Analysis of the Effects of Structural Parameters on the Thermal Performance and System Stability of Ventilation Air Methane-Fueled Reverse-Flow Oxidation Reactors
Abstract
:1. Introduction
2. Numerical Model
2.1. Geometric Model of the Computational Domain
2.2. Numerical Scheme
2.3. Description of the Critical Evaluation Indices
2.4. Mesh Independence Analysis
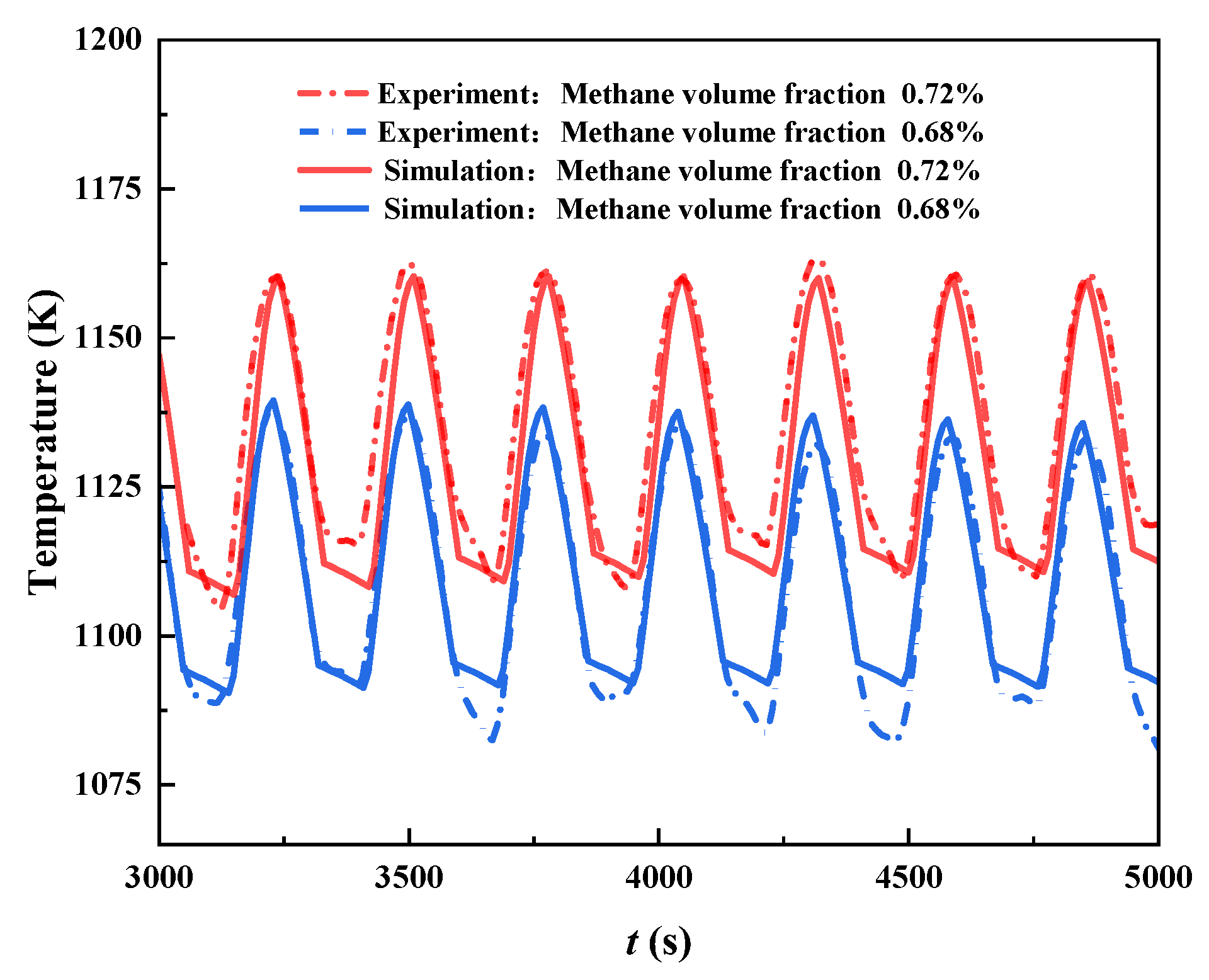
2.5. Model Validation
3. Results and Discussion
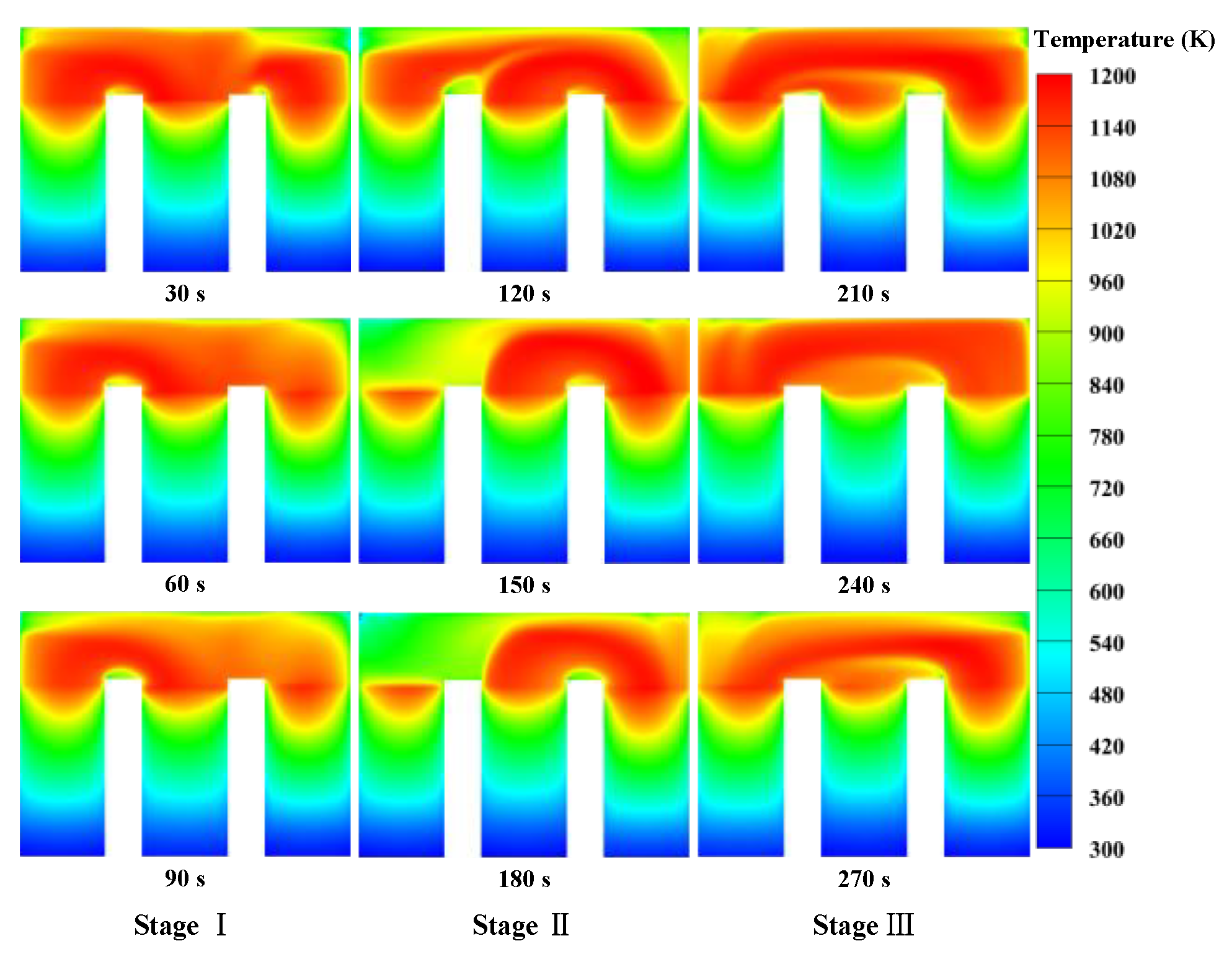
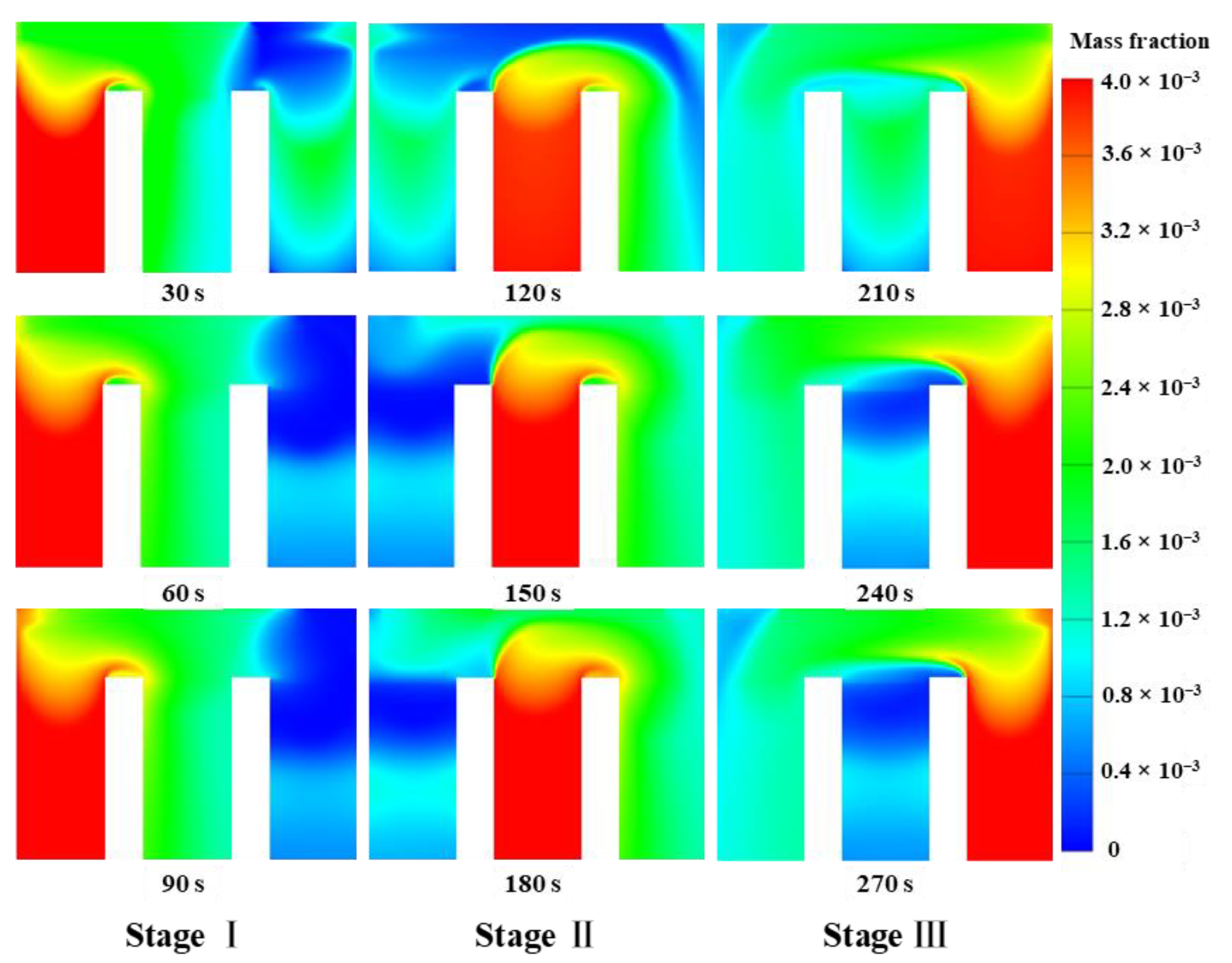
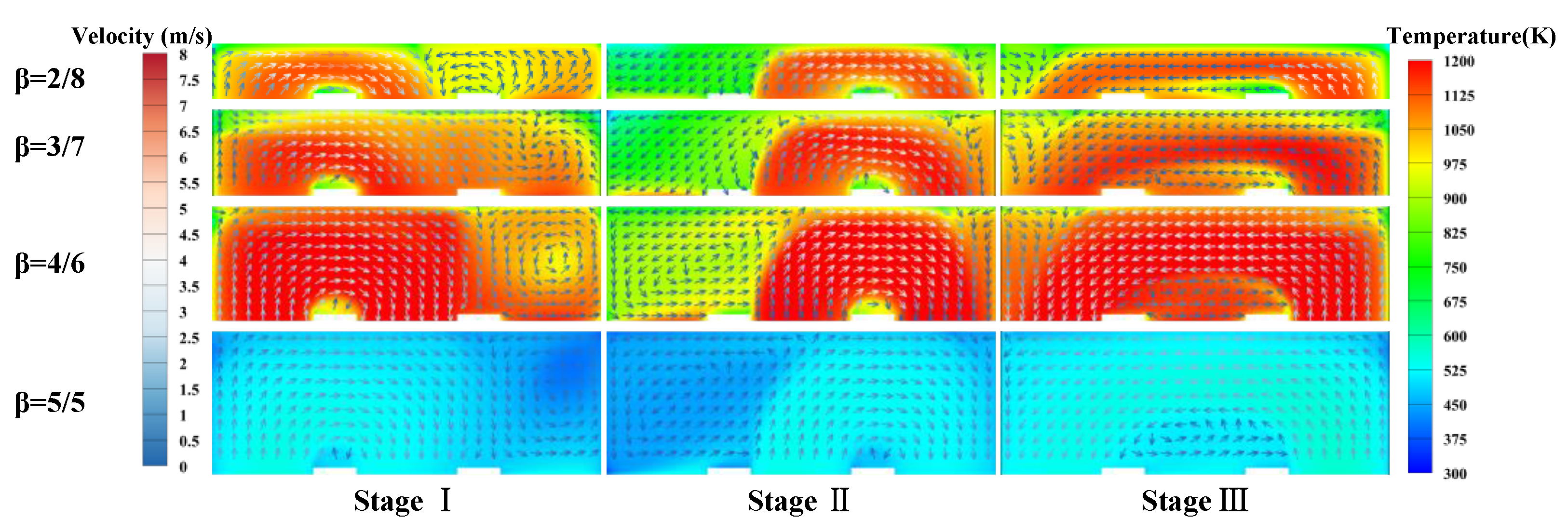
3.1. Basic Combustion Performance of the Thermal Reversal Oxidation Reactor
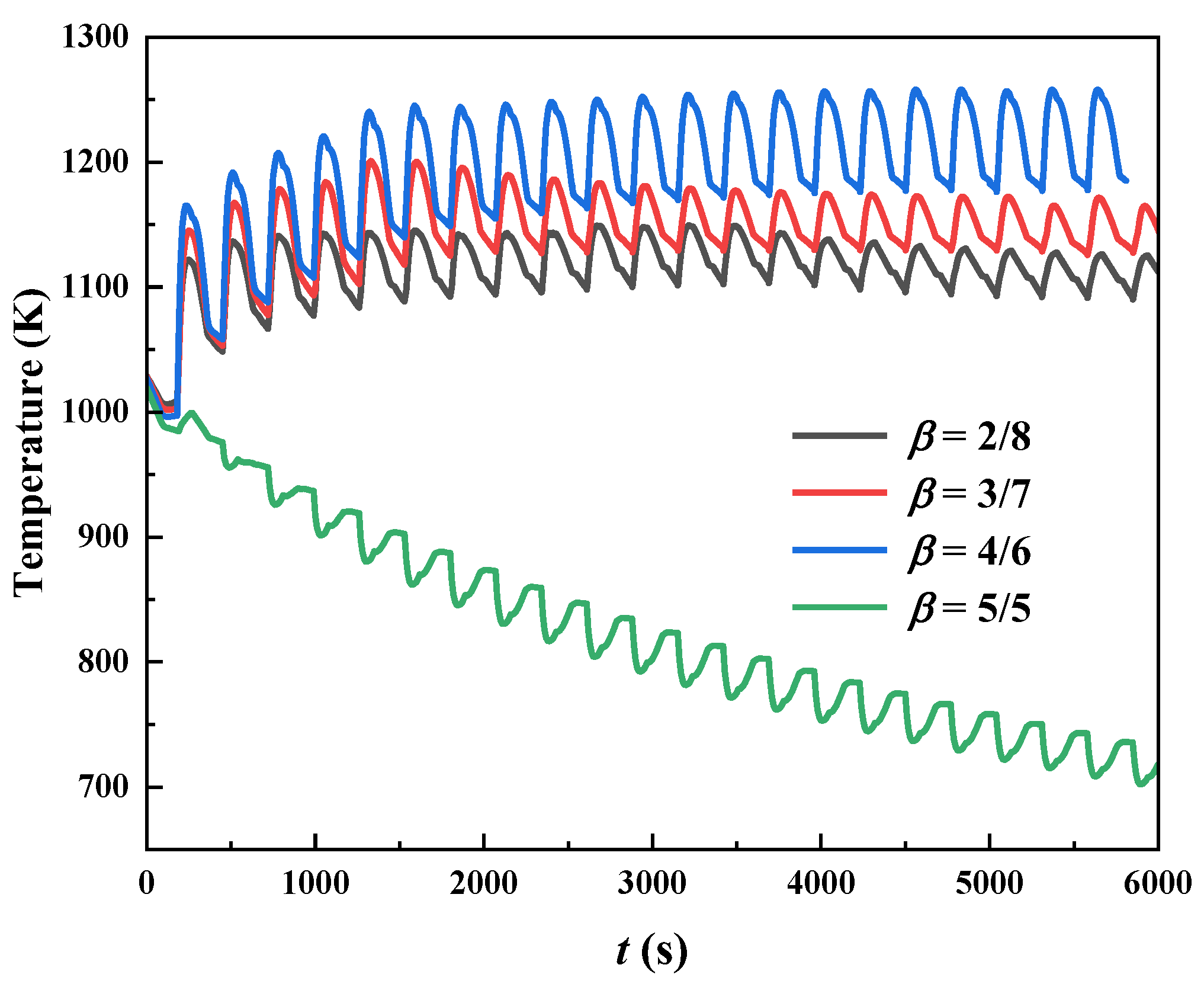
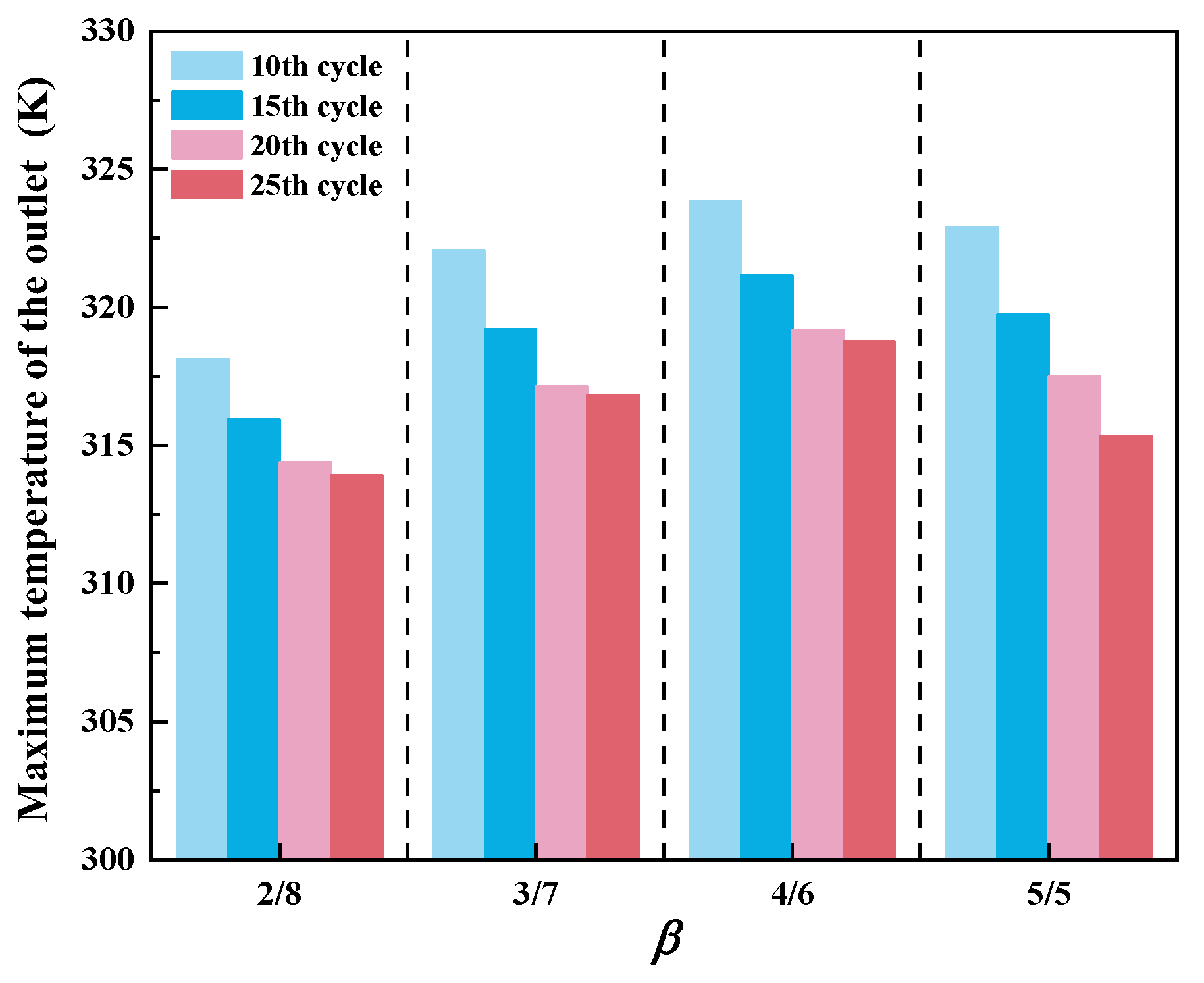
3.2. Effect of Chamber/Regenerator Height Ratio
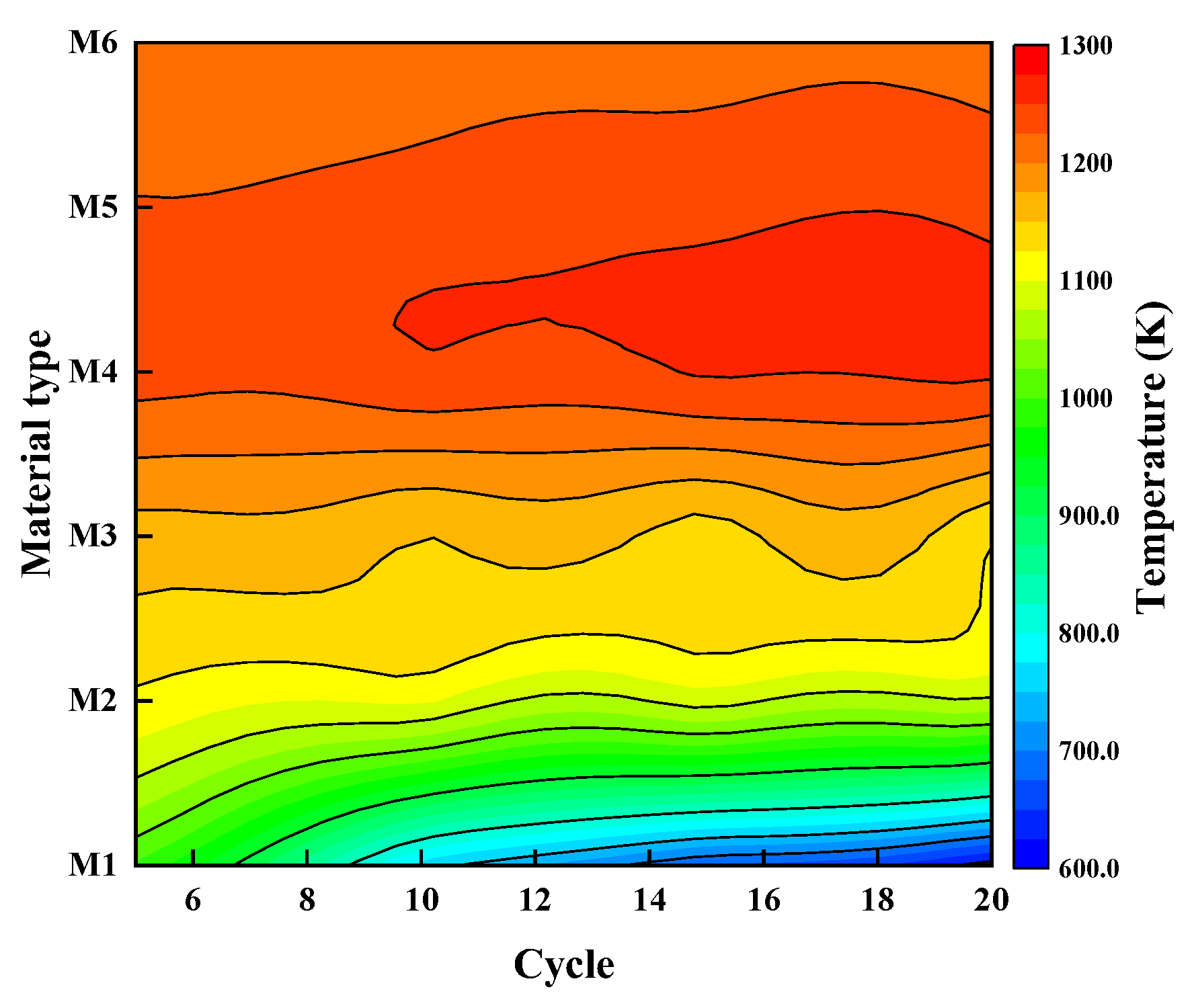
3.3. Effect of the Heat Storage Material in the Regenerator
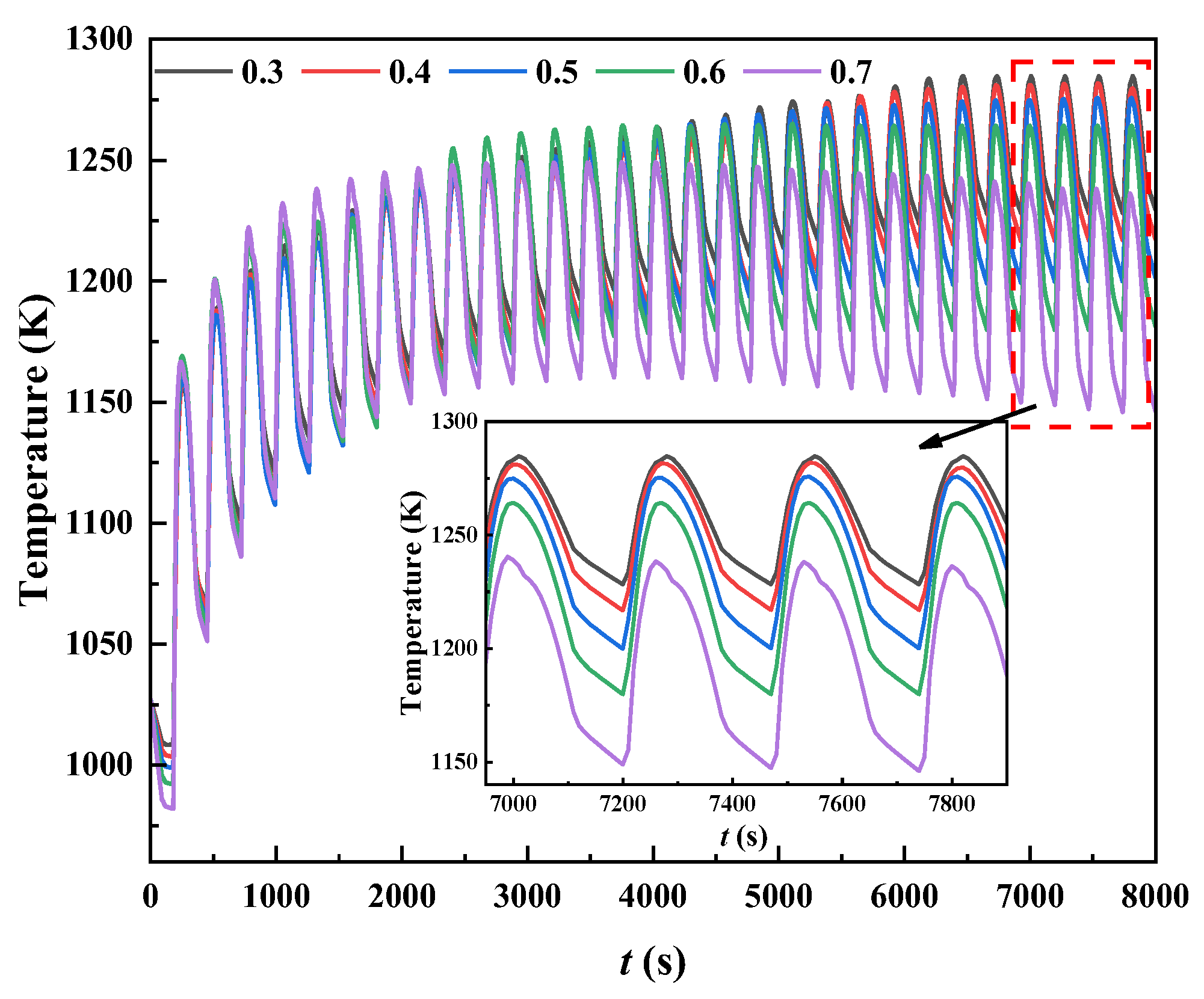
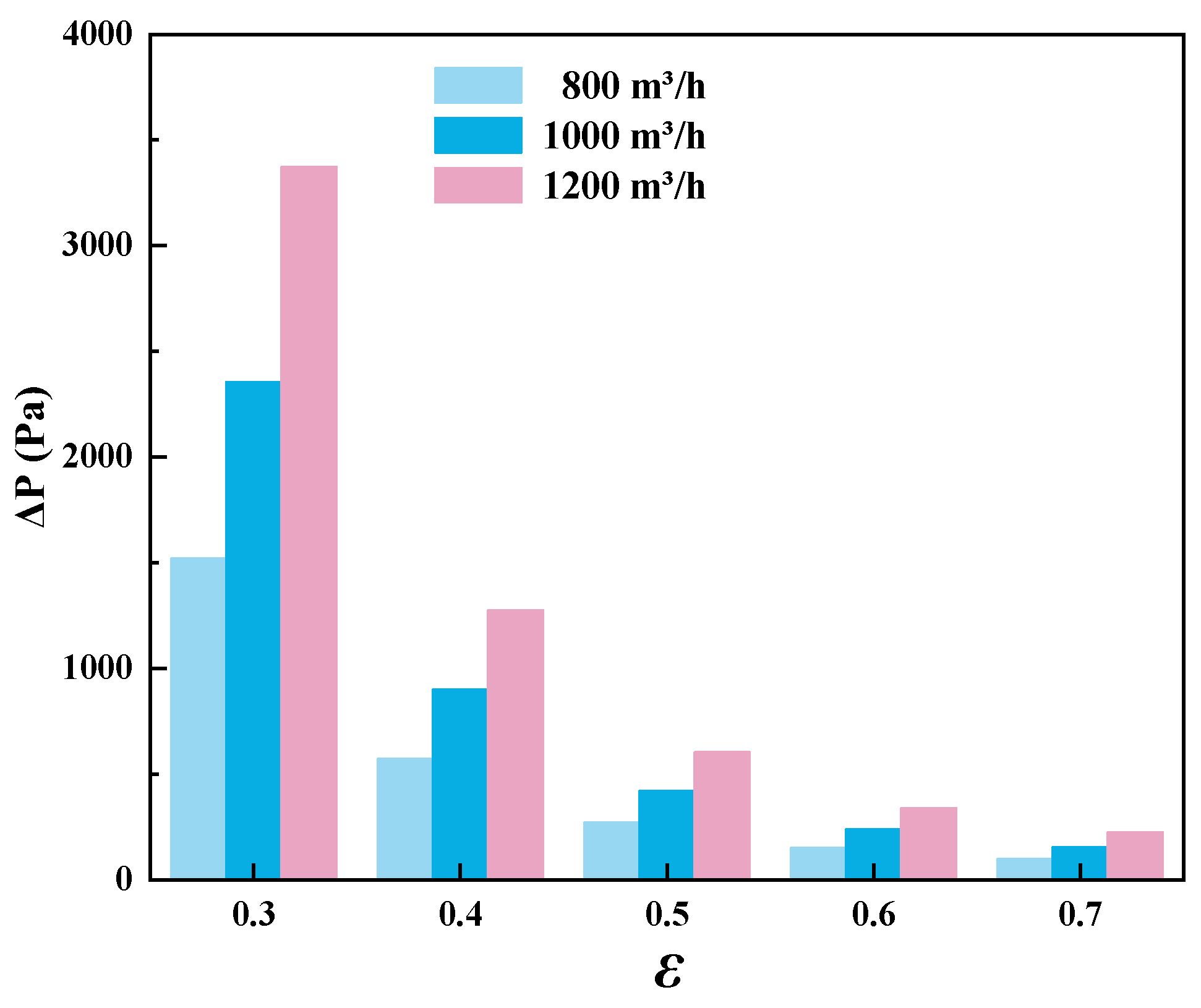
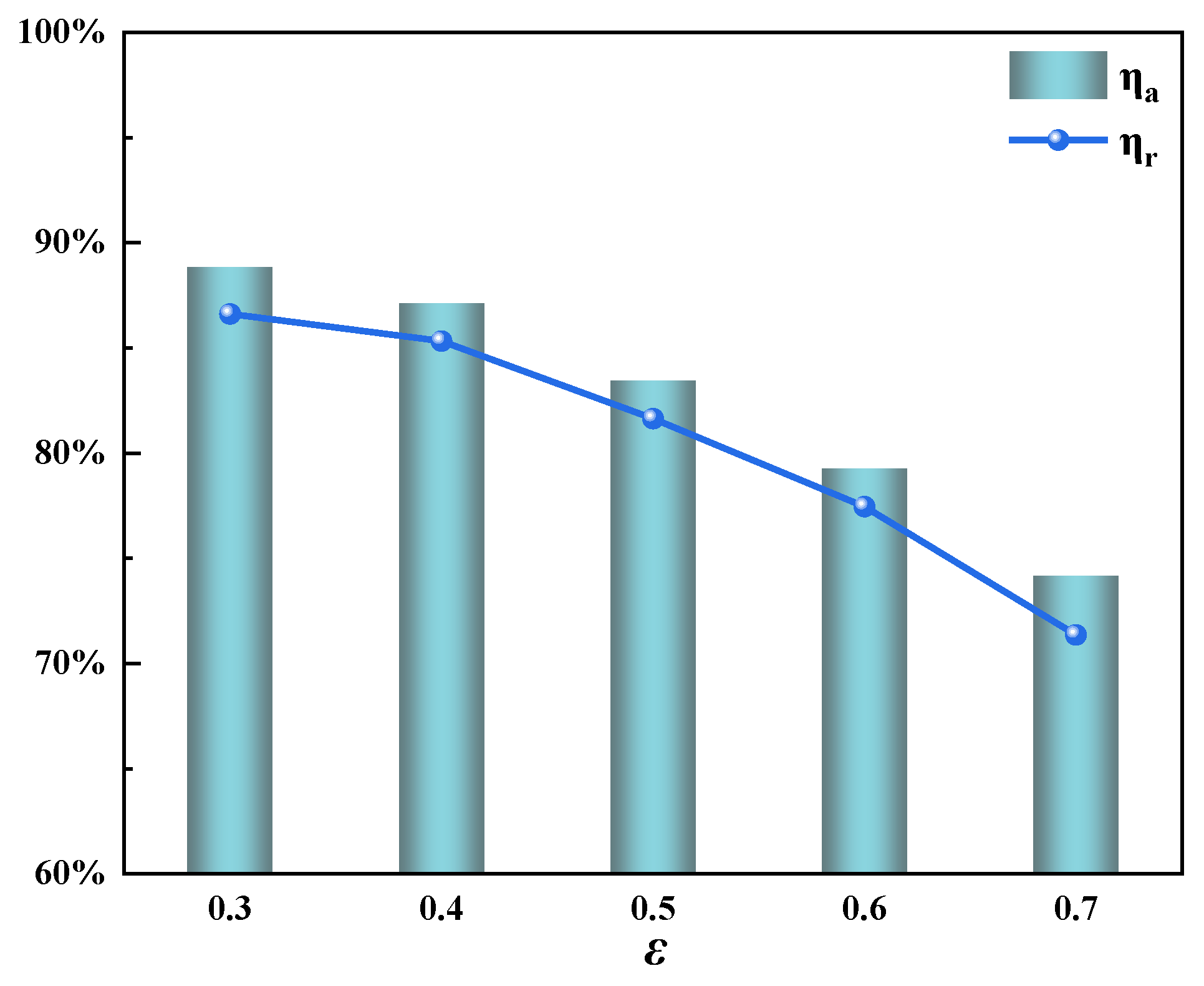
3.4. Effect of the Porosity in the Regenerator
4. Conclusions
- (1)
- The combustion characteristics of each stage during a cycle exhibit significant differences. There could be a discontinuity in the temperature distribution when the gas is introduced from Regenerator 2 and swept from Regenerator 3 because of the different flow directions.
- (2)
- The regenerator temperature presents a non-monotonic change trend with the height ratio. The highest temperature tends to occur at the ratio of 4/6, due to the relatively high methane conversion rate, signifying a much stronger ability to resist system destabilization. However, below this value, the combustion system may become unstable.
- (3)
- Among the heat storage materials investigated, the regenerator with mullite inserted is associated with the highest temperature regardless of the methane volume fraction. With the other materials, the temperature gradually decreases, and the system becomes unstable as SiC is adopted, indicating the importance of choosing a proper thermal diffusivity.
- (4)
- The porosity of the heat storage materials was shown to have little effect on the system’s stability. Decreasing the porosity can effectively reduce the oscillation amplitude of the regenerator temperature, but it also results in greater pressure losses.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karakurt, I.; Aydin, G.; Aydiner, K. Mine ventilation air methane as a sustainable energy source. Renew. Sustain. Energy Rev. 2011, 15, 1042–1049. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Pan, M.; Lv, J.; Lu, K.; Ye, Y.; Tan, D. Utilization of hydrogen-diesel blends for the improvements of a dual-fuel engine based on the improved Taguchi methodology. Energy 2024, 292, 130474. [Google Scholar] [CrossRef]
- Tan, D.; Dong, R.; Zhang, Z.; Zhang, B.; Jiang, F.; Ye, Y.; Li, D.; Liu, H. Multi-objective impact mechanism on the performance characteristic for a diesel particulate filter by RF-NSGA III-TOPSIS during soot loading. Energy 2024, 286, 129582. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Shi, Y.; Zhang, Y.; Shi, J.; Sun, P. Strategies for heat recovery in a reverse flow reactor designed to lessen coal mine methane emissions test. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 8665–8685. [Google Scholar] [CrossRef]
- Zhang, Y.; Doroodchi, E.; Moghtaderi, B. Utilization of ventilation air methane as an oxidizing agent in chemical looping combustion. Energy Convers. Manag. 2014, 85, 839–847. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Gutmark, E. Overview of fundamental kinetic mechanisms and emission mitigation in ammonia combustion. Chem. Eng. J. 2023, 458, 141391. [Google Scholar] [CrossRef]
- Ostrowski, P.; Pronobis, M.; Remiorz, L. Mine emissions reduction installations. Appl. Therm. Eng. 2015, 84, 390–398. [Google Scholar] [CrossRef]
- Su, S.; Beath, A.; Guo, H.; Mallett, C. An assessment of mine methane mitigation and utilisation technologies. Prog. Energy Combust. Sci. 2005, 31, 123–170. [Google Scholar] [CrossRef]
- You, C.; Xu, X. Utilization of ventilation air methane as a supplementary fuel at a circulating fluidized bed combustion boiler. Environ. Sci. Technol. 2008, 42, 2590–2593. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.; Li, Y.-R. Numerical simulation of thermodynamic performance in a honeycomb ceramic channel. In Proceedings of the 3rd International Symposium on Mine Safety Science and Engineering, Montreal, QC, Canada, 13–19 August 2016; pp. 219–224. [Google Scholar]
- Marín, P.; Ho, W.; Ordóñez, S.; Díez, F.V. Demonstration of a control system for combustion of lean hydrocarbon emissions in a reverse flow reactor. Chem. Eng. Sci. 2010, 65, 54–59. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Qin, Z.; Zhu, H.; Wu, J.; Wang, R.; Lei, L.; Chen, J.; Dong, M.; Fan, W. Demonstration of mitigation and utilization of ventilation air methane in a pilot scale catalytic reverse flow reactor. Fuel Process. Technol. 2017, 160, 102–108. [Google Scholar] [CrossRef]
- Zhou, F.; Xia, T.; Wang, X.; Zhang, Y.; Sun, Y.; Liu, J. Recent developments in coal mine methane extraction and utilization in China: A review. J. Nat. Gas Sci. Eng. 2016, 31, 437–458. [Google Scholar] [CrossRef]
- Li, Z.; Qin, Z.; Zhang, Y.; Wu, Z.; Wang, H.; Li, S.; Dong, M.; Fan, W.; Wang, J. A logic-based controller for the mitigation of ventilation air methane in a catalytic flow reversal reactor. Front. Chem. Sci. Eng. 2013, 7, 347–356. [Google Scholar] [CrossRef]
- Su, S.; Yu, X. A 25 kWe low concentration methane catalytic combustion gas turbine prototype unit. Energy 2015, 79, 428–438. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A. Catalytic or thermal reversed flow combustion of coal mine ventilation air methane: What is better choice and when? Chem. Eng. J. 2014, 238, 78–85. [Google Scholar] [CrossRef]
- Gosiewski, K.; Matros, Y.S.; Warmuzinski, K.; Jaschik, M.; Tanczyk, M. Homogeneous vs. catalytic combustion of lean methane—Air mixtures in reverse-flow reactors. Chem. Eng. Sci. 2008, 63, 5010–5019. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Y.; Gao, Z. Heat extraction characteristic of embedded heat exchanger in honeycomb ceramic packed bed. Int. Commun. Heat Mass Transf. 2012, 39, 1526–1534. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Y.; Gao, Z. Influence of packed honeycomb ceramic on heat extraction rate of packed bed embedded heat exchanger and heat transfer modes in heat transfer process. Int. Commun. Heat Mass Transf. 2015, 65, 76–81. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Cao, Q.; Wang, C.A.; Che, D. Homogeneous combustion of fuel ultra-lean methane–air mixtures: Experimental study and simplified reaction mechanism. Energy Fuels 2011, 25, 3437–3445. [Google Scholar] [CrossRef]
- Shahamiri, S.; Wierzba, I. Modeling catalytic oxidation of lean mixtures of methane–air in a packed-bed reactor. Chem. Eng. J. 2009, 149, 102–109. [Google Scholar] [CrossRef]
- Chaouki, J.; Guy, C.; Sapundzhiev, C.; Kusohorsky, D.; Klvana, D. Combustion of methane in a cyclic catalytic reactor. Ind. Eng. Chem. Res. 1994, 33, 2957–2963. [Google Scholar] [CrossRef]
- Marín, P.; Díez, F.V.; Ordóñez, S. Reverse flow reactors as sustainable devices for performing exothermic reactions: Applications and engineering aspects. Chem. Eng. Process. Process Intensif. 2019, 135, 175–189. [Google Scholar] [CrossRef]
- Qi, X.; Liu, Y.; Xu, H.; Liu, Z.; Liu, R. Modeling thermal oxidation of coal mine methane in a non-catalytic reverse-flow reactor. J. Mech. Eng. Stroj. Vestn. 2014, 60, 495–505. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, Y.; Liu, Y.; Sun, P.; Mao, M. Experimental and simulation research on heat extraction characteristics from a pilot-scale reverse flow reactor for low-grade energy. Appl. Therm. Eng. 2023, 220, 119761. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A.; Jaschik, M. Energy recovery from ventilation air methane via reverse-flow reactors. Energy 2015, 92, 13–23. [Google Scholar] [CrossRef]
- Banerjee, A.; Saveliev, A.V. High temperature heat extraction from counterflow porous burner. Int. J. Heat Mass Transf. 2018, 127, 436–443. [Google Scholar] [CrossRef]
- Lan, B.; Li, Y.-R. Numerical study on thermal oxidation of lean coal mine methane in a thermal flow-reversal reactor. Chem. Eng. J. 2018, 351, 922–929. [Google Scholar] [CrossRef]
- Deng, H.X.; Wen, Y.X.; Xiao, Q.; Xiao, Y.H. Parametric study of thermal flow-reversal reactor for ventilation air methane oxidation. Appl. Mech. Mater. 2013, 372, 406–409. [Google Scholar] [CrossRef]
- Chen, G.; Chi, Y.; Yan, J.-H.; Ni, M.-J. Effect of periodic variation of the inlet concentration on the performance of reverse flow reactors. Ind. Eng. Chem. Res. 2011, 50, 5448–5458. [Google Scholar] [CrossRef]
- Mao, M.; Liu, Y.; Shi, J.; Li, C.; Zhang, Q. Experimental study on control of flame inclination in a thermal flow reversal reactor with extra lean premixed methane/air intake. Fuel 2022, 318, 123722. [Google Scholar] [CrossRef]
- Mao, M.; Shi, J.; Liu, Y.; Gao, M.; Chen, Q. Experimental investigation on control of temperature asymmetry and nonuniformity in a pilot scale thermal flow reversal reactor. Appl. Therm. Eng. 2020, 175, 115375. [Google Scholar] [CrossRef]
- Marín, P.; Ordóñez, S.; Díez, F.V. Monoliths as suitable catalysts for reverse-flow combustors: Modeling and experimental validation. AIChE J. 2010, 56, 3162–3173. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Y.; Zhou, Y.; Sun, P.; Mao, M.; Zhang, Y. Study on dynamic heat extraction characteristics of heat exchanger tube embedded in thermal flow reverse reactor for heat recovery. Process Saf. Environ. Prot. 2022, 162, 846–858. [Google Scholar] [CrossRef]
- Litto, R.; Hayes, R.; Sapoundjiev, H.; Fuxman, A.; Forbes, F.; Liu, B.; Bertrand, F. Optimization of a flow reversal reactor for the catalytic combustion of lean methane mixtures. Catal. Today 2006, 117, 536–542. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, M.; Li, H.; Liu, Y.; Shang, Q. Study on thermal stress of honeycomb ceramic regenerators with different parameters. Strength Mater. 2014, 46, 256–261. [Google Scholar] [CrossRef]
- Rao, K.; Liu, Y.; An, T.; Shen, Y. Numerical analysis of oxidation performance of basalt fiber bundle thermal flow-reversal reactor. Appl. Therm. Eng. 2022, 215, 118886. [Google Scholar]
- Zazhigalov, S.; Elyshev, A.; Zagoruiko, A. Catalytic reverse-flow oxidation process in reactors of various designs: Axial, side and tangential gas inlet. Chem. Eng. Res. Des. 2023, 191, 364–374. [Google Scholar] [CrossRef]
- Kushwaha, A.; Poirier, M.; Hayes, R.; Sapoundjiev, H. Heat extraction from a flow reversal reactor in lean methane combustion. Chem. Eng. Res. Des. 2005, 83, 205–213. [Google Scholar] [CrossRef]
- Mei, H.; Li, C.; Liu, H.; Ji, S. Simulation of catalytic combustion of methane in a monolith honeycomb reactor. Chin. J. Chem. Eng. 2006, 14, 56–64. [Google Scholar] [CrossRef]
- Cai, T.; Tang, A.; Li, C. Experimental and kinetic analyses on the flame dynamics and stabilization of ammonia/hydrogen-air mixtures in a micro-planar combustor. Chem. Eng. J. 2023, 477, 147038. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, A.; Cai, T.; Huang, Q. Analysis of entropy generation and exergy efficiency of the methane/dimethyl ether/air premixed combustion in a micro-channel. Int. J. Heat Mass Transf. 2024, 218, 124801. [Google Scholar] [CrossRef]
- Pu, G.; Li, X.; Yuan, F. Numerical study on heat transfer efficiency of regenerative thermal oxidizers with three canisters. Processes 2021, 9, 1621. [Google Scholar] [CrossRef]
- Zou, W.F.; Lan, B. The numerical simulation research on thermal flow-reversal reactor of mine ventilation gas. Adv. Mater. Res. 2014, 926, 1627–1631. [Google Scholar] [CrossRef]
- Colombo, P.; Degischer, H. Highly porous metals and ceramics. Mater. Sci. Technol. 2010, 26, 1145–1158. [Google Scholar] [CrossRef]


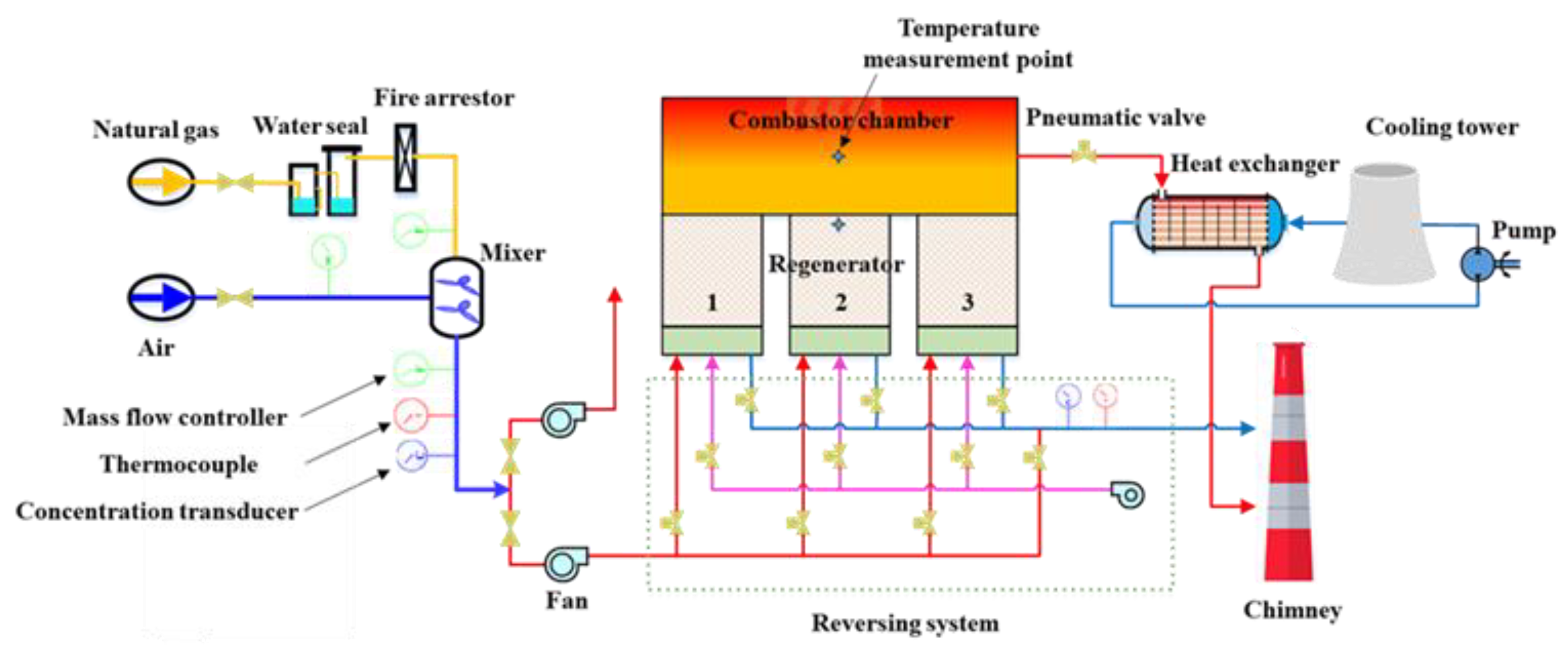



| No. | Regenerator 1 | Regenerator 2 | Regenerator 3 |
|---|---|---|---|
| Stage I | Inlet 1 (VAM) | Outlet 2 | Inlet 3 (Air) |
| Stage II | Inlet 1 (Air) | Inlet 2 (VAM) | Outlet 3 |
| Stage III | Outlet 1 | Inlet 2 (Air) | Inlet 3 (VAM) |
| No. | Reaction Steps | Ar (1/s) | Er (J/mol) |
|---|---|---|---|
| 1 | 2CH4 + 3O2 → 2CO + 4H2O | 5.012 × 1011 | 0 |
| 2 | 2CO + O2 → 2CO2 | 2.239 × 1012 | 0 |
| 3 | 2CO2 → 2CO + O2 | 5 × 108 | 0 |
| No. | Material Name | Density (kg/m3) | Heat Capacity (J/(g·K)) | Thermal Conductivity (W/(m·K)) | Thermal Diffusivity (mm2/s) |
|---|---|---|---|---|---|
| M1 | SiC [18] | 3160 | 1195 | 87 | 23 |
| M2 | Steel [18] | 7854 | 1169 | 30 | 3.3 |
| M3 | Si4N3 [18] | 2400 | 1155 | 8.8 | 3.2 |
| M4 | Mullite [44] | 2700 | 1000 | 3 | 1.1 |
| M5 | Cordierite [37] | 2300 | 981 | 2 | 0.9 |
| M6 | Basalt [45] | 2870 | 898 | 1.6 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yang, J.; Shao, S.; Cai, T.; Tang, A.; Xiao, L. Analysis of the Effects of Structural Parameters on the Thermal Performance and System Stability of Ventilation Air Methane-Fueled Reverse-Flow Oxidation Reactors. Processes 2024, 12, 1193. https://doi.org/10.3390/pr12061193
Zhang Z, Yang J, Shao S, Cai T, Tang A, Xiao L. Analysis of the Effects of Structural Parameters on the Thermal Performance and System Stability of Ventilation Air Methane-Fueled Reverse-Flow Oxidation Reactors. Processes. 2024; 12(6):1193. https://doi.org/10.3390/pr12061193
Chicago/Turabian StyleZhang, Zhigang, Jiaze Yang, Shanshan Shao, Tao Cai, Aikun Tang, and Lu Xiao. 2024. "Analysis of the Effects of Structural Parameters on the Thermal Performance and System Stability of Ventilation Air Methane-Fueled Reverse-Flow Oxidation Reactors" Processes 12, no. 6: 1193. https://doi.org/10.3390/pr12061193





