Ibuprofen Formulations: Permeability and Biodegradability Comparison Depending on the Type of Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel, Organogel, Eucerin Ointment, Silicone Ointment, and Zinc Ointment Preparation
2.3. Stability of Formulations
2.4. The Skin Permeability for Formulations
2.5. The Release Test
- f(t)—fraction of drug released in t time;
- k—the release constant incorporated structural and geometric dosage form;
- n—the release exponent indicating the drug release mechanism.
2.6. Skin Accumulation Studies
2.7. High-Performance Liquid Chromatography (HPLC)
2.8. Elemental Analysis
2.9. Formulation Biodegradation
2.9.1. The Testing Medium
2.9.2. Origin of Samples of Active Sludge
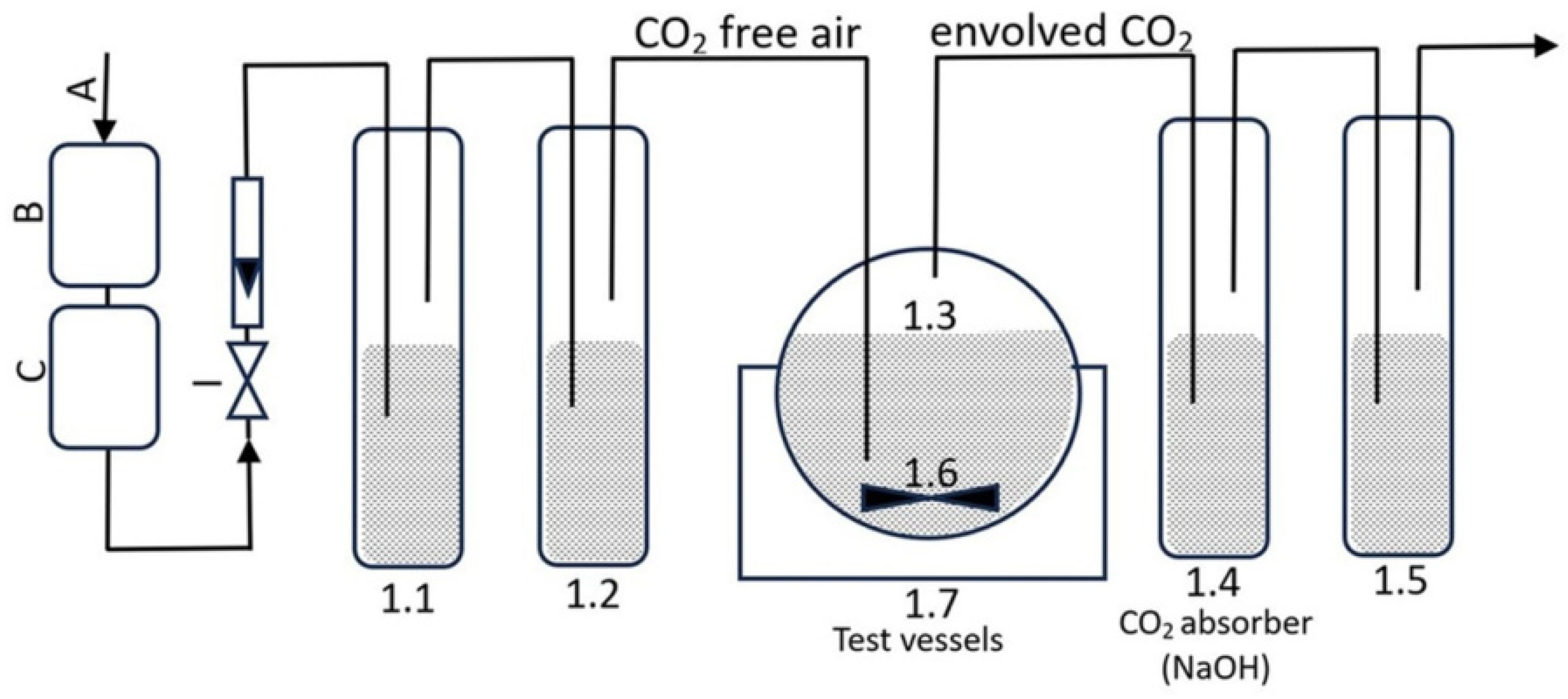
2.9.3. Aerobic Biodegradation Potential Assessment Method for Formulations
- I: air with an aeration rate of 50–100 mL·min−1, employed to oxygenate the entire testing system;
- B and C: CO2 absorber (KOH);
- 1.1: The CO2 absorber consists of potassium hydroxide with a concentration of 5 mol·L−1;
- 1.2: The CO2 indication is performed using barium hydroxide with a concentration of 0.01 mol·L−1;
- 1.3: The test containers have a capacity of 500 mL and are stirred using a magnetic stirrer 1.6;
- 1.4: Sodium hydroxide is used as the CO2 absorber (the concentration of sodium hydroxide used as a CO2 absorber is 0.05 mol·L−1);
- 1.5: Distilled water is used as the O2 absorber;
- 1.6: A container filled with distilled water, in which test vessels with a capacity of 500 mL were placed;
- 1.7: A cryostat that accurately controls the temperature of the distilled water in the container. The incubation process was conducted at 23 ± 0.5 °C for 28 days.
- %B—degree of biodegradation;
- CIC—concentration of inorganic carbon in the test vessel 1.4, obtained by TOC analysis of the test sample corrected by blank (mg·L−1);
- R—dilution of the sample collected from the test vessel 1.4 (2.5);
- V0—the initial volume of NaOH solution in the test vessel 1.4 (0.25 L);
- i—sample number;
- Vp—the volume of the sample taken from the test vessel 1.4 (0.01 L);
- m—mass of test compound injected into the test vessel 1.3 (mg);
- U—the proportion of carbon in the test compound introduced into the test vessel 1.3 (-).
2.10. Swelling Assessment of Hydrogel Formulations
- W1 (g)—weight of the swollen hydrogels F1-IBU and F1-IBUNa;
- W0 (g)—weight of the obtained hydrogels F1-IBU and F1-IBUNa.
2.11. Statistical Analysis
3. Results and Discussion
3.1. Permeations of Active Compound Studies
3.2. Elemental Analysis of Formulations Studies
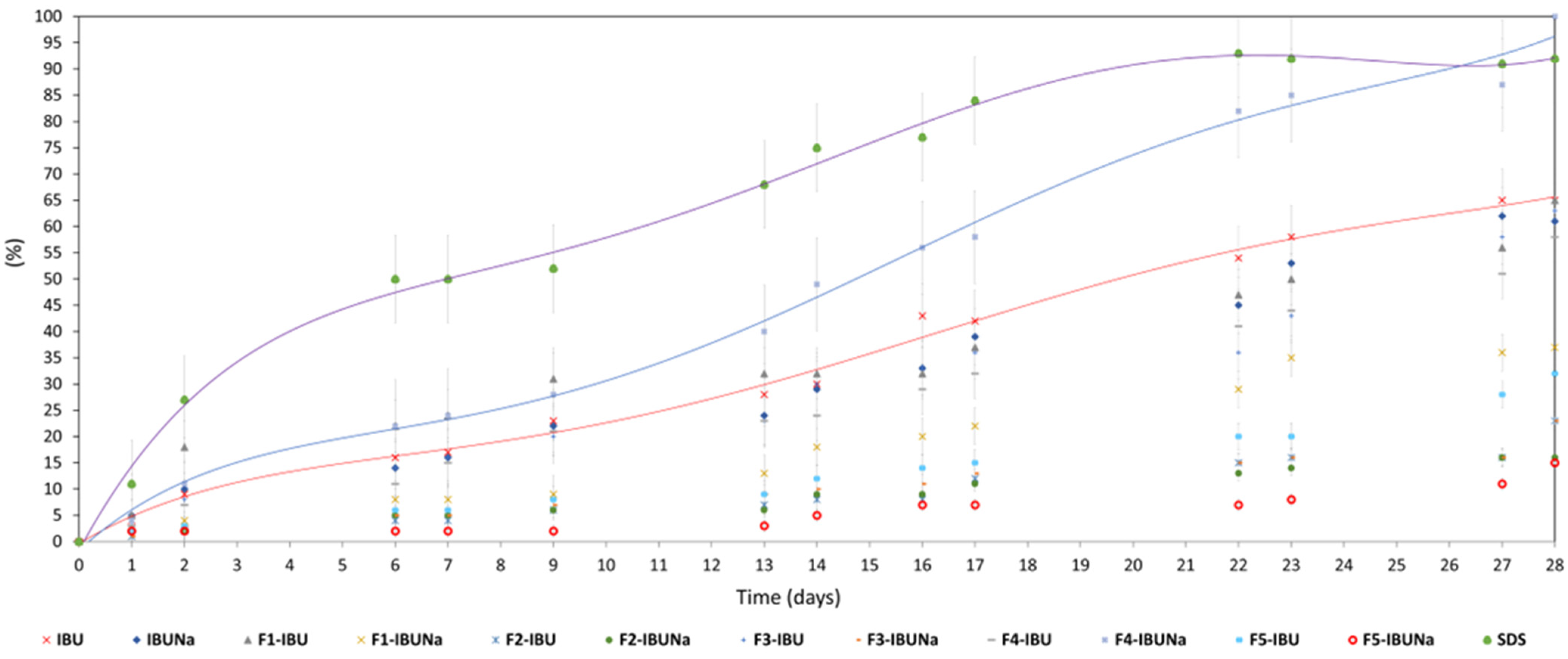
3.3. Biodegradation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngo, V.T.H.; Bajaj, T. Ibuprofen. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Show, S.; Chakraborty, P.; Karmakar, B.; Halder, G. Sorptive and Microbial Riddance of Micro-Pollutant Ibuprofen from Contaminated Water: A State of the Art Review. Sci. Total Environ. 2021, 786, 147327. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Adenle, A.A.; Sowe, S.K.; Parayil, G.; Aginam, O. Analysis of Open Source Biotechnology in Developing Countries: An Emerging Framework for Sustainable Agriculture. Technol. Soc. 2012, 34, 256–269. [Google Scholar] [CrossRef]
- Makuch, E.; Ossowicz-Rupniewska, P.; Klebeko, J.; Janus, E. Biodegradation of L-Valine Alkyl Ester Ibuprofenates by Bacterial Cultures. Materials 2021, 14, 3180. [Google Scholar] [CrossRef] [PubMed]
- Adamczuk, M. Environmentally Realistic Concentrations of Ibuprofen Influence Life Histories but Not Population Dynamics of Daphnia Magna. Sci. Total Environ. 2022, 848, 157783. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, M.A.C.; Migdal, C.W.; Tembhekar, A.R.; Harris, J.A.; DeMesa, C. Topical Administration of Ibuprofen for Injured Athletes: Considerations, Formulations, and Comparison to Oral Delivery. Sports Med. Open 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef] [PubMed]
- Ouranidis, A.; Gkampelis, N.; Vardaka, E.; Karagianni, A.; Tsiptsios, D.; Nikolakakis, I.; Kachrimanis, K. Overcoming the Solubility Barrier of Ibuprofen by the Rational Process Design of a Nanocrystal Formulation. Pharmaceutics 2020, 12, 969. [Google Scholar] [CrossRef]
- Rastogi, A.; Tiwari, M.K.; Ghangrekar, M.M. A Review on Environmental Occurrence, Toxicity and Microbial Degradation of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). J. Environ. Manag. 2021, 300, 113694. [Google Scholar] [CrossRef]
- Salgado, R.; Brito, D.; Noronha, J.P.; Almeida, B.; Bronze, M.R.; Oehmen, A.; Carvalho, G.; Barreto Crespo, M.T. Metabolite Identification of Ibuprofen Biodegradation by Patulibacter Medicamentivorans under Aerobic Conditions. Environ. Technol. 2020, 41, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Jan-Roblero, J.; Cruz-Maya, J.A. Ibuprofen: Toxicology and Biodegradation of an Emerging Contaminant. Molecules 2023, 28, 2097. [Google Scholar] [CrossRef] [PubMed]
- Mussa, Z.H.; Al-Qaim, F.F.; Jawad, A.H.; Scholz, M.; Yaseen, Z.M. A Comprehensive Review for Removal of Non-Steroidal Anti-Inflammatory Drugs Attained from Wastewater Observations Using Carbon-Based Anodic Oxidation Process. Toxics 2022, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Abbot, V.; Paliwal, D.; Sharma, A.; Sharma, P. A Review on the Physicochemical and Biological Applications of Biosurfactants in Biotechnology and Pharmaceuticals. Heliyon 2022, 8, e10149. [Google Scholar] [CrossRef] [PubMed]
- El-Gammal, M.A.; Elsaeidy, A.S.; Ashry, H.; Jobran, A.W.M. Biodegradation Method of Pharmaceuticals and Personal Care Products. In Handbook of Biodegradable Materials; Ali, G.A.M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1093–1131. ISBN 978-3-031-09710-2. [Google Scholar]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-Based Wastewater Treatment: Mechanisms, Challenges, Recent Advances, and Future Prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Kumar, D. Ibuprofen as an Emerging Organic Contaminant in Environment, Distribution and Remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Malik, M.Y.; Singh, S.K.; Chaturvedi, S.; Gayen, J.R.; Wahajuddin, M. Bioavailability Enhancement of Poorly Soluble Drugs: The Holy Grail in Pharma Industry. Curr. Pharm. Des. 2019, 25, 987–1020. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, D.; Chen, J.; Zhao, C.; Pour Shahid Saeed Abadi, P. Synthesis and Biomedical Applications of Functional Polymers. Int. J. Polym. Sci. 2021, 2021, e9763105. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Ossowicz-Rupniewska, P.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Adamiak-Giera, U.; Prowans, P.; Czapla, N.; Bargiel, P.; et al. Epilobium angustifolium L. Extracts as Valuable Ingredients in Cosmetic and Dermatological Products. Molecules 2021, 26, 3456. [Google Scholar] [CrossRef]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. The Effect of Cream and Gel Vehicles on the Percutaneous Absorption and Skin Retention of a New Eugenol Derivative with Antioxidant Activity. Front. Pharmacol. 2021, 12, 658381. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.M.; Essa, H.; El-Ammawi, T.; Abdelkader, H.; Hussein, A.K. Formulation and Clinical Evaluation of Silymarin Pluronic-Lecithin Organogels for Treatment of Atopic Dermatitis. Drug Des. Dev. Ther. 2016, 10, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, E.; Xiao, M.; Chen, C.; Xu, W. Enhanced Chemical and Biological Activities of a Newly Biosynthesized Eugenol Glycoconjugate, Eugenol α-d-Glucopyranoside. Appl. Microbiol. Biotechnol. 2013, 97, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. Enhancement of the Antioxidant and Skin Permeation Properties of Eugenol by the Esterification of Eugenol to New Derivatives. AMB Expr. 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, Ł.; Cybulska, K.; Kucharska, E.; Nowak, A.; Pełech, R.; Klimowicz, A. Biologically Active Preparations from the Leaves of Wild Plant Species of the Genus Rubus. Molecules 2022, 27, 5486. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- ISO 9308-1:2014; Water Quality—Enumeration of Escherichia coli and Coliform bacteria. International Organization for Standardization: Geneva, Switzerland, 2014.
- Adejokun, D.A.; Dodou, K. A Novel Method for the Evaluation of the Long-Term Stability of Cream Formulations Containing Natural Oils. Cosmetics 2020, 7, 86. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, W.; Jin, C. Protocol Efficiently Measuring the Swelling Rate of Hydrogels. MethodsX 2020, 7, 100779. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Date, P.; Tanwar, A.; Ladage, P.; Kodam, K.M.; Ottoor, D. Biodegradable and Biocompatible Agarose–Poly (Vinyl Alcohol) Hydrogel for the in Vitro Investigation of Ibuprofen Release. Chem. Pap. 2020, 74, 1965–1978. [Google Scholar] [CrossRef]
- Bukhari, S.M.H.; Khan, S.; Rehanullah, M.; Ranjha, N.M. Synthesis and Characterization of Chemically Cross-Linked Acrylic Acid/Gelatin Hydrogels: Effect of pH and Composition on Swelling and Drug Release. Int. J. Polym. Sci. 2015, 2015, e187961. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Bednarczyk, P.; Nowak, M.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klebeko, J.; Świątek, E.; Bilska, K.; Rokicka, J.; et al. Evaluation of the Structural Modification of Ibuprofen on the Penetration Release of Ibuprofen from a Drug-in-Adhesive Matrix Type Transdermal Patch. Int. J. Mol. Sci. 2022, 23, 7752. [Google Scholar] [CrossRef] [PubMed]
- Jipa, I.M.; Stoica-Guzun, A.; Stroescu, M. Controlled Release of Sorbic Acid from Bacterial Cellulose Based Mono and Multilayer Antimicrobial Films. LWT 2012, 47, 400–406. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of Environmental Wastes: The Role of Microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Albers, E.; Muller, B.W. Cyclodextrin Derivatives in Pharmaceutics. CRT 1995, 12, 311–337. [Google Scholar] [CrossRef]
- Agboola, A.A.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Story, A.; Story, G.; Struk, Ł.; Antosik, A.K.; Ossowicz-Rupniewska, P. Emulsion-Based Gel Loaded with Ibuprofen and Its Derivatives. Gels 2023, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Tauxe-Wuersch, A.; De Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Occurrence of Several Acidic Drugs in Sewage Treatment Plants in Switzerland and Risk Assessment. Water Res. 2005, 39, 1761–1772. [Google Scholar] [CrossRef]
- González, S.; Müller, J.; Petrovic, M.; Barceló, D.; Knepper, T.P. Biodegradation Studies of Selected Priority Acidic Pesticides and Diclofenac in Different Bioreactors. Environ. Pollut. 2006, 144, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.B.; Weiss, S.; Reemtsma, T. Pathways and Metabolites of Microbial Degradation of Selected Acidic Pharmaceutical and Their Occurrence in Municipal Wastewater Treated by a Membrane Bioreactor. Water Res. 2005, 39, 2654–2664. [Google Scholar] [CrossRef]
- Gu, J.-D. Biodegradation Testing: So Many Tests but Very Little New Innovation. Appl. Environ. Biotechnol. 2016, 1, 92–95. [Google Scholar] [CrossRef]
- Qu, H.; Barrett, H.; Wang, B.; Han, J.; Wang, F.; Gong, W.; Wu, J.; Wang, W.; Yu, G. Co-Occurrence of Antiseptic Triclocarban and Chiral Anti-Inflammatory Ibuprofen in Environment: Association between Biological Effect in Sediment and Risk to Human Health. J. Hazard. Mater. 2021, 407, 124871. [Google Scholar] [CrossRef] [PubMed]
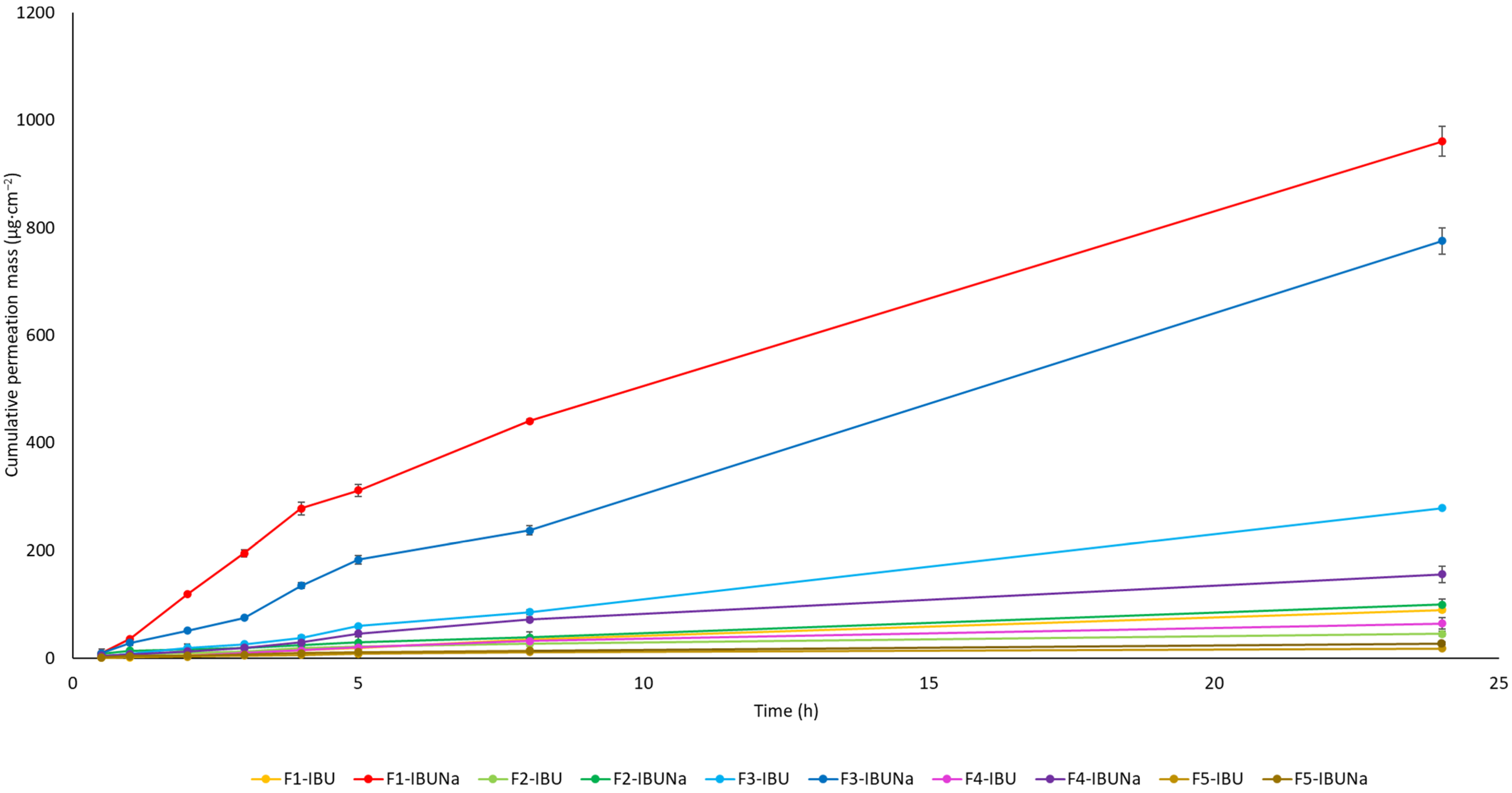
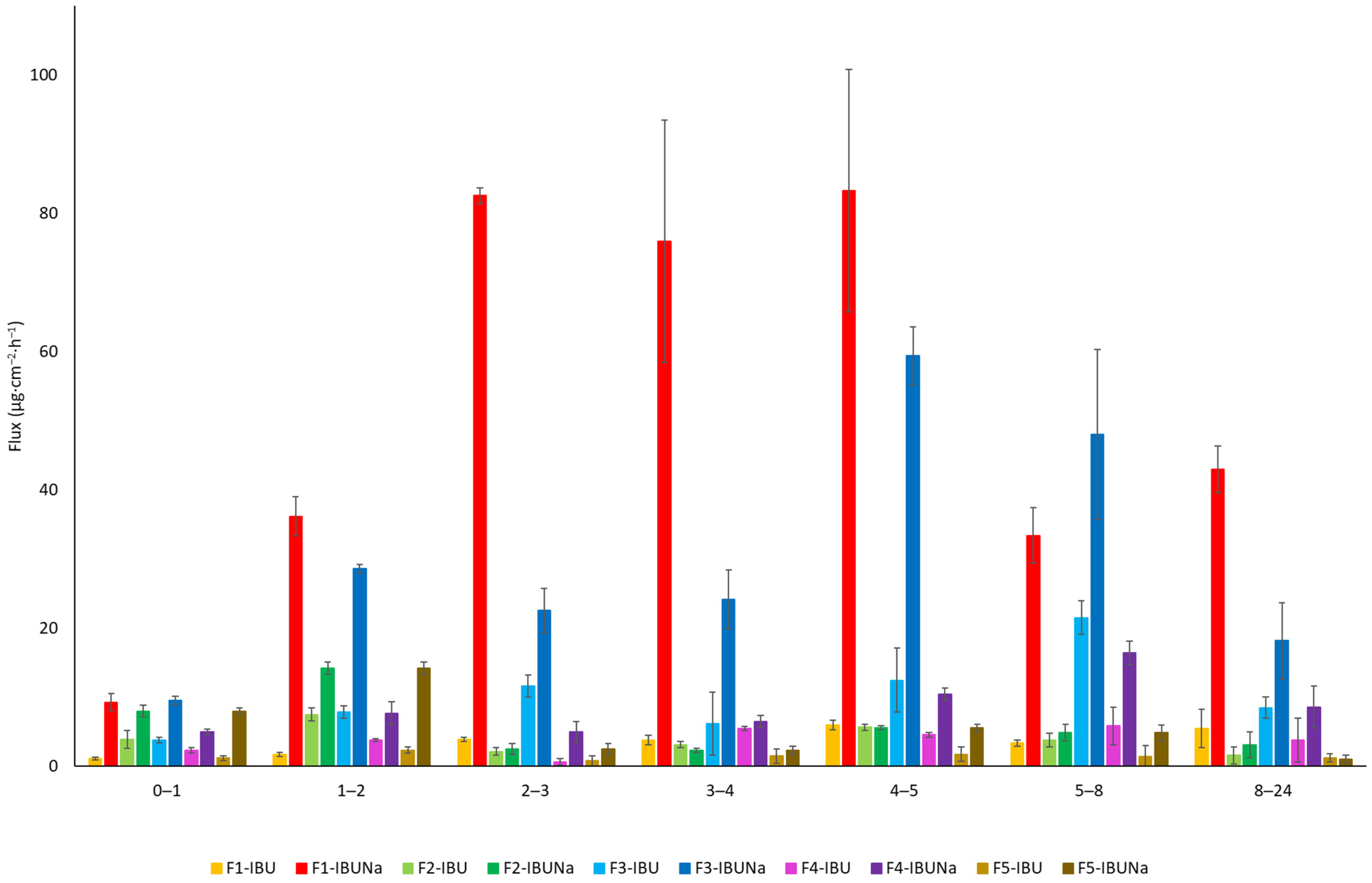
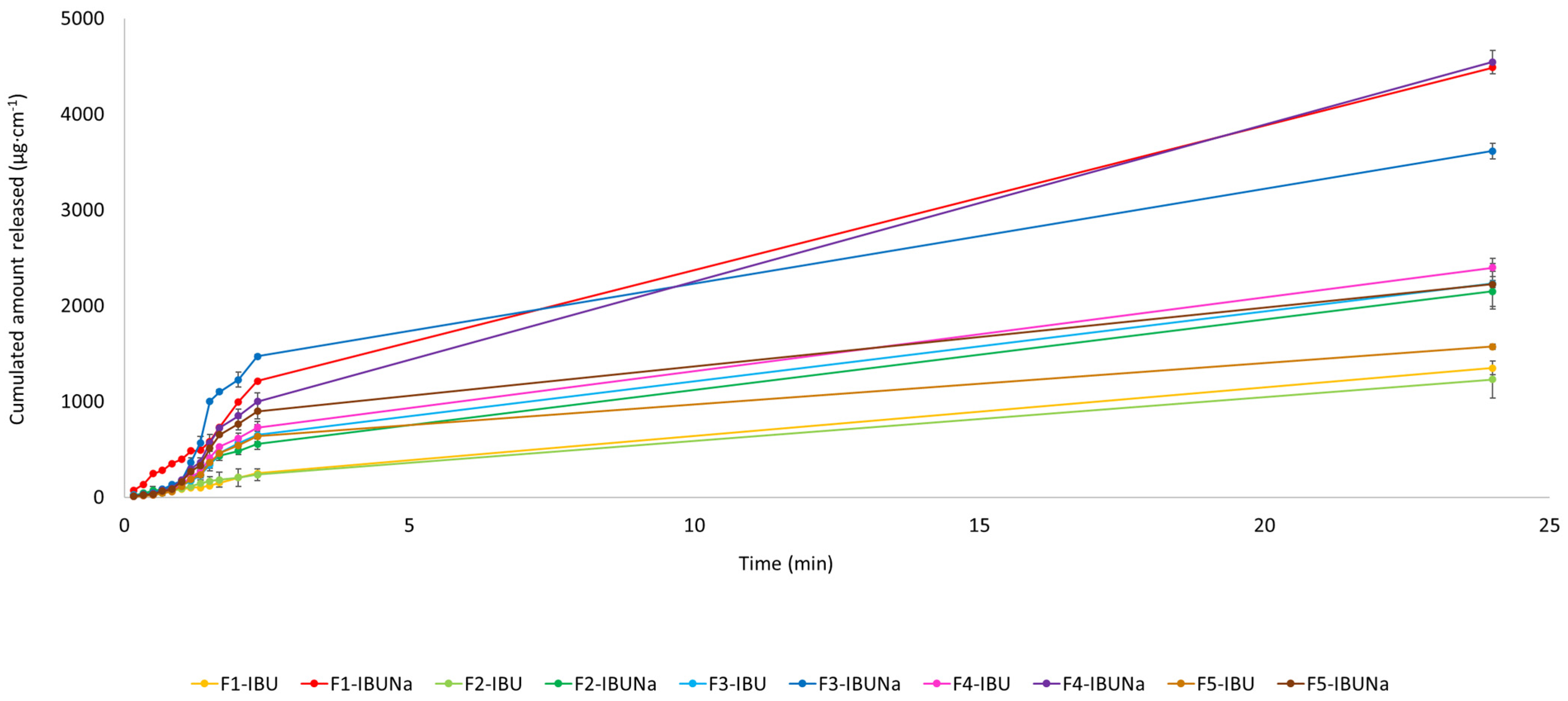

| Vehicle | The Cumulative Permeation Mass (µg·cm−2) |
|---|---|
| F1-IBU | 89.208 ± 3.334 c |
| F1-IBUNa | 960.092 ± 27.776 g |
| F2-IBU | 44.976 ± 4.851 ab |
| F2-IBUNa | 99.431 ± 11.165 c |
| F3-IBU | 278.691 ± 1.6612 e |
| F3-IBUNa | 775.016 ± 24.641 f |
| F4-IBU | 64.531 ± 10.584 bc |
| F4-IBUNa | 155.243 ± 14.898 d |
| F5-IBU | 18.325 ± 2.848 a |
| F5-IBUNa | 27.387 ± 1.156 ab |
| Vehicle | |
|---|---|
| F1-IBU/F1-IBUNa | Z = −2.4881 p = 0.0128 * |
| F2-IBU/F2-IBUNa | Z = 0.7522 p = 0.4519 |
| F3-IBU/F3-IBUNa | Z = −1.0994 p = 0.2715 |
| F4-IBU/F4-IBUNa | Z = −2.0252 p = 0.0401 * |
| F5-IBU/F5-IBUNa | Z = −0.5207 p = 0.6025 |
| Vehicle | |
|---|---|
| F1-IBU/F1-IBUNa | Z = −3.1282 p = 0.0017 * |
| F2-IBU/F2-IBUNa | Z = 1.3846 p = 0.1661 |
| F3-IBU/F3-IBUNa | Z = 0.6230 p = 0.3559 |
| F4-IBU/F4-IBUNa | Z = 0.5641 p = 0.5726 |
| F5-IBU/F5-IBUNa | Z = −0.6153 p = 0.5383 |
| Vehicle/ Formulation | JSS, µg·cm−2·h−1 | KP∙103, cm·h−1 | LT, h | D∙104, cm2·h−1 | Km | Q%24 h |
|---|---|---|---|---|---|---|
| F1-IBU | 4.653 ± 0.4779 | 0.093 ± 0.009 | 0.884 ± 0.320 | 4.715 ± 1.859 | 0.009 ± 0.001 | 0.18 ± 0.01 |
| F1-IBUNa | 80.127 ± 3.269 | 1.603 ± 0.065 | 0.543 ± 0.082 | 7.678 ± 1.248 | 0.104 ± 0.019 | 1.92 ± 0.06 |
| F2-IBU | 4.707 ± 0.276 | 0.094 ± 0.006 | 0.213 ± 0.117 | 19.538 ± 1.145 | 0.002 ± 0.001 | 0.09 ± 0.01 |
| F2-IBUNa | 5.218 ± 0.454 | 0.104 ± 0.009 | * | * | * | 0.20 ± 0.02 |
| F3-IBU | 16.973 ± 1.417 | 0.339 ± 0.028 | 1.573 ± 0.186 | 2.689 ± 0.326 | 0.064 ± 0.012 | 0.56 ± 0.01 |
| F3-IBUNa | 53.687 ± 4.888 | 1.074 ± 0.098 | 1.566 ± 0.201 | 2.711 ± 0.334 | 0.202 ± 0.045 | 1.55 ± 0.05 |
| F4-IBU | 5.201 ± 0.961 | 0.104 ± 0.019 | 1.132 ± 0.281 | 3.679 ± 0.413 | 0.014 ± 0.001 | 0.13 ± 0.01 |
| F4-IBUNa | 13.425 ± 1.278 | 0.269 ± 0.026 | 1.647 ± 0.154 | 2.530 ± 0.242 | 0.053 ± 0.001 | 0.31 ± 0.03 |
| F5-IBU | 1.599 ± 0.254 | 0.032 ± 0.005 | 0.078 ± 0.012 | 53.339 ± 10.427 | 0.003 ± 0.001 | 0.04 ± 0.01 |
| F5-IBUNa | 2.301 ± 0.419 | 0.046 ± 0.008 | * | * | * | 0.05 ± 0.01 |
| IBU | IBUNa | F1-IBU | F1-IBUNa | F2-IBU | F2-IBUNa | F3-IBU |
| (h/days) | (h/days) | (h/days) | (h/days) | (h/days) | (h/days) | (h/days) |
| 492/21 | 552/23 | 552/23 | 931/39 | 1136/60 | 2175/91 | 622/26 |
| F3-IBUNa | F4-IBU | F4-IBUNa | F5-IBU | F5-IBUNa | SDS | |
| (h/days) | (h/days) | (h/days) | (h/days) | (h/days) | (h/days) | |
| 1440/60 | 619/26 | 336/14 | 1067/44 | 2262/94 | 162/7 |
| Compound Name | Phase of Degradation (%/h) | ||
|---|---|---|---|
| Lag Phase | Degradation Phase | Plateau Phase | |
| IBU | 0–7/0–33 | 7–59/33–556 | 59–65/556–672 |
| IBUNa | 0–6/0–25 | 6–55/25–576 | 55–61/576–672 |
| F1-IBU | 0–6/0–30 | 6–58/30–652 | 58–65/652–672 |
| F1-IBUNa | 0–4/0–48 | 4–33/48–550 | 33–37/550–672 |
| F2-IBU | 0–2/0–48 | 2–21/48–662 | 21–23/662–672 |
| F2-IBUNa | 0–2/0–24 | 2–14/24–336 | 14–15/336–672 |
| F3-IBU | 0–6/0–40 | 6–56/40–632 | 56–62/632–672 |
| F3-IBUNa | 0–2/0–40 | 2–21/40–665 | 21–23/665–672 |
| F4-IBU | 0–6/0–47 | 6–53/47–649 | 53–58/649–672 |
| F4-IBUNa | 0–10/0–47 | 10–90/47–652 | 90–100/652–672 |
| F5-IBU | 0–3/0–48 | 3–28/48–648 | 28–32/648–672 |
| F5-IBUNa | 0–1/0–24 | 1–13/24–312 | 13–15/312–672 |
| SDS | 0–9/0–15 | 9–78/15–537 | 78–87/537–672 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharska, E.; Ok, B.; Nowak, A.; Kucharski, Ł.; Muzykiewicz-Szymańska, A.; Ossowicz-Rupniewska, P. Ibuprofen Formulations: Permeability and Biodegradability Comparison Depending on the Type of Formulation. Processes 2024, 12, 1236. https://doi.org/10.3390/pr12061236
Kucharska E, Ok B, Nowak A, Kucharski Ł, Muzykiewicz-Szymańska A, Ossowicz-Rupniewska P. Ibuprofen Formulations: Permeability and Biodegradability Comparison Depending on the Type of Formulation. Processes. 2024; 12(6):1236. https://doi.org/10.3390/pr12061236
Chicago/Turabian StyleKucharska, Edyta, Berin Ok, Anna Nowak, Łukasz Kucharski, Anna Muzykiewicz-Szymańska, and Paula Ossowicz-Rupniewska. 2024. "Ibuprofen Formulations: Permeability and Biodegradability Comparison Depending on the Type of Formulation" Processes 12, no. 6: 1236. https://doi.org/10.3390/pr12061236
APA StyleKucharska, E., Ok, B., Nowak, A., Kucharski, Ł., Muzykiewicz-Szymańska, A., & Ossowicz-Rupniewska, P. (2024). Ibuprofen Formulations: Permeability and Biodegradability Comparison Depending on the Type of Formulation. Processes, 12(6), 1236. https://doi.org/10.3390/pr12061236









