Drying Kinetics of Industrial Pineapple Waste: Effective Diffusivity and Thermodynamic Properties Resulting from New Mathematical Models Derived from the Fick Equation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pineapple Peel Treatment
2.3. Drying Kinetics Determination
2.4. Mathematical Models
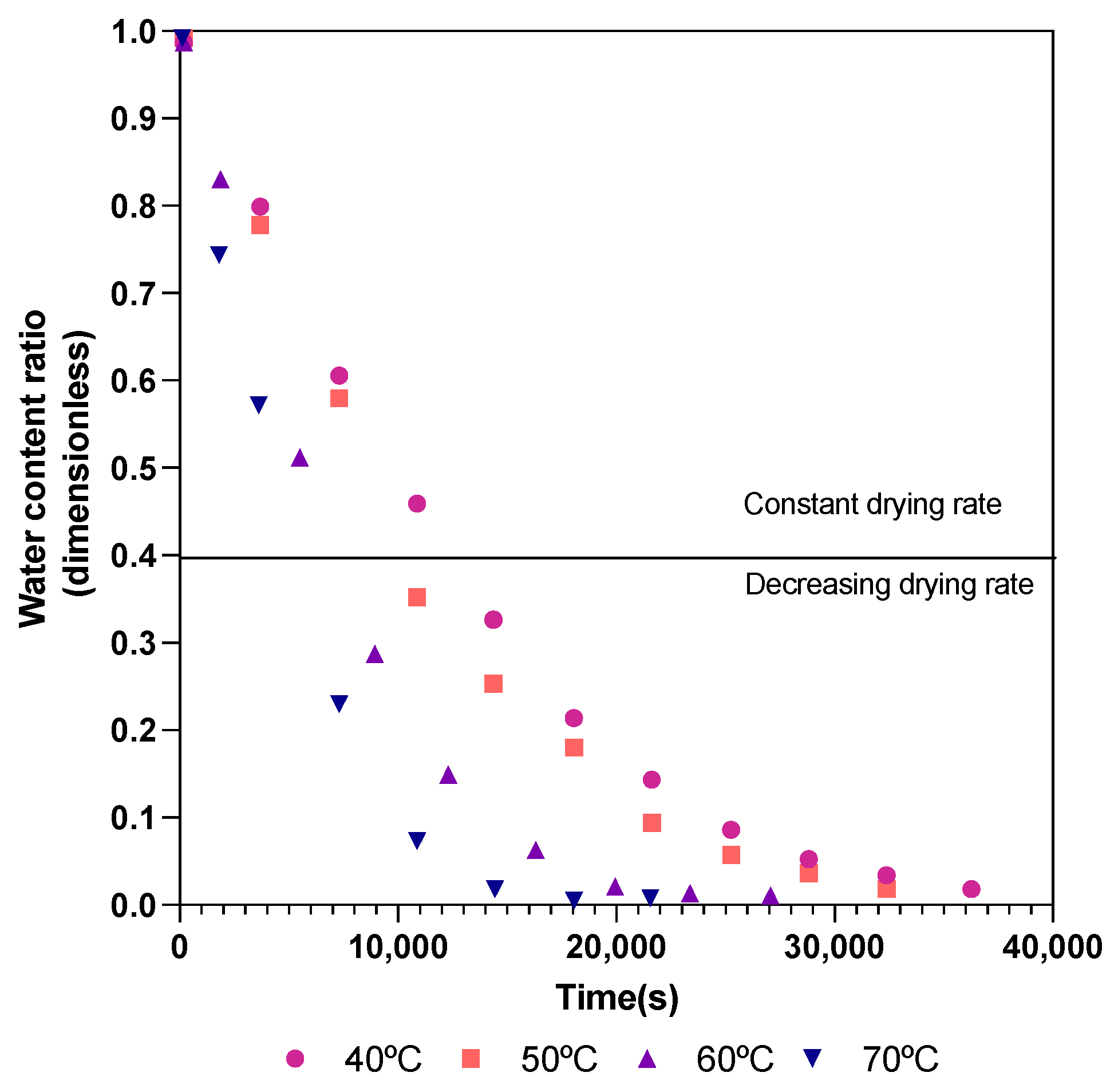
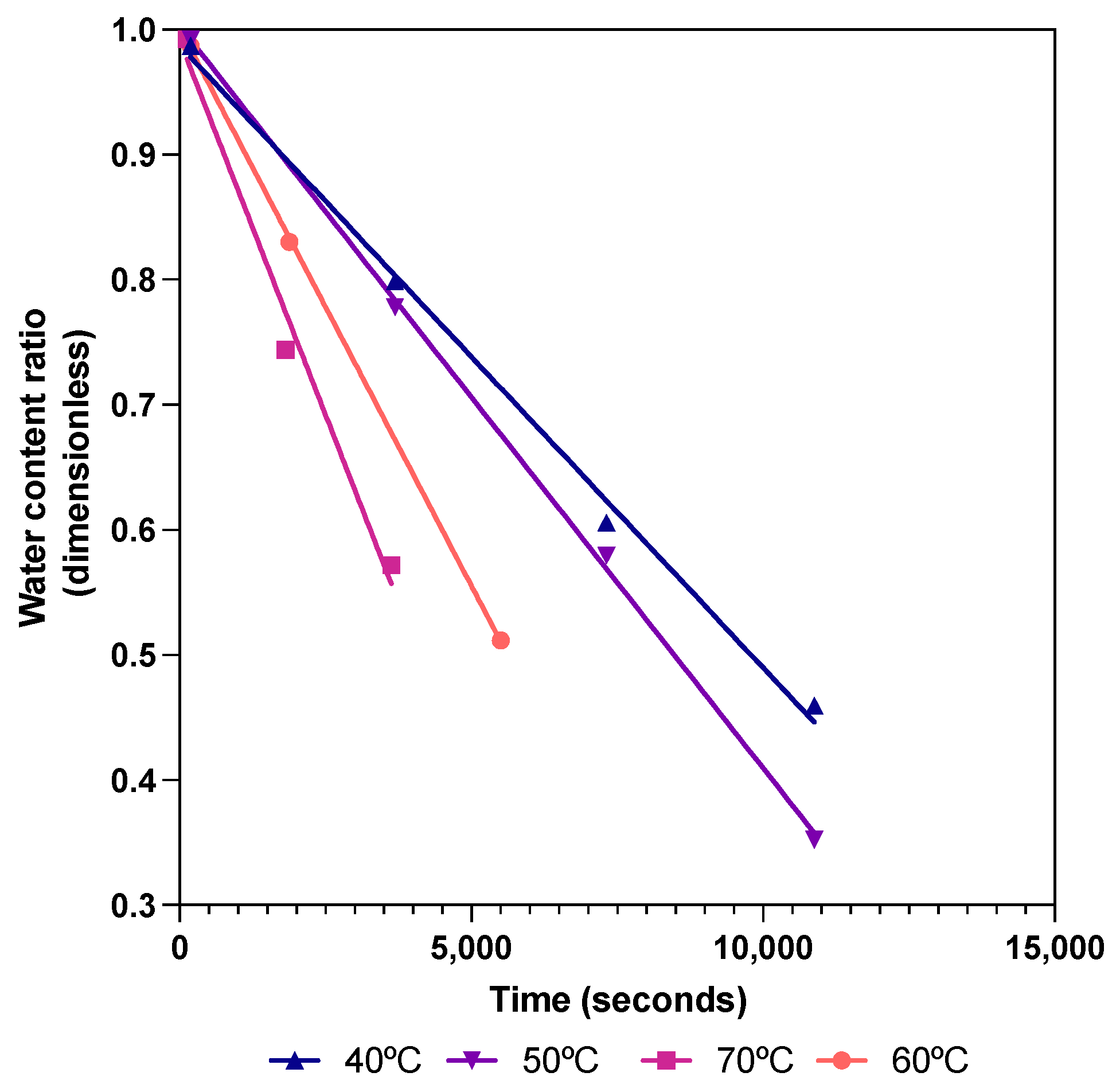
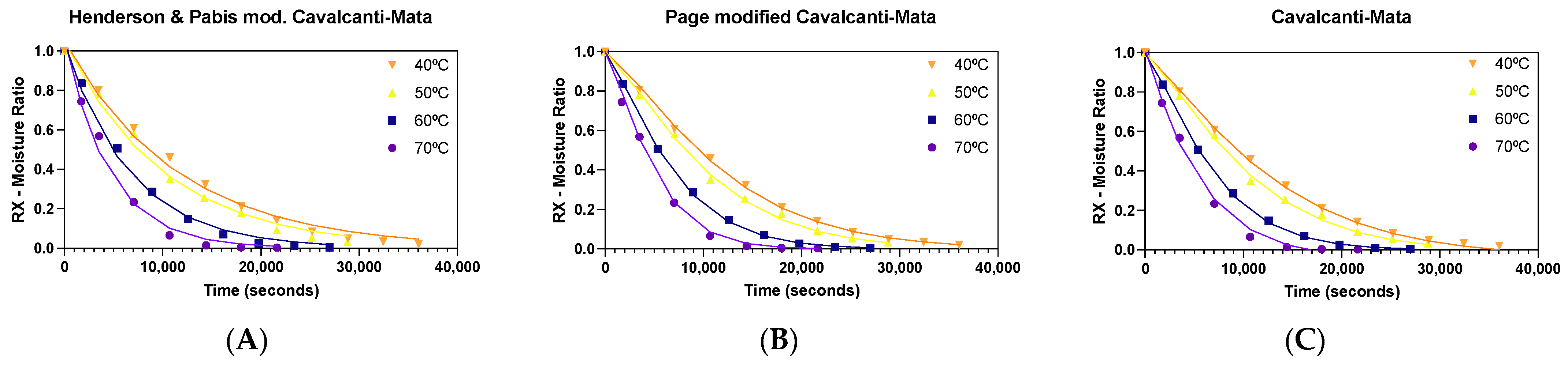
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costa, B.P.; de Carvalho, A.O.; Júnior, E.K.M.; Tsutsumi, C.Y.; de Moraes Rego, C.A.R.; da Costa, N.V. Fruit culture growth in Northeast Brazil and its relationship with work. Sci. Agrar. Parana. 2022, 21, 57–65. [Google Scholar] [CrossRef]
- Silva, L.R.; Almeida, E.I.B.; de Sousa Ferreira, L.; Figueirinha, K.T.; da Costa Ferreira, A.G.; da Silva Sousa, W. Estimates and causes of fresh fruit post-harvest losses in the Chapadinha Microregion, Maranhão, Brazil. Rev. Agro Mbiente Online 2018, 12, 288–299. [Google Scholar] [CrossRef]
- Aili Hamzah, A.F.; Hamzah, M.H.; Che Man, H.; Jamali, N.S.; Siajam, S.I.; Ismail, M.H. Recent Updates on the Conversion of Pineapple Waste (Ananas comosus) to Value-Added Products, Future Perspectives and Challenges. Agronomy 2021, 11, 2221. [Google Scholar] [CrossRef]
- De França Silva, A.P.; de Sousa, A.P.M.; de Macedo, A.D.B.; Dantas, D.L.; Costa, J.D.; de Almeida, A.F.; de Santana, R.A.C.; Campos, A.R.N. Obtenção de produto farináceo a partir de resíduos agroindustriais por diferentes métodos de Secagem. Res. Soc. Dev. 2020, 9, e405997334. [Google Scholar] [CrossRef]
- Banerjee, S.; Ranganathan, V.; Patti, A.; Arora, A. Valorisation of pineapple wastes for food and therapeutic applications. Trends Food Sci. Technol. 2018, 82, 60–70. [Google Scholar] [CrossRef]
- Kassab, Z.; Abdellaoui, Y.; Salim, M.H.; El Achaby, M. Cellulosic materials from pea (Pisum sativum) and broad beans (Vicia faba) pods agro-industrial residues. Mater. Lett. 2020, 280, 128539. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Duarte, M.E.; Cavalcanti-Mata, M.E. Modeling of food drying processes in industrial spray dryers. Food Bioprod. Process. 2018, 107, 49–60. [Google Scholar] [CrossRef]
- Nascimento, A.; Cavalcanti-Mata, M.E.; Martins Duarte, M.E.; Pasquali, M.; Lisboa, H.M. Construction of a design space for goat milk powder production using moisture sorption isotherms. J. Food Process Eng. 2019, 42, e13228. [Google Scholar] [CrossRef]
- Sukri, S.A.M.; Andu, Y.; Sarijan, S.; Khalid, H.-N.M.; Kari, Z.A.; Harun, H.C.; Rusli, N.D.; Mat, K.; Khalif, R.I.A.R.; Wei, L.S. Pineapple waste in animal feed: A review of nutritional potential, impact and prospects. Ann. Anim. Sci. 2023, 23, 339–352. [Google Scholar] [CrossRef]
- Idayanti, R.W.; Arifin, M.; Purbowati, E.; Purnomoadi, A. Utilization of pineapple waste as a roughage source diets for ruminant: A review. In Proceedings of the International Conference on Improving Tropical Animal Production for Food Security (ITAPS 2021), Kendari, Indonesia, 20–21 November 2021; pp. 123–130. [Google Scholar]
- Meena, L.; Sengar, A.S.; Neog, R.; Sunil, C.K. Pineapple processing waste (PPW): Bioactive compounds, their extraction, and utilisation: A review. J. Food Sci. Technol. 2022, 59, 4152–4164. [Google Scholar] [CrossRef]
- Del Valle, J.; Aránguiz, V.; Díaz, L. Volumetric procedure to assess infiltration kinetics and porosity of fruits by applying a vacuum pulse. J. Food Eng. 1998, 38, 207–221. [Google Scholar] [CrossRef]
- Klomklao, P.; Kuntinugunetanon, S.; Wongkokua, W. Moisture content measurement in paddy. J. Phys. Conf. Ser. 2017, 901, 012068. [Google Scholar] [CrossRef]
- Moura, H.V.; de Figueirêdo, R.M.F.; de Melo Queiroz, A.J.; de Vilela Silva, E.T.; Esmero, J.A.D.; Lisbôa, J.F. Mathematical modeling and thermodynamic properties of the drying kinetics of trapiá residues. J. Food Process Eng. 2021, 44, e13768. [Google Scholar] [CrossRef]
- Cavalcanti-Mata, M.E.R.M.; Duarte, M.E.M.; Lira, V.V.; de Oliveira, R.F.; Costa, N.L.; Oliveira, H.M.L. A new approach to the traditional drying models for the thin-layer drying kinetics of chickpeas. J. Food Process Eng. 2020, 43, e13569. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Vega, A.; Fito, P.; Andrés, A.; Lemus, R. Mathematical modeling of hot-air drying kinetics of red bell pepper (var. Lamuyo). J. Food Eng. 2007, 79, 1460–1466. [Google Scholar] [CrossRef]
- Radojčin, M.; Pavkov, I.; Bursać Kovačević, D.; Putnik, P.; Wiktor, A.; Stamenković, Z.; Kešelj, K.; Gere, A. Effect of Selected Drying Methods and Emerging Drying Intensification Technologies on the Quality of Dried Fruit: A Review. Processes 2021, 9, 132. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Araujo, H.; Paiva, G.; Oriente, S.; Pasquali, M.; Duarte, M.E.; Mata, M.E.C. Determination of characteristic properties of mulatto beans (Phaseolus vulgaris L.) during convective drying. J. Agric. Food Res. 2019, 1, 100003. [Google Scholar] [CrossRef]
- Santos, H.H.; Rodovalho, R.S.; da SILVA, D.P.; de Moraes Morgado, V.N. Drying kinetics of passion fruit seeds. Científica 2018, 46, 49–56. [Google Scholar] [CrossRef][Green Version]
- Botelho, F.M.; Hoscher, R.H.; Hauth, M.R.; Botelho, S. Cinética de secagem de grãos de soja: Influência varietal. Rev. Eng. Agric. 2018, 26, 13–25. [Google Scholar]
- Nadi, F.; Tzempelikos, D. Vacuum drying of apples (cv. Golden Delicious): Drying characteristics, thermodynamic properties, and mass transfer parameters. Heat Mass Transf. 2018, 54, 1853–1866. [Google Scholar] [CrossRef]
- Cruz, A.C.; Guiné, R.P.; Gonçalves, J.C. Drying kinetics and product quality for convective drying of apples (cvs. Golden Delicious and Granny Smith). Int. J. Fruit Sci. 2015, 15, 54–78. [Google Scholar] [CrossRef]
- De Oliveira Sousa Wanderley, R.; Feirosa de Figueirêdo, R.M.; Jose de Melo Queiroz, A.; Suelia dos Santos, F.; Paiva, Y.F.; de Lima Ferreira, J.P.; Barbosa de Lima, A.G.; Gomes, J.P.; Costa, C.C.; da Silva, W.P.; et al. The Temperature Influence on Drying Kinetics and Physico-Chemical Properties of Pomegranate Peels and Seeds. Foods 2023, 12, 286. [Google Scholar] [CrossRef]
- Madamba, P.S.; Driscoll, R.H.; Buckle, K.A. The thin-layer drying characteristics of garlic slices. J. Food Eng. 1996, 29, 75–97. [Google Scholar] [CrossRef]
- Gilago, M.C.; Mugi, V.R.; Chandramohan, V.P. Evaluation of drying kinetics of carrot and thermal characteristics of natural and forced convection indirect solar dryer. Results Eng. 2023, 18, 101196. [Google Scholar] [CrossRef]
- Zogzas, N.; Maroulis, Z.; Marinos-Kouris, D. Moisture diffusivity data compilation in foodstuffs. Dry. Technol. 1996, 14, 2225–2253. [Google Scholar] [CrossRef]
- Gomes, F.P.; Osvaldo, R.; Sousa, E.P.; de Oliveira, D.E.; de Araújo Neto, F.R. Drying kinetics of crushed mass of ‘jambu’: Effective diffusivity and activation energy. Rev. Bras. Eng. Agríc. Ambient. 2018, 22, 499–505. [Google Scholar] [CrossRef]
- De Oliveira, D.E.C.; Resende, O.; Bessa, J.F.V.; Kester, A.N.; Smaniotto, T.A.S. Mathematical modeling and thermodynamic properties for drying soybean grains. Afr. J. Agric. Res. 2015, 10, 31–38. [Google Scholar]
- Martins, E.A.; Lage, E.Z.; Goneli, A.L.; Hartmann Filho, C.P.; Lopes, J.G. Cinética de secagem de folhas de timbó (Serjania marginata Casar). Rev. Bras. Eng. Agríc. Ambient. 2015, 19, 238–244. [Google Scholar] [CrossRef]
- Araujo, W.D.; Goneli, A.L.D.; Corrêa, P.C.; Hartmann, C.P.; Martins, E.A.S. Modelagem matemática da secagem dos frutos de amendoim em camada delgada1. Rev. Ciência Agronôm. 2017, 48, 448–457. [Google Scholar]
- Guimarães, R.M.; de Oliveira, D.E.; Resende, O.; de Silva, J.S.; de Rezende, T.A.; Egea, M.B. Thermodynamic properties and drying kinetics of ‘okara’. Rev. Bras. Eng. Agríc. Ambient. 2018, 22, 418–423. [Google Scholar] [CrossRef]
- Quequeto, W.D.; Resende, O.; Silva, P.C.; Silva, F.; de Silva, L.M. Drying kinetics of noni seeds. J. Agric. Sci. 2019, 11, 250–258. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Santos, N.C.; dos Santos Pereira, T.; de Queiroga, A.P.R.; de Alcântara Silva, V.M.; de Alcântara Ribeiro, V.H.; Araújo, R.D.A.; Cabral, M.B.; da Silva, L.R.I.; Borges, E.M.E.S. Cinética de secagem do feijão azuki: Modelagem matemática e propriedades termodinâmicas. Res. Soc. Dev. 2020, 9, e27932316. [Google Scholar] [CrossRef]
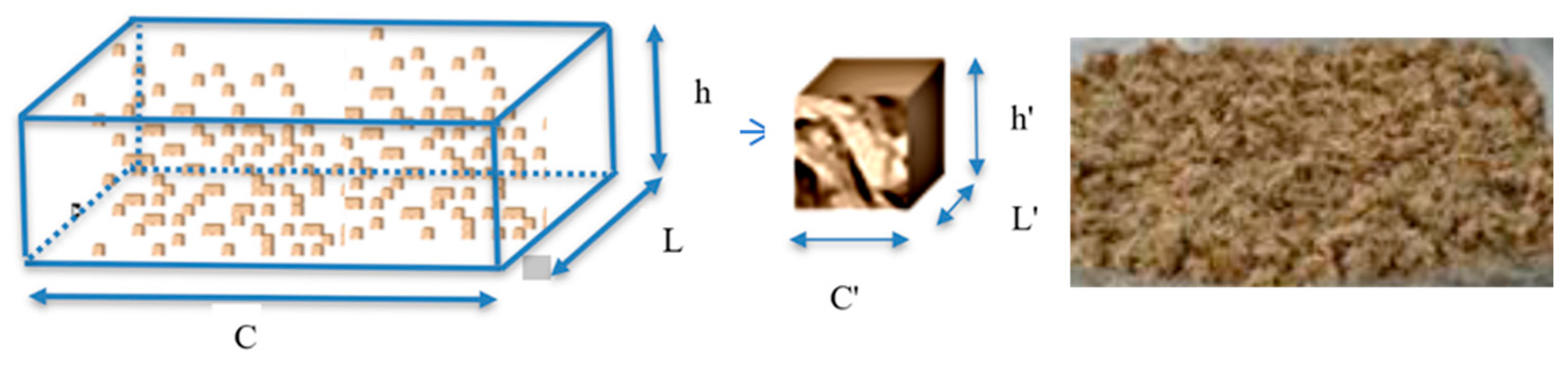

| Temperature | C | L | h | Asf | As | Rv | Pvs | Pv | dX/dt | hd | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | K | mm | Mm | mm | mm2 | mm2 | kJ/kg K | kPa | kPa | g/s | m s−1 |
| 40 | 313 | 130 | 70 | 50 | 903 × 103 | 1358 × 103 | 0.462 | 7.38 | 1.826 | 2.96 × 10−5 | 5.69 × 10−7 |
| 50 | 323 | 130 | 70 | 50 | 899 × 103 | 1354 × 103 | 0.462 | 12.34 | 1.966 | 4.12 × 10−5 | 4.37 × 10−7 |
| 60 | 333 | 130 | 70 | 50 | 934 × 103 | 1389 × 103 | 0.462 | 19.93 | 1.995 | 5.47 × 10−5 | 3.38 × 10−7 |
| 70 | 343 | 130 | 70 | 50 | 954 × 103 | 1409 × 103 | 0.462 | 31.18 | 2.051 | 7.22 × 10−5 | 2.79 × 10−7 |
| Temp. | First Term | Second Term | Third Term | Fourth Term | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | Def | R2 (%) | SE | P | Def | R2 (%) | SE | P | Def | R2 (%) | SE | P | Def | R2 (%) | SE | P |
| mm2/s | mm2/s | mm2/s | mm2/s | |||||||||||||
| 40 | 0.017368 | 91.23 | 0.0092 | 19.60 | 0.017361 | 93.51 | 0.00683 | 18.80 | 0.017355 | 94.31 | 0.0060 | 18.35 | 0.017346 | 97.24 | 0.00573 | 18.18 |
| 50 | 0.019950 | 91.38 | 0.0964 | 26.49 | 0.019960 | 93.79 | 0.0818 | 25.63 | 0.019948 | 94.57 | 0.0765 | 25.26 | 0.019937 | 97.38 | 0.0747 | 25.12 |
| 60 | 0.029992 | 92.51 | 0.0983 | 21.38 | 0.029981 | 94.75 | 0.0822 | 20.39 | 0.029960 | 95.57 | 0.0755 | 19.88 | 0.029937 | 97.91 | 0.0732 | 19.68 |
| 70 | 0.039956 | 92.29 | 0.1020 | 17.61 | 0.039934 | 94.71 | 0.0845 | 16.50 | 0.039924 | 95.44 | 0.0785 | 16.18 | 0.039916 | 97.81 | 0.0764 | 16.02 |
| Temperature | Parameters—Henderson–Pabis Model Modified by Cavalcanti-Mata | |||||||
|---|---|---|---|---|---|---|---|---|
| h mm | A | K | m2/s | R² | SE | P | ||
| 40 °C | 25 | 1.04817 | 0.000087 | 0.882 × 10−9 | 0.9868 | 0.00139 | 10.09 | |
| 50 °C | 25 | 1.04746 | 0.000099 | 1.003 × 10−9 | 0.9874 | 0.0369 | 22.79 | |
| 60 °C | 25 | 1.03908 | 0.000150 | 1.520 × 10−9 | 0.9921 | 0.0083 | 13.65 | |
| 70 °C | 25 | 1.05893 | 0.000220 | 2.229 × 10−9 | 0.9855 | 0.0176 | 11.75 | |
| Temperature | Parameters—Page model modified by Cavalcanti-Mata | |||||||
| h mm | K | N | m2/s | R² | SE | P | ||
| 40 °C | 25 | 5.4 × 10−6 | 1.2842 | 5.47 × 10−9 | 0.9985 | 0.00015 | 11.61 | |
| 50 °C | 25 | 6.5 × 10−6 | 1.28504 | 6.59 × 10−9 | 0.9984 | 0.0131 | 5.74 | |
| 60 °C | 25 | 1.3 × 10−5 | 1.26474 | 13.2 × 10−9 | 0.9995 | 0.0083 | 13.65 | |
| 70 °C | 25 | 1.5 × 10−5 | 1.29495 | 15.2 × 10−9 | 0.9977 | 0.0176 | 11.75 | |
| Temperature | Parameters—Cavalcanti-Mata Model | |||||||
| A1 | N1 | A2 | N2 | m2/s | R² | SE | P | |
| 40 °C | 1.698121 | 1.118394 | −0.700480 | 0.976141 | 3.620 × 10−9 | 0.9996 | 0.00005 | 13.07 |
| 50 °C | 2.292974 | 1.114945 | −1.29365 | 0.997683 | 4.34 × 10−9 | 0.9985 | 0.0132 | 8.15 |
| 60 °C | 1.688064 | 1.128602 | −0.688994 | 0.989407 | 4.62 × 10−9 | 0.9996 | 0.0083 | 13.65 |
| 70 °C | 1.252784 | 1.209190 | −0.262371 | 1.157325 | 8.849 × 10−9 | 0.9951 | 0.0176 | 11.75 |
| Enthalpy (J mol−1) | ||||
|---|---|---|---|---|
| Temperature (K) | Fick Four Terms | Modified Henderson–Pabis | Page | Modified Cavalcanti-Mata |
| 313.15 | 22,725.03 | 25,218.94 | 30,294.14 | 21,153.89 |
| 323.15 | 22,643.69 | 25,137.60 | 30,212.80 | 21,072.55 |
| 333.15 | 22,562.35 | 25,056.26 | 30,131.46 | 20,991.21 |
| 343.15 | 22,481.01 | 24,974.92 | 30,050.12 | 20,909.87 |
| Entropy (Jmol−1K−1) | ||||
| 313.15 | −192.71 | −209.21 | −215.34 | −223.58 |
| 323.15 | −192.96 | −209.46 | −215.59 | −223.84 |
| 333.15 | −193.21 | −209.71 | −215.84 | −224.08 |
| 343.15 | −193.45 | −209.95 | −216.08 | −224.32 |
| Gibbs Free Energy (Jmol−1) | ||||
| 313.15 | 83,070.87 | 90,732.20 | 97,726.94 | 91,167.99 |
| 323.15 | 84,999.21 | 92,825.56 | 99,881.60 | 93,405.07 |
| 333.15 | 86,930.07 | 94,921.44 | 102,038.77 | 95,644.68 |
| 343.15 | 88,863.37 | 97,019.75 | 104,198.39 | 97,886.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcanti-Mata, M.E.; Duarte, M.E.; Tolentino, M.; Mendes, F.A.; Batista, L.; de Lima, J.M.; Lúcio, A.; Nascimento, A.P.; Almeida, R.D.; Lisboa, H.M. Drying Kinetics of Industrial Pineapple Waste: Effective Diffusivity and Thermodynamic Properties Resulting from New Mathematical Models Derived from the Fick Equation. Processes 2024, 12, 1198. https://doi.org/10.3390/pr12061198
Cavalcanti-Mata ME, Duarte ME, Tolentino M, Mendes FA, Batista L, de Lima JM, Lúcio A, Nascimento AP, Almeida RD, Lisboa HM. Drying Kinetics of Industrial Pineapple Waste: Effective Diffusivity and Thermodynamic Properties Resulting from New Mathematical Models Derived from the Fick Equation. Processes. 2024; 12(6):1198. https://doi.org/10.3390/pr12061198
Chicago/Turabian StyleCavalcanti-Mata, Mário Eduardo, Maria Elita Duarte, Manoel Tolentino, Francisco Assis Mendes, Leonardo Batista, Janaína Maria de Lima, Alexandre Lúcio, Amanda Priscila Nascimento, Rafaela D. Almeida, and Hugo M. Lisboa. 2024. "Drying Kinetics of Industrial Pineapple Waste: Effective Diffusivity and Thermodynamic Properties Resulting from New Mathematical Models Derived from the Fick Equation" Processes 12, no. 6: 1198. https://doi.org/10.3390/pr12061198
APA StyleCavalcanti-Mata, M. E., Duarte, M. E., Tolentino, M., Mendes, F. A., Batista, L., de Lima, J. M., Lúcio, A., Nascimento, A. P., Almeida, R. D., & Lisboa, H. M. (2024). Drying Kinetics of Industrial Pineapple Waste: Effective Diffusivity and Thermodynamic Properties Resulting from New Mathematical Models Derived from the Fick Equation. Processes, 12(6), 1198. https://doi.org/10.3390/pr12061198












