Innovative Solution for Invasive Species and Water Pollution: Hydrochar Synthesis from Pleco Fish Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Enzymatic Hydrolysis
2.3. Microwave Hydrothermal Carbonization
2.4. MHTC Optimization Protocol
2.5. Hydrochar Characterization
2.6. Chemical and Energy Properties of Hydrochar
2.7. Adsorption Experiments
3. Results and Discussion
3.1. Hydrochar Yield
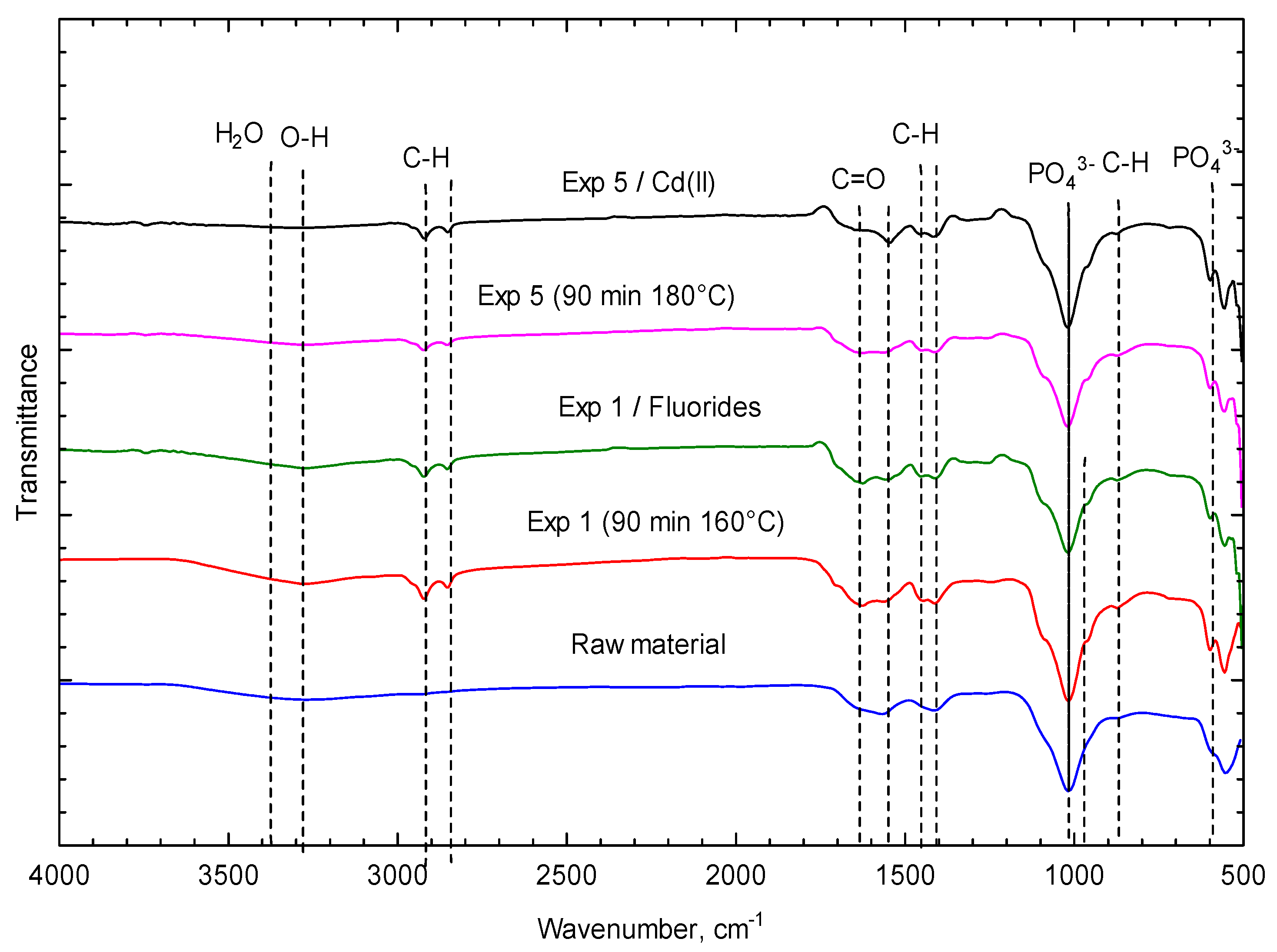
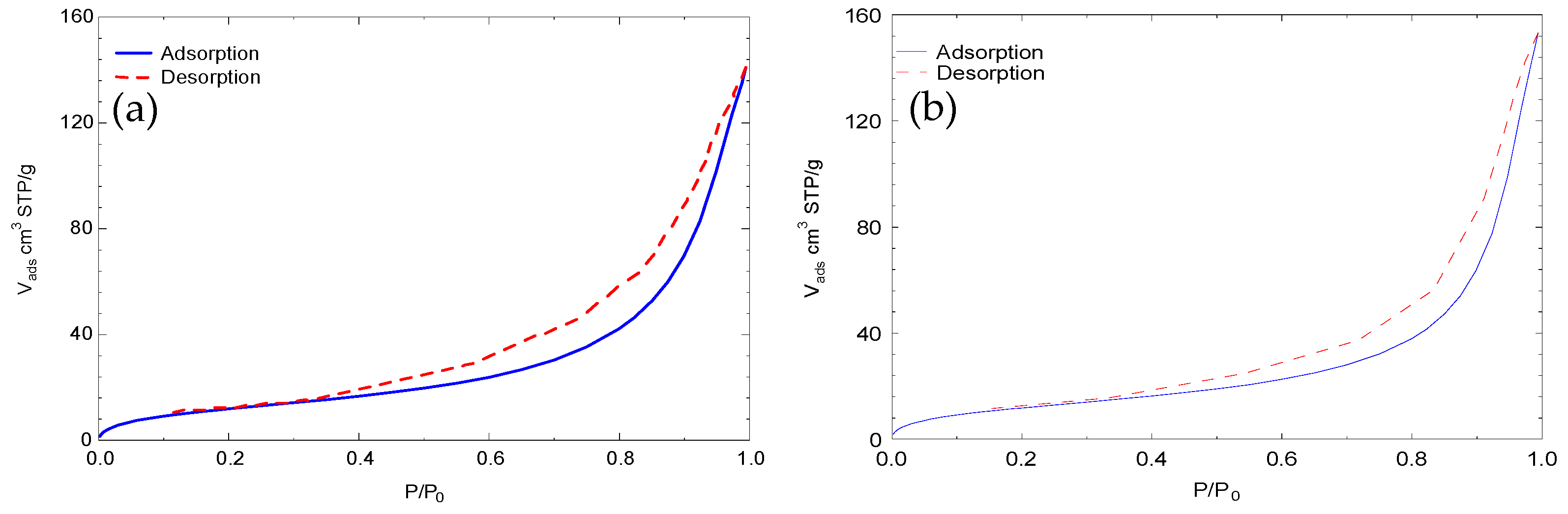
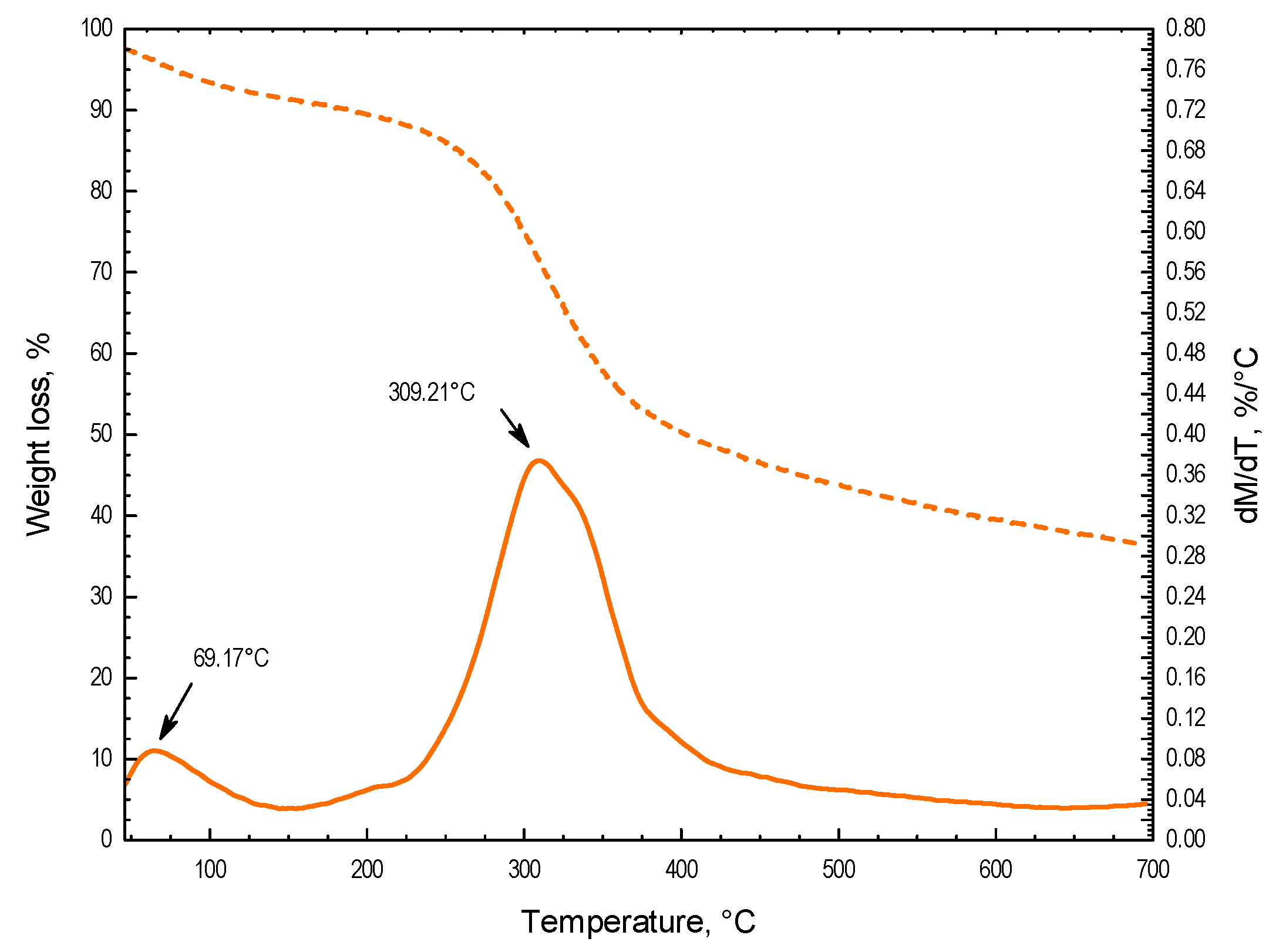
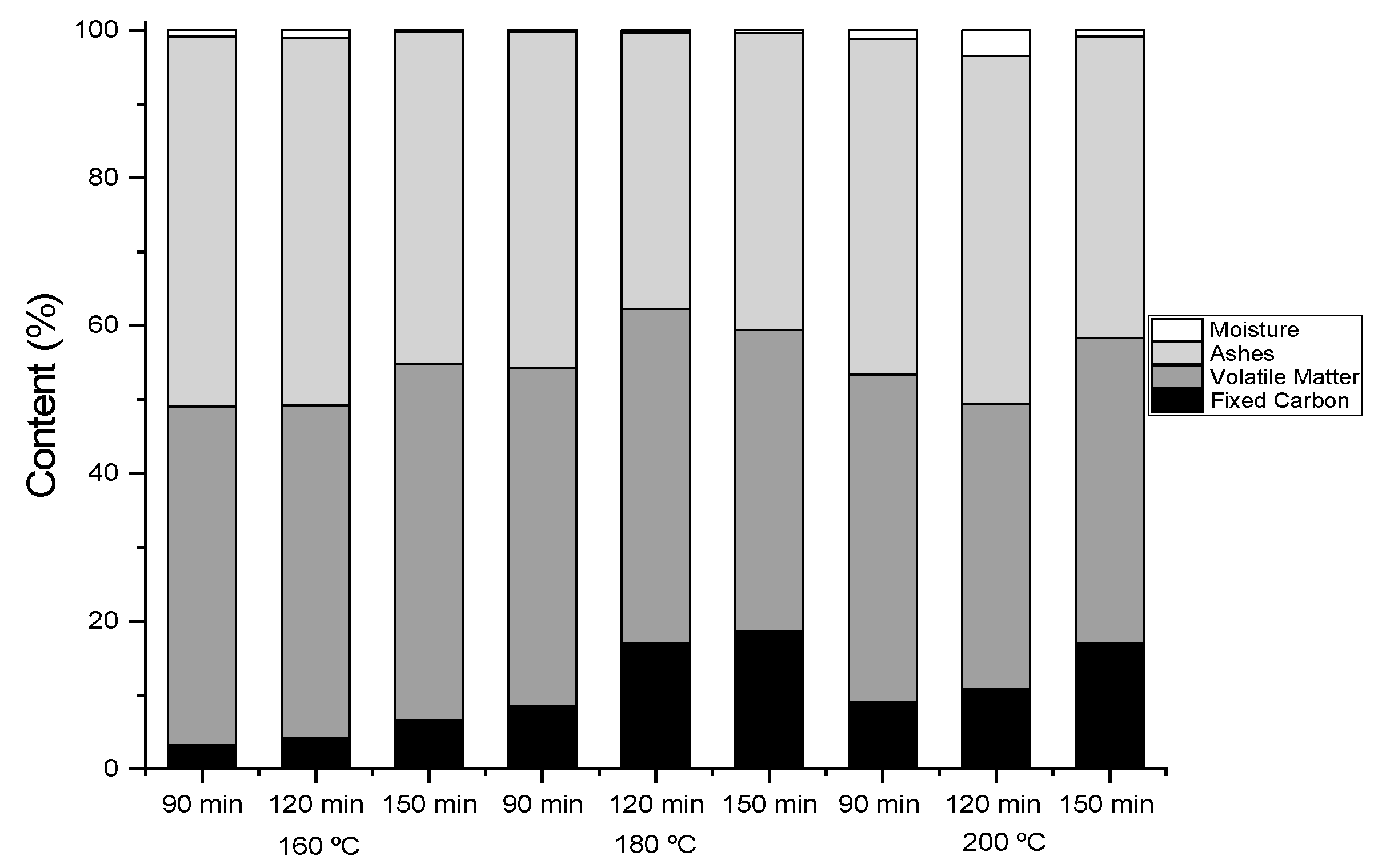
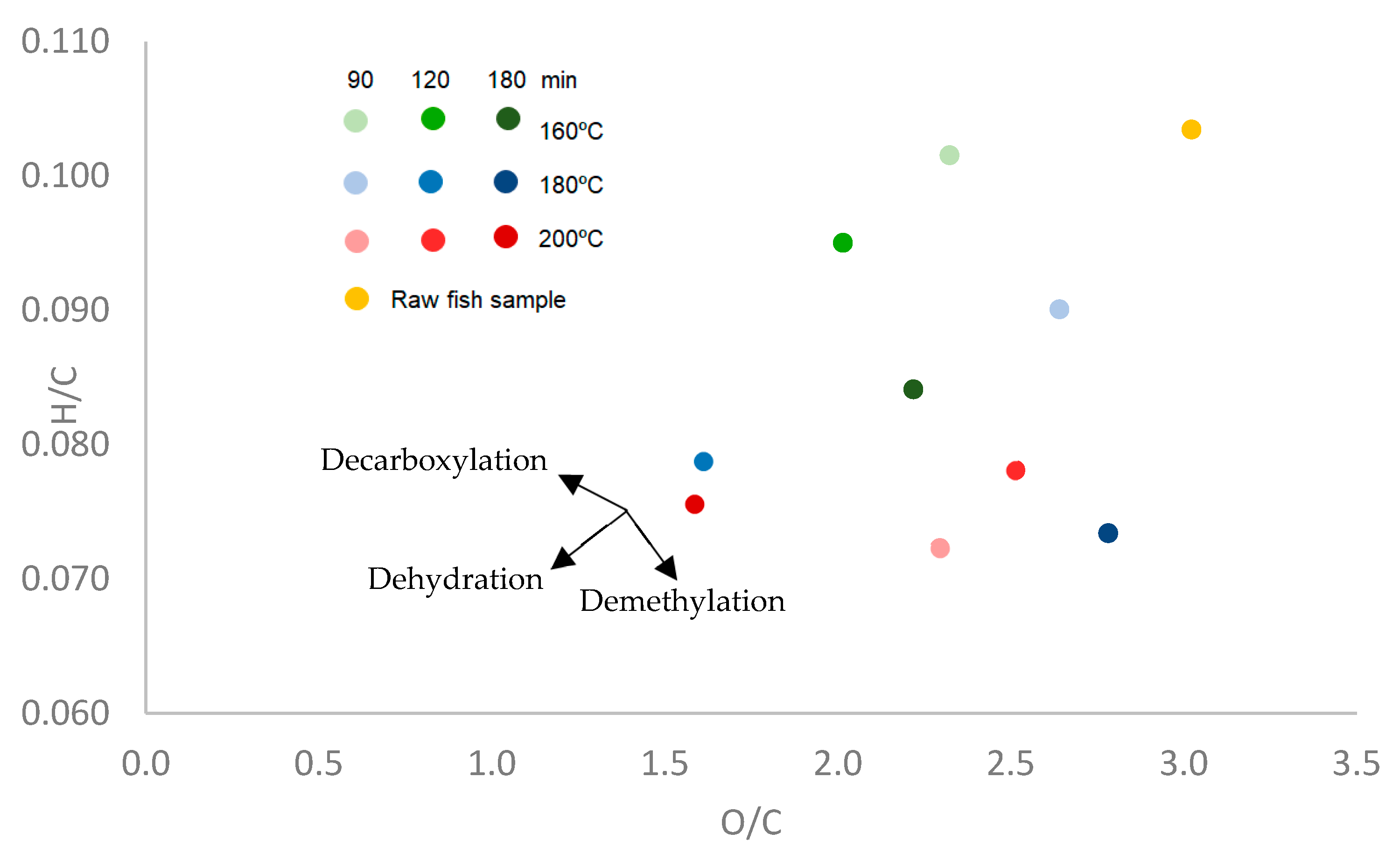
3.2. Hydrochar Characterization
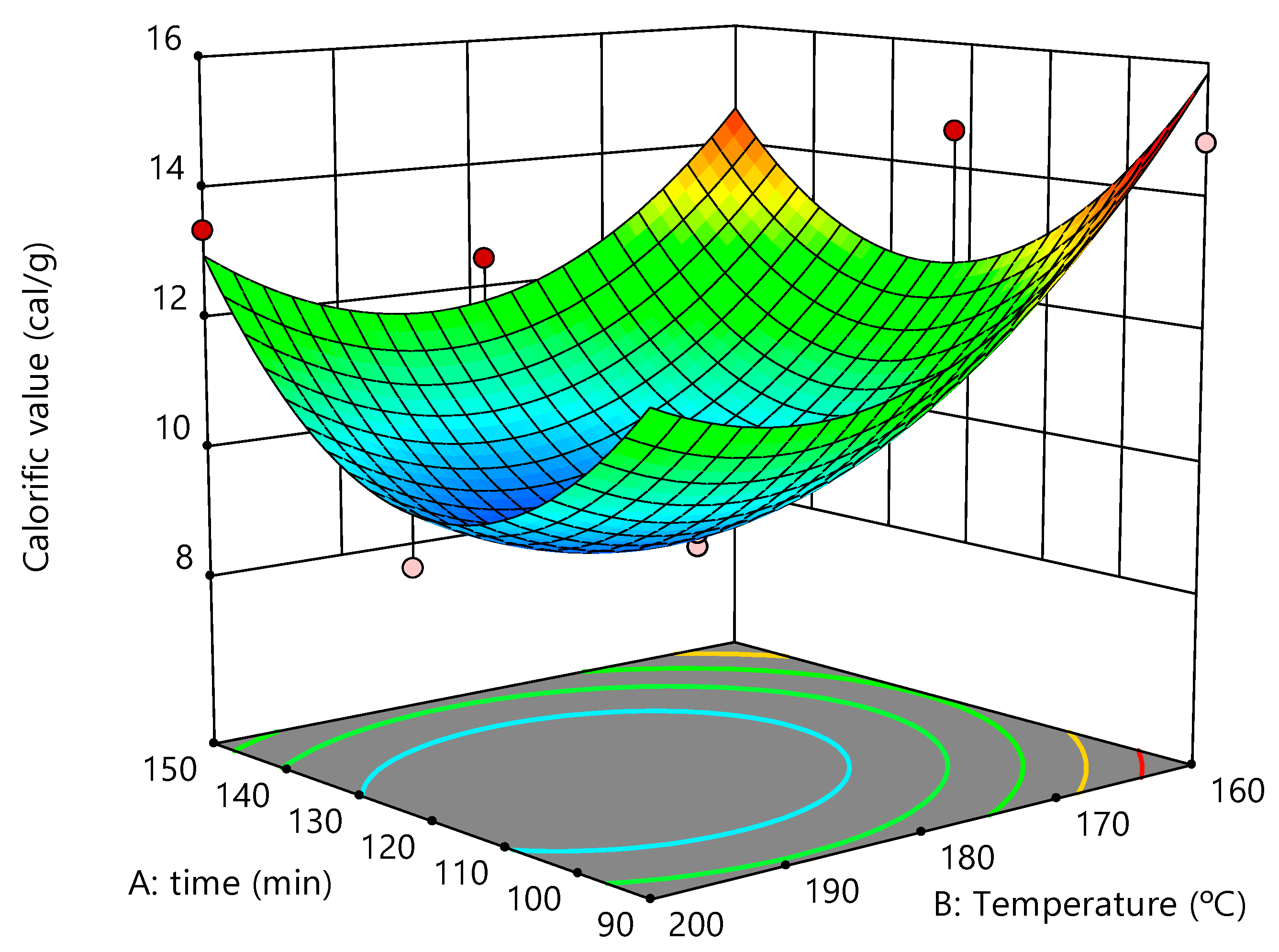
3.3. Calorific Value
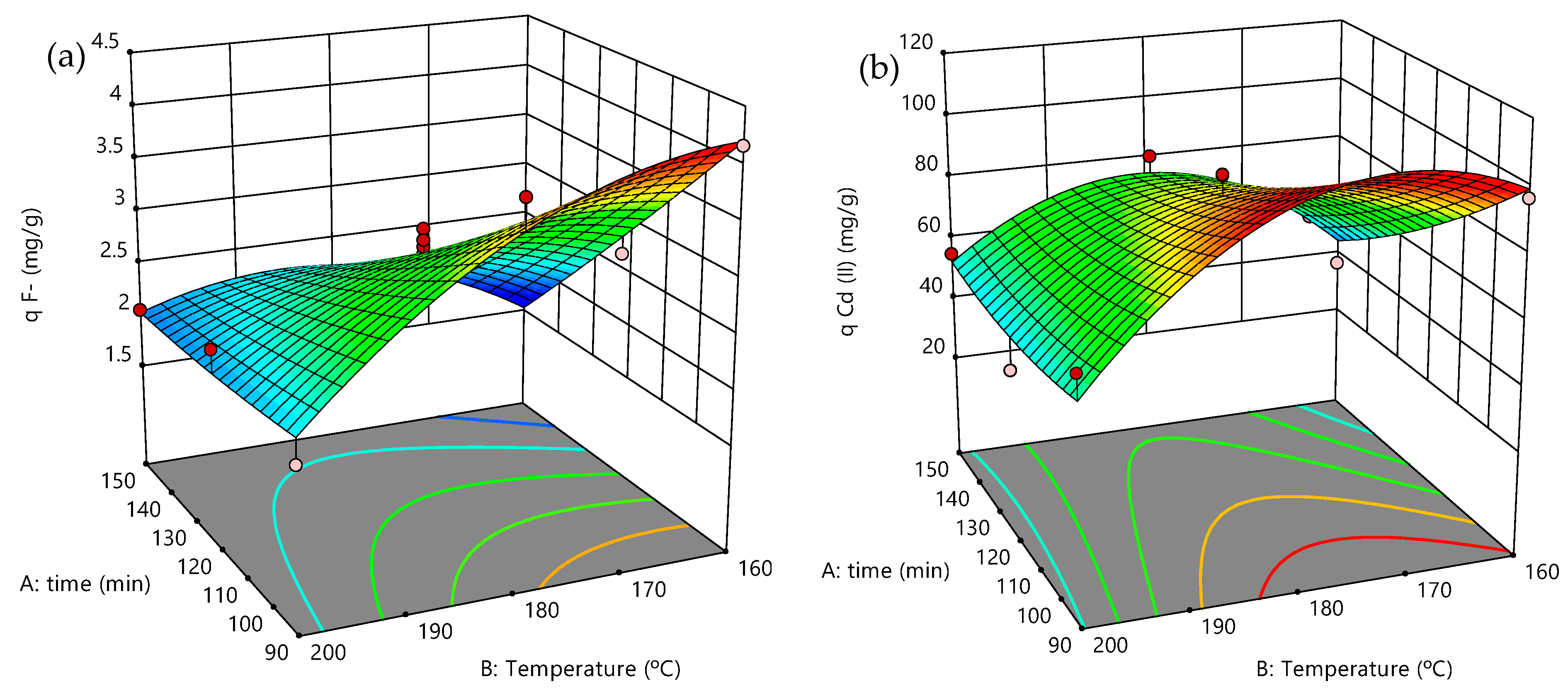
3.4. Adsorption Experiments
| Adsorbent | Adsorbate | pH | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|
| Pine wood biochar | Fluoride | NR | 7.66 | [41] |
| Activated alumina | Fluoride | 7.0 | 2.41 | [42] |
| Activated carbon | Fluoride | 2.0 | 4.71 | [43] |
| Bone char | Fluoride | 7.0 | 4.51 | [44] |
| 7.0 | 7.53 | [45] | ||
| 5.0 | 13.49 | [39] | ||
| 7.0 | 10.0 | [46] | ||
| 5.0 | 11.2 | [47] | ||
| Cd(II) | 3.0 | 85.5 | [48] | |
| 5.0 | 64.07 | [49] | ||
| 7.0 | 213 | [50] | ||
| Charcoal | Fluoride | 7.0 | 13.64 | [51] |
| Hydroxyapatite | Fluoride | NR | 4.54 | [52] |
| Waste carbon slurry | Fluoride | 7.58 | 4.31 | [53] |
| Sawdust hydrochar | Cd(II) | 7.0 | 20.18 | [54] |
| Chicken manure hydrochar | Cd(II) | 6.0 | 30.0 | [55] |
| Hydrochar/glucose | Cd(II) | 4.0 | 88.8 | [56] |
| Bamboo powder hydrochar | Cd(II) | 4.0 | 90.37 | [57] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Howard, L.; van Rees, C.B.; Dahlquist, Z.; Luikart, G.; Hand, B.K. A Review of Invasive Species Reporting Apps for Citizen Science and Opportunities for Innovation. NeoBiota 2022, 71, 165–188. [Google Scholar] [CrossRef]
- Cruz-Briano, S.A.; Medellín-Castillo, N.A.; Torres-Dosal, A.; Leyva-Ramos, R.; Moreno-Piraján, J.C.; Giraldo-Gutiérrez, L.; Díaz-Flores, P.E.; Reyes-López, S.Y.; Ocampo-Pérez, R. Bone Char from an Invasive Aquatic Specie as a Green Adsorbent for Fluoride Removal in Drinking Water. Water Air Soil Pollut. 2021, 232, 346. [Google Scholar] [CrossRef]
- Haedar, K.A.; Ainurridho, M.; Pagdee, A. Pleco-based Feedstock for Black Soldier Fly Maggot: Potential Management for Invasive Pleco Fish (Glyptoperichthys gibbiceps) in Tempe Lake, Sulawesi Selatan, Indonesia. EnvironmentAsia 2022, 15, 152–158. [Google Scholar]
- Mendoza, R.; Contreras, S.; Ramírez, C.; Koleff, P.; Alvarez, P.; Aguilar, V. Los peces diablo: Especies Invasoras de Alto Impacto. Biodiversitas 2007, 70, 1–5. [Google Scholar]
- CCA (Comisión para la Cooperación Ambiental de América del Norte). Directrices Trinacionales Para la Evaluación de Riesgos de las Especies Acuáticas Exóticas Invasoras: Casos de Prueba Para el Pez Cabeza de Serpiente (Channidae) y el Pleco (Loricariidae) en Aguas Continentales de América del Norte; Comisión para la Cooperación Ambiental: Montreal, QC, Canada, 2009. [Google Scholar]
- Unggang, J.A.F.; Bakar, M.N.; Khair, A.B.A. The Potential of Several Wild Invasive Fish Species as Fish-Based Organic Fertilizers on the Growth of Two Common Vegetables in Malaysia. Sains Malays. 2023, 52, 71–81. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Cisneros-Ontiveros, H.G.; Carranza-Álvarez, C.; Ilizaliturri-Hernandez, C.A.; Yánez-Estrada, L.G.; Rodríguez-López, A.G. Evaluation of the Fish Invasiveness Scoring Kit (FISK v2) for Pleco Fish or Devil Fish. In Bacterial Fish Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 205–227. [Google Scholar]
- Kannan, S.; Burelle, I.; Orsat, V.; Vijaya Raghavan, G.S. Characterization of Bio-crude Liquor and Bio-oil Produced by Hydrothermal Carbonization of Seafood Waste. Waste Biomass Valorization 2020, 11, 3553–3565. [Google Scholar] [CrossRef]
- Kannan, S.; Gariepy, Y.; Raghavan, G.S.V. Conventional Hydrothermal Carbonization of Shrimp Waste. Energ. Fuel 2018, 32, 3532–3542. [Google Scholar] [CrossRef]
- Kang, K.; Nanda, S.; Sun, G.; Qiu, L.; Gu, Y.; Zhang, T. Microwave-assisted Hydrothermal Carbonization of Corn Stalk for Solid Biofuel Production: Optimization of Process Parameters and Characterization of Hydrochar. Energy 2019, 186, 115795. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A Review on Conventional and Novel Materials Towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of Potential Applications of Hydrochar Derived from Hydrothermal Carbonization of Biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, G.; Chi, T.; Li, Y.; Zhang, Y.; Wang, J.; Li, P.; Liu, J.; Yu, Z.; Wang, Q. Insights into the Enhanced Effect of Biochar on Cadmium Removal in Vertical Flow Constructed Wetlands. J. Hazard. Mater. 2023, 443, 130148. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.J.; Sankannavar, R.; Madhu, G.M. Fluoride Sources, Toxicity and Fluorosis Management Techniques—A Brief Review. J. Hazard. Mater. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global Analysis and Prediction of Fluoride in Groundwater. Nat. Commun. 2022, 13, 4232. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Gariepy, Y.; Raghavan, G.S.V. Optimization and Characterization of Hydrochar Produced from Microwave Hydrothermal Carbonization of Fish Waste. Waste Manag. 2017, 65, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.A.; Bradley, C.; Kibbee, R.; Wesgate, R.; Wilkinson, M.A.C.; Sharpe, T.; Maillard, J.Y. Disinfectant Wipes are Appropriate to Control Microbial Bioburden from Surfaces: Use of a New ASTM Standard Test Protocol to Demonstrate Efficacy. J. Hosp. Infect. 2015, 91, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.O.D.; Sohail, M. Comparative Evaluation of Conventional and Microwave Hydrothermal Carbonization of Human Biowaste for Value Recovery. Water Sci. Technol. 2017, 75, 2852–2863. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska-Miecznik, J.; Haberko, K.; Sitarz, M.; Bućko, M.M.; Macherzyńska, B. Hydroxyapatite from Animal Bones–extraction and Properties. Ceram. Int. 2015, 41, 4841–4846. [Google Scholar] [CrossRef]
- Lee, J.; Hong, J.; Jang, D.; Park, K.Y. Hydrothermal Carbonization of Waste from Leather Processing and Feasibility of Produced Hydrochar as an Alternative Solid Fuel. J. Environ. Manag. 2019, 247, 115–120. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Handbook of Vibrational Spectroscopy, 6th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Guo, S.; Dong, X.; Wu, T.; Shi, F.; Zhu, C. Characteristic Evolution of Hydrochar from Hydrothermal Carbonization of Corn Stalk. J. Anal. Appl. Pyrolysis 2015, 116, 1–9. [Google Scholar] [CrossRef]
- Cisneros-Ontiveros, H.G.; Medellín-Castillo, N.A.; Aldama-Aguilera, C.; Ilizaliturri-Hernández, C.A.; Cruz-Briano, S.A.; Labrada-Delgado, G.J.; Flores-Rojas, A.I. Optimization of Synthesis Variables and Characterization of Devilfish Bone Chars for the Removal of Cadmium (II) from Water. MRS Adv. 2022, 7, 997–1003. [Google Scholar] [CrossRef]
- Muttakin, M.; Mitra, S.; Thu, K.; Ito, K.; Saha, B.B. Theoretical Framework to Evaluate Minimum Desorption Temperature for IUPAC Classified Adsorption Isotherms. Int. J. Heat Mass. Transf. 2018, 122, 795–805. [Google Scholar] [CrossRef]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of Micro/Mesoporous Materials by Physisorption: Concepts and Case Studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Tuning Hydrochar Properties for Enhanced Mesopore Development in Activated Carbon by Hydrothermal Carbonization. Micropor. Mesopor. Mat. 2015, 203, 178–185. [Google Scholar] [CrossRef]
- Eisa, M.Y.; Al Dabbas, M.; Abdulla, F.H. Quantitative Identification of Phosphate Using X-Ray Diffraction and Fourier Transform Infrared (FTIR) Spectroscopy. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 270–283. [Google Scholar]
- Wang, J.; Wang, Y.; Wang, J.; Du, G.; Khan, K.Y.; Song, Y.; Cui, X.; Cheng, Z.; Yan, B.; Chen, G. Comparison of Cadmium Adsorption by Hydrochar and Pyrochar Derived from Napier Grass. Chemosphere 2022, 308, 136389. [Google Scholar] [CrossRef] [PubMed]
- Wądrzyk, M.; Janus, R.; Lewandowski, M.; Magdziarz, A. On Mechanism of Lignin Decomposition–Investigation using Microscale Techniques: Py-GC-MS, Py-FT-IR and TGA. Renew. Energy 2021, 177, 942–952. [Google Scholar] [CrossRef]
- Steven, S.; Hernowo, P.; Restiawaty, E.; Irawan, A.; Rasrendra, C.B.; Riza, A.; Bindar, Y. Thermodynamics Simulation Performance of Rice Husk Combustion with a Realistic Decomposition Approach on the Devolatilization Stage. Waste Biomass Valorization 2022, 13, 2735–2747. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Hernández-Ramírez, M.G.; Salazar-Rábago, J.J.; Labrada-Delgado, G.J.; Aragón-Piña, A. Bioadsorción de Plomo (II) Presente en Solución Acuosa Sobre Residuos de Fibras Naturales Procedentes de la Industria Ixtlera (Agave lechuguilla Torr. Y Yucca carnerosana (Trel.) McKelvey). Rev. Int. Contam. Ambie 2017, 33, 269–280. [Google Scholar] [CrossRef]
- Patel, S.; Han, J.; Qiu, W.; Gao, W. Synthesis and Characterisation of Mesoporous Bone Char Obtained by Pyrolysis of Animal Bones, for Environmental Application. J. Environ. Chem. Eng. 2015, 3, 2368–2377. [Google Scholar] [CrossRef]
- Huezo, L.; Vasco-Correa, J.; Shah, A. Hydrothermal Carbonization of Anaerobically Digested Sewage Sludge for Hydrochar Production. Bioresour. Technol. Rep. 2021, 15, 100795. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Ab Rahim, M.H.; Park, J.W.; Kim, H.J. Hydrothermal Carbonization of Lignocellulosic Biomass for Carbon Rich Material Preparation: A Review. Biomass Bioenerg. 2019, 130, 105384. [Google Scholar] [CrossRef]
- Yu, S.; Yang, X.; Zhao, P.; Li, Q.; Zhou, H.; Zhang, Y. From Biomass to Hydrochar: Evolution on Elemental Composition, Morphology, and Chemical Structure. J. Energy Inst. 2022, 101, 194–200. [Google Scholar] [CrossRef]
- Alkurdi, S.S.A.; Al-Juboori, R.A.; Bundschuh, J.; Bowtell, L.; McKnight, S. Effect of Pyrolysis Conditions on Bone Char Characterization and Its Ability for Arsenic and Fluoride Removal. Environ. Pollut. 2020, 262, 114221. [Google Scholar] [CrossRef] [PubMed]
- Polikovsky, M.; Gillis, A.; Steinbruch, E.; Robin, A.; Epstein, M.; Kribus, A.; Golberg, A. Biorefinery for The Co-production of Protein, Hydrochar and Additional Co-products from a Green Seaweed Ulva Sp. with Subcritical Water Hydrolysis. Energy Convers. Manag. 2020, 225, 113380. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Cruz-Briano, S.A.; Leyva-Ramos, R.; Moreno-Piraján, J.C.; Torres-Dosal, A.; Giraldo-Gutiérrez, L.; Labrada-Delgado, G.J.; Pérez, R.O.; Rodriguez-Estupiñan, J.P.; Lopez, S.Y.R. Use of Bone Char Prepared from an Invasive Species, Pleco Fish (Pterygoplichthys Spp.), to Remove Fluoride and Cadmium (II) in Water. J. Environ. Manag. 2020, 256, 109956. [Google Scholar]
- Nguyen, M.H.; Zbair, M.; Dutournié, P.; Gervasini, A.; Vaulot, C.; Bennici, S. Toward New Low-Temperature Thermochemical Heat Storage Materials: Investigation of Hydration/Dehydration Behaviors of Mgso4/Hydroxyapatite Composite. Sol. Energy Mater. Sol. Cells 2022, 240, 111696. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and Inorganic Contaminants Removal from Water With Biochar, a Renewable, Low Cost and Sustainable Adsorbent—A Critical Review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, S.; Pant, K.K. Equilibrium, Kinetics and Breakthrough Studies for Adsorption of Fluoride on Activated Alumina. Sep. Purif. Technol. 2005, 42, 265–271. [Google Scholar] [CrossRef]
- Sahu, N.; Bhan, C.; Singh, J. Removal of Fluoride from an Aqueous Solution by Batch and Column Process Using Activated Carbon Derived from Iron Infused Pisum Sativum Peel: Characterization, Isotherm, Kinetics Study. Environ. Eng. Res. 2021, 26, 200241. [Google Scholar] [CrossRef]
- Tovar-Gómez, R.; Moreno-Virgen, M.R.; Dena-Aguilar, J.A.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A. Modeling of Fixed-Bed Adsorption of Fluoride on Bone Char Using a Hybrid Neural Network Approach. Chem. Eng. J. 2013, 228, 1098–1109. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A.; Hernandez-Montoya, V.; Moreno-Virgen, M.R.; Tovar-Gómez, R.; Montes-Morán, M.A. Optimization of Pyrolysis Conditions and Adsorption Properties of Bone Char for Fluoride Removal from Water. J. Anal. Appl. Pyrolysis 2013, 104, 10–18. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kim, J.Y.; Choi, Y.G. Synthesis of Bone Char from Cattle Bones and its Application for Fluoride Removal from the Contaminated Water. Groundw. Sustain. Dev. 2019, 8, 324–331. [Google Scholar] [CrossRef]
- Herath, H.M.A.S.; Kawakami, T.; Tafu, M. The Extremely High Adsorption Capacity of Fluoride by Chicken Bone Char (CBC) in Defluoridation of Drinking Water in Relation to its Finer Particle Size for Better Human Health. Healthcare 2018, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.I.; Órfão, J.J.M.; Soares, O. Sorption of Copper, Nickel and Cadmium on Bone Char. Prot. Met. Phys. Chem. Surf. 2017, 53, 618–627. [Google Scholar] [CrossRef]
- Cheung, C.W.; Porter, J.F.; Mckay, G. Sorption Kinetic Analysis for the Removal of Cadmium Ions from Effluents Using Bone Char. Water Res. 2001, 35, 605–612. [Google Scholar] [CrossRef]
- Guo, Q.; Tang, H.; Jiang, L.; Chen, M.; Zhu, N.; Wu, P. Sorption of Cd2+ on Bone Chars with or without Hydrogen Peroxide Treatment under Various Pyrolysis Temperatures: Comparison of Mechanisms and Performance. Processes 2022, 10, 618. [Google Scholar] [CrossRef]
- Tchomgui-Kamga, E.; Alonzo, V.; Nanseu-Njiki, C.P.; Audebrand, N.; Ngameni, E.; Darchen, A. Preparation and Characterization of Charcoals that Contain Dispersed Aluminum Oxide as Adsorbents for Removal of Fluoride from Drinking Water. Carbon. 2010, 48, 333–343. [Google Scholar] [CrossRef]
- Fan, X.; Parker, D.J.; Smith, M.D. Adsorption Kinetics of Fluoride on Low Cost Materials. Water Res. 2003, 37, 4929–4937. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saini, V.K. Defluoridation of Wastewaters Using Waste Carbon Slurry. Water Res. 2007, 41, 3307–3316. [Google Scholar] [CrossRef]
- Hua, Y.; Zheng, X.; Xue, L.; Han, L.; He, S.; Mishra, T.; Feng, Y.; Yang, L.; Xing, B. Microbial Aging of Hydrochar as a Way to Increase Cadmium Ion Adsorption Capacity: Process and Mechanism. Bioresour. Technol. 2020, 300, 122708. [Google Scholar] [CrossRef]
- Han, L.; Sun, H.; Ro, K.S.; Sun, K.; Libra, J.A.; Xing, B. Removal of Antimony (III) and Cadmium (II) from Aqueous Solution Using Animal Manure-Derived Hydrochars and Pyrochars. Bioresour. Technol. 2017, 234, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Demir-Cakan, R.; Baccile, N.; Antonietti, M.; Titirici, M.M. Carboxylate-rich Carbonaceous Materials Via One-Step Hydrothermal Carbonization of Glucose in the Presence of Acrylic Acid. Chem. Mater. 2009, 21, 484–490. [Google Scholar] [CrossRef]
- Li, B.; Guo, J.; Lv, K.; Fan, J. Adsorption of Methylene Blue and Cd (II) onto Maleylated Modified Hydrochar from Water. Environ. Pollut. 2019, 254, 113014. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.M.; Mo, C.H.; Li, H.; Zhang, Y.N.; Huang, W.X.; Wong, M.H. Comparison of Physicochemical Properties of Biochars and Hydrochars Produced from Food Wastes. J. Clean. Prod. 2019, 236, 117637. [Google Scholar] [CrossRef]
- Maletić, S.; Isakovski, M.K.; Sigmund, G.; Hofmann, T.; Hüffer, T.; Beljin, J.; Rončević, S. Comparing Biochar and Hydrochar for Reducing the Risk of Organic Contaminants in Polluted River Sediments used for Growing Energy Crops. Sci. Total Environ. 2022, 843, 157122. [Google Scholar] [CrossRef] [PubMed]
- Dhull, S.B.; Rose, P.K.; Rani, J.; Goksen, G.; Bains, A. Food Waste to Hydrochar: A Potential Approach Towards the Sustainable Development Goals, Carbon Neutrality, and Circular Economy. J. Chem. Eng. 2024, 490, 151609. [Google Scholar] [CrossRef]
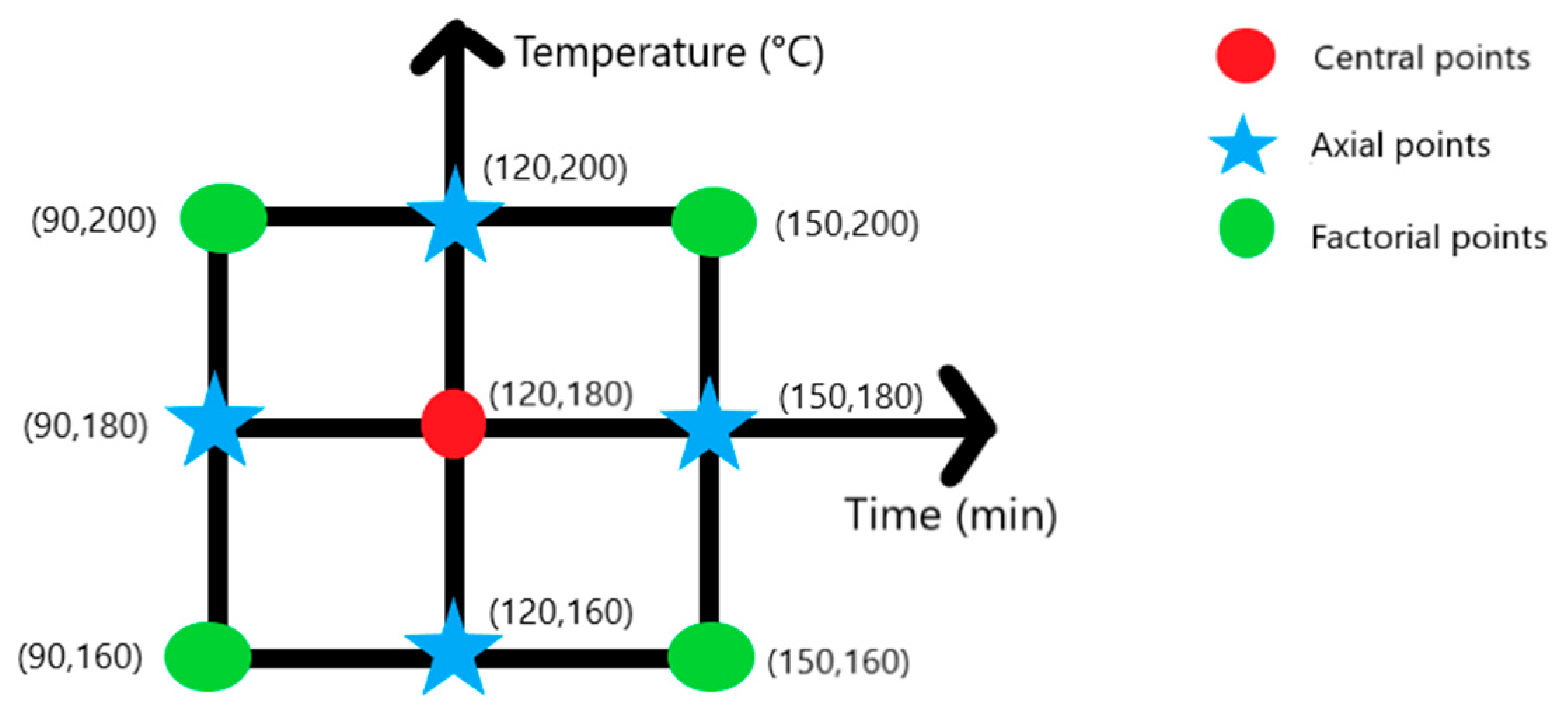

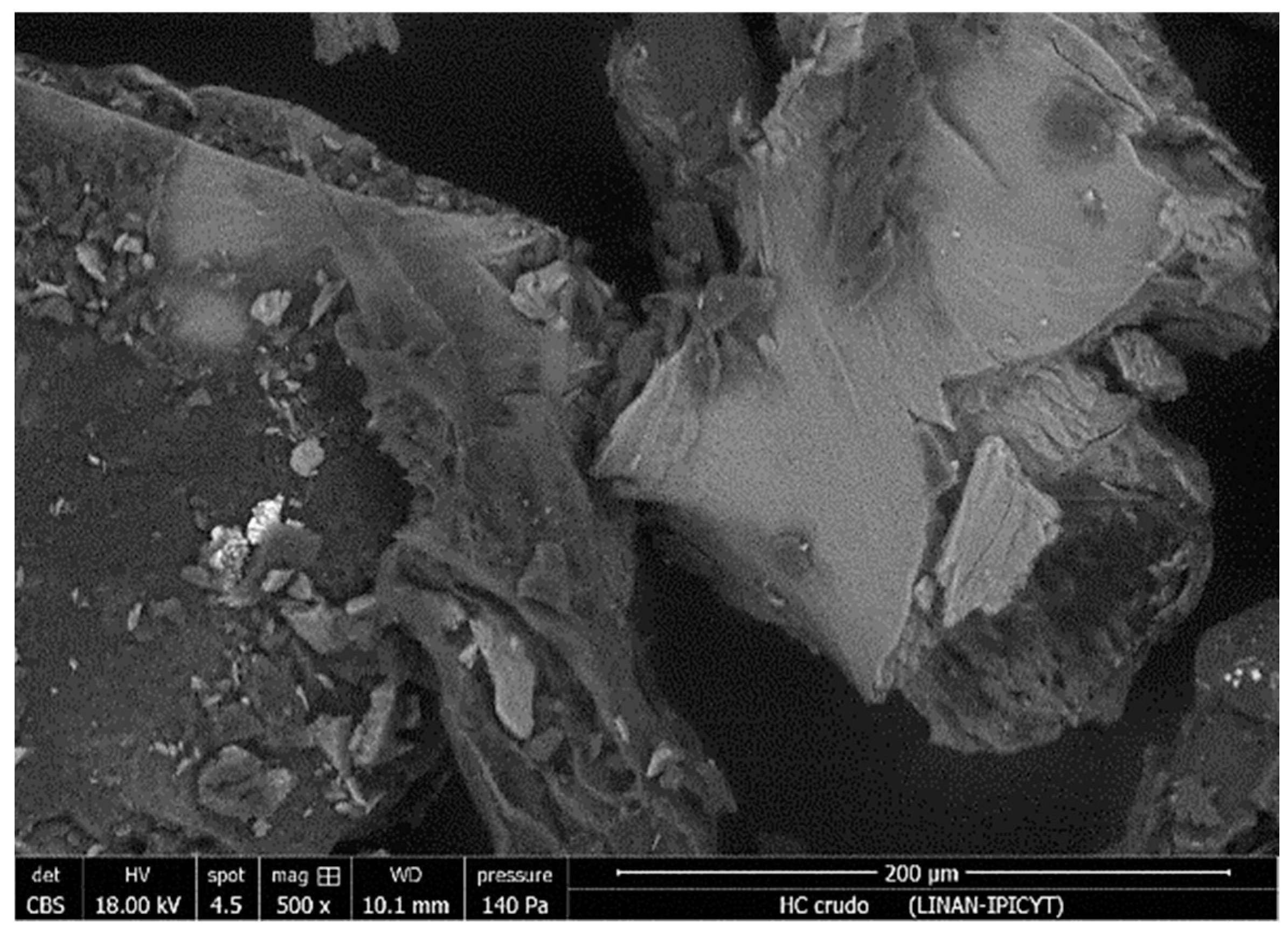
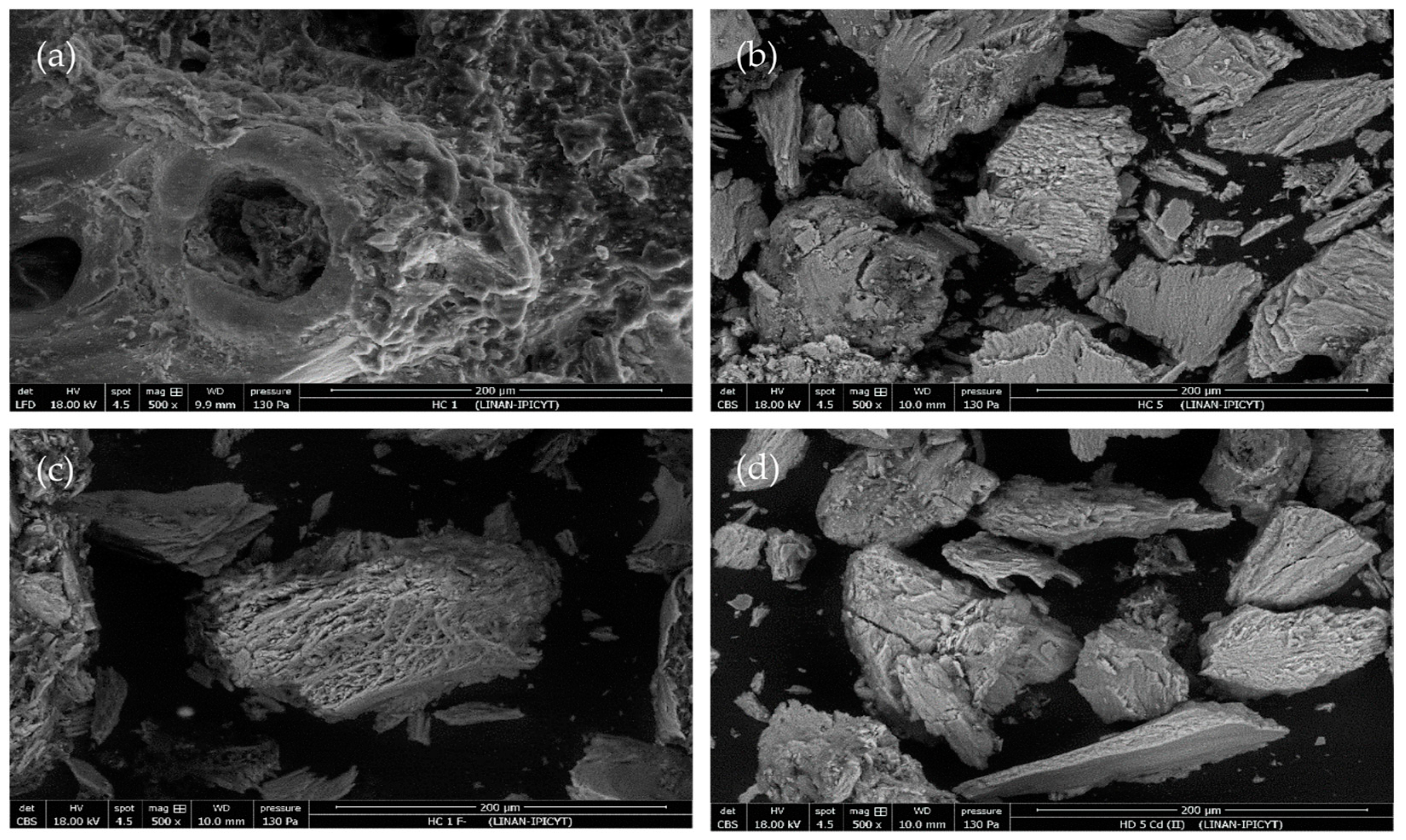
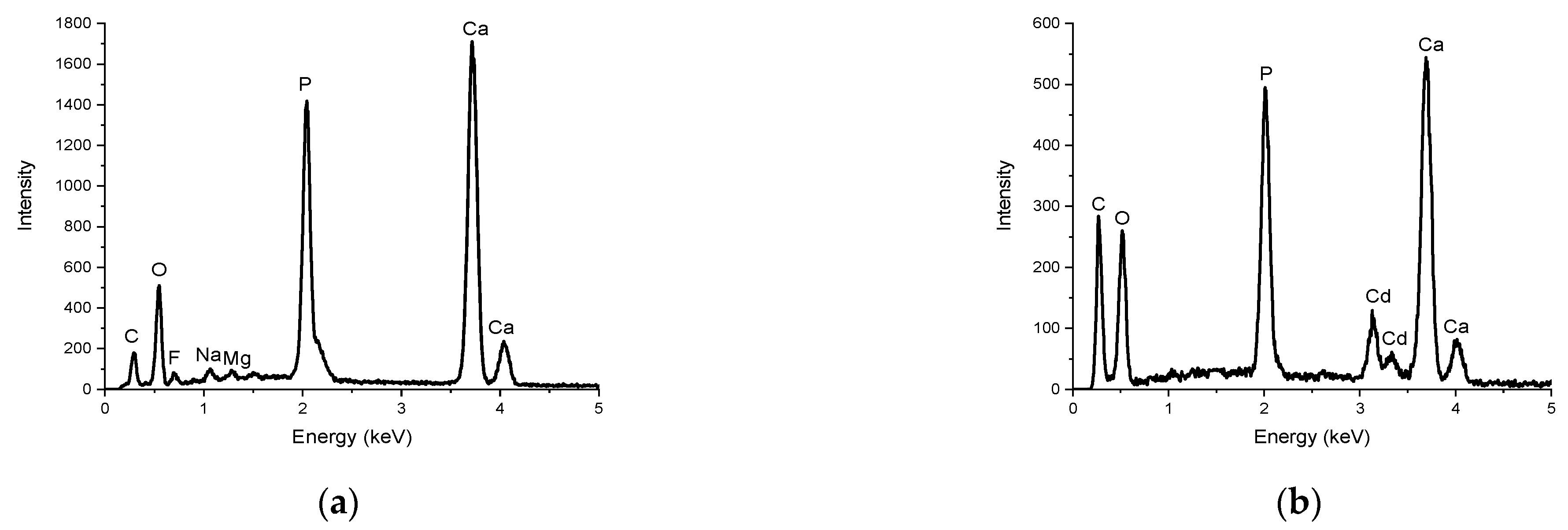


| Experiment | Synthesis Conditions | Yield (%) | |
|---|---|---|---|
| Time (min) | Temperature (°C) | ||
| 1 | 90 | 160 | 60.3 |
| 2 | 150 | 160 | 34.8 |
| 3 | 90 | 200 | 51.0 |
| 4 | 150 | 200 | 30.8 |
| 5 | 90 | 180 | 53.5 |
| 6 | 150 | 180 | 32.4 |
| 7 | 120 | 160 | 45.8 |
| 8 | 120 | 200 | 37.0 |
| 9 | 120 | 180 | 40.7 |
| 10 | 120 | 180 | 40.3 |
| 11 | 120 | 180 | 40.3 |
| 12 | 120 | 180 | 40.2 |
| 13 | 120 | 180 | 40.4 |
| Sample | BET Surface (m2 g−1) | Pore Volume (cm3 g−1) | Pore Diameter (nm) |
|---|---|---|---|
| Raw material | ND | ND | ND |
| Exp 1 (90 min 160 °C) | 47 | 0.21 | 17.7 |
| Exp 5 (90 min 180 °C) | 46 | 0.23 | 19.9 |
| Experiment | Synthesis Conditions | qexp (mg/g) | ||
|---|---|---|---|---|
| Time (min) | Temperature (°C) | Fluorides | Cd(II) | |
| 1 | 90 | 160 | 4.16 | 96.55 |
| 2 | 150 | 160 | 1.74 | 46.40 |
| 3 | 90 | 200 | 2.79 | 62.14 |
| 4 | 150 | 200 | 2.05 | 54.90 |
| 5 | 90 | 180 | 3.95 | 98.49 |
| 6 | 150 | 180 | 2.00 | 80.40 |
| 7 | 120 | 160 | 2.66 | 79.26 |
| 8 | 120 | 200 | 2.31 | 39.17 |
| 9 | 120 | 180 | 3.14 | 82.03 |
| 10 | 120 | 180 | 2.98 | 85.91 |
| 11 | 120 | 180 | 2.91 | 82.39 |
| 12 | 120 | 180 | 2.98 | 79.75 |
| 13 | 120 | 180 | 3.04 | 92.26 |
| Contaminant | Time (min) | Temperature (°C) | qmax Exp (mg/g) | qmax Pred (mg/g) | %D |
|---|---|---|---|---|---|
| Fluoride | 90 | 160 | 4.16 | 3.87 | 7.0 |
| Cd(II) | 90 | 171.5 | 98.5 | 108.3 | 9.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Cárdenas, M.; Medellín-Castillo, N.A.; González-Fernández, L.A.; Leyva-Ramos, R.; Gómez-Duran, C.F.A.; Gariepy, Y.; Pou, K.R.J.; Raghavan, V. Innovative Solution for Invasive Species and Water Pollution: Hydrochar Synthesis from Pleco Fish Biomass. Processes 2024, 12, 1158. https://doi.org/10.3390/pr12061158
Castro-Cárdenas M, Medellín-Castillo NA, González-Fernández LA, Leyva-Ramos R, Gómez-Duran CFA, Gariepy Y, Pou KRJ, Raghavan V. Innovative Solution for Invasive Species and Water Pollution: Hydrochar Synthesis from Pleco Fish Biomass. Processes. 2024; 12(6):1158. https://doi.org/10.3390/pr12061158
Chicago/Turabian StyleCastro-Cárdenas, Marisol, Nahum Andrés Medellín-Castillo, Lázaro Adrián González-Fernández, Roberto Leyva-Ramos, Cesar Fernando Azael Gómez-Duran, Yvan Gariepy, K. R. Jolvis Pou, and Vijaya Raghavan. 2024. "Innovative Solution for Invasive Species and Water Pollution: Hydrochar Synthesis from Pleco Fish Biomass" Processes 12, no. 6: 1158. https://doi.org/10.3390/pr12061158
APA StyleCastro-Cárdenas, M., Medellín-Castillo, N. A., González-Fernández, L. A., Leyva-Ramos, R., Gómez-Duran, C. F. A., Gariepy, Y., Pou, K. R. J., & Raghavan, V. (2024). Innovative Solution for Invasive Species and Water Pollution: Hydrochar Synthesis from Pleco Fish Biomass. Processes, 12(6), 1158. https://doi.org/10.3390/pr12061158








