeneity and compression on liquid water transport in gas diffusion layer coated with microporous layer of PEMFC. Int. J. Hydrogen Energy 2021, 46, 17397–17413. [Google Scholar] [CrossRef]
- Shum, A.D.; Liu, C.P.; Lim, W.H.; Parkinson, D.Y.; Zenyuk, I.V. Using Machine Learning Algorithms for Water Segmentation in Gas Diffusion Layers of Polymer Electrolyte Fuel Cells. Transp. Porous Media 2022, 144, 715–737. [Google Scholar] [CrossRef]
- Mahdaviara, M.; Shojaei, M.J.; Siavashi, J.; Sharifi, M.; Blunt, M.J. Deep learning for multiphase segmentation of X-ray images of gas diffusion layers. Fuel 2023, 345, 128180. [Google Scholar] [CrossRef]
- Froning, D.; Wirtz, J.; Hoppe, E.; Lehnert, W. Flow Characteristics of Fibrous Gas Diffusion Layers Using Machine Learning Methods. Appl. Sci. 2022, 12, 12193. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Piuleac, C.-G.; Curteanu, S.; Linares, J.J. Direct and inverse neural networks modelling applied to study the influence of the gas diffusion layer properties on PBI-based PEM fuel cells. Int. J. Hydrogen Energy 2010, 35, 7889–7897. [Google Scholar] [CrossRef]
- Pourrahmani, H. Water management of the proton exchange membrane fuel cells: Optimizing the effect of microstructural properties on the gas diffusion layer liquid removal. Energy 2022, 256, 124712. [Google Scholar] [CrossRef]
- Lei, H.; Xing, L.; Jiang, H.; Wang, Y.; Bin Xu, B.; Xuan, J.; Liu, T.X. Designing graded fuel cell electrodes for proton exchange membrane (PEM) fuel cells with recurrent neural network (RNN) approaches. Chem. Eng. Sci. 2023, 267, 118350. [Google Scholar] [CrossRef]
- Nagulapati, V.M.; Kumar, S.S.; Annadurai, V.; Lim, H. Machine learning based fault detection and state of health estimation of proton exchange membrane fuel cells. Energy AI 2023, 12, 100237. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Z.; Ji, M.; Shan, J.; Ni, M.; Hou, Z.; Cai, J.; Gu, X.; Yuan, X.; Gong, Z.; et al. Long short-term memory deep learning model for predicting the dynamic performance of automotive PEMFC system. Energy AI 2023, 14, 100278. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Tang, F.; Ming, P.; Li, B.; Zhang, C. Power evolution of fuel cell stack driven by anode gas diffusion layer degradation. Appl. Energy 2022, 313, 118858. [Google Scholar] [CrossRef]
- Marappan, M.; Palaniswamy, K.; Velumani, T.; Chul, K.B.; Velayutham, R.; Shivakumar, P.; Sundaram, S. Performance Studies of Proton Exchange Membrane Fuel Cells with Different Flow Field Designs—Review. Chem. Rec. 2021, 21, 663–714. [Google Scholar] [CrossRef]
- Li, X.; Sabir, I. Review of bipolar plates in PEM fuel cells: Flow-field designs. Int. J. Hydrogen Energy 2005, 30, 359–371. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Cunningham, B.; Baird, D.G. The development of economical bipolar plates for fuel cells. J. Mater. Chem. 2006, 16, 4385–4388. [Google Scholar] [CrossRef]
- Tawfik, H.; Hung, Y.; Mahajan, D. Metal bipolar plates for PEM fuel cell—A review. J. Power Sources 2007, 163, 755–767. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Contact resistance prediction of proton exchange membrane fuel cell considering fabrication characteristics of metallic bipolar plates. Energy Convers. Manag. 2018, 169, 334–344. [Google Scholar]
- Ramos-Alvarado, B.; Hernandez-Guerrero, A.; Elizalde-Blancas, F.; Ellis, M.W. Constructal flow distributor as a bipolar plate for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 12965–12976. [Google Scholar] [CrossRef]
- Atyabi, S.A.; Afshari, E. A numerical multiphase CFD simulation for PEMFC with parallel sinusoidal flow fields. J. Therm. Anal. Calorim. 2019, 135, 1823–1833. [Google Scholar] [CrossRef]
- Choi, K.-S.; Kim, H.-M.; Moon, S.-M. Numerical studies on the geometrical characterization of serpentine flow-field for efficient PEMFC. Int. J. Hydrogen Energy 2011, 36, 1613–1627. [Google Scholar] [CrossRef]
- Perng, S.-W.; Wu, H.-W. A three-dimensional numerical investigation of trapezoid baffles effect on non-isothermal reactant transport and cell net power in a PEMFC. Appl. Energy 2015, 143, 81–95. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Berg, P. On the modeling of water transport in polymer electrolyte membrane fuel cells. Electrochim. Acta 2009, 54, 6913–6927. [Google Scholar] [CrossRef]
- Kakaç, S.; Shah, R.K.; Aung, W. Handbook of Single-Phase Convective Heat Transfer; Wiley-Interscience: Hoboken, NJ, USA, 1987. [Google Scholar]
- Hartnig, C.; Schmidt, T.J. On a new degradation mode for high-temperature polymer electrolyte fuel cells: How bipolar plate degradation affects cell performance. Electrochim. Acta 2011, 56, 4237–4242. [Google Scholar] [CrossRef]
- Eom, K.; Cho, E.; Nam, S.-W.; Lim, T.-H.; Jang, J.H.; Kim, H.-J.; Hong, B.K.; Yang, Y.C. Degradation behavior of a polymer electrolyte membrane fuel cell employing metallic bipolar plates under reverse current condition. Electrochim. Acta 2012, 78, 324–330. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, Z.; Tao, W.-Q.; Wang, X.; Wu, L.; Wu, S.; Xie, X.; Tongsh, C.; Huo, W.; Bao, Z.; et al. Porous flow field for next-generation proton exchange membrane fuel cells: Materials, characterization, design, and challenges. Chem. Rev. 2022, 123, 989–1039. [Google Scholar] [CrossRef]
- Ahn, C.; Lim, M.S.; Hwang, W.; Kim, S.; Park, J.E.; Lim, J.; Choi, I.; Cho, Y.; Sung, Y. Effect of porous metal flow field in polymer electrolyte membrane fuel cell under pressurized condition. Fuel Cells 2017, 17, 652–661. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, L.; Xu, G.; Wang, C.; Wang, D. Improvement of the three-dimensional fine-mesh flow field of proton exchange membrane fuel cell (PEMFC) using CFD modeling, artificial neural network and genetic algorithm. Int. J. Hydrogen Energy 2022, 47, 35038–35054. [Google Scholar] [CrossRef]
- Zheng, J.; Qin, Y.; Guo, Q.; Dong, Z.; Zhu, C.; Wang, Y. Block structure optimization in PEMFC flow channels using a data-driven surrogate model based on random forest. Int. J. Green Energy 2023, 20, 816–822. [Google Scholar] [CrossRef]
- Wilberforce, T.; Biswas, M. A study into proton exchange membrane fuel cell power and voltage prediction using artificial neural network. Energy Rep. 2022, 8, 12843–12852. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Y.; Liu, X. Proton Exchange Membrane Fuel Cell Power Prediction Based on Ridge Regression and Convolutional Neural Network Data-Driven Model. Sustainability 2023, 15, 11010. [Google Scholar] [CrossRef]
- Cao, J.; Yin, C.; Feng, Y.; Su, Y.; Lu, P.; Tang, H. A Dimension-Reduced Artificial Neural Network Model for the Cell Voltage Consistency Prediction of a Proton Exchange Membrane Fuel Cell Stack. Appl. Sci. 2022, 12, 11602. [Google Scholar] [CrossRef]
- Li, H.-W.; Liu, J.-N.; Yang, Y.; Lu, G.-L.; Qiao, B.-X. Coupling flow channel optimization and Bagging neural network to achieve performance prediction for proton exchange membrane fuel cells with varying imitated water-drop block channel. Int. J. Hydrogen Energy 2022, 47, 39987–40007. [Google Scholar] [CrossRef]
- Li, H.-W.; Liu, J.-N.; Yang, Y.; Lu, G.-L.; Qiao, B.-X. A coupled and interactive influence of operational parameters for optimizing power output of cleaner energy production systems under uncertain conditions. Int. J. Energy Res. 2019, 43, 1294–1302. [Google Scholar]








| Sector | Application (Power) | Potential | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Portable | Laptops, cell phones, and military radio/communication devices. (5 to 50 W) | -Provide continuous power as long as hydrogen fuel is available. -Can be fabricated in small sizes without efficiency loss. | -Low acoustic and thermal signatures, high reliability, quick recharging, and high energy density. | -Complex system with water and heat management issues. -Hydrogen storage system. -Costly. | [59] |
| Stationary | Backup power system, off grid power supply, combined heat and power unit (CHP) (to 300 kW) | -Can be co-located other renewable power sources. -Significant cost benefits compared to battery-generator systems for shorter durations. | High energy and power densities, high modularity, longer operation times, compact size, and ability to operate under unkind ambient conditions. | -Coolant leakage for longer run. -Coolant and bipolar plate compatibility. -Reliability of components. | [60,61] |
| Vehicle | Passenger car, utility vehicles, and bus. (20 to 250 kW) | -Can be used in hybrid power system in addition to battery and supercapacitor. | -Efficiency is higher than the vehicles powered by internal combustion engines. -Low refueling time (<5 min) -No noise. -Zero emissions. | -Cost of the components (catalyst). -High operation cost. -Low durability (2500–3000 h). | [59,62] |
| Marine | Container, demonstrator, yachts, ferries, submarine (12–300 kW) | -Can be used both as main and auxiliary power system. | -High power to weight ratio. -Low maintenance cost. -Low noise. -Good modularity/part load performance | -Low power capacity. -Safety and reliability. -Durability. -High cost. -Storage issue. | [63] |
| Aviation | Small scaled manned/unmanned aircraft, drones. (100 W–33 kW) | Main power source of unmanned aerial vehicle (UAV), auxiliary power unit (APU) for large aircrafts. | -High power output.-Light weight. -Simple design. | -Additional space requirements for hydrogen storage. -Heat and water management. | [59,64,65] |
Physical Insight | ||||
|---|---|---|---|---|
| Category | Black Box (ML) | Grey Box (Semi-Empirical) | White Box (Mechanistic) | |
| Features | ||||
| Basis | Based on input and output data from the experiment. | Based on empirical equations validated by the experimental data. | Based on complicated algebraic and differential equations. | |
| Dependency of test data | High | Medium | Low | |
| Computational cost | Low | Medium | High | |
| Accuracy of findings | Reasonable | Reasonable | High | |
| Applications | Stack level | Stack level | Cell level | |
| Online simulation | Possible | Possible | Not possible | |
 Granularity | ||||
| Components | Functions | Materials (Present) | Materials (Future) | Reference |
|---|---|---|---|---|
| Polymer electrolyte Membrane | -Conduction of protons -Separating barriers of reactants. -Blockade of electrons. | -Perfluorosulfonic acid (PFSA) membranes (~90 °C operations). | -Composite membranes such as Polytetrafluoroethylene (PTFE) and Polyvinylidene difluoride (PDDF) membranes (>100 °C operations). -PEM with ionogels | [23,76,77] |
| Catalyst layer | Site of electrochemical reactions. | -Platinum (Pt) loading (0.1–0.2 g kW−1). -Heterogenous coverage of ionomer. | -Platinum (Pt) loading (<0.1 g kW−1). -Lower and better distribution of ionomer. -Pt monolayer catalysts, nanometer film catalysts, controlled crystal shape catalysts, and non-PGM catalysts. | [23,76] |
| Gas diffusion layer | -Distribution of reactants. -Water management. -Electricity and heat distribution through conduction. | -Carbon paper with fiber structure -Carbon black and hydrophobic agents | -Carbon paper with modified fiber configurations. -Carbon black with super hydrophobic additives. -Metal foam. | [23,76] |
| Bi-Polar plate | -Transport of reactants. -Water management. -Dissipation of heat. -Current collector. | -Graphite (~1 mm thick). -Metal (~0.6 mm thick). | -Metal foam. -Metal (~0.5 mm thick). | [23,76] |
| Research Method | Software/Code | Findings | Reference |
|---|---|---|---|
| MD simulation | Materials studio (MS) 4.3 COMPASS | Instead of Nafion, most widely used membrane material, a natural polymer Chitosan (a derivative of chitin a natural polysaccharide found in insects, fungi, and yeast) was used. The highest conductivity of 7.14 × 10−2 S/cm was observed when the system contained 40% of water. | [86] |
| MD simulation | Materials studio (MS) 4.4 COMPASS | The diffusion of water molecules and hydronium ions in Nafion 117 are measured using MD simulation. They found that the diffusion coefficient of both water molecules and hydrogen ions increase with the water content when the temperature in constant. Similar to this, both the diffusion coefficient increases with the temperature at constant water content. | [87] |
| MD simulation | COMPASS | Proton conductivity of three different types of polymer membrane; Dow, Nafion and Aciplex was compared. The Aciplex membrane has the optimum proton mobility and best water molecules and hydronium mobility at 350 K. | [88] |
| MD simulation | Materials Studio COMPASS II | Sulfonated polynorbornene-based (SPNB) membranes swell upon after getting hydrated and divided into hydrophobic and hydrophilic regions having sulfonic acid groups at their interfaces. Also, more water molecules can absorb the sulfur atoms and hydronium due to the rise of water at increasing temperature. | [89] |
| MD simulation | LAMPPS | Pt/C models were investigated with various PTFE-binder content in the presence of H3PO4 at 298.15 K and 433.15 K. It was found that the coverage of H3PO4 is higher at high temperature in comparison to lower temperature as the H3PO4 could still manage to contact the Pt surface through PTFE-binder due to favorable interactions. | [90] |
| Equation | Remarks | Ref. |
|---|---|---|
| This equation is obtained from the fitting experimental data of water uptake in Nafion membrane at 80 °C | [97] | |
| [98,99] | ||
| Although the relation in > 1 obtained from 80 °C, the polynomial relation comes at 30 °C | [96] | |
| A fit data obtained from the experiment under VE conditions at 80 °C | [100] |
| Variable | Porous Electrode Model | Agglomerate Model |
|---|---|---|
| Overall liquid water flux, NL | ||
| Overall membrane water flux, NW | Equation above. | |
| Gas-phase component flux, NG,i | Equation above. | |
| Gas-phase component partial pressure, pG,i | ||
| Liquid pressure, pL | ||
| Membrane water chemical potential, μw | Equation (14)/Equation (16) | |
| Electronic-phase current density, i1 | ||
| Membrane current density, i2 | Equation (9)/Equation (13) | |
| Electronic-phase potential, Φ1 | ||
| Membrane potential, ΦL | Where | |
| Temperature, T | Or | |
| Total gas pressure, pG | ||
| Liquid saturation, S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaiser, R.; Ahn, C.-Y.; Kim, Y.-H.; Park, J.-C. Towards Reliable Prediction of Performance for Polymer Electrolyte Membrane Fuel Cells via Machine Learning-Integrated Hybrid Numerical Simulations. Processes 2024, 12, 1140. https://doi.org/10.3390/pr12061140
Kaiser R, Ahn C-Y, Kim Y-H, Park J-C. Towards Reliable Prediction of Performance for Polymer Electrolyte Membrane Fuel Cells via Machine Learning-Integrated Hybrid Numerical Simulations. Processes. 2024; 12(6):1140. https://doi.org/10.3390/pr12061140
Chicago/Turabian StyleKaiser, Rashed, Chi-Yeong Ahn, Yun-Ho Kim, and Jong-Chun Park. 2024. "Towards Reliable Prediction of Performance for Polymer Electrolyte Membrane Fuel Cells via Machine Learning-Integrated Hybrid Numerical Simulations" Processes 12, no. 6: 1140. https://doi.org/10.3390/pr12061140
APA StyleKaiser, R., Ahn, C.-Y., Kim, Y.-H., & Park, J.-C. (2024). Towards Reliable Prediction of Performance for Polymer Electrolyte Membrane Fuel Cells via Machine Learning-Integrated Hybrid Numerical Simulations. Processes, 12(6), 1140. https://doi.org/10.3390/pr12061140








