Comparison of Mechanical, Fatigue, and Corrosion Properties of Fusion-Welded High-Strength AA6011 Alloy Using Three Filler Wires
Abstract
:1. Introduction
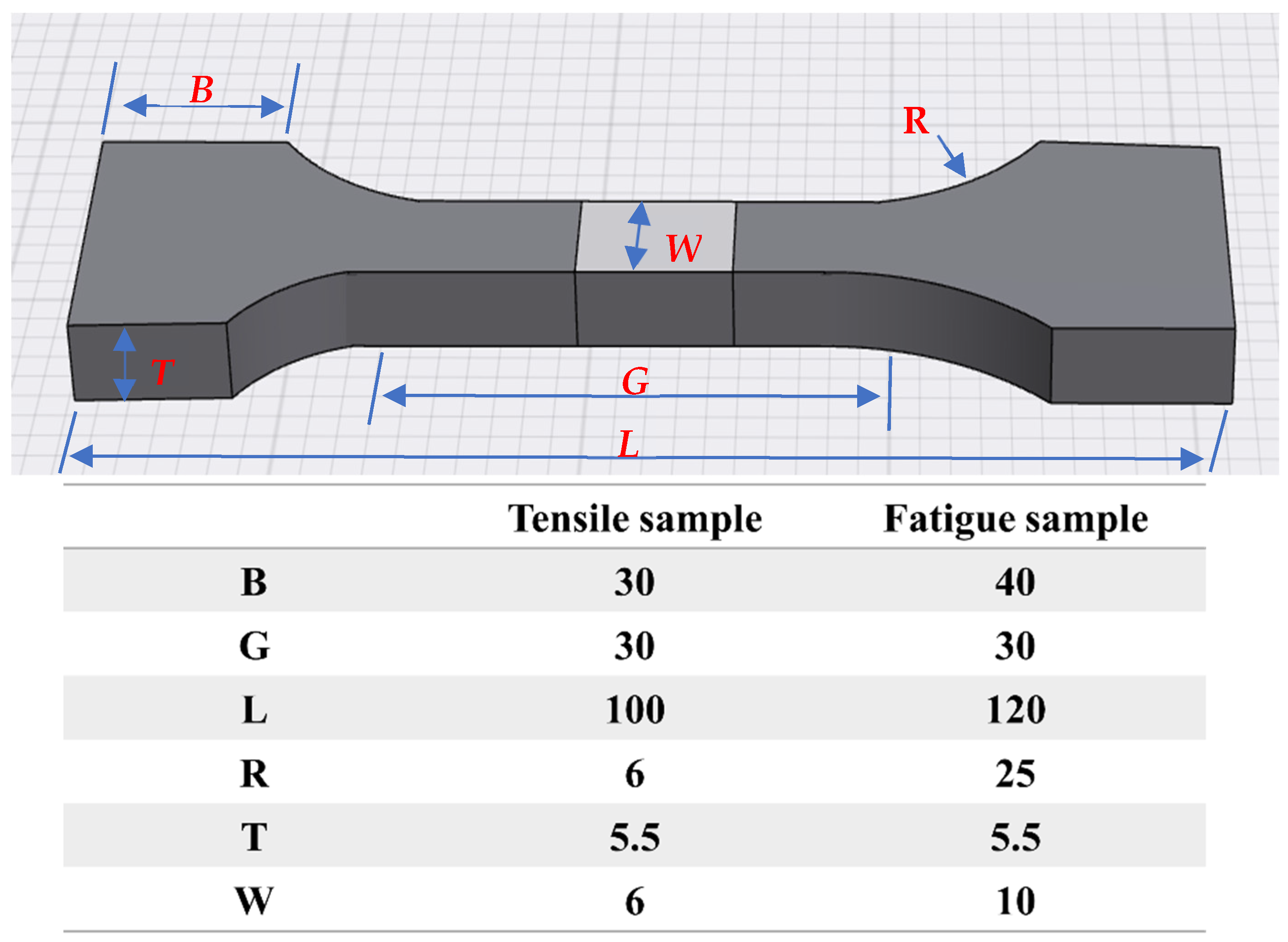
2. Materials and Methods
3. Results and Discussion
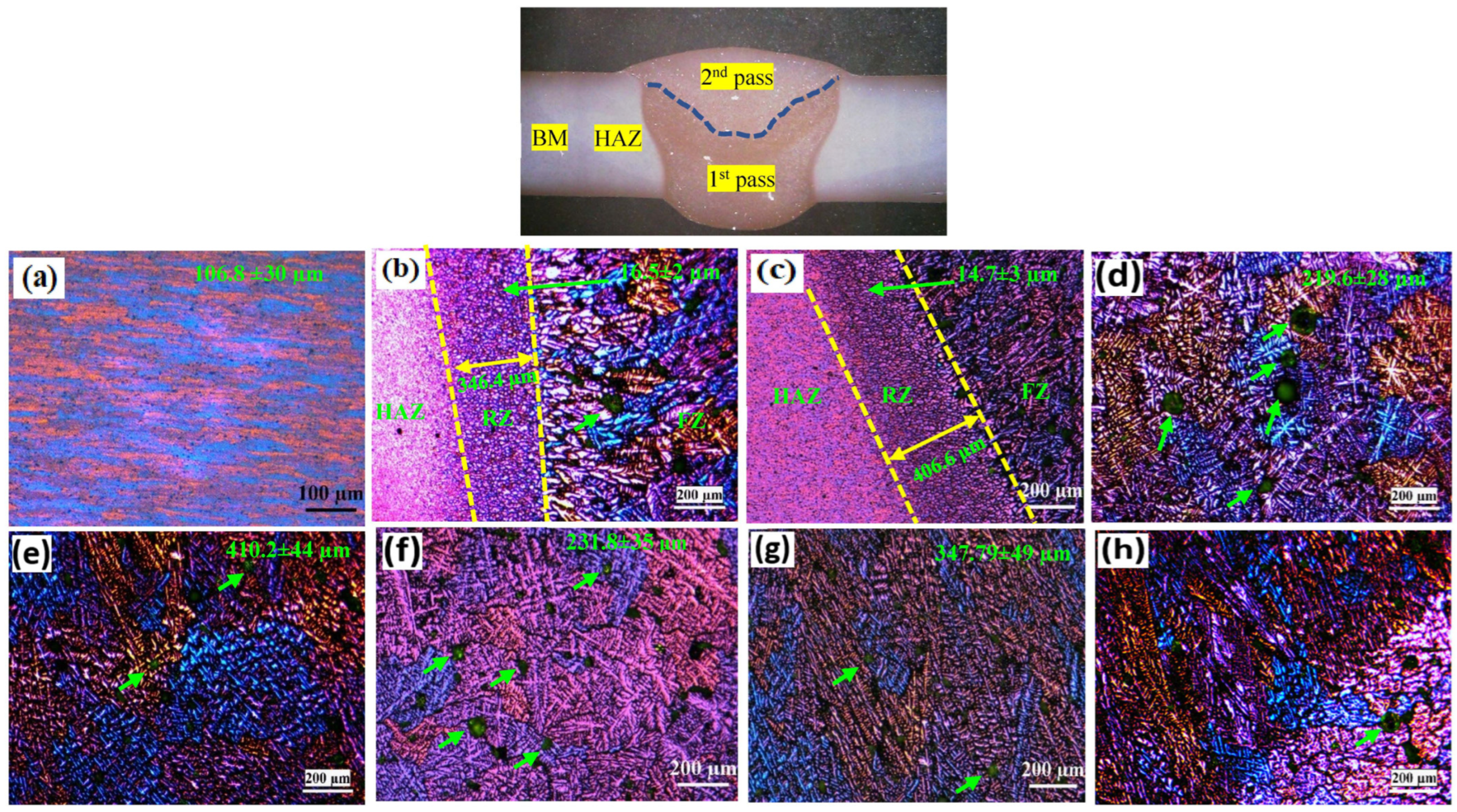
3.1. Microstructures
3.2. Microhardness
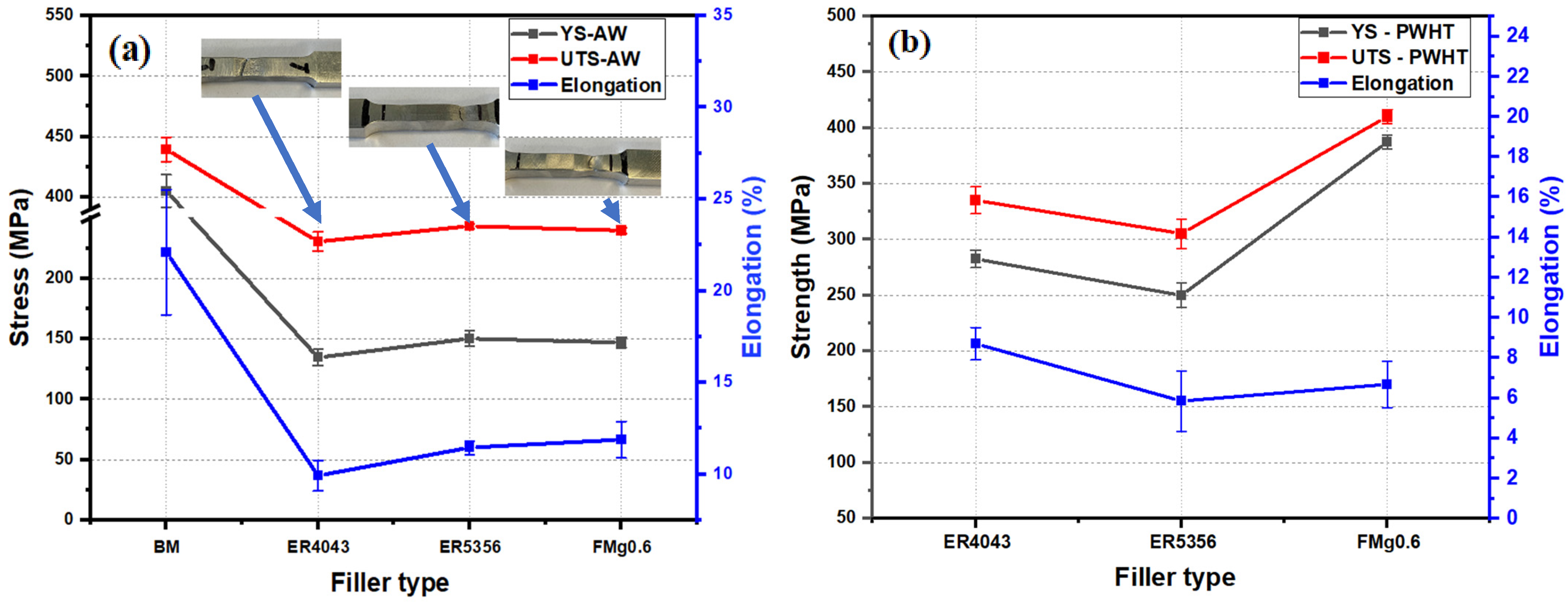
3.3. Tensile Properties
3.4. Fatigue Properties
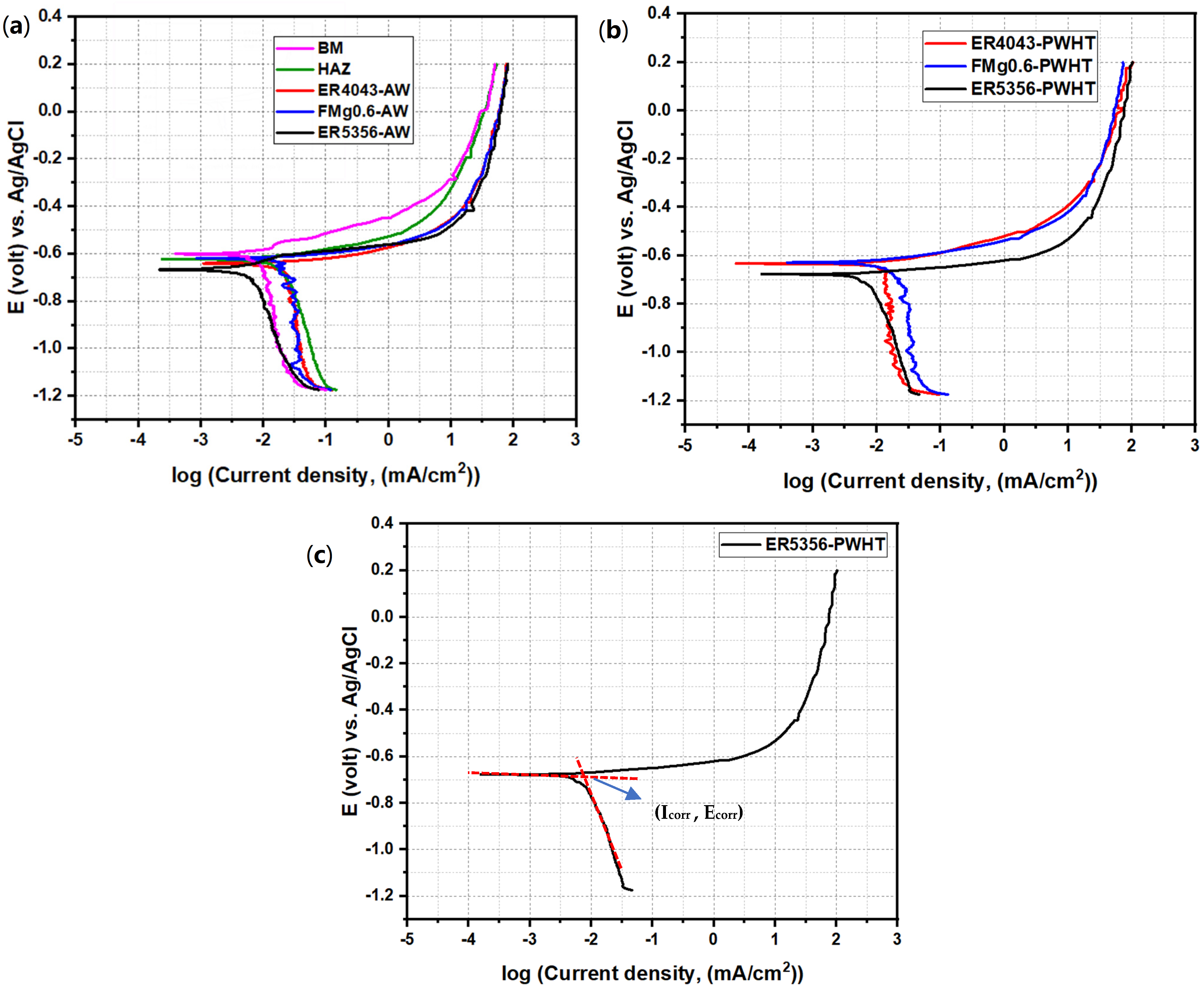
3.5. Corrosion Resistance
3.5.1. Potentiodynamic Polarization
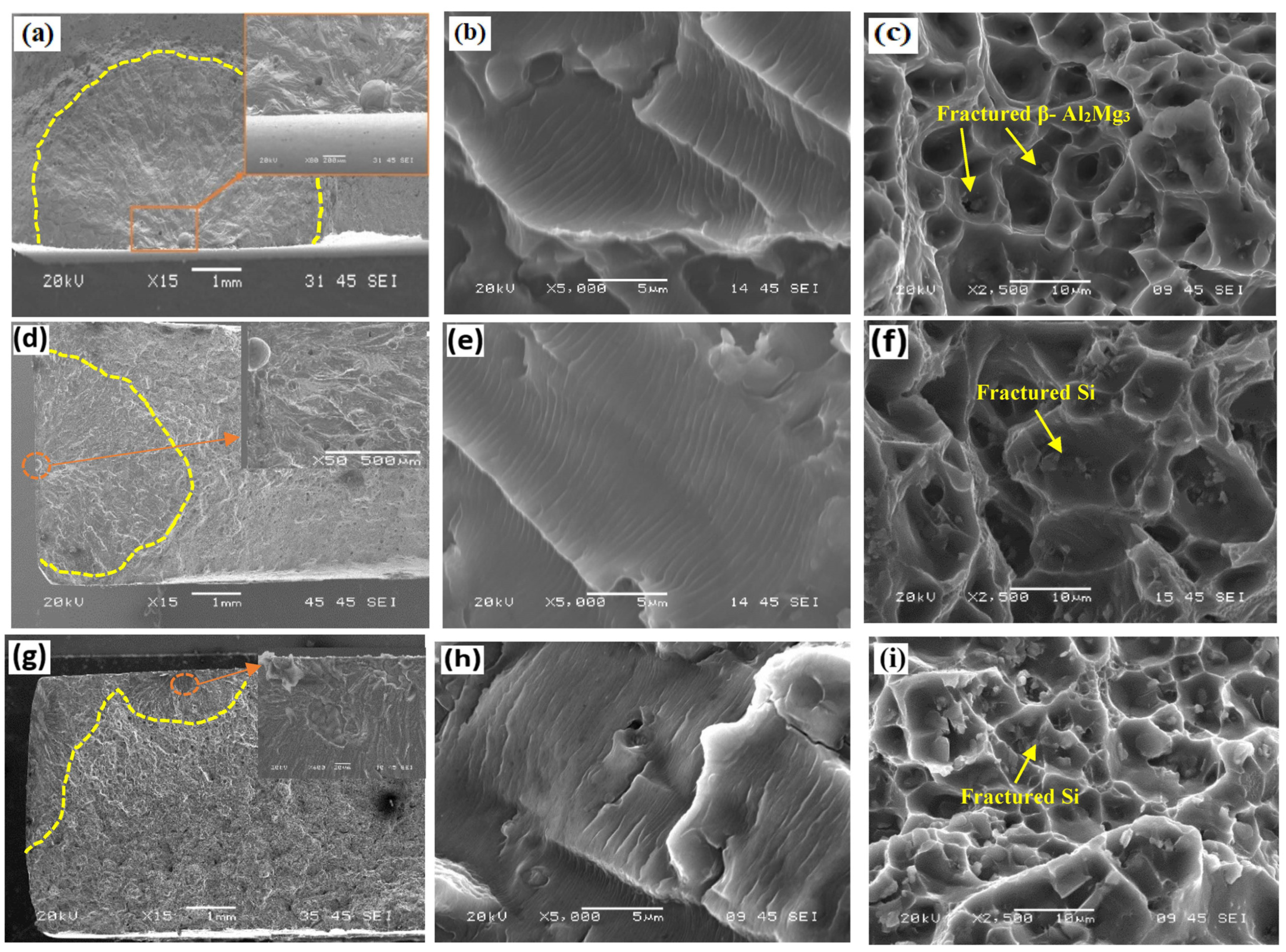
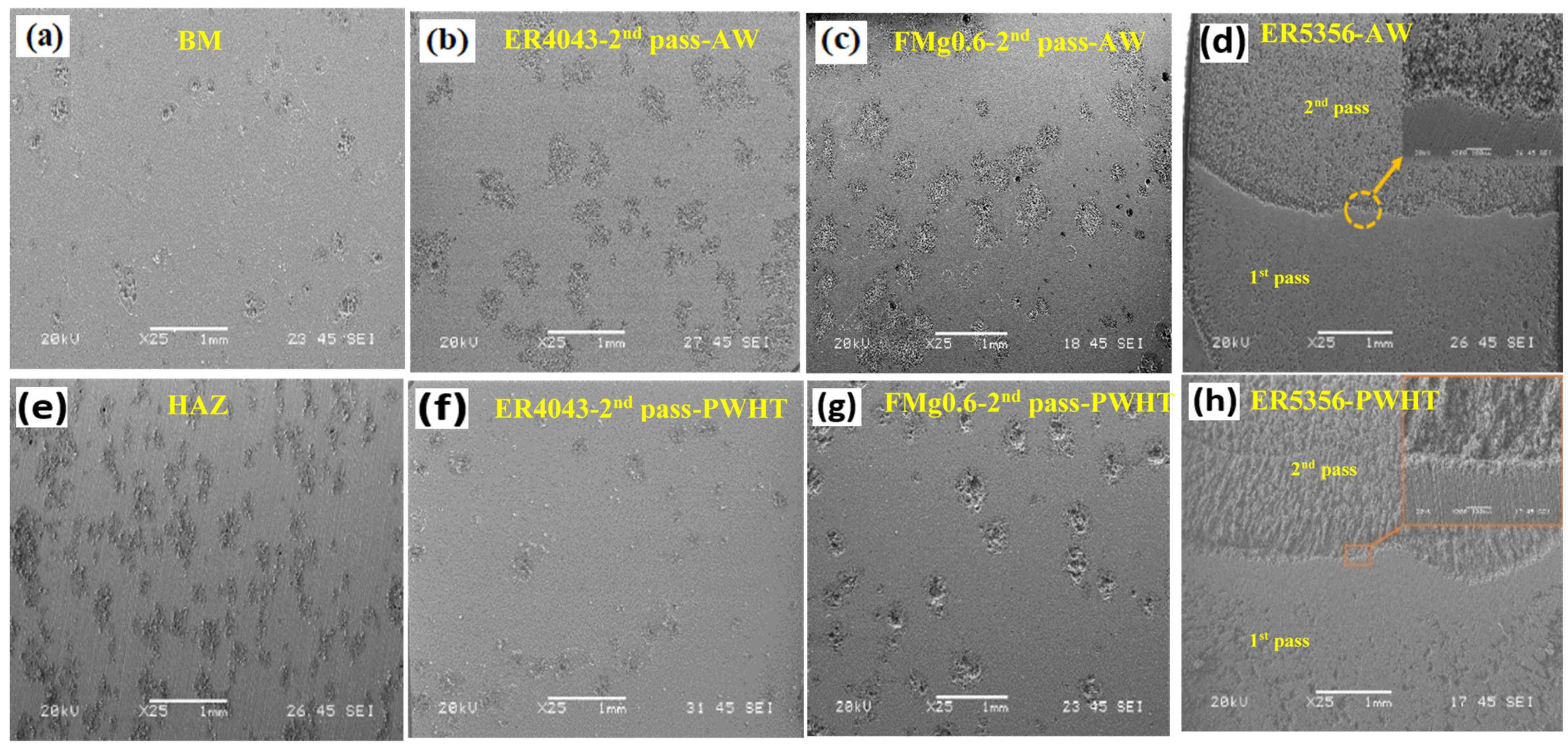
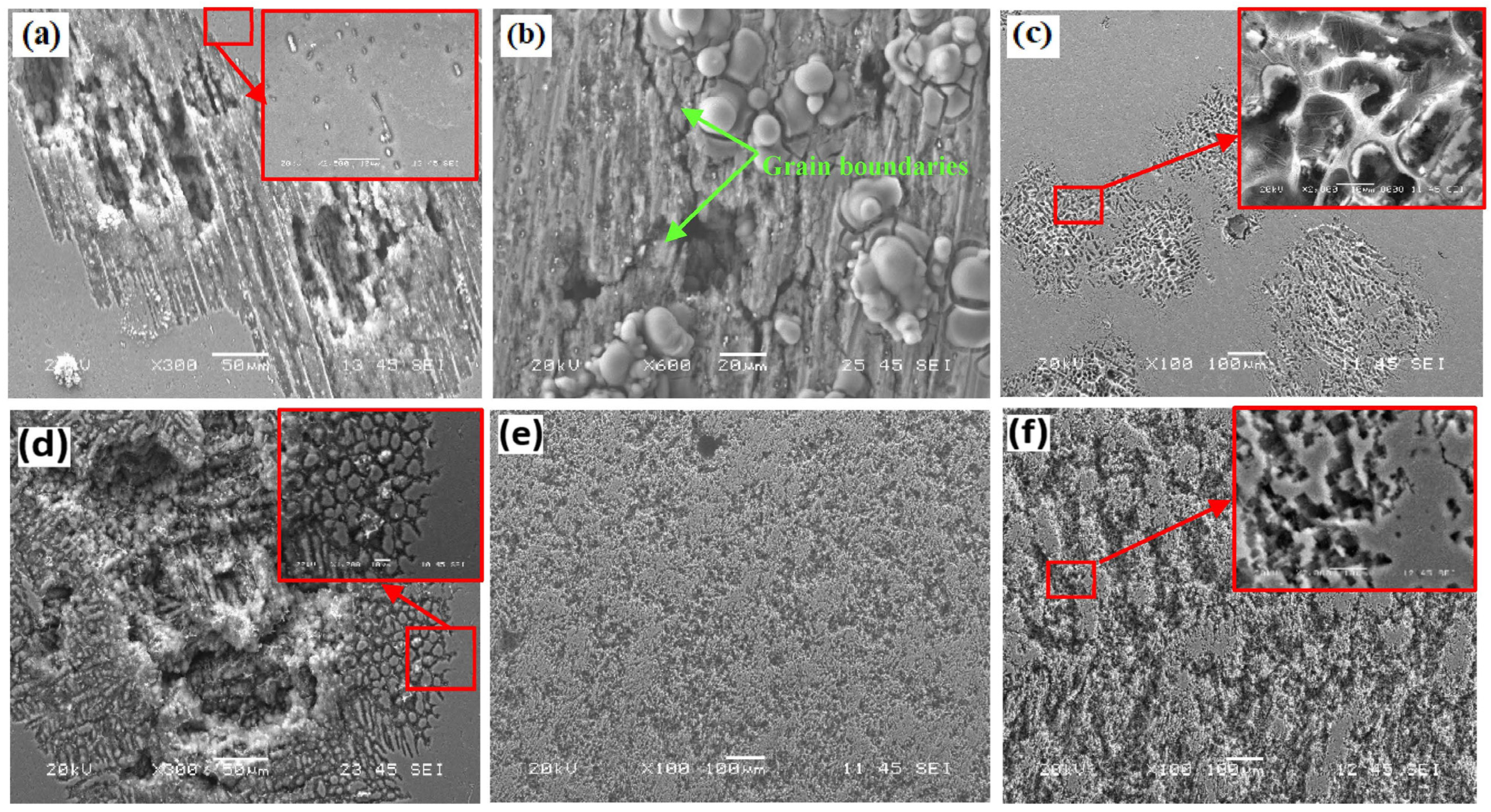
3.5.2. Surface Morphologies of Corroded Samples
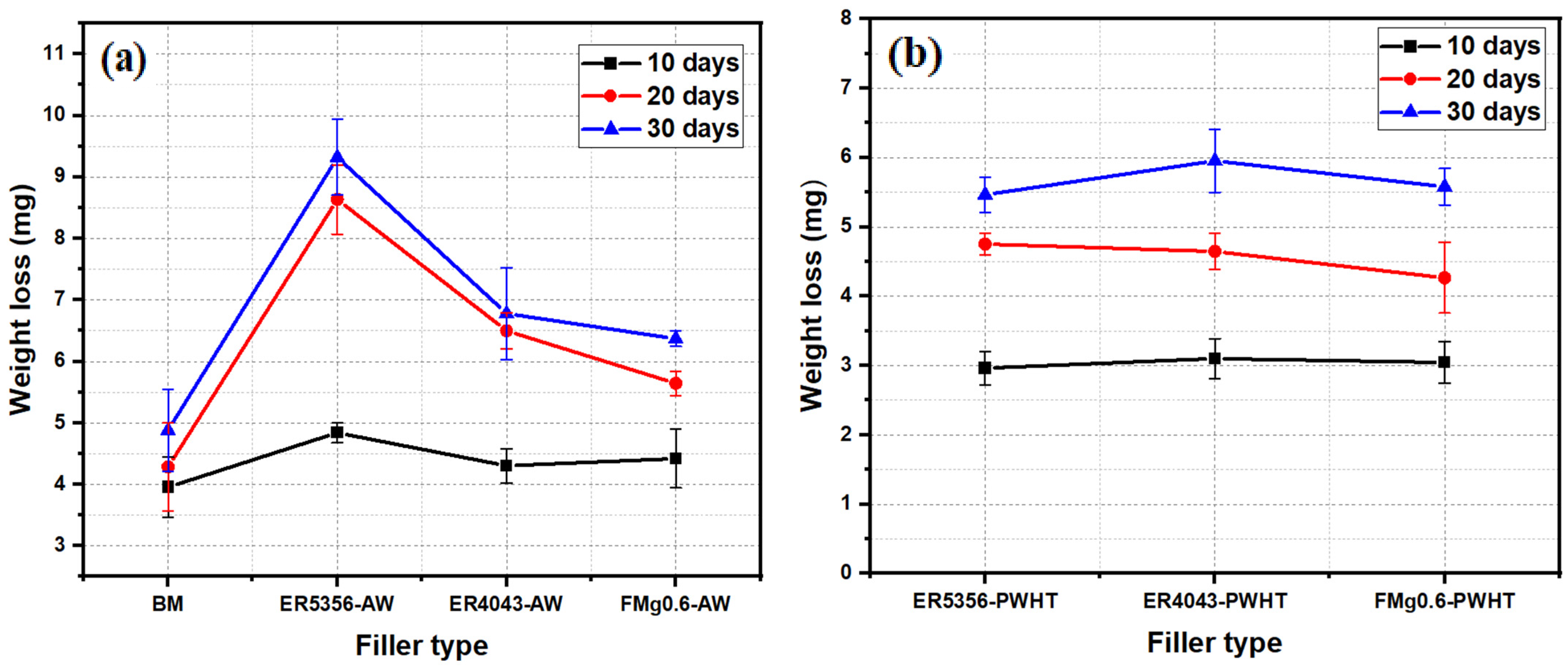
3.5.3. Immersion Tests
4. Discussion
5. Conclusions
- The ER4043 and FMg0.6 joints exhibited finer grain sizes in the fusion zone than the ER5356 joint in the AW condition. After PWHT, precipitation of brittle β-Al2Mg3 was observed at the grain boundaries and inside of grains in the ER5356 joint. In contrast, the ER4043 and FMg0.6 joints underwent spheroidization of eutectic Si and partial fragmentation of the Fe-rich intermetallics.
- Owing to the high Mg content in the FZs, the AW ER5356 and FMg0.6 joints exhibited higher microhardness values and tensile strengths than the ER4043 joint. The fractures in the former occurred in the softest HAZ, whereas the fractures in the ER4043 joint occurred in the FZ. After PWHT, the FMg0.6 joint exhibited the highest tensile strength among the three joints studied.
- The FMg0.6 joint presented the highest fatigue strength and best fatigue life compared with the commercial ER4043 and ER5356 joints in both the AW and PWHT conditions, which was attributed to the modification of its filler composition with the addition of Mg. The striation widths of the PWHT samples were in the order of FMg0.6 < ER4043 < ER5356, indicating the lowest fatigue crack propagation rate in the FMg0.6 joint. The fatigue strength and life of the PWHT joints were significantly improved compared with those of their AW counterparts.
- The ER5356 joint exhibited the lowest corrosion resistance among the three joints studied under all conditions. In the AW state, the FMg0.6 joint exhibited a slightly higher corrosion rate than the ER4043 joint, which was attributed to the high-volume fraction of eutectic Mg2Si. After PWHT, the corrosion resistances of both the ER4043 and FMg0.6 joints improved to a similar level.
- Considering the overall welding performance, including high tensile strength, superior fatigue properties, and satisfactory corrosion resistance, the FMg0.6 joint outperformed the commercial ER4043 and ER5356 joints studied. Therefore, the newly developed FMg0.6 filler wire is a promising filler metal for welding high-strength 6xxx alloys.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benedyk, J. Aluminum alloys for lightweight automotive structures. In Materials, Design and Manufacturing for Lightweight Vehicles; Elsevier: Amsterdam, The Netherlands, 2010; pp. 79–113. [Google Scholar]
- Sokoluk, M.; Yao, G.; Pan, S.; Cao, C.; Li, X. High Strength Nanotreated Filler Material for TIG Welding of AA6061. In Light Metals 2020; Springer: Berlin/Heidelberg, Germany, 2020; pp. 380–385. [Google Scholar]
- Verma, R.P.; Pandey, K.; Sharma, Y. Effect of ER4043 and ER5356 filler wire on mechanical properties and microstructure of dissimilar aluminium alloys, 5083-O and 6061-T6 joint, welded by the metal inert gas welding. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2015, 229, 1021–1028. [Google Scholar] [CrossRef]
- Fattahi, M.; Rostami, M.; Amirkhanlu, F.; Arabian, N.; Ahmadi, E.; Moayedi, H.J.D. Fabrication of aluminum TIG welding filler rods reinforced by ZrO2/reduced graphene oxide hybrid nanoparticles via accumulative roll bonding. DRM 2019, 99, 107518. [Google Scholar] [CrossRef]
- Naing, T.H.; Muangjunburee, P. Metallurgical and mechanical characterization of MIG welded repair joints for 6082-T6 aluminum alloy with ER 4043 and ER 5356. Trans. Indian Inst. Met. 2022, 75, 1583–1593. [Google Scholar] [CrossRef]
- Sokoluk, M.; Cao, C.; Pan, S.; Li, X. Nanoparticle-enabled phase control for arc welding of unweldable aluminum alloy 7075. Nat. Commun. 2019, 10, 98. [Google Scholar] [CrossRef]
- Bethea, J.F. Gas Tungsten Arc Welding of Aluminum Alloys with Nanocomposite 4943 Filler Material. Master’s Thesis, University of California, Los Angeles, CA, USA, 2019. [Google Scholar]
- Hu, Z.; Wan, L.; Lü, S.; Zhu, P.; Wu, S. Research on the microstructure, fatigue and corrosion behavior of permanent mold and die cast aluminum alloy. Mater. Des. 2014, 55, 353–360. [Google Scholar] [CrossRef]
- Yan, S.; Xing, B.; Zhou, H.; Xiao, Y.; Qin, Q.-H.; Chen, H. Effect of filling materials on the microstructure and properties of hybrid laser welded Al-Mg-Si alloys joints. Mater. Charact. 2018, 144, 205–218. [Google Scholar] [CrossRef]
- Bach, L.; Son, D.; Phong, M.; Thang, L.; Bian, M.; Nam, N. A study on Mg and AlN composite in microstructural and electrochemical characterizations of extruded aluminum alloy. Compos. B. Eng. 2019, 156, 332–343. [Google Scholar] [CrossRef]
- Zhang, D.-Q.J.; Gao, X.; Joo, L.-X.; Lee, H.G.; Yong, K. Effect of laser–arc hybrid welding on fracture and corrosion behaviour of AA6061-T6 alloy. Mater. Sci. Eng. A 2011, 528, 2748–2754. [Google Scholar] [CrossRef]
- Majeed, T.; Mehta, Y.; Siddiquee, A.N. Precipitation-dependent corrosion analysis of heat treatable aluminum alloys via friction stir welding, a review. Proc. Inst. Mech. Eng., Part C 2021, 235, 7600–7626. [Google Scholar] [CrossRef]
- Nam, N.; Dai, L.; Mathesh, M.; Bian, M.; Thu, V. Role of friction stir welding–traveling speed in enhancing the corrosion resistance of aluminum alloy. Mater. Chem. Phys. 2016, 173, 7–11. [Google Scholar] [CrossRef]
- Osório, W.R.; Freire, C.M.; Garcia, A. The role of macrostructural morphology and grain size on the corrosion resistance of Zn and Al castings. Mater. Sci. Eng. A 2005, 402, 22–32. [Google Scholar] [CrossRef]
- Rahman, A.M.; Kumar, S.; Gerson, A. Galvanic corrosion of laser weldments of AA6061 aluminium alloy. Corros. Sci. 2007, 49, 4339–4351. [Google Scholar] [CrossRef]
- Donatus, U.; Thompson, G.; Omotoyinbo, J.; Alaneme, K.; Aribo, S.; Agbabiaka, O. Corrosion pathways in aluminium alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 55–62. [Google Scholar] [CrossRef]
- Fadaeifard, F.; Matori, K.A.; Garavi, F.; Al-Falahi, M.; Sarrigani, G.V. Effect of post weld heat treatment on microstructure and mechanical properties of gas tungsten arc welded AA6061-T6 alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 3102–3114. [Google Scholar] [CrossRef]
- Haryadi, G.D.; Kim, S.J. Influences of post weld heat treatment on fatigue crack growth behavior of TIG welding of 6013 T4 aluminum alloy joint (Part 1. Fatigue crack growth across the weld metal). J. Mech. Sci. Technol. 2011, 25, 2161–2170. [Google Scholar] [CrossRef]
- Ahmed, M.; Javidani, M.; Mirakhorli, F.; Maltais, A.; Chen, X.G. Developing High-Strength Al-Si-Mg Filler Metals for Aluminum Fusion Welding. J. Mater. Eng. Perform. 2023, 32, 2218–2227. [Google Scholar] [CrossRef]
- ASTM E8-04; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM E466-96; Standard Practice for Conducting Force Controlled Constant Amplitude Axial Fatigue Tests of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2002.
- ISO 12107:2003; Metallic Materials, Fatigue Testing, Statistical Planning and Analysis of Data. ISO Standard: Geneva, Switzerland, 2003.
- ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 1999.
- GB/T 19746-2005; Corrosion of Metals and Alloys, Alternate Immersion Test in Salt Solution. GBT Standard: Beijing, China, 2005.
- GB/T 16545-1996; Corrosion of Metals and Alloys, Removal of Corrosion Products from Corrosion Test Specimens. GBT Standard: Beijing, China, 1996.
- Peng, W.; Wu, G.; Lu, R.; Lian, Q.; Zhang, J. The Evaluation on Corrosion Resistance and Dross Formation of Zn–23 wt% Al–0.3 wt% Si–x wt% Mg Alloy. Coatings 2019, 9, 199. [Google Scholar] [CrossRef]
- Wang, B.; Xue, S.; Ma, C.; Wang, J.; Lin, Z. Effects of porosity, heat input and post-weld heat treatment on the microstructure and mechanical properties of TIG welded joints of AA6082-T6. Metals 2017, 7, 463. [Google Scholar] [CrossRef]
- Easton, M.A.S.; David, H. Improved prediction of the grain size of aluminum alloys that includes the effect of cooling rate. Mater. Sci. Eng. A 2008, 486, 8–13. [Google Scholar] [CrossRef]
- Ahmed, M.; Javidani, M.; Maltais, A.; Chen, X.-G. Welding of AA6061-T6 Sheets Using High-Strength 4xxx Fillers: Effect of Mg on Mechanical and Fatigue Properties. Materials 2023, 16, 3832. [Google Scholar] [CrossRef]
- Seifeddine, S.; Svensson, I.L. The influence of Fe and Mn content and cooling rate on the microstructure and mechanical properties of A380-die casting alloys. Metall. Res. Technol. 2009, 27, 11–20. [Google Scholar]
- Kumar, S.; Grant, P.S.; O’Reilly, K.A.Q. Fe bearing intermetallic phase formation in a wrought Al–Mg–Si alloy. Trans. Indian Inst. Met. 2012, 65, 553–557. [Google Scholar] [CrossRef]
- Moustafa, M.A. Effect of iron content on the formation of β-Al5FeSi and porosity in Al–Si eutectic alloys. Mater. Process. Technol. 2009, 209, 605–610. [Google Scholar] [CrossRef]
- Ahmed, M.; Javidani, M.; Maltais, A.; Chen, X.-G. Microstructure and Mechanical Properties of High-Strength AA6011 Aluminum Alloy Welding with Novel 4xxx Filler Metals. Materials 2024, 17, 380. [Google Scholar] [CrossRef]
- Pérez, J.S.; Ambriz, R.R.; López, F.F.C. Recovery of mechanical properties of a 6061-T6 aluminum weld by heat treatment after welding. Metall. Mater. Trans. A 2016, 47, 3412–3422. [Google Scholar] [CrossRef]
- Poznak, A.; Freiberg, D.; Sanders, P. Automotive wrought aluminium alloys. In Fundamentals of Aluminium Metallurgy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 333–386. [Google Scholar]
- Leo, P.; D’Ostuni, S.; Casalino, G. Hybrid welding of AA5754 annealed alloy: Role of post weld heat treatment on microstructure and mechanical properties. Mater. Des. 2016, 90, 777–786. [Google Scholar] [CrossRef]
- Liu, K.; Algendy, A.; Gu, J.; Chen, X.-G. Evolution of Microstructure and Dispersoids in Al-Mg 5xxx Alloys Under Wire+ Arc Additive Manufacturing and Permanent Mold Casting. In TMS 2021 150th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Berlin/Heidelberg, Germany, 2021; pp. 165–175. [Google Scholar]
- Ha, S.H.; Yoon, Y.O.; Kim, S.K. Effects of Si Addition on the Microstructure and Tensile Property of Al-xMg Alloys with Al2Ca during Heat Treatment (x: 5 and 7 Mass%). In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2017; pp. 109–113. [Google Scholar]
- Johannesson, B.; Cáceres, C. Effect of Si additions and heat treatment on the mechanical behaviour of an Al–5Mg casting alloy. Int. J. Cast Met. Res. 2004, 17, 94–98. [Google Scholar] [CrossRef]
- Coniglio, N.; Cross, C.E.; Michael, T.; Lammers, M. Defining a critical weld dilution to avoid solidification cracking in aluminum. Weld. J. 2008, 87, 237s–247s. [Google Scholar]
- Mousavi, M.G.; Cross, C.E.; Grong, Ø.; Hval, M. Controlling weld metal dilution for optimised weld performance in aluminium. Sci. Technol. Weld. Join. 1997, 2, 275–278. [Google Scholar] [CrossRef]
- Wen, W.; Zhao, Y.; Morris, J.G. The effect of Mg precipitation on the mechanical properties of 5xxx aluminum alloys. Mater. Sci. Eng. A 2005, 392, 136–144. [Google Scholar] [CrossRef]
- Ma, M.; Lai, R.; Qin, J.; Wang, B.; Liu, H.; Yi, D. Effect of weld reinforcement on tensile and fatigue properties of 5083 aluminum metal inert gas (MIG) welded joint: Experiments and numerical simulations. Int. J. Fatigue 2021, 144, 106046. [Google Scholar] [CrossRef]
- Ren, L.; Gu, H.; Wang, W.; Wang, S.; Li, C.; Wang, Z.; Zhai, Y.; Ma, P. Effect of Mg content on microstructure and properties of Al–Mg alloy produced by the wire arc additive manufacturing method. Materials 2019, 12, 4160. [Google Scholar] [CrossRef]
- ASTM G59-97; Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. ASTM International: West Conshohocken, PA, USA, 2003.
- Won, S.; Seo, B.; Park, J.M.; Kim, H.K.; Song, K.H.; Min, S.H.; Ha, S.-K.; Park, T.K. Corrosion behaviors of friction welded dissimilar aluminum alloys. Mater. Charact. 2018, 144, 652–660. [Google Scholar] [CrossRef]
- Adeosun, S.; Sekunowo, O.; Balogun, S.; Obiekea, V. Corrosion behaviour of heat-treated aluminum-magnesium alloy in Chloride and exco environments. Int. J. Corros. 2012, 2012, 927380. [Google Scholar] [CrossRef]
- Wei, R.P.; Liao, C.-M.; Gao, M. A transmission electron microscopy study of constituent-particle-induced corrosion in 7075-T6 and 2024-T3 aluminum alloys. Metall. Mater. Trans. A 1998, 29, 1153–1160. [Google Scholar] [CrossRef]
- Choi, I.-K.; Cho, S.-H.; Kim, S.-J.; Jo, Y.-S.; Kim, S.-H. Improved corrosion resistance of 5xxx aluminum alloy by homogenization heat treatment. Coatings 2018, 8, 39. [Google Scholar] [CrossRef]
- Gupta, R.K.; Zhang, R.; Davies, C.H.J.; Birbilis, N. Influence of Mg content on the sensitization and corrosion of Al-xMg (-Mn) alloys. Corrosion 2013, 69, 1081–1087. [Google Scholar] [CrossRef]
- Lin, Y.K.; Wang, S.H.; Chen, R.Y.; Hsieh, T.S.; Tsai, L.; Chiang, C.C. The effect of heat treatment on the sensitized corrosion of the 5383-H116 Al-Mg alloy. Materials 2017, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Nikseresht, Z.; Karimzadeh, F.; Golozar, M.; Heidarbeigy, M. Effect of heat treatment on microstructure and corrosion behavior of Al6061 alloy weldment. Mater. Des. 2010, 31, 2643–2648. [Google Scholar] [CrossRef]
- Abuaisha, R. Corrosion Behaviour of Friction Stir Welded AA5xxx Aluminium Alloys; The University of Manchester: Manchester, UK, 2013. [Google Scholar]
- Chen, G.C.; Wang, Q.; Du, B.; Ming, Z. Microstructure evolution and tensile mechanical properties of thixoformed high performance Al-Zn-Mg-Cu alloy. Met. Mater. Int. 2015, 21, 897–906. [Google Scholar] [CrossRef]
- Xie, W.H.; Yang, T.; Fan, C.; Lin, S.; Xu, W. Comparison of microstructure, mechanical properties, and corrosion behavior of Gas Metal Arc (GMA) and Ultrasonic-wave-assisted GMA (U-GMA) welded joints of Al–Zn–Mg alloy. J. Mater. Process. Technol. 2020, 277, 116470. [Google Scholar] [CrossRef]
- Zaid, B.; Saidi, D.; Benzaid, A.; Hadji, S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corros. Sci. 2008, 50, 1841–1847. [Google Scholar] [CrossRef]
- Cramer, S.D.; Covino, B.S., Jr. Corrosion: Fundamentals, Testing, and Protection, Volume 13A, ASM Handbook. J. Therm. Spray Technol. 2003, 12, 459. [Google Scholar]




| Si | Mg | Mn | Fe | Cu | |
|---|---|---|---|---|---|
| BM (6011) | 0.92 | 0.75 | 0.45 | 0.17 | 0.64 |
| ER4043 | 4.97 | 0.024 | 0.02 | 0.16 | - |
| ER5356 | 0.06 | 5 | 0.1 | 0.15 | 0.01 |
| FMg0.6 | 6.23 | 0.6 | 0.23 | 0.14 | 0.011 |
| Current (Amp.) | Voltage (V) | Travel Speed (m/min) | Wire Feed Speed (m/min) | |||||
|---|---|---|---|---|---|---|---|---|
| Pass 1 | Pass 2 | Pass 1 | Pass 2 | Pass 1 | Pass 2 | Pass 1 | Pass 2 | |
| ER5356 | 132 | 87 | 18.8 | 17.2 | 0.5 | 0.27 | 5 | 3.2 |
| ER4043 & FMg0.6 | 163 ± 4 | 110 ± 5 | 21.5 ± 0.5 | 22 ± 0.7 | 0.5 | 0.27 | 4.5 ± 0.3 | 3.2 |
| Samples | Ecorr (mV) | Icorr (µA/cm2) | Corrosion Rate (µm/y) |
|---|---|---|---|
| BM | −602.91 | 2.23 | 24.31 |
| HAZ | −623.2 | 10.42 | 113.58 |
| ER5356-AW | −666.15 | 13.35 | 145.52 |
| ER4043-AW | −635.02 | 7.19 | 78.37 |
| FMg0.6-AW | −620.27 | 9.13 | 99.52 |
| ER5356-PWHT | −680.35 | 16.4 | 178.76 |
| ER4043-PWHT | −630.07 | 5.51 | 60.06 |
| FMg0.6-PWHT | −628.12 | 4.85 | 52.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Javidani, M.; Maltais, A.; Chen, X.-G. Comparison of Mechanical, Fatigue, and Corrosion Properties of Fusion-Welded High-Strength AA6011 Alloy Using Three Filler Wires. Processes 2024, 12, 1172. https://doi.org/10.3390/pr12061172
Ahmed M, Javidani M, Maltais A, Chen X-G. Comparison of Mechanical, Fatigue, and Corrosion Properties of Fusion-Welded High-Strength AA6011 Alloy Using Three Filler Wires. Processes. 2024; 12(6):1172. https://doi.org/10.3390/pr12061172
Chicago/Turabian StyleAhmed, Mohamed, Mousa Javidani, Alexandre Maltais, and X.-Grant Chen. 2024. "Comparison of Mechanical, Fatigue, and Corrosion Properties of Fusion-Welded High-Strength AA6011 Alloy Using Three Filler Wires" Processes 12, no. 6: 1172. https://doi.org/10.3390/pr12061172






