Identification and Absorption–Distribution–Metabolism–Excretion–Toxicity Prediction of Potential MTHFD2 Enzyme Inhibitors from Urtica dioica Ethanolic Leaf Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Extraction
2.2. GC–MS Method
2.3. HPLC Method
2.4. Cell Cultures
2.4.1. Cell Viability
2.4.2. Cell Viability (%)
2.5. Molecular Docking and ADMET Prediction
2.5.1. Protein Structure Preparation
2.5.2. Ligand Preparation
2.5.3. Molecular Docking Preparation
2.6. ADMET Prediction
2.7. Statistical Analysis
3. Results
3.1. GC–MS Analysis of the Extract
3.2. HPLC Profiles of the Extracts
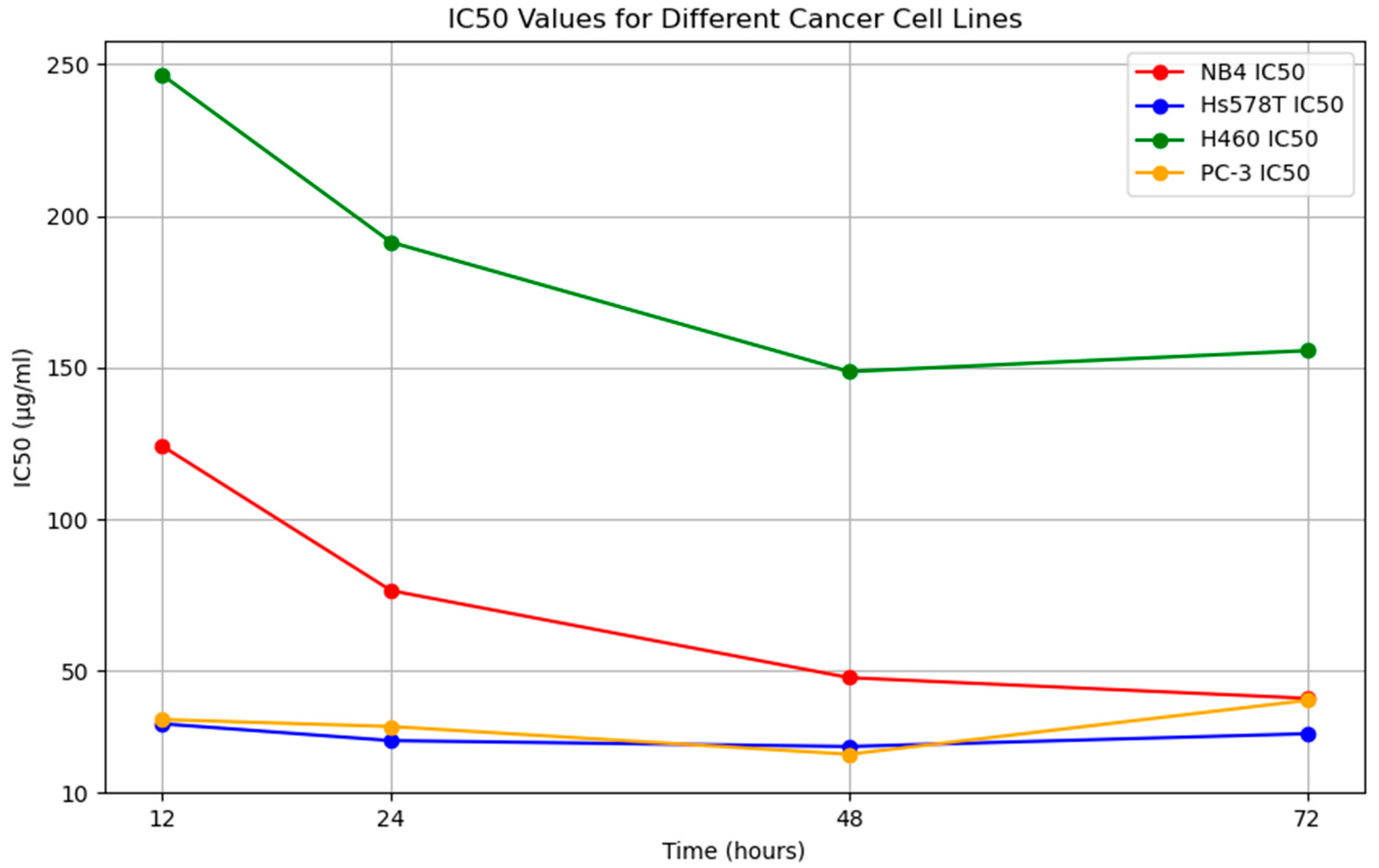
3.3. Anticancer Activity
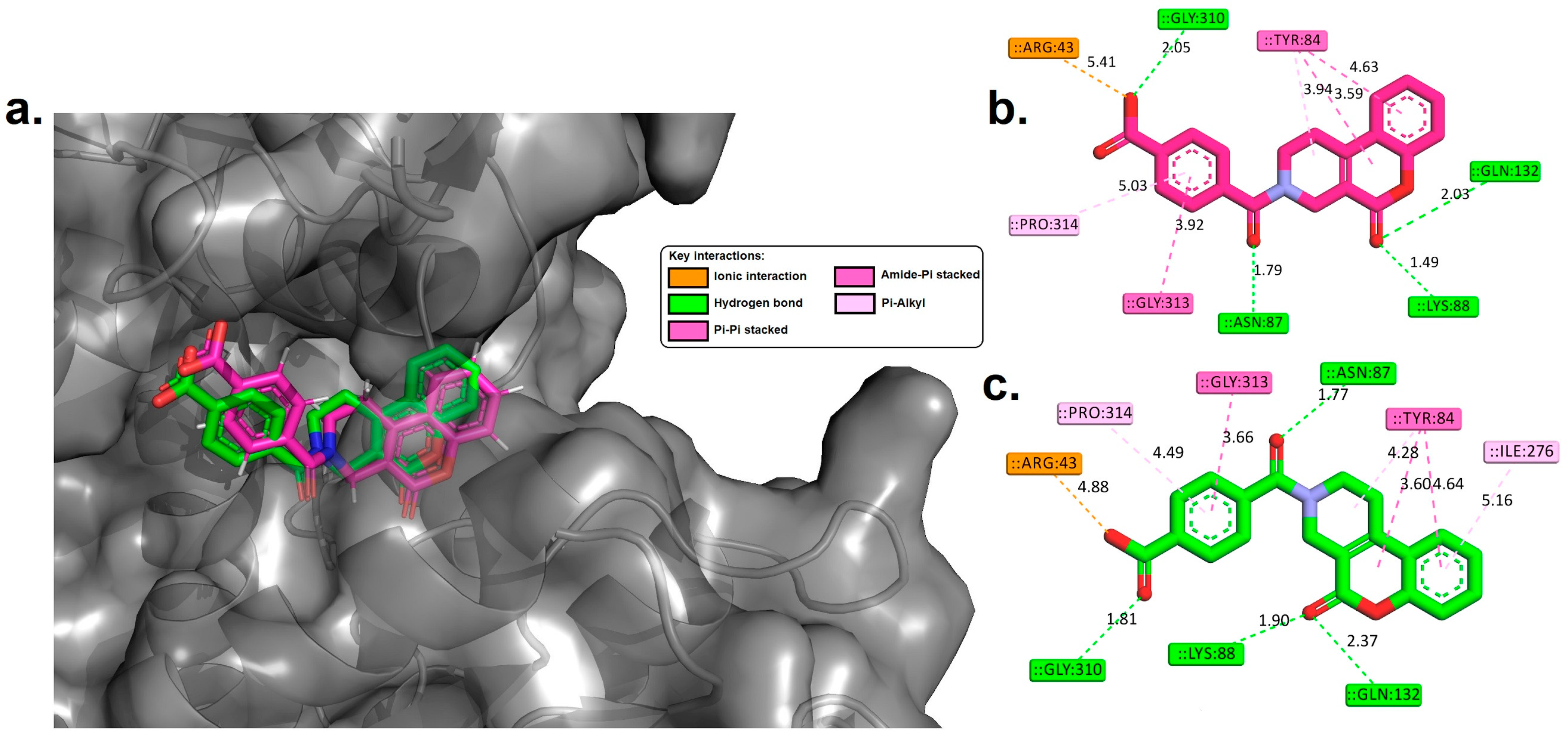
3.4. Molecular Docking Analysis
3.5. Drug-Likeness and ADMET Properties
4. Discussion
4.1. GC–MS and HPLC Analyses of the Extract
4.2. Molecular Docking
4.3. Drug-Likeness and ADMET Properties
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seyfried, T.N.; Shelton, L.M. Cancer as a metabolic disease. Nutr. Metab. 2010, 7, 7. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- Tamburrino, A.; Piro, G.; Carbone, C.; Tortora, G.; Melisi, D. Mechanisms of resistance to chemotherapeutic and anti-angiogenic drugs as novel targets for pancreatic cancer therapy. Front. Pharmacol. 2013, 4, 56. [Google Scholar] [CrossRef]
- Du, G.-H. Natural Small Molecule Drugs from Plants; Springer: Singapore, 2018. [Google Scholar]
- Wangchuk, P. Therapeutic applications of natural products in herbal medicines, biodiscovery programs, and biomedicine. J. Biol. Act. Prod. Nat. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Discovery and development of antineoplastic agents from natural sources. Cancer Investig. 1999, 17, 153–163. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K. Stinging nettle (Urtica dioica L.): Nutritional composition, bioactive compounds, and food functional properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef]
- Karakol, P.; Saraydin, S.U.; Bozkurt, M.; Hepokur, C.; Inan, Z.D.S.; Turan, M. Anticancer Effects of Urtica Dioica in Breast Cancer. Asian Pac. J. Cancer Prev. 2022, 23, 673–681. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mansoori, B.; Aghapour, M.; Baradaran, B. Urtica dioica dichloromethane extract induce apoptosis from intrinsic pathway on human prostate cancer cells (PC3). Cell. Mol. Biol. 2016, 62, 78–83. [Google Scholar]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P.V. Therapeutic Perspectives of Molecules from Urtica dioica Extracts for Cancer Treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mansoori, B.; Baradaran, P.C.; Khaze, V.; Aghapour, M.; Farhadi, M.; Baradaran, B. Urtica dioica extract inhibits proliferation and induces apoptosis and related gene expression of breast cancer cells in vitro and in vivo. Clin. Breast Cancer 2017, 17, 463–470. [Google Scholar] [CrossRef]
- Semwal, P.; Rauf, A.; Olatunde, A.; Singh, P.; Zaky, M.Y.; Islam, M.M.; Khalil, A.A.; Aljohani, A.S.; Al Abdulmonem, W.; Ribaudo, G. The medicinal chemistry of Urtica dioica L.: From preliminary evidence to clinical studies supporting its neuroprotective activity. Nat. Prod. Bioprospecting 2023, 13, 16. [Google Scholar] [CrossRef]
- Fattahi, S.; Ghadami, E.; Asouri, M.; Ardekanid, A.M.; Akhavan-Niaki, H. Urtica dioica inhibits cell growth and induces apoptosis by targeting Ornithine decarboxylase and Adenosine deaminase as key regulatory enzymes in adenosine and polyamines homeostasis in human breast cancer cell lines. Cell. Mol. Biol. 2018, 64, 97–102. [Google Scholar] [CrossRef]
- Rashidbaghan, A.; Mostafaie, A.; Yazdani, Y.; Mansouri, K. Urtica dioica agglutinin (a plant lectin) has a caspase-dependent apoptosis induction effect on the acute lymphoblastic leukemia cell line. Cell. Mol. Biol. 2020, 66, 121–126. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Ciaramella, V.; Graziani, V.; Papaccio, F.; Della Corte, C.M.; Potenza, N.; Fiorentino, A.; Ciardiello, F.; Morgillo, F. Urtica dioica L. inhibits proliferation and enhances cisplatin cytotoxicity in NSCLC cells via Endoplasmic Reticulum-stress mediated apoptosis. Sci. Rep. 2019, 9, 4986. [Google Scholar] [CrossRef]
- Wagner, H.; Geiger, W.; Boos, G.; Samtleben, R. Studies on the binding of Urtica dioica agglutinin (UDA) and other lectins in an in vitro epidermal growth factor receptor test. Phytomedicine 1995, 1, 287–290. [Google Scholar] [CrossRef]
- Ghasemi, S.; Moradzadeh, M.; Mousavi, S.; Sadeghnia, H.R. Cytotoxic effects of Urtica dioica radix on human colon (HT29) and gastric (MKN45) cancer cells mediated through oxidative and apoptotic mechanisms. Cell. Mol. Biol. 2016, 62, 90–96. [Google Scholar]
- Uyar, A.; Doğan, A.; Yaman, T.; Keleş, Ö.F.; Yener, Z.; Çelik, İ.; Alkan, E.E. The protective role of Urtica dioica seed extract against azoxymethane-induced colon carcinogenesis in rats. Nutr. Cancer 2022, 74, 306–319. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, L.; Zhang, L.; Geng, J.; Zhang, E. ATF4/MYC Regulates MTHFD2 to Promote NSCLC Progression by Mediating Redox Homeostasis. Dis. Markers 2022, 2022, 7527996. [Google Scholar] [CrossRef]
- Zhu, Z.; Leung, G.K.K. More than a metabolic enzyme: MTHFD2 as a novel target for anticancer therapy? Front. Oncol. 2020, 10, 658. [Google Scholar] [CrossRef]
- Zhou, F.; Yuan, Z.; Gong, Y.; Li, L.; Wang, Y.; Wang, X.; Ma, C.; Yang, L.; Liu, Z.; Wang, L. Pharmacological targeting of MTHFD2 suppresses NSCLC via the regulation of ILK signaling pathway. Biomed. Pharmacother. 2023, 161, 114412. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Y.; Lin, C.; Huang, X.; Zhang, F. MTHFD2 facilitates breast cancer cell proliferation via the AKT signaling pathway. Exp. Ther. Med. 2021, 22, 703. [Google Scholar] [CrossRef]
- Zhao, R.; Feng, T.; Gao, L.; Sun, F.; Zhou, Q.; Wang, X.; Liu, J.; Zhang, W.; Wang, M.; Xiong, X. PPFIA4 promotes castration-resistant prostate cancer by enhancing mitochondrial metabolism through MTHFD2. J. Exp. Clin. Cancer Res. 2022, 41, 125. [Google Scholar] [CrossRef]
- Pikman, Y.; Puissant, A.; Alexe, G.; Furman, A.; Chen, L.M.; Frumm, S.M.; Ross, L.; Fenouille, N.; Bassil, C.F.; Lewis, C.A. Targeting MTHFD2 in acute myeloid leukemia. J. Exp. Med. 2016, 213, 1285–1306. [Google Scholar] [CrossRef]
- Hertweck, K.L.; Vikramdeo, K.S.; Galeas, J.N.; Marbut, S.M.; Pramanik, P.; Yunus, F.; Singh, S.; Singh, A.P.; Dasgupta, S. Clinicopathological significance of unraveling mitochondrial pathway alterations in non-small-cell lung cancer. FASEB J. 2023, 37, e23018. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, P.; Li, Q.; Du, J.; Chen, Y.; Wang, Y.; Shi, H.; Wang, Y.; Zhang, H.; Xue, W. The effect of MTHFD2 on the proliferation and migration of colorectal cancer cell lines. OncoTargets Ther. 2019, 12, 6361–6370. [Google Scholar] [CrossRef]
- Gustafsson Sheppard, N.; Jarl, L.; Mahadessian, D.; Strittmatter, L.; Schmidt, A.; Madhusudan, N.; Tegnér, J.; Lundberg, E.K.; Asplund, A.; Jain, M. The folate-coupled enzyme MTHFD2 is a nuclear protein and promotes cell proliferation. Sci. Rep. 2015, 5, 15029. [Google Scholar]
- Li, G.; Wu, J.; Li, L.; Jiang, P. p53 deficiency induces MTHFD2 transcription to promote cell proliferation and restrain DNA damage. Proc. Natl. Acad. Sci. USA 2021, 118, e2019822118. [Google Scholar] [CrossRef]
- Bonagas, N.; Gustafsson, N.M.; Henriksson, M.; Marttila, P.; Gustafsson, R.; Wiita, E.; Borhade, S.; Green, A.C.; Vallin, K.S.; Sarno, A. Pharmacological targeting of MTHFD2 suppresses acute myeloid leukemia by inducing thymidine depletion and replication stress. Nat. Cancer 2022, 3, 156–172. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Liao, M.; Yang, Y.; Wang, Y.; Yuan, Y.; Ouyang, L. Folate-mediated one-carbon metabolism: A targeting strategy in cancer therapy. Drug Discov. Today 2021, 26, 817–825. [Google Scholar] [CrossRef]
- Zhao, L.N.; Björklund, M.; Caldez, M.J.; Zheng, J.; Kaldis, P. Therapeutic targeting of the mitochondrial one-carbon pathway: Perspectives, pitfalls, and potential. Oncogene 2021, 40, 2339–2354. [Google Scholar] [CrossRef]
- Albanesi, J.; Noguera, N.I.; Banella, C.; Colangelo, T.; De Marinis, E.; Leone, S.; Palumbo, O.; Voso, M.T.; Ascenzi, P.; Nervi, C. Transcriptional and metabolic dissection of ATRA-induced granulocytic differentiation in NB4 acute promyelocytic leukemia cells. Cells 2020, 9, 2423. [Google Scholar] [CrossRef]
- Ugur, D.; Gungul, T.B.; Yucel, S.; Ozcivici, E.; Yalcin-Ozuysal, O.; Mese, G. Connexin 32 overexpression increases proliferation, reduces gap junctional intercellular communication, motility and epithelial-to-mesenchymal transition in Hs578T breast cancer cells. J. Cell Commun. Signal. 2022, 16, 361–376. [Google Scholar] [CrossRef]
- Lei, Z.-N.; Teng, Q.-X.; Zhang, W.; Fan, Y.-F.; Wang, J.-Q.; Cai, C.-Y.; Lu, K.W.; Yang, D.-H.; Wurpel, J.N.; Chen, Z.-S. Establishment and characterization of a topotecan resistant non-small cell lung cancer NCI-H460/TPT10 cell line. Front. Cell Dev. Biol. 2020, 8, 607275. [Google Scholar] [CrossRef]
- Rapuano, R.; Riccio, A.; Mercuri, A.; Madera, J.R.; Dallavalle, S.; Moricca, S.; Lupo, A. Proliferation and migration of PC-3 prostate cancer cells is counteracted by PPARγ-cladosporol binding-mediated apoptosis and a decreased lipid biosynthesis and accumulation. Biochem. Pharmacol. 2024, 222, 116097. [Google Scholar] [CrossRef]
- Sodde, V.K.; Lobo, R.; Kumar, N.; Maheshwari, R.; Shreedhara, C.S. Cytotoxic activity of Macrosolen parasiticus (L.) Danser on the growth of breast cancer cell line (MCF-7). Pharmacogn. Mag. 2015, 11, S156–S160. [Google Scholar] [CrossRef]
- Savić, M.; Nikolic, D.V.; Savic-Gajic, M.I.; Kundakovic, D.T.; Stanojkovic, P.T.; Najman, J.S. Chemical composition and biological activity of the plum seed extract. Adv. Technol. 2016, 5, 38–45. [Google Scholar] [CrossRef]
- Popolin, C.P.; Reis, J.P.; Becceneri, A.B.; Graminha, A.E.; Almeida, M.A.; Correa, R.S.; Colina-Vegas, L.A.; Ellena, J.; Batista, A.A.; Cominetti, M.R. Cytotoxicity and anti-tumor effects of new ruthenium complexes on triple negative breast cancer cells. PLoS ONE 2017, 12, e0183275. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, M.; Chen, S.; Li, G.; Zhang, F.; Yang, N.; Huang, L. A cell viability assessment method based on area-normalized impedance spectrum (ANIS). Biosens. Bioelectron. 2018, 110, 193–200. [Google Scholar] [CrossRef]
- Gudipaty, S.A.; Conner, C.M.; Rosenblatt, J.; Montell, D.J. Unconventional ways to live and die: Cell death and survival in development, homeostasis, and disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 311–332. [Google Scholar] [CrossRef]
- Kawai, J.; Ota, M.; Ohki, H.; Toki, T.; Suzuki, M.; Shimada, T.; Matsui, S.; Inoue, H.; Sugihara, C.; Matsuhashi, N. Structure-based design and synthesis of an isozyme-selective MTHFD2 inhibitor with a tricyclic coumarin scaffold. ACS Med. Chem. Lett. 2019, 10, 893–898. [Google Scholar] [CrossRef]
- Westbrook, J.; Feng, Z.; Chen, L.; Yang, H.; Berman, H.M. The protein data bank and structural genomics. Nucleic Acids Res. 2003, 31, 489–491. [Google Scholar] [CrossRef]
- Dassault Systèmes (BIOVIA). Discovery Studio Visualizer; Release 2019; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]
- Alhawarri, M.B.; Al-Thiabat, M.G.; Dubey, A.; Tufail, A.; Fouad, D.; Alrimawi, B.H.; Dayoob, M. ADME profiling, molecular docking, DFT, and MEP analysis reveal cissamaline, cissamanine, and cissamdine from Cissampelos capensis Lf as potential anti-Alzheimer’s agents. RSC Adv. 2024, 14, 9878–9891. [Google Scholar] [CrossRef]
- Alhawarri, M.B.; Olimat, S. Potential Serotonin 5-HT2A Receptor Agonist of Psychoactive Components of Silene undulata Aiton: LC-MS/MS, ADMET, and Molecular Docking Studies. Curr. Pharm. Biotechnol. 2024, 25, 1–15. [Google Scholar] [CrossRef]
- Yunos, N.M.; Al-Thiabat, M.G.; Sallehudin, N.J. Quassinoids from Eurycoma longifolia as Potential Dihydrofolate Reductase Inhibitors: A Computational Study. Curr. Pharm. Biotechnol. 2024, 25, 1–12. [Google Scholar] [CrossRef]
- Alhawarri, M.; Dianita, R.; Rawa, M.; Nogawa, T.; Wahab, H. Potential Anti-Cholinesterase Activity of Bioactive Compounds Extracted from Cassia grandis Lf and Cassia timoriensis DC. Plants 2023, 12, 344. [Google Scholar] [CrossRef]
- Amir Rawa, M.S.; Al-Thiabat, M.G.; Nogawa, T.; Futamura, Y.; Okano, A.; Wahab, H.A. Naturally Occurring 8ß, 13ß-kaur-15-en-17-al and Anti-Malarial Activity from Podocarpus polystachyus Leaves. Pharmaceuticals 2022, 15, 902. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A tool to obtain structural guidance in biocatalytic investigations. In Protein Engineering: Methods and Protocols; Humana Press: New York, NY, USA, 2018; pp. 43–67. [Google Scholar]
- Shalayel, M.H.F.; Al-Mazaideh, G.M.; Alanezi, A.A.; Almuqati, A.F.; Alotaibi, M. Diosgenin and Monohydroxy Spirostanol from Prunus amygdalus var amara Seeds as Potential Suppressors of EGFR and HER2 Tyrosine Kinases: A Computational Approach. Pharmaceuticals 2023, 16, 704. [Google Scholar] [CrossRef]
- Abdelbagi, M.E.; Al-Mazaideh, G.M.; Ahmed, A.E.; Al-Rimawi, F.; Ayyal Salman, H.; Almutairi, A.; Abuilaiwi, F.A.; Wedian, F. Exploring Securigera securidaca Seeds as a Source of Potential CDK1 Inhibitors: Identification of Hippeastrine and Naringenin as Promising Hit Candidates. Processes 2023, 11, 1478. [Google Scholar] [CrossRef]
- Gordon, J.C.; Myers, J.B.; Folta, T.; Shoja, V.; Heath, L.S.; Onufriev, A. H++: A server for estimating p K as and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005, 33, W368–W371. [Google Scholar] [CrossRef]
- Norgan, A.P.; Coffman, P.K.; Kocher, J.-P.A.; Katzmann, D.J.; Sosa, C.P. Multilevel parallelization of AutoDock 4.2. J. Cheminformatics 2011, 3, 12. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Bryant, S.H. PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009, 37, W623–W633. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Dar, S.A.; Yousuf, A.; Ganai, F.A.; Sharma, P.; Kumar, N.; Singh, R. Bioassay guided isolation and identification of anti-inflammatory and anti-microbial compounds from Urtica dioica L.(Urticaceae) leaves. Afr. J. Biotechnol. 2012, 11, 12910–12920. [Google Scholar]
- Asgarpanah, J.; Mohajerani, R. Phytochemistry and pharmacologic properties of Urtica dioica L. J. Med. Plants Res. 2012, 6, 5714–5719. [Google Scholar]
- Gülçin, I.; Küfrevioǧlu, Ö.İ.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201. [Google Scholar]
- Krajewska, A.; Mietlińska, K. Determining the parameters of the Stinging Nettle (Urtica dioica L.) hydrolate distillation process. Molecules 2022, 27, 3912. [Google Scholar] [CrossRef]
- Al-Tameme, H.J.; Hadi, M.Y.; Hameed, I.H. Phytochemical analysis of Urtica dioica leaves by fourier-transform infrared spectroscopy and gas chromatography-mass spectrometry. J. Pharmacogn. Phytother. 2015, 7, 238–252. [Google Scholar]
- Otles, S.; Yalcin, B. Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci. World J. 2012, 2012, 564367. [Google Scholar] [CrossRef]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Common nettle (Urtica dioica L.) as an active filler of natural rubber biocomposites. Materials 2021, 14, 1616. [Google Scholar] [CrossRef]
- Silva, J.d.O.; Dias, M.I.; Barros, L.; Tanamati, A.A.; Ferreira, I.C.; Amaral, J.S. Bioactive and nutritional characterization of stinging nettle (Urtica dioica L.) harvested in Portugal. In Proceedings of the XX EuroFoodChem Conference, Porto, Portugal, 17–19 June 2019. [Google Scholar]
- Pinelli, P.; Ieri, F.; Vignolini, P.; Bacci, L.; Baronti, S.; Romani, A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. J. Agric. Food Chem. 2008, 56, 9127–9132. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Abu-Reidah, I.M.; Altamimi, A.; Jaradat, N. Hydroethanolic extract of Urtica dioica L.(stinging nettle) leaves as disaccharidase inhibitor and glucose transport in Caco-2 hinderer. Molecules 2022, 27, 8872. [Google Scholar] [CrossRef]
- Levy, A.; Sivanesan, D.; Murugan, R.K.; Jornadal, J.; Quiñónez, Y.B.; Jaffe, M.; Rathinavelu, A. Urtica dioica Induces Cytotoxicity in Human Prostate Carcinoma LNCaP Cells: Involvement of Oxidative Stress, Mitochondrial Depolarization and Apoptosis. Trop. J. Pharm. Res. 2014, 13, 711–717. [Google Scholar] [CrossRef]
- Lim, J.N.; Oh, J.J.; Wang, T.; Lee, J.S.; Kim, S.H.; Kim, Y.J.; Lee, H.G. trans-11 18:1 vaccenic acid (TVA) has a direct anti-carcinogenic effect on MCF-7 human mammary adenocarcinoma cells. Nutrients 2014, 6, 627–636. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; Alqarni, A.O.; Ahmed Alshrahili, M.; Abou-Salim, M.A.; Alqahtani, M.N.; Mushtaq, S.; Sadiq, A.; Jan, M.S. GC-MS Analysis and Various In Vitro and In Vivo Pharmacological Potential of Habenaria plantaginea Lindl. Evid.-Based Complement. Altern. Med. 2022, 2022, 7921408. [Google Scholar] [CrossRef]
- Silva, G.; Marques, J.N.J.; Linhares, E.P.M.; Bonora, C.M.; Costa, É.T.; Saraiva, M.F. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem. Biol. Interact. 2022, 362, 109994. [Google Scholar] [CrossRef]
- Yao, S.; Wang, X.; Li, C.; Zhao, T.; Jin, H.; Fang, W. Kaempferol inhibits cell proliferation and glycolysis in esophagus squamous cell carcinoma via targeting EGFR signaling pathway. Tumor Biol. 2016, 37, 10247–10256. [Google Scholar] [CrossRef]
- Ziaee, A.; Zamansoltani, F.; Nassiri-Asl, M.; Abbasi, E. Effects of rutin on lipid profile in hypercholesterolaemic rats. Basic Clin. Pharmacol. Toxicol. 2009, 104, 253–258. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef]
- Naqvi, A.A.; Mohammad, T.; Hasan, G.M.; Hassan, M.I. Advancements in docking and molecular dynamics simulations towards ligand-receptor interactions and structure-function relationships. Curr. Top. Med. Chem. 2018, 18, 1755–1768. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Hou, X.; Du, J.; Zhang, J.; Du, L.; Fang, H.; Li, M. How to improve docking accuracy of AutoDock4. 2: A case study using different electrostatic potentials. J. Chem. Inf. Model. 2013, 53, 188–200. [Google Scholar] [CrossRef]
- Yunos, N.M.; Wahab, H.A.; Al-Thiabat, M.G.; Sallehudin, N.J.; Jauri, M.H. In Vitro and In Silico Analysis of the Anticancer Effects of Eurycomanone and Eurycomalactone from Eurycoma longifolia. Plants 2023, 12, 2827. [Google Scholar] [CrossRef]
- Larue, L.; Kenzhebayeva, B.; Al-Thiabat, M.G.; Jouan–Hureaux, V.; Mohd–Gazzali, A.; Wahab, H.A.; Boura, C.; Yeligbayeva, G.; Nakan, U.; Frochot, C. tLyp–1: A peptide suitable to target NRP–1 receptor. Bioorg. Chem. 2023, 130, 106200. [Google Scholar] [CrossRef]
- Alidmat, M.M.; Khairuddean, M.; Kamal, N.N.S.N.M.; Muhammad, M.; Wahab, H.A.; Althiabat, M.G.; Alhawarri, M.B. Synthesis, Characterization, Molecular Docking and Cytotoxicity Evaluation of New Thienyl Chalcone Derivatives against Breast Cancer Cells. Syst. Rev. Pharm. 2022, 13, 1–11. [Google Scholar]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in food: Cancer prevention and apoptosis induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef]
- Imran, M.; Insaf, A.; Hasan, N.; Sugandhi, V.V.; Shrestha, D.; Paudel, K.R.; Jha, S.K.; Hansbro, P.M.; Dua, K.; Devkota, H.P. Exploring the Remarkable Chemotherapeutic Potential of Polyphenolic Antioxidants in Battling Various Forms of Cancer. Molecules 2023, 28, 3475. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Sun, J.; Liu, R.H. Cranberry phytochemical extracts induce cell cycle arrest and apoptosis in human MCF-7 breast cancer cells. Cancer Lett. 2006, 241, 124–134. [Google Scholar] [CrossRef]
- Kar, S.; Roy, K.; Leszczynski, J. In silico tools and software to predict ADMET of new drug candidates. In In Silico Methods for Predicting Drug Toxicity; Humana: New York, NY, USA, 2022; pp. 85–115. [Google Scholar]
- Kar, S.; Leszczynski, J. Open access in silico tools to predict the ADMET profiling of drug candidates. Expert Opin. Drug Discov. 2020, 15, 1473–1487. [Google Scholar] [CrossRef]




| Peak No. | Component Name | RT (min) | Area % |
|---|---|---|---|
| 1 | Hexadecane | 4.708 | 0.832 |
| 2 | Geraniol | 5.236 | 3.101 |
| 3 | Geranyl Acetate | 6.483 | 0.724 |
| 4 | β-cis-Caryophyllene | 6.612 | 8.309 |
| 5 | Isoledene | 7.237 | 4.574 |
| 6 | Hexyl 2-[(4-butylbenzoyl) amino] propanoate | 7.483 | 5.72 |
| 7 | Isocadinene | 7.823 | 3.387 |
| 8 | Hexahydrofarnesyl acetone | 8.033 | 4.089 |
| 9 | 3,7,11,15-Tetramethyl-2-Hexadecen-1-ol | 8.232 | 9.043 |
| 10 | Methyl hexadecanoate | 8.734 | 14.16 |
| 11 | Ethyl hexadecanoate | 9.173 | 5.144 |
| 12 | Pentadecanoic Acid | 9.468 | 0.866 |
| 13 | Methyl (Z)-octadec-13-enoate | 10.72 | 0.527 |
| 14 | Phytol | 12.32 | 1.332 |
| 15 | Cis-Vaccenic Acid | 12.74 | 12.04 |
| 16 | Octadecanal | 15.47 | 4.871 |
| 17 | Oleyl acetate | 16.3 | 8.321 |
| 18 | γ-Tocopherol | 17.5 | 4.651 |
| 19 | β-Sitosteryl stearate | 20.51 | 8.301 |
| Anticancer Impacts | Time (Hours) | Cancer Cell Line | |||
|---|---|---|---|---|---|
| NB4 | Hs578T | H460 | PC-3 | ||
| IC50 (µg/mL) | 12 | 124.20 | 32.60 | 246.50 | 34.00 |
| 24 | 76.50 | 27.06 | 191.33 | 31.70 | |
| 48 | 47.80 | 25.11 | 148.70 | 22.57 | |
| 72 | 41.00 | 29.33 | 155.60 | 40.33 | |
| Cell Viability % | 12 | 82.00 | 83.50 | 82.08 | 80.20 |
| 24 | 70.07 | 76.90 | 78.51 | 76.50 | |
| 48 | 64.60 | 65.88 | 71.00 | 68.90 | |
| 72 | 61.80 | 81.02 | 74.30 | 78.90 | |
| Cell Viability % Untreated (Control) | 12 | 98.44 | 98.00 | 98 | 98.78 |
| 24 | 98.44 | 98.00 | 98 | 98.66 | |
| 48 | 98.44 | 98.00 | 98 | 98.55 | |
| 72 | 98.44 | 98.00 | 98 | 98.9 | |
| Identified Compound | *ΔGbind (kcal/mol) |
|---|---|
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | −4.93 |
| β-cis-Caryophyllene | −6.84 |
| β-Sitosteryl stearate | −6.84 |
| γ-Tocopherol | −7.06 |
| Caffeic acid | −6.76 |
| Catechin | −9.31 |
| Cis-Vaccenic Acid | −3.49 |
| Ethyl hexadecanoate | −6.06 |
| Ferulic acid | −6.55 |
| Gallic acid | −6.72 |
| Geraniol | −4.93 |
| Geranyl Acetate | −6.85 |
| Hexahydrofarnesyl acetone | −5.91 |
| Hexyl 2-[(4-butylbenzoyl) amino] propanoate | −7.12 |
| Isocadinene | −6.42 |
| Isoledene | −7.06 |
| Kaempferol | −9.44 |
| Methyl (Z)-octadec-13-enoate | −4.87 |
| Methyl hexadecanoate | −6.11 |
| Octadecanal | −2.68 |
| Oleyl acetate | −2.53 |
| Pentadecanoic Acid | −5.99 |
| Phytol | −5.25 |
| Quercetin-3-O-rutinoside | −9.82 |
| Quinic acid | −6.73 |
| Co-crystalized ligand (control) | −9.89 |
| Property | Model Name | Predicted Value | ||
|---|---|---|---|---|
| Catechin | Kaempferol | Quercetin-3-O-rutinoside | ||
| Drug-Likeness | *Lipinski Rule | Accepted | Accepted | Rejected |
| *Pfizer Rule | Accepted | Accepted | Accepted | |
| *Golden Triangle | Accepted | Accepted | Rejected | |
| *GSK Rule | Accepted | Accepted | Rejected | |
| Absorption | Papp (Caco-2 Permeability) cm/s | −4.65 | −4.97 | −6.33 |
| MDCK Permeability (cm/s) | 5 × 10−6 | 9 × 10−6 | 30 × 10−6 | |
| HIA (Human Intestinal Absorption) | 0.035 | 0.004 | 0.920 | |
| Distribution | PPB (Plasma Protein Binding) % | 89.23 | 97.86 | 83.81 |
| BBB (Blood–Brain Barrier) | Cannot cross | Cannot cross | Can penetrate | |
| VD (Volume Distribution) L/kg | 0.66 | 0.52 | 0.75 | |
| Fu (Fraction unbound in plasms) % | 12.91 | 4.41 | 20.86 | |
| Metabolism | CYP1A2 substrate | No | No | No |
| CYP2C19 substrate | No | No | No | |
| CYP2C9 substrate | No | No | Yes | |
| CYP2D6 substrate | No | No | No | |
| CYP1A2 inhibitor | No | No | Yes | |
| CYP2C19 inhibitor | No | No | Yes | |
| CYP2C9 inhibitor | No | No | No | |
| CYP3A4 inhibitor | No | No | No | |
| Excretion | *CL (Clearance Rate) mL/min/kg | 17.30 | 6.86 | 1.34 |
| T ½ (Half-Lifetime) hr | 0.89 | 0.91 | 0.52 | |
| Toxicity | H-HT (Human Hepatotoxicity) | - | - | - |
| AMES (Ames Mutagenicity) | - | - | + | |
| Carcinogenicity | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, S.O. Identification and Absorption–Distribution–Metabolism–Excretion–Toxicity Prediction of Potential MTHFD2 Enzyme Inhibitors from Urtica dioica Ethanolic Leaf Extract. Processes 2024, 12, 1177. https://doi.org/10.3390/pr12061177
Alshammari SO. Identification and Absorption–Distribution–Metabolism–Excretion–Toxicity Prediction of Potential MTHFD2 Enzyme Inhibitors from Urtica dioica Ethanolic Leaf Extract. Processes. 2024; 12(6):1177. https://doi.org/10.3390/pr12061177
Chicago/Turabian StyleAlshammari, Shifaa O. 2024. "Identification and Absorption–Distribution–Metabolism–Excretion–Toxicity Prediction of Potential MTHFD2 Enzyme Inhibitors from Urtica dioica Ethanolic Leaf Extract" Processes 12, no. 6: 1177. https://doi.org/10.3390/pr12061177
APA StyleAlshammari, S. O. (2024). Identification and Absorption–Distribution–Metabolism–Excretion–Toxicity Prediction of Potential MTHFD2 Enzyme Inhibitors from Urtica dioica Ethanolic Leaf Extract. Processes, 12(6), 1177. https://doi.org/10.3390/pr12061177







