Bioremediation of Smog: Current Trends and Future Perspectives
Abstract
1. Introduction
2. Methodology
2.1. Literature Selection
2.2. Data Extraction and Analysis
3. Geographical Aspect of Smog
| Geographical Region | Concentration of PM (μg/m3) | NO2 (μg/m3) | Air Quality Index | Smog Status | Eradication Measures | References |
|---|---|---|---|---|---|---|
| Saudi Arabia (Jeddah) | 102 | 15.35 | 110 | Moderate | Biological air purification system to purify air in large buildings and industries | [18] |
| India (Delhi) | 60 | 15.2 | 136 (Unhealthy) | Intense | Phytoremediation using transgenic plants | [19] |
| China | 38.15 | 33 | 144 (Unhealthy) | Intense | Sulfate-reducing bacteria-assisted remediation of SOx; microbial electrochemical technology | [20,21] |
| Afghanistan | 60 | 17 | 157 (Unhealthy) | Intense | Algal bioremediation | [22] |
| Iran | 30 | - | 92 (Moderate) | Intense | Phytoremediation | [23,24] |
| South Africa | 21 | - | 89 (Moderate) | Moderate | Microbial remediation | [25] |
| United States of America | 9.6 | 11.3 | 10 (Good) | Moderate | Installment of effluent treatment plants to prevent the escape of industrial effluents into air; public transportation to reduce vehicular emissions; bioremediation of e-waste sites; recycling; CRISPR-Cas9-based bioremediation | [26] |
| Japan | 10.84 | 10 | 89 (Moderate) | Moderate | Phytoremediation; green buildings; transgenic microbial remediation; microalgae-based treatment; carbon filtering using phylloremediation | [27] |
| Australia | 3.1 | 8 | 40 (Good) | Moderate | Microbial remediation; genome editing-based bioremediation | [26] |
4. Health Effects of Smog
| Pollution | Chemical | Effects on Body System | Mechanism of Action | Citation |
|---|---|---|---|---|
| Smog | Ozone | Menstrual and pregnancy-related disturbances in females | Decline in progesterone and increment in estrogen level to alter luteinizing hormones | [45] |
| Smog | Particulate matter2.5 | Reduction in sperm count and disturbance in its motility | Increment in thiobarbituric acid in testes and decline of superoxide dismutase to increase SO2 levels | [46] |
| Smog | Persistent organic compounds | Obesity | Disruption in metabolic pathways, increment in oxidative stress leading to hormonal instability | [47] |
| Smog | Nitrogen and sulfur oxides | Acute coronary problems | Increment in blood pressure and obstruction in blood flow, leading to blood clotting | [48] |
| Smog | Genotoxic carcinogens (benzene) | Brain tumors | Interference with the growth and differentiation of cells at DNA level | [49] |
| Smog | Non-genotoxic carcinogen (dichlorobenzene) | Brain tumors | Increment of inflammation in the brain along with assisting the accumulation of arsenic to enhance oxidative stress in the brain | [49] |
| Smog | Ozone, NO2 | Bronchitis | Inhibition of β-catenin levels and production of IL-6 and interferon, causing inflammation in lungs | [50] |
| Smog | Ozone, PM2.5 | Intellectual and psychological problems | Impairment in cognitive abilities and social withdrawal due to anxiety and stress posed by air pollutants | [51] |
| Smog | Volatile organic compounds, SOx, ozone | Retinal problems and rhinorrhea | Release of histamine and neuropeptides to enhance mucous production in nose and dryness in eyes | [52] |
| Smog | PM2.5, SO2 | Alzheimer’s disease | Neuroinflammation due to the passage of cytokines from blood into brain | [53] |
5. Bioremediation of Smog via Bacteria
| Name of Bacteria | Effects | References |
|---|---|---|
| Corynebacterium sp. | 55% reduction in VOCs | [60] |
| Pseudomonas aeruginosa | 60% reduction in hydrocarbons | [61] |
| Flavobacterium sp. | 50% reduction in NOx | [62] |
| Azotobacter sp. | Sulfur compounds decreased by a whopping 70% | [63] |
| Nocardia sp. | 60% reduction in VOCs | [64] |
| Burkholderia sp. | 55% reduction in hydrocarbons | [65] |
| Nitrosomonas sp. | 65% decrease in nitrogen oxides | [66] |
| Sphingomonas sp. | 60% reduction in VOCs | [67] |
| Streptomyces sp. | 70% drop in carbon monoxide | [68] |
| Rhodococcus sp. | 50% reduction in NOx | [69] |
| Alcaligenes sp. | 45% reduction in VOCs | [70] |
| Micrococcus luteus | Sulfur compounds reduced by 40% | [71] |
| Acinetobacter sp. | Up to 55% less sulfur compounds | [72] |
| Bacillus subtilis | Nitric oxide levels also dropped by 60% | [73] |
| Pseudomonas putida | 50% reduction in hydrocarbons | [74] |
5.1. Mechanism of Air Pollutant Degradation by Bacteria
| Bacterial Species | Pollutant Type | Mechanism of Degradation | Effectiveness | References |
|---|---|---|---|---|
| Pseudomonas putida | Hydrocarbons | Oxygenase enzymes convert hydrocarbons to alcohols, acids, and CO2 | 50% reduction in hydrocarbons | [74] |
| Pseudomonas putida | Nitrogen oxides (NOx) | Nitrate reductase converts NOx to nitrogen gas (N2) via denitrification | 60% reduction in NOx | [73] |
| Acinetobacter sp. | Sulfur compounds | Sulfur-oxidizing enzymes oxidize sulfur compounds into sulfates | 55% reduction in sulfur compounds | [72] |
| Acinetobacter sp. | Ammonia | Ammonia monooxygenase converts ammonia (NH3) into nitrite (NO2−) | 65% reduction in ammonia | [76] |
| Pseudomonas fluorescens | BTEX (benzene, toluene, etc.) | Toluene dioxygenase degrades BTEX compounds in oxygen-limited conditions | Significant reduction in BTEX | [76] |
| Flavobacterium sp. | Nitrogen oxides (NOx) | Utilizes nitrate reductase to convert NOx into nitrogen gas | 50% reduction in NOx | [72] |
| Burkholderia sp. | Hydrocarbons | Oxygenase enzymes break down hydrocarbons | 55% reduction in hydrocarbons | [76] |
| Sphingomonas sp. | Volatile organic compounds (VOCs) | Degradation of VOCs was complemented by biofilms and biosurfactants | VOCs reduced to 60% | [82] |
| Streptomyces sp. | Carbon monoxide | Carbon monoxide dehydrogenase oxidizes CO to CO2 | 70% reduction in CO | [72] |
| Corynebacterium sp. | Volatile organic compounds (VOCs) | Hydrocarbon-degrading enzymes degrade VOCs into non-toxic byproducts | 55% reduction in VOCs | [76] |
| Rhodococcus sp. | Nitrogen oxides (NOx) | Nitrate reduction enzymes convert NOx into nitrogen gas | 50% reduction in NOx | [80] |
| Micrococcus luteus | Sulfur compounds | Sulfur oxidase enzymes degrade sulfur compounds into less harmful sulfates | 40% reduction in sulfur compounds | [72] |
| Sphingomonas sp. | Polycyclic aromatic hydrocarbons (PAHs) | Enhanced by organic matter in manure compost; utilizes oxygenase enzymes | 40% reduction in PAHs | [84] |
| Thiobacillus ferrooxidans | Inorganic sulfur compounds | Oxidation of sulfur compounds to sulfates using sulfur-oxidizing enzymes | Rapid oxidation of sulfur compounds | [83] |
| Pseudomonas fluorescens | BTEX (benzene, toluene, etc.) | Toluene dioxygenase (oxidation); breakdown under hypoxic conditions | Significant reduction in BTEX | [76] |
| Burkholderia sp. | Hydrocarbons | Oxygenase enzymes (oxidation) | 55% reduction in hydrocarbons | [82] |
| Methylosinus trichosporium OB3b | Nitrous oxide (N2O) | Methanobactin inhibits reduction in N2O (inhibition of denitrification) | Reduced N2O production by denitrifying bacteria | [85] |
| Sphingomonas sp. | PAHs | Oxygenation enhanced by water-extractable organic matter | Significant degradation of PAHs | [84] |
| Pseudomonas aeruginosa | Toluene | Unique metabolic route for toluene degradation (oxidation) | 70% degradation of toluene | [86] |
| Methanobactin OB3b | Nitrous oxide | Inhibits N2O reduction in denitrifiers | Inhibition of denitrification process | [87] |
5.2. Limiting Factors in Bacterial Bioremediation
| Limiting Factor | Impact on Bioremediation Efficiency | Bacteria | References |
|---|---|---|---|
| Pollutant concentration | High concentrations can inhibit bacterial growth and enzymatic activity | Pseudomonas aeruginosa | [82] |
| [86] | |||
| Bioavailability of pollutants | Limited solubility of hydrophobic pollutants reduces bacterial access | Pseudomonas fluorescens study on BTEX | [76] |
| Environmental pH | Extreme pH levels can denature bacterial enzymes, reducing effectiveness | Bacillus subtilis nitrogen removal study | [77] |
| Temperature fluctuations | Decreases bacterial metabolism and enzyme activity | Biofilter study for VOC removal | [81] |
| Cold temperatures slow down bacterial metabolic rates | Degradation of hydrocarbons in cold conditions | [88] | |
| Oxygen levels | Anaerobic conditions limit oxygen-dependent bacterial degradation | Study on BTEX degradation by oxygenase enzymes | [80] |
| Nutrient availability | Lack of nitrogen or phosphorus limits bacterial growth and activity | Study on phosphorus-enhanced hydrocarbon degradation | [79] |
| Presence of multiple pollutants | Bacteria may not effectively degrade mixed contaminants simultaneously | Inhibition of nitrous oxide reduction by Methylosinus | [86] |
| Bioremediation of CO and sulfur compounds | [72] | ||
| Toxicity of pollutants | Highly toxic compounds inhibit bacterial enzyme production and growth | Naphthalene degradation by Pseudomonas spp. | [20] |
| Inhibition of nitrous oxide reduction by Methylosinus | [85] | ||
| Moisture content | Low moisture reduces bacterial metabolic rates | Study on BTEX in soil bioreactors | [89] |
| Study on PAH degradation by Sphingomonas spp. | [84] | ||
| Aeration | Poor aeration limits aerobic degradation processes | Bacillus cereus and CO2 removal in bioreactor | [76] |
6. Mycoremediation of Smog
| Type of Bioremediation | Type of Microorganism | Effects | References |
|---|---|---|---|
| Vapor-phase bioreactors for VOC removal | Exophiala lecanii-corni, Cladosporium sphaerospermum, Cladosporium resinae, Mucor rouxii, Phanerochaete chrysosporium | Degradation of VOC | [95] |
| Biotrickling and biofilters for BTEX removal | Candida subhashii, Fusarium solani | BTEX removal 37.7 ± 3.3 g/m3 h | [96] |
| Soil bioremediation of TNT | Phanerochaete velutina | 70% TNT degradation in 49 days | [97] |
| Degradation of HMW-PAHs | Fusarium sp. strain ZH-H2 | Achieved 85.9% reduction in HMW-PAHs | [98] |
| Chlorobenzene removal by white-rot fungus | Phanerochaete chrysosporium | Achieved 95% chlorobenzene removal at 550 mg/m3 | [99] |
| Perchloroethylene degradation by white-rot fungus | Trametes versicolor | PCE degradation rates were 0.20 and 0.28 nmol/h mg | [100] |
| Hydrocarbon degradation | Purpureocillium lilacinum | Up to 15.3% weight loss | [101] |
| Hydrocarbon degradation | Penicillium chrysogenum | 7.6% degradation of hydrocarbons | [101] |
| VOC removal in biofilters | Arizona cypress, Pseudomonas fluorescens | Co-inoculation showed enhanced bioremediation; effective in reducing fuel pollution | [102] |
6.1. In Situ Strategies and Ex Situ Strategies
| Strategy | Mechanism | Pollutants Targeted | Control over Environmental Factors | Efficiency | Research Findings | References |
|---|---|---|---|---|---|---|
| In situ: bioventing | Oxygen introduced into the subsurface to stimulate aerobic fungal degradation. | VOCs, hydrocarbons, and organic pollutants in shallow soil. | Limited control over temperature, moisture, and airflow. | Moderate | Phanerochaete chrysosporium for PAH remediation (85.9% in 50 days). | [106] |
| In situ: bio-sparging | Air and nutrients injected into groundwater or soil to stimulate fungal biodegradation. | Hydrocarbons, VOCs, PAHs, and volatile pollutants. | Limited control; dependent on nutrient diffusion and oxygen levels. | Moderate | Pleurotus pulmonarius for dioxin degradation (96% degradation). | [108] |
| In situ: bio-stimulation | Nutrient addition (e.g., phosphorus) to stimulate fungal activity for pollutant degradation. | Hydrocarbons, nitrogenous compounds, and organic pollutants. | Minimal control; depends on soil nutrient distribution. | Moderate | Phosphorus-enhanced hydrocarbon degradation by Trichoderma in petroleum-contaminated soil. | [79] |
| Ex situ: bioreactors | Contaminated materials placed in bioreactors with controlled conditions for fungal degradation. | Persistent organic pollutants (POPs), PAHs, dioxins, and VOCs. | High control over temperature, pH, and oxygen levels. | High | White-rot fungi for PAH degradation in bioreactors, showing efficient removal of PAHs. | [109] |
| Ex situ: composting | Organic matter mixed with fungi in a controlled compost environment to enhance degradation. | Organic pollutants, hydrocarbons, heavy metals, and solid waste. | High control over temperature, moisture, and aeration. | High | Fungal treatment of compost using Trichoderma species shows enhanced pollutant breakdown. | [110] |
| Ex situ: landfarming | Contaminated soil spread over a designated area and tilled regularly for fungal degradation. | Hydrocarbons, PAHs, and semi-volatile organic compounds. | Moderate control over moisture and aeration. | Moderate | Mixed fungal remediation of hydrocarbon-contaminated soils; significant hydrocarbon degradation achieved. | [111] |
| Ex situ: biopiling | Contaminated soil piled and treated with controlled aeration and irrigation systems to enhance fungal activity. | Hydrocarbons, PAHs, and organic pollutants in solid waste. | High control over oxygen and moisture. | High | Mixed white-rot fungi used in biopiles for lignite degradation, showing increased floatation efficiency. | [112] |
| Ex situ: biofilters | Air or gas containing pollutants passed through a biofilter inoculated with fungi for degradation. | VOCs, hydrocarbons, BTEX, and α-pinene. | High control over air flow, moisture, and pollutant concentration. | High | Pseudomonas fluorescens and Alcaligenes xylosoxidans showed complete remediation. | [79] |
| Ex situ: vapor-phase bioreactors | Airborne pollutants treated in a vapor-phase bioreactor inoculated with fungal strains. | VOCs, hydrocarbons, and BTEX. | High control over all environmental conditions. | High | Fusarium solani for BTEX removal in a vapor-phase bioreactor (37.7 ± 3.3 g/m³ h). | [113] |
6.2. Advantages and Limitations of Fungal Methods
| Remediation Approach | Advantages | Limitations | Organism | References |
|---|---|---|---|---|
| Fungal bioremediation | High efficiency in degrading complex organic pollutants like dioxins, PAHs, and pesticides; enzymatic versatility | Slower growth rate; sensitive to environmental factors (pH, temperature); limited for mixed pollutants | Phanerochaete chrysosporium for PAHs | [118] |
| Bacterial bioremediation | Faster degradation rates; capable of handling mixed pollutants; efficient under various conditions | Limited to simpler organic pollutants; requires specific conditions like oxygen and nutrients | Pseudomonas putida for hydrocarbons | [74] |
| Phytoremediation | Cost-effective; improves soil structure; long-term solution | Slow process; limited to shallow-rooted plants; not effective for volatile pollutants | Study on heavy metal removal by plants | [120] |
| Nano-remediation | Highly effective in removing small concentrations of pollutants; rapid degradation | Potential environmental toxicity; high cost; limited large-scale applications | Study on ZnS nanoparticles with A. niger | [115] |
| Chemical remediation | Immediate pollutant breakdown; effective for a wide range of pollutants | High cost; secondary pollution; not environmentally friendly | General chemical treatment for VOCs | [118] |
| Phytoremediation (enhanced) | Bio-stimulation can enhance phytoremediation, making it more effective for metal removal | Slow process; dependent on environmental conditions; limited scope | Phyto-enhanced bioremediation | [103] |
| Nanoparticle-enhanced remediation | Increases the efficiency of microbial degradation by improving bioavailability and pollutant breakdown | Environmental risks due to nanoparticles; potential toxicity | Study with ZnS nanoparticles | [115] |
7. Nano-Remediation of Smog
| Type of Nanomaterial | Efficiency | References |
|---|---|---|
| Silver nanoparticles | Cutting particulates by 75% | [133] |
| Titanium dioxide-loaded platinum nanoparticles | 2.4-fold increase in CO oxidation to CO2 | [134] |
| Zinc oxide nanoparticles | 45–50% decrease in sulfur compounds | [135] |
| Gold nanoparticles | 55% drop in carbon monoxide | [136] |
| Copper nanoparticles | 65% reduction in hydrocarbons | [137] |
| Silica nanoparticles | 60% reduction in VOCs | [138] |
| Aluminum oxide nanoparticles | Lowered sulfur compounds by 55% | [139] |
| Platinum nanoparticles | Nitrogen oxides down by 70% | [140] |
| Nickel nanoparticles | 50% reduction in VOCs | [141] |
| Cobalt nanoparticles | 55% reduction in NOx | [142] |
| Graphene oxide nanoparticles | 65% reduction in hydrocarbons | [143] |
| Cerium oxide nanoparticles | Lowered CO by 60% | [144] |
| Manganese oxide nanoparticles | 55% less sulfur compounds | [145] |
| Palladium nanoparticles | 60% reduction in VOCs | [146] |
8. Phytoremediation of Smog
| Type of Plants | Effects | References |
|---|---|---|
| Dracaena fragrans (Golden Coast) | Removal of up to 3 ppb NO2 per m2 of leaf area over a 1 h test period. | [173] |
| Caesalpinia gilliesii and Robinia pseudoacacia | The air pollution tolerance index (APTI) was species-specific; ascorbic acid was crucial for Robinia pseudoacacia (88.1%) and Caesalpinia gilliesii (78.9%). | [174] |
| Eucalyptus camaldulensis | pH of leaf extract was dominant in Eucalyptus camaldulensis (45.7%). | [174] |
| Clerics siliquastrum | Total chlorophyll content was most significant in Clerics siliquastrum (56.1%). | [174] |
| Portable active green wall with unspecified plant species | The active green wall achieved single-pass removal efficiencies of 56.42 ± 21.02% for PM2.5 and 20.73 ± 0.87% for O3. | [175] |
| Nephrolepis exaltata and Spathiphyllum wallisii | Nephrolepis exaltata and Spathiphyllum wallisii removed CO2 by 45.4–51% and VOCs by 36.2–42.7%. | [176] |
| Dypsis lutescens and Latania Livistona | Dypsis lutescens and Latania Livistona achieved CO2 removal of 40.9–41.8% and VOC removal of 46–47.8%. | [176] |
| Epipremnum aureum | Epipremnum aureum removed CO2 by 35.6–38.6% and VOCs by 32–34.3%. | [176] |
| Vigna radiata | Formaldehyde removal rates increased with microbial addition. Vigna radiata showed the highest enhancement, with 97.6 ± 0.9 μg/h/g and an 88.7% increase over the 25.1 ± 4.2 μg/h/g without microbes. | [177] |
| Tradescantia zebrina | Tradescantia zebrina had a removal rate of 86.4 ± 0.7 μg/h/g with microbes compared to 59.3 ± 0.2 μg/h/g without, showing a 45.6% increase. | [177] |
| Aloe vera | Aloe vera achieved 23.1 ± 0.1 μg/h/g with microbes versus 18.5 ± 0.21 μg/h/g without, a 24.9% improvement. | [177] |
| A vegetation biofilter | The vegetation biofilter achieved an average single-pass removal efficiency of 20% for isobutylene at 5000 ppm. | [178] |
| Agave americana | 18.40 for the air pollution tolerance index (APTI). | [179] |
| Cassia roxburghii | Tolerance index (APTI) for selected plants is Cassia roxburghii at 17.63. | [179] |
| Anacardium occidentale | Tolerance index (APTI) is 11.97. | [179] |
| Cassia fistula | Tolerance index (APTI) for selected plants is Cassia fistula at 11.60. | [179] |
| Mangifera indica | Tolerance index (APTI) is 11.59. | [179] |
| Saraca asoca | Tolerance index (APTI) is 10.88. | [179] |
| Spathiphyllum wallisii | 70% reduction in benzene. | [180] |
| Sansevieria trifasciata | 60% reduction in toluene level. | [180] |
| Gerbera jamesonii | Decreased xylene concentrations by approximately 50–60%. | [180] |
| No specific particular types | Plant clean air delivery rates (CADRs) were low, with a median value of 0.023 m3/h. | [181] |
| Madhuca longifolia | Madhuca longifolia had the highest APTI values based on pH, ascorbic acid content, relative water content, and total chlorophyll content | [182] |
| Cyperus and Brachiaria spp. | Cyperus and Brachiaria spp. showed significant potential in phytoremediation processes. | [183] |
| Nephrolepis sp. | Nephrolepis also yielded favorable results for organic contaminants. | [183] |
| Acacia sp. | Oil emulsion: 48% oil, suspension: 23%, settled emulsion: 42%, and sludge emulsion: 36%. | [184] |
| Lactuca sativa | Dieldrin removal rates: 50–78%. | [185] |
| Raphanus sativus | 50–78% | [185] |
8.1. Phytoremediation Mechanism
8.1.1. Phytoextraction
8.1.2. Phytovolatilization
8.1.3. Phytodegradation
8.1.4. Phytostabilization
8.1.5. Rhizodegradation
8.1.6. Rhizo-Filtration
8.2. Phytoremediation of Particular Matter
8.3. Phytoremediation of Inorganic Air Pollutants
8.4. Phytoremediation of VOCs
8.5. Phytoremediation and CRISPR-Cas9
8.6. Efficacy of Plants in Urban Phytoremediation and Major Challenges
8.7. Major Challenges in Scaling up Phytoremediation for Urban Air Pollution
9. Phylloremediation of Smog
10. Cost-Effectiveness and Challenges of Bioremediation
10.1. Limitations of Bacteria
10.2. Limitations of Fungi
10.3. Limitations of Nano-Remediation
10.4. Limitations and Challenges for Phytoremediation
10.5. Ethical and Ecological Concerns of GMOs
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| VOC | Volatile organic compound |
| NOx | Nitrogen oxides |
| SOx | Sulfur oxides |
| AQI | Air quality index |
| PM | Particulate matter |
| CO | Carbon monoxide |
| O3 | Ozone |
| SO2 | Sulfur dioxide |
| COPD | Chronic obstructive pulmonary disease |
| WHO | World Health Organization |
| POCs | Persistent organic compounds |
| EPA | Environmental Protection Agency |
| MBR | Membrane bioreactor system |
| BTX | Benzene, toluene, and xylene |
| PAHs | Polyaromatic hydrocarbons |
| IONPs | Iron oxide nanoparticles |
| MF | Modified Fenton |
| APTI | Air pollution tolerance index |
| PHB | Polyhydroxy butyrate |
| PWHCs | Petroleum waste hydrocarbons |
| HGT | Horizontal gene transfer |
| GMOs | Genetically modified organisms |
| DDT | 1,1-dichloro-2,2-bis(p-chlorophenyl)ethane |
| HVAC | Heating, ventilation, and air conditioning |
References
- Suruchi; Singh, S. Removal of Toxic Chemicals from Air Through Phytoremediation. In Phytoremediation: Biological Treatment of Environmental Pollution; Springer Nature: Cham, Switzerland, 2024; pp. 75–100. [Google Scholar]
- Woodrow, J.E.; Gibson, K.A.; Seiber, J.N. Pesticides and related toxicants in the atmosphere. Rev. Environ. Contam. Toxicol. 2019, 247, 147–196. [Google Scholar]
- Whittaker, A.; BeruBe, K.; Jones, T.; Maynard, R.; Richards, R. Killer smog of London, 50 years on: Particle properties and oxidative capacity. Sci. Total Environ. 2004, 334, 435–445. [Google Scholar] [CrossRef]
- Gaffney, J.S.; Marley, N.A.; Frederick, J.E. Formation and Effects of Smog. Environmental and Ecological Chemistry; Sabljic, A., Ed.; Eolss Publishers Co., Ltd.: Oxford, UK, 2009; Volume 2, pp. 25–51. [Google Scholar]
- Wu, Y.; Zhang, L.; Wang, J.; Mou, Y. Communicating air quality index information: Effects of different styles on individuals’ risk perception and precaution intention. Int. J. Environ. Res. Public Health 2021, 18, 10542. [Google Scholar] [CrossRef]
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Beelen, R.; Wang, M.; Hoek, G.; Andersen, Z.J.; Hoffmann, B.; Stafoggia, M.; Samoli, E.; Weinmayr, G.; Dimakopoulou, K.; et al. Particulate matter air pollution components and risk for lung cancer. Environ. Int. 2016, 87, 66–73. [Google Scholar] [CrossRef]
- Khaltaev, N.; Axelrod, S. Chronic respiratory diseases global mortality trends, treatment guidelines, life style modifications, and air pollution: Preliminary analysis. J. Thorac. Dis. 2019, 11, 2643. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques—Classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 1–18. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines. 22 September 2021. Available online: https://www.who.int/news-room/questions-and-answers/item/who-global-air-quality-guidelines (accessed on 15 July 2024).
- Moyebi, O.D.; Fatmi, Z.; Carpenter, D.O.; Santoso, M.; Siddique, A.; Khan, K.; Zeb, J.; Hussain, M.M.; Khwaja, H.A. Fine particulate matter and its chemical constituents’ levels: A troubling environmental and human health situation in Karachi, Pakistan. Sci. Total Environ. 2023, 868, 161474. [Google Scholar] [CrossRef]
- Mandal, M.; Popek, R.; Przybysz, A.; Roy, A.; Das, S.; Sarkar, A. Breathing fresh air in the city: Implementing avenue trees as a sustainable solution to reduce particulate pollution in urban agglomerations. Plants 2023, 12, 1545. [Google Scholar] [CrossRef]
- Basak, P.; Dey, S.; Elahi, K.M. Air Pollution in Urban Bangladesh from Climate Change and Public Health Perspectives. In Climate Change and Human Health Scenarios: International Case Studies; Springer Nature: Cham, Switzerland, 2024; pp. 129–149. [Google Scholar]
- Liaqut, A.; Tariq, S.; Younes, I. A study on optical properties, classification, and transport of aerosols during the smog period over South Asia using remote sensing. Environ. Sci. Pollut. Res. 2023, 30, 69096–69121. [Google Scholar] [CrossRef]
- Hooftman, N.; Messagie, M.; Van Mierlo, J.; Coosemans, T. A review of the European passenger car regulations—Real driving emissions vs local air quality. Renew. Sustain. Energy Rev. 2018, 86, 1–2. [Google Scholar] [CrossRef]
- Wrightson, S. Associations between Population Density, Air Pollution Exposure and Related Health Outcomes in Auckland. Ph.D. Thesis, The University of Auckland, Auckland, New Zealand, 2023. [Google Scholar]
- Khan, W.A.; Sharif, F.; Khokhar, M.F.; Shahzad, L.; Ehsan, N.; Jahanzaib, M. Monitoring of ambient air quality patterns and assessment of air pollutants’ correlation and effects on ambient air quality of Lahore, Pakistan. Atmosphere 2023, 14, 1257. [Google Scholar] [CrossRef]
- Tazeem, S.B.; Nabeel, A.; Athar, H. Spatio-temporal co-variability of air pollutants and meteorological variables over Haqel and Jeddah, Saudi Arabia. Atmósfera 2023, 37, 131–158. [Google Scholar] [CrossRef]
- Gupta, L.; Bansal, M.; Nandi, P.; Habib, G.; Raman, R.S. Source apportionment and potential source regions of size-resolved particulate matter at a heavily polluted industrial city in the Indo-Gangetic Plain. Atmos. Environ. 2023, 298, 119614. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Q.; Niu, Y.; Kan, H.; Chen, R. Fine particulate matter and cardiorespiratory health in China: A systematic review and meta-analysis of epidemiological studies. J. Environ. Sci. 2023, 123, 306–316. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, X.; Wang, Y.; Cheng, Y.; Fan, W. Long-term bioremediation of cadmium contaminated sediment using sulfate reducing bacteria: Perspective on different depths of the sediment profile. Chem. Eng. J. 2023, 451, 138697. [Google Scholar] [CrossRef]
- Salehie, O.; Jamal, M.H.B.; Shahid, S. Characterization and prediction of PM2.5 levels in Afghanistan using machine learning techniques. Theor. Appl. Climatol. 2024, 155, 9081–9097. [Google Scholar] [CrossRef]
- Kazemi, Z.; Kazemi, Z.; Jafari, A.J.; Farzadkia, M.; Hosseini, J.; Amini, P.; Shahsavani, A.; Kermani, M. Estimating the health impacts of exposure to Air pollutants and the evaluation of changes in their concentration using a linear model in Iran. Toxicol. Rep. 2024, 12, 56–64. [Google Scholar] [CrossRef]
- Taghizadeh, F.; Mokhtarani, B.; Rahmanian, N. Air pollution in Iran: The current status and potential solutions. Environ. Monit. Assess. 2023, 195, 737. [Google Scholar] [CrossRef]
- Ndletyana, O.; Madonsela, B.S.; Maphanga, T. Spatial Distribution of PM10 and NO2 in Ambient Air Quality in Cape Town CBD, South Africa. Nat. Environ. Pollut. Technol. 2023, 22, 1–13. [Google Scholar] [CrossRef]
- Air Quality Database. n.d. Available online: https://www.who.int/data/gho/data/themes/air-pollution/who-air-quality-database (accessed on 21 July 2024).
- Samudro, G.; Samudro, H.; Mangkoedihardjo, S. Healthy building phytoarchitecture requires essential criteria for sustainable phylloremediation of contaminated indoor air. Int. J. Adv. Appl. Sci. 2024, 13, 662–672. [Google Scholar] [CrossRef]
- Jin, S.; Fallgren, P.H. Feasibility of using bioelectrochemical systems for bioremediation. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 493–507. [Google Scholar]
- Vardoulakis, S.; Marks, G.; Abramson, M.J. Lessons learned from the Australian bushfires: Climate change, air pollution, and public health. JAMA Intern. Med. 2020, 180, 635–636. [Google Scholar] [CrossRef]
- Khan, M.J.; Wibowo, A.; Karim, Z.; Posoknistakul, P.; Matsagar, B.M.; Wu, K.C.W.; Sakdaronnarong, C. Wastewater Treatment Using Membrane Bioreactor Technologies: Removal of Phenolic Contaminants from Oil and Coal Refineries and Pharmaceutical Industries. Polymers 2024, 16, 443. [Google Scholar] [CrossRef]
- Hussain, S.; Hoque, R.R. Ecological and natural-based solutions as green growth strategies for disaster and emergency management of air pollution extremes. In Extremes in Atmospheric Processes and Phenomenon: Assessment, Impacts and Mitigation; Springer Nature: Singapore, 2022; pp. 369–395. [Google Scholar]
- Usman, N.; Atta, H.I.; Tijjani, M.B. Biodegradation studies of benzene, toluene, ethylbenzene and xylene (BTEX) compounds by Gliocladium sp. and Aspergillus terreus. J. Appl. Sci. Environ. Manag. 2020, 24, 1063–1069. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent insights into particulate matter (PM2.5)-mediated toxicity in humans: An overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Gavett, S.H.; Koren, H.S. The role of particulate matter in exacerbation of atopic asthma. Int. Arch. Allergy Immunol. 2001, 124, 109–112. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; Skosana, B.T.; Ferguson, L.M.; Ramsunder, Y.; Ayad, B.M.; Du Plessis, S.S. Implications of Exposure to Air Pollution on Male Reproduction: The Role of Oxidative Stress. Antioxidants 2024, 13, 64. [Google Scholar] [CrossRef]
- Fan, W.; Zlatnik, M.G. Climate change and pregnancy: Risks, mitigation, adaptation, and resilience. Obstet. Gynecol. Surv. 2023, 78, 223–236. [Google Scholar] [CrossRef]
- Headon, K.S. The Association between Air Pollution Exposure and the Risk of Postpartum Depression and Gestational Diabetes Mellitus during the COVID-19 Pandemic. Ph.D. Thesis, UC Irvine, Irvine, CA, USA, 2024. [Google Scholar]
- Sheppard, N.; Carroll, M.; Gao, C.; Lane, T. Particulate matter air pollution and COVID-19 infection, severity, and mortality: A systematic review and meta-analysis. Sci. Total Environ. 2023, 880, 163272. [Google Scholar] [CrossRef]
- Qayyum, F.; Tariq, S.; Nawaz, H.; ul-Haq, Z.; Mehmood, U.; Babar, Z.B. Variation of air pollutants during COVID-19 lockdown phases in the mega-city of Lahore (Pakistan); Insights into meteorological parameters and atmospheric chemistry. Acta Geophys. 2024, 72, 2083–2096. [Google Scholar] [CrossRef]
- Naveed, Z.; Khayyam, U. Smog and cognitive issues in the school going children of Lahore and Islamabad, Pakistan. Int. J. Environ. Sci. Technol. 2023, 20, 4151–4166. [Google Scholar] [CrossRef]
- Berg, C.D.; Schiller, J.H.; Boffetta, P.; Cai, J.; Connolly, C.; Kerpel-Fronius, A.; Kitts, A.B.; Lam, D.C.L.; Mohan, A.; Myers, R.; et al. Air pollution and lung cancer: A review by International Association for the Study of Lung Cancer Early Detection and Screening Committee. J. Thorac. Oncol. 2023, 18, 1277–1289. [Google Scholar] [CrossRef]
- Aslam, R.; Sharif, F.; Baqar, M.; Nizami, A.S.; Ashraf, U. Role of ambient air pollution in asthma spread among various population groups of Lahore City: A case study. Environ. Sci. Pollut. Res. 2023, 30, 8682–8697. [Google Scholar] [CrossRef]
- Edgar, V.N.; Hermes, P.H.; Denisse, V.G.J.; Andrea, P.M.; Roberto, S.C.C.; Ileana, V.R.; Fabián, F.L. Microorganisms, Plants, and Nanotechnology for Environmental Remediation: A Sustainable Prospect. In Nano-Bioremediation for Water and Soil Treatment; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 43–97. [Google Scholar]
- Mocek-Płóciniak, A.; Mencel, J.; Zakrzewski, W.; Roszkowski, S. Phytoremediation as an effective remedy for removing trace elements from eco-systems. Plants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Saleem, A.; Awan, T.; Akhtar, M.F. A comprehensive review on endocrine toxicity of gaseous components and particulate matter in smog. Front. Endocrinol. 2024, 15, 1294205. [Google Scholar] [CrossRef]
- Meng, Z.; Bai, W. Oxidation damage of sulfur dioxide on testicles of mice. Environ. Res. 2024, 96, 298–304. [Google Scholar] [CrossRef]
- Ćurčić, M.; Esteban, J.; Cakmak, G.; Durgo, K.; Baralić, K.; Živanović, J.; Marić, D.; Đorđević, A.B.; Miljaković, E.A.; Bulat, Z.; et al. Environmental pollutants and the obesity: Proven causalities and open questions. Arch. Pharm. 2024, 74, 426–435. [Google Scholar] [CrossRef]
- Kurasz, A.; Święczkowski, M.; Dąbrowski, E.J.; Bachórzewska-Gajewska, H.; Tomaszuk-Kazberuk, A.; Roszkowska, S.; Dobrzycki, S.; Kuźma, Ł. The impact of polish smog on public regional health–baseline results of the EP-PARTICLES study. J. Health Inequalities 2023, 9, 73–80. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Savarese, B.; Avilia, G.; Giarra, A.; Pagano, G.; Marano, A.; Trifuoggi, M.; Bifulco, M.; et al. Impacts of environmental pollution on brain tumorigenesis. Int. J. Mol. Sci. 2023, 24, 5045. [Google Scholar] [CrossRef]
- Usman, M.; Amjad, S.; Khan, A. Clearing the Air: Legal Strategies for Combating Smog and Pollution. J. Strateg. Policy Glob. Aff. 2023, 4, 15–21. [Google Scholar] [CrossRef]
- Abubakar, M.; Fatima, Z.; Riaz, N.; Khursheed, A.; Imtiaz, A. Psychological and Behavioral Effects of Smog on University Students. J. Appl. Res. Multidiscip. Stud. 2023, 4, 67–78. [Google Scholar]
- Mahmood, S.; Ali, A.; Jumaah, H.J. Geo-visualizing the hotspots of smog-induced health effects in district Gujranwala, Pakistan: A community perspective. Environ. Monit. Assess. 2024, 196, 457. [Google Scholar] [CrossRef]
- Olloquequi, J.; Díaz-Peña, R.; Verdaguer, E.; Ettcheto, M.; Auladell, C.; Camins, A. From Inhalation to Neurodegeneration: Air Pollution as a Modifiable Risk Factor for Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 6928. [Google Scholar] [CrossRef]
- Antony, S.; Antony, S.; Rebello, S.; George, S.; Biju, D.T.; Reshmy, R.; Madhavan, A.; Binod, P.; Pandey, A.; Sindhu, R.; et al. Bioremediation of endocrine disrupting chemicals-advancements and challenges. Environ. Res. 2022, 213, 113509. [Google Scholar] [CrossRef]
- Apollon, W.; Flores-Breceda, H.; Méndez-Zamora, G.; Gómez-Leyva, J.F.; Luna-Maldonado, A.I.; Kamaraj, S.K. Importance of Genetically Engineered Microbes (GEMs) in Bioremediation of Environmental Pollutants: Recent Advances and Challenges. In Omics for Environmental Engineering and Microbiology Systems; CRC Press: Boca Raton, FL, USA, 2022; pp. 203–219. [Google Scholar]
- Olak-Kucharczyk, M.; Festinger, N.; Smułek, W. Application of Ozonation-Biodegradation Hybrid System for Polycyclic Aromatic Hydrocarbons Degradation. Int. J. Environ. Res. Public Health 2023, 20, 5347. [Google Scholar] [CrossRef]
- Bai, S.; Du, S.; Liu, H.; Lin, S.; Zhao, X.; Wang, Z.; Wang, Z. The causal and independent effect of ozone exposure during pregnancy on the risk of preterm birth: Evidence from northern China. Environ. Res. 2022, 214, 113879. [Google Scholar] [CrossRef]
- Umar, M.F.; Rafatullah, M.; Abbas, S.Z.; Mohamad Ibrahim, M.N.; Ismail, N. Advancement in benthic microbial fuel cells toward sustainable bioremediation and renewable energy production. Int. J. Environ. Res. Public Health 2021, 18, 3811. [Google Scholar] [CrossRef]
- Bhandari, S. Environmental Pollution Caused by Organic Pollutants, Their Harmful Impacts, and Treatment through a Microbiological Approach. In Nano-Phytoremediation and Environmental Pollution; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–36. [Google Scholar]
- Cui, Y.; Zhang, H.; Zhang, J.; Lv, B.; Xie, B. The emission of volatile organic compounds during the initial decomposition stage of food waste and its relationship with the bacterial community. Environ. Technol. Innov. 2022, 27, 102443. [Google Scholar] [CrossRef]
- Hamza, L.H.; Adly, T.A.; Afifi, S. Bioremediation—The Journey from Hazardous to Green. In Proceedings of the SPE International Conference and Exhibition on Health, Safety, Environment, and Sustainability, Nice, France, 15–17 April 2008; SPE: Kuala Lumpur, Malaysia, 2008; p. SPE-111620. [Google Scholar]
- Ranalli, G.; Matteini, M.; Tosini, I.; Zanardini, E.; Sorlini, C. Bioremediation of cultural heritage: Removal of sulphates, nitrates and organic substances. In Of Microbes and Art: The Role of Microbial Communities in the Degradation and Protection of Cultural Heritage; Springer: Boston, MA, USA, 2000; pp. 231–245. [Google Scholar]
- Nikiema, J.; Dastous, P.A.; Heitz, M. Elimination of volatile organic compounds by bioflltration: A review. Rev. Environ. Health 2007, 22, 273–294. [Google Scholar] [CrossRef]
- Maurya, A.; Sharma, D.; Partap, M.; Kumar, R.; Bhargava, B. Microbially-assisted phytoremediation toward air pollutants: Current trends and future directions. Environ. Technol. Innov. 2023, 31, 103140. [Google Scholar] [CrossRef]
- Khalid, F.E.; Lim, Z.S.; Sabri, S.; Gomez-Fuentes, C.; Zulkharnain, A.; Ahmad, S.A. Bioremediation of diesel contaminated marine water by bacteria: A review and bibliometric analysis. J. Mar. Sci. Eng. 2021, 9, 155. [Google Scholar] [CrossRef]
- Khamis, M. Pollution and its effects on life. Microreviews Cell Mol. Biol. 2017, 2. [Google Scholar]
- Rahul Saxena, R.; Kumar, S.; Pal, D.B. Volatile Organic Compounds Impacts on Environment: Biofiltration as an Effective Control Method. In Sustainable Valorization of Agriculture & Food Waste Biomass: Application in Bioenergy & Useful Chemicals; Springer Nature: Singapore, 2023; pp. 51–69. [Google Scholar]
- Barla, R.J.; Gupta, S.; Raghuvanshi, S. Sustainable synergistic approach to chemolithotrophs—Supported bioremediation of wastewater and flue gas. Sci. Rep. 2024, 14, 16529. [Google Scholar] [CrossRef]
- Sheoran, K.; Siwal, S.S.; Kapoor, D.; Singh, N.; Saini, A.K.; Alsanie, W.F.; Thakur, V.K. Air pollutants removal using biofiltration technique: A challenge at the frontiers of sustainable environment. ACS Eng. Au 2022, 2, 378–396. [Google Scholar] [CrossRef]
- Gopinath, M.; Pulla, R.H.; Rajmohan, K.S.; Vijay, P.; Muthukumaran, C.; Gurunathan, B. Bioremediation of volatile organic compounds in biofilters. In Bioremediation: Applications for Environmental Protection and Management; Springer: Singapore, 2018; pp. 301–330. [Google Scholar]
- Adepoju, A.O.; Omotoso, I.O.; Tiamiyu, O.G. Air pollution: Prevention and control strategies. In Environmental Pollution and Public Health; Elsevier: Amsterdam, The Netherlands, 2024; pp. 49–62. [Google Scholar]
- Ganguly, P.; Mandal, J.; Sengupta, S.; Sinha, B. Basics of Environment, Pollution, and Bioremediation Techniques. In Environmental Contaminants; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 1–17. [Google Scholar]
- Kour, D.; Khan, S.S.; Kour, H.; Kaur, T.; Devi, R.; Rai, P.K.; Judy, C.; McQuestion, C.; Bianchi, A.; Spells, S.; et al. Microbe-mediated bioremediation: Current research and future challenges. J. Appl. Biol. Biotechnol. 2022, 10, 6–24. [Google Scholar] [CrossRef]
- Qamar, S.A.; Bhatt, P.; Ghotekar, S.; Bilal, M. Nanomaterials for bioremediation of air pollution. In Nano-Bioremediation: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–261. [Google Scholar]
- Anand, A.; Raghuvanshi, S.; Gupta, S. Assessing the bacterial consortium’s potential to bio-mitigate CO2 and SO2 from simulated flue gas, wastewater bioremediation, and product characterization. Process Biochem. 2024, 147, 86–100. [Google Scholar] [CrossRef]
- Lu, G.; Clement, T.P.; Zheng, C.; Wiedemeier, T.H. Natural attenuation of BTEX compounds: Model development and field-scale application. Groundwater 1999, 37, 707–717. [Google Scholar] [CrossRef]
- Yang, T.; Xin, Y.; Zhang, L.; Gu, Z.; Li, Y.; Ding, Z.; Shi, G. Characterization on the aerobic denitrification process of Bacillus strains. Biomass Bioenergy 2020, 140, 105677. [Google Scholar] [CrossRef]
- Kleinheinz, G.T.; Bagley, S.T.; St John, W.P.; Rughani, J.R.; McGinnis, G.D. Characterization of alpha-pinene-degrading microorganisms and application to a bench-scale biofiltration system for VOC degradation. Arch. Environ. Contam. Toxicol. 1999, 37, 151–157. [Google Scholar] [CrossRef]
- Moehlman, L.M. Biostimulatory Solutions for PHC Contaminated Sites: Effects of C: N: P Ratios on Degrader Prevalence and Potential Activity. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2018. [Google Scholar]
- Hu, F.; Wang, P.; Li, Y.; Ling, J.; Ruan, Y.; Yu, J.; Zhang, L. Bioremediation of environmental organic pollutants by Pseudomonas aeruginosa: Mechanisms, methods and challenges. Environ. Res. 2023, 239, 117211. [Google Scholar] [CrossRef]
- Sun, C.; Yuan, J.; Xu, H.; Huang, S.; Wen, X.; Tong, N.; Zhang, Y. Simultaneous removal of nitric oxide and sulfur dioxide in a biofilter under micro-oxygen thermophilic conditions: Removal performance, competitive relationship and bacterial community structure. Bioresour. Technol. 2019, 290, 121768. [Google Scholar] [CrossRef]
- Karimi, B.; Habibi, M.; Esvand, M. Biodegradation of naphthalene using Pseudomonas aeruginosa by up flow anoxic–aerobic continuous flow combined bioreactor. J. Environ. Health Sci. Eng. 2015, 13, 26. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Landesman, J.; Duncan, D.W.; Walden, C.C. Oxidation of inorganic sulfur compounds by washed cell suspensions of Thiobacillus ferrooxidans. Can. J. Microbiol. 1966, 12, 957–964. [Google Scholar] [CrossRef]
- Kobayashi, T.; Murai, Y.; Tatsumi, K.; Iimura, Y. Biodegradation of polycyclic aromatic hydrocarbons by Sphingomonas sp. enhanced by water-extractable organic matter from manure compost. Sci. Total Environ. 2009, 407, 5805–5810. [Google Scholar] [CrossRef]
- Chang, J.; Peng, P.; DiSpirito, A.A.; Semrau, J.D. Variable inhibition of nitrous oxide reduction in denitrifying bacteria by different forms of methanobactin. Appl. Environ. Microbiol. 2022, 88, e02346-21. [Google Scholar] [CrossRef]
- Muccee, F.; Ejaz, S.; Riaz, N. Toluene degradation via a unique metabolic route in indigenous bacterial species. Arch. Microbiol. 2019, 201, 1369–1383. [Google Scholar] [CrossRef]
- Chang, J.; Gu, W.; Park, D.; Semrau, J.D.; DiSpirito, A.A.; Yoon, S. Methanobactin from Methylosinus trichosporium OB3B inhibits N2O reduction in denitrifiers. ISME J. 2018, 12, 2086–2089. [Google Scholar] [CrossRef]
- Isola, D.; Scano, A.; Orrù, G.; Prenafeta-Boldú, F.X.; Zucconi, L. Hydrocarbon-contaminated sites: Is there something more than Exophiala xenobiotica? New insights into black fungal diversity using the long cold incubation method. J. Fungi 2021, 7, 817. [Google Scholar] [CrossRef]
- Prenafeta-Boldú, F.X.; Ballerstedt, H.; Gerritse, J.; Grotenhuis, J.T.C. Bioremediation of BTEX hydrocarbons: Effect of soil inoculation with the toluene-growing fungus Cladophialophora sp. strain T1. Biodegradation 2004, 15, 59–65. [Google Scholar] [CrossRef]
- Soares, P.R.S.; Birolli, W.G.; Ferreira, I.M.; Porto, A.L.M. Biodegradation pathway of the organophosphate pesticides chlorpyrifos, methyl parathion and profenofos by the marine-derived fungus Aspergillus sydowii CBMAI 935 and its potential for methylation reactions of phenolic compounds. Mar. Pollut. Bull. 2021, 166, 112185. [Google Scholar] [CrossRef]
- Pointing, S. Feasibility of bioremediation by white-rot fungi. Appl. Microbiol. Biotechnol. 2001, 57, 20–33. [Google Scholar]
- Kumari, M.; Ghosh, P.; Joshi, S.; Thakur, I. Microcosmic study of endosulfan degradation by Paenibacillus sp. ISTP10 and its toxicological evaluation using mammalian cell line. Int. Biodeterior. Biodegrad. 2014, 96, 33–40. [Google Scholar] [CrossRef]
- Nadhman, A.; Hasan, F.; Shah, Z.; Hameed, A.; Shah, A. Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) depolymerase from Aspergillus sp. NA-25. Appl. Biochem. Microbiol. 2012, 48, 482–487. [Google Scholar] [CrossRef]
- Qi, B.; Moe, W.; Kinney, K. Biodegradation of volatile organic compounds by five fungal species. Appl. Microbiol. Biotechnol. 2002, 58, 684–689. [Google Scholar]
- Marycz, M.; Brillowska-Dąbrowska, A.; Cantera, S.; Gębicki, J.; Muñoz, R. Fungal co-culture improves the biodegradation of hydrophobic VOCs gas mixtures in conventional biofilters and biotrickling filters. Chemosphere 2023, 313, 137609. [Google Scholar] [CrossRef]
- Anasonye, F.; Winquist, E.; Räsänen, M.; Kontro, J.; Björklöf, K.; Vasilyeva, G.; Jørgensen, K.S.; Steffen, K.T.; Tuomela, M. Bioremediation of TNT contaminated soil with fungi under laboratory and pilot scale conditions. Int. Biodeterior. Biodegrad. 2015, 105, 7–12. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Li, C.; Zhang, L.; Ning, G.; Shi, W.; Zhang, X.; Yang, Z. Ligninolytic enzyme involved in removal of high molecular weight polycyclic aromatic hydrocarbons by Fusarium strain ZH-H2. Environ. Sci. Pollut. Res. 2020, 27, 42969–42978. [Google Scholar] [CrossRef]
- Wang, C.; Xi, J.-Y.; Hu, H.-Y.; Wen, X.-H. Biodegradation of gaseous chlorobenzene by white-rot fungus Phanerochaete chrysosporium. Biomed. Environ. Sci. 2008, 21, 474–478. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Gabarrell, X.; Sarrà, M.; Caminal, G.; Vicent, T.; Reddy, C.A. Novel aerobic perchloroethylene degradation by the white-rot fungus Trametes versicolor. Environ. Sci. Technol. 2006, 40, 7796–7802. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Liu, Y.; Feng, W. Biodegradation of hydrocarbons by Purpureocillium lilacinum and Penicillium chrysogenum from heavy oil sludge and their potential for bioremediation of contaminated soils. Int. Biodeterior. Biodegrad. 2023, 178, 105566. [Google Scholar] [CrossRef]
- Aalipour, H.; Nikbakht, A.; Etemadi, N. Co-inoculation of Arizona cypress with arbuscular mycorrhiza fungi and Pseudomonas fluorescens under fuel pollution. Mycorrhiza 2019, 29, 277–289. [Google Scholar] [CrossRef]
- Wang, C.; Yu, L.; Zhang, Z.; Wang, B.; Sun, H. Tourmaline combined with Phanerochaete chrysosporium to remediate agricultural soil contaminated with PAHs and OCPs. J. Hazard. Mater. 2014, 264, 439–448. [Google Scholar] [CrossRef]
- Devi, M.S.; Mishra, V.K.; Shyam, R.; Pankaj, U. Microbial remediation of hazardous chemical pesticides toward sustainable agriculture. In Microbial Based Land Restoration Handbook; CRC Press: Boca Raton, FL, USA, 2022; Volume 1, pp. 245–272. [Google Scholar]
- Prakash, J.; Shukla, A.; Yadav, R. Bioremediation and Information Technologies for Sustainable Management. Int. J. Biol. Med. Res. 2023, 14, 7702–7711. [Google Scholar]
- Molina, L.; Segura, A. Biochemical and metabolic plant responses toward polycyclic aromatic hydrocarbons and heavy metals present in atmospheric pollution. Plants 2021, 10, 2305. [Google Scholar] [CrossRef]
- Butnariu, M.; Butu, A. Viability of in situ and ex situ bioremediation approaches for degradation of noxious substances in stressed environs. In Bioremediation and Biotechnology, Vol 4: Techniques for Noxious Substances Remediation; Springer: Cham, Switzerland, 2020; pp. 167–193. [Google Scholar]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar] [CrossRef]
- Kaewlaoyoong, A.; Cheng, C.Y.; Lin, C.; Chen, J.R.; Huang, W.Y.; Sriprom, P. White rot fungus Pleurotus pulmonarius enhanced bioremediation of highly PCDD/F-contaminated field soil via solid state fermentation. Sci. Total Environ. 2020, 738, 139670. [Google Scholar] [CrossRef]
- Tekere, M.; Read, J.S.; Mattiasson, B. Polycyclic aromatic hydrocarbon biodegradation by a subtropical white rot fungus in packed bed and suspended carrier bioreactor systems. Environ. Technol. 2007, 28, 683–691. [Google Scholar] [CrossRef]
- Fan, J.; Ai, S.; Zheng, G.; Yin, T.; Zhang, H.; Tao, D.; Wang, S. Insight into effects of pyrolysis products and white-rot fungi on co-composting of pig manure and corn stalk. Biomass Convers. Biorefinery 2024, 14, 15937–15947. [Google Scholar] [CrossRef]
- Liu, Q.; He, X.; Wang, K.; Li, D. Biochar drives humus formation during composting by regulating the specialized metabolic features of microbiome. Chem. Eng. J. 2023, 458, 141380. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Y.; Chen, P.; Li, Y. Contribution of lignin peroxidase, manganese peroxidase, and laccase in lignite degradation by mixed white-rot fungi. Waste Biomass Valorizat. 2021, 12, 3753–3763. [Google Scholar] [CrossRef]
- Quintella, C.M.; Mata, A.M.; Lima, L.C. Overview of bioremediation with technology assessment and emphasis on fungal bioremediation of oil contaminated soils. J. Environ. Manag. 2019, 241, 156–166. [Google Scholar] [CrossRef]
- Priyanka, U.; Lens, P.N. Enhanced removal of hydrocarbons BTX by light-driven Aspergillus niger ZnS nanobiohybrids. Enzym. Microb. Technol. 2022, 157, 110020. [Google Scholar] [CrossRef]
- Baron, N.C.; Pagnocca, F.C.; Otsuka, A.A.; Prenafeta-Boldú, F.X.; Vicente, V.A.; Attili de Angelis, D. Black fungi and hydrocarbons: An environmental survey for alkylbenzene assimilation. Microorganisms 2021, 9, 1008. [Google Scholar] [CrossRef]
- Babar, Z.; Khan, M.; Chotana, G.A.; Murtaza, G.; Shamim, S. Evaluation of the potential role of Bacillus altitudinis MT422188 in nickel bioremediation from contaminated industrial effluents. Sustainability 2021, 13, 7353. [Google Scholar] [CrossRef]
- Polli, A.D.; Oliveira, V.A., Jr.; Ribeiro, M.A.D.S.; Polonio, J.C.; Rosini, B.; Oliveira, J.A.D.S.; Bini, R.D.; Golias, H.C.; Fávaro-Polonio, C.Z.; Orlandelli, R.C.; et al. Synthesis, characterization, and reusability of novel nanobiocomposite of endophytic fungus Aspergillus flavus and magnetic nanoparticles (Fe3O4) with dye bioremediation potential. Chemosphere 2023, 340, 139956. [Google Scholar] [CrossRef]
- He, Y.; Li, C.; Sun, Z.; Zhang, W.; He, J.; Zhao, Y.; Xu, Z.; Zhao, W. Penicillium spp. XK10, fungi with potential to repair cadmium and antimony pollution. Appl. Sci. 2023, 13, 1228. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Bioremediation mechanism and potential of copper by actively growing fungus Trichoderma lixii CR700 isolated from electroplating wastewater. J. Environ. Manag. 2021, 277, 111370. [Google Scholar] [CrossRef]
- Ryan, D.R.; Leukes, W.D.; Burton, S.G. Fungal bioremediation of phenolic wastewaters in an airlift reactor. Biotechnol. Prog. 2005, 21, 1068–1074. [Google Scholar] [CrossRef]
- Sabuda, M.C.; Rosenfeld, C.E.; DeJournett, T.D.; Schroeder, K.; Wuolo-Journey, K.; Santelli, C.M. Fungal bioremediation of selenium-contaminated industrial and municipal wastewaters. Front. Microbiol. 2020, 11, 2105. [Google Scholar] [CrossRef]
- Rodrigues, A.D.; Dos Santos Montanholi, A.; Shimabukuro, A.A.; Yonekawa, M.K.A.; Cassemiro, N.S.; Silva, D.B.; Marchetti, C.R.; Weirich, C.E.; Beatriz, A.; Zanoelo, F.F.; et al. N-acetylation of toxic aromatic amines by fungi: Strain screening, cytotoxicity and genotoxicity evaluation, and application in bioremediation of 3, 4-dichloroaniline. J. Hazard. Mater. 2023, 441, 129887. [Google Scholar] [CrossRef]
- Sofia, P.; Asgher, M.; Shahid, M.; Randhawa, M.A. Chitosan beads immobilized Schizophyllum commune IBL-06 lignin peroxidase with novel thermo stability, catalytic and dye removal properties. JAPS J. Anim. Plant Sci. 2016, 26, 1451–1463. [Google Scholar]
- Stanaszek-Tomal, E. Anti-smog building and civil engineering structures. Processes 2021, 9, 1446. [Google Scholar] [CrossRef]
- Somanathan, A.; Mathew, N.; Arfin, T. Environmental impacts and developments in waste-derived nanoparticles for air pollution control. In Waste-Derived Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2024; pp. 281–318. [Google Scholar]
- Ren, H.; Koshy, P.; Chen, W.F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Demir, M.; Yu, Q.; Hu, X.; Jiang, W.; Wang, L. Polyacrylonitrile-derived nitrogen enriched porous carbon fiber with high CO2 capture performance. Sep. Purif. Technol. 2022, 303, 122299. [Google Scholar] [CrossRef]
- Das, D.; Chakraborty, D.; Barman, S. Emerging Role of Nanotechnology-Based Devices for Detection of Environmental Contaminants. In Functionalized Smart Nanomaterials for Point-of-Care Testing; Springer Nature: Singapore, 2023; pp. 199–209. [Google Scholar]
- Weon, S. Photocatalytic Oxidation of Carbon Monoxide Using Synergy of Redox-Separated Photocatalyst and Ozone. Molecules 2022, 27, 8482. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J.; Ismail, A.F.; Goh, P.S. Advances of nanomaterials for air pollution remediation and their impacts on the environment. Chemosphere 2021, 287, 132083. [Google Scholar] [CrossRef]
- Choi, H.; Seo, J.H.; Weon, S. Visualizing indoor ozone exposures via o-dianisidine based colorimetric passive sampler. J. Hazard. Mater. 2023, 460, 132510. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Pramanik, P.; Mazumdar, S.P.; Dubey, R. Nanobioremediation Technologies for Clean Environment. In Bioremediation Science; CRC Press: Boca Raton, FL, USA, 2021; pp. 298–309. [Google Scholar]
- Weon, S.; Suh, M.; Chu, C.; Huang, D.; Stavitski, E.; Kim, J. Site-Selective loading of Single-Atom PT on TIO2 for photocatalytic oxidation and reductive hydrodefluorination. ACS EST Eng. 2021, 1, 512–522. [Google Scholar] [CrossRef]
- Sharma, K.; Tyagi, S.; Vikal, S.; Devi, A.; Gautam, Y.K.; Singh, B.P. Green nanomaterials for remediation of environmental air pollution. In Handbook of Green and Sustainable Nanotechnology: Fundamentals, Developments and Applications; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–26. [Google Scholar]
- Rather, M.A.; Bhuyan, S.; Chowdhury, R.; Sarma, R.; Roy, S.; Neog, P.R. Nano-remediation strategies to address environmental problems. Sci. Total Environ. 2023, 886, 163998. [Google Scholar] [CrossRef]
- Panichikkal, J.; Krishnankutty, R.E. Application of Biogenic Nanoparticles for a Clean Environment. In Sustainable Bioprocessing for a Clean and Green Environment; CRC Press: Boca Raton, FL, USA, 2021; pp. 147–161. [Google Scholar]
- Palencia, M.; García-Quintero, A. Green synthesis of nanomaterials and their use in bio-and nano-remediation. In Bio and Nano-Remediation of Hazardous Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2023; pp. 195–229. [Google Scholar]
- Hashemi, M.; Maleky, S.; Rajabi, S. Nanoadsorbents in Air Pollution Control. In Adsorption through Advanced Nanoscale Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 289–324. [Google Scholar]
- Mohamed, E.F.; Awad, G. Advanced Nano-biotechnology for Chlorinated Volatile Compound Pollutants Control. Environ. Manag. Sustain. Dev. 2023, 12, 34–66. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, S.; Kumar, A.V.; Hajam, Y.A. Biofunctionalized Nanomaterials a Zero Waste Approach for the Remediation of Pollutants. In Zero Waste Management Technologies; Springer: Cham, Switzerland, 2024; pp. 285–308. [Google Scholar]
- Vrvić, M. Technologies for Remediation of Polluted Environments: Between Classic Processes and the Challenges of New Approaches. In International Conference “New Technologies, Development and Applications”; Springer Nature: Cham, Switzerland, 2023; pp. 205–219. [Google Scholar]
- Ramadevi, D.; Ushasri, K. Nanotechnology Applications in the Environment, India. In Proceedings of the book of National Conference on Pollution Control and Sustainable Environment (NCPCSE-2023), Online, 24–25 February 2023. [Google Scholar]
- Yang, L.; Yang, L.; Ding, L.; Deng, F.; Luo, X.B.; Luo, S.L. Principles for the application of nanomaterials in environmental pollution control and resource reutilization. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–23. [Google Scholar]
- Rocha, A. Development of a Hybrid Photo-Bioreactor Coupled with Nano–and Micro-Interfaces for Air Pollution Remediation; McGill University: Montréal, QC, Canada, 2016. [Google Scholar]
- El Khawaja, R.; Sonar, S.; Barakat, T.; Heymans, N.; Su, B.L.; Löfberg, A.; Lamonier, J.F.; Giraudon, J.M.; De Weireld, G.; Poupin, C.; et al. VOCs catalytic removal over hierarchical porous zeolite NaY supporting Pt or Pd nanoparticles. Catal. Today 2022, 405, 212–220. [Google Scholar] [CrossRef]
- Ahmadian, M.; Anbia, M.; Rezaie, M. Sulfur dioxide removal from flue gas by supported CuO nanoparticle adsorbents. Ind. Eng. Chem. Res. 2020, 59, 21642–21653. [Google Scholar] [CrossRef]
- Saucedo-Lucero, J.O.; Arriaga, S. Photocatalytic degradation of hexane vapors in batch and continuous systems using impregnated ZnO nanoparticles. Chem. Eng. J. 2013, 218, 358–367. [Google Scholar] [CrossRef]
- Kim, T.H.; Choi, B.H.; Kang, M.S.; Lee, H.J. Removal of iron oxide from indoor air at a subway station using a vegetation biofilter: A case study of Seoul, Korea. Atmosphere 2021, 12, 1463. [Google Scholar] [CrossRef]
- Changsuphan, A.; Wahab, M.I.B.; Oanh, N.T.K. Removal of benzene by ZnO nanoparticles coated on porous adsorbents in presence of ozone and UV. Chem. Eng. J. 2012, 181, 215–221. [Google Scholar] [CrossRef]
- Hussain, M.; Russo, N.; Saracco, G. Photocatalytic abatement of VOCs by novel optimized TiO2 nanoparticles. Chem. Eng. J. 2011, 166, 138–149. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Z.; Duan, Y.; Ma, R.; Zheng, S. Synthesis of nano-TiO2/diatomite composite and its photocatalytic degradation of gaseous formaldehyde. Appl. Surf. Sci. 2017, 412, 105–112. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aazam, E. Synthesis and characterization of Pt–ZnO-hydroxyapatite nanoparticles for photocatalytic degradation of benzene under visible light. Desalinat. Water Treat. 2013, 51, 6082–6090. [Google Scholar] [CrossRef]
- Khiadani, M.; Foroughi, M.; Amin, M.M. Improving urban run-off quality using iron oxide nanoparticles with magnetic field. Desalinat. Water Treat. 2014, 52, 678–682. [Google Scholar] [CrossRef]
- Pei, L.; Zhou, J.; Zhang, L. Preparation and properties of Ag-coated activated carbon nanocomposites for indoor air quality control. Build. Environ. 2013, 63, 108–113. [Google Scholar] [CrossRef]
- Eltouny, N.A.; Ariya, P.A. Fe3O4 nanoparticles and carboxymethyl cellulose: A green option for the removal of atmospheric benzene, toluene, ethylbenzene, and o-xylene (BTEX). Ind. Eng. Chem. Res. 2012, 51, 12787–12795. [Google Scholar] [CrossRef]
- Méndez, L.; Muñoz, R. Enhancing microalgae-based bioremediation technologies with carbon-coated zero valent iron nanoparticles. Algal Res. 2024, 79, 103448. [Google Scholar] [CrossRef]
- Osadebe, A.U.; Ogugbue, C.J.; Okpokwasili, G.C. Bioremediation of crude oil polluted surface water using specialised alginate-based nanocomposite beads loaded with hydrocarbon-degrading bacteria and inorganic nutrients. Bioremediat. J. 2024, 1–23. [Google Scholar] [CrossRef]
- Gholami, F.; Mosmeri, H.; Shavandi, M.; Dastgheib, S.M.M.; Amoozegar, M.A. Application of encapsulated magnesium peroxide (MgO2) nanoparticles in permeable reactive barrier (PRB) for naphthalene and toluene bioremediation from groundwater. Sci. Total Environ. 2019, 655, 633–640. [Google Scholar] [CrossRef]
- Mosmeri, H.; Gholami, F.; Shavandi, M.; Dastgheib, S.M.M.; Alaie, E. Bioremediation of benzene-contaminated groundwater by calcium peroxide (CaO2) nanoparticles: Continuous-flow and biodiversity studies. J. Hazard. Mater. 2019, 371, 183–190. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Mu, Y.-J.; Zhu, Y.-G.; Ding, H.; Arens, N.C. Which ornamental plant species effectively remove benzene from indoor air? Atmos. Environ. 2007, 41, 650–654. [Google Scholar] [CrossRef]
- Oanh, N.T.K.; Hung, Y.T. Indoor air pollution control. In Advanced Air and Noise Pollution Control; Humana Press: Totowa, NJ, USA, 2005; pp. 237–272. [Google Scholar]
- MacDonald Gibson, J.; Brammer, A.; Davidson, C.; Folley, T.; Launay, F.; Thomsen, J. Burden of disease from indoor air pollution. In Environmental Burden of Disease Assessment; Springer: Chapel Hill, NC, USA, 2013; pp. 109–132. [Google Scholar]
- Pipal, A.S.; Kumar, A.; Jan, R.; Taneja, A. Role of plants in removing indoor air pollutants. In Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives; Springer: Berlin/Heidelberg, Germany, 2012; pp. 319–321. [Google Scholar]
- Borchers, A.T.; Chang, C.; Keen, C.L.; Gershwin, M.E. Airborne environmental injuries and human health. Clin. Rev. Allergy Immunol. 2006, 31, 1–101. [Google Scholar] [CrossRef]
- Jafta, N.; Barregard, L.; Jeena, P.M.; Naidoo, R.N. Indoor air quality of low and middle income urban households in Durban, South Africa. Environ. Res. 2017, 156, 47–56. [Google Scholar] [CrossRef]
- Jones, B.; Molina, C. Indoor air quality. In Encyclopedia of Sustainable Technologies; Abraham, M.A., Ed.; Elsevier: Oxford, UK, 2017; Volume 17, pp. 197–207. [Google Scholar]
- Austin, K.F.; Mejia, M.T. Household air pollution as a silent killer: Women’s status and solid fuel use in developing nations. Popul. Environ. 2017, 39, 1–25. [Google Scholar] [CrossRef]
- Cociorva, S.; Iftene, A. Indoor air quality evaluation in intelligent building. Energy Procedia 2017, 112, 261–268. [Google Scholar] [CrossRef]
- Dela Cruz, M.; Christensen, J.H.; Thomsen, J.D.; Müller, R. Can ornamental potted plants remove volatile organic compounds from indoor air?—A review. Environ. Sci. Pollut. Res. 2014, 21, 13909–13928. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Mitigation of air pollution by greenness: A narrative review. Eur. J. Intern. Med. 2018, 55, 1–5. [Google Scholar] [CrossRef]
- Gawronski, S.W.; Gawronska, H.; Lomnicki, S.; Saebo, A.; Vangronsveld, J. Plants in air phytoremediation. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2017; Volume 83, pp. 319–346. [Google Scholar]
- Gubb, C.; Blanusa, T.; Griffiths, A.; Pfrang, C. Potted plants can remove the pollutant nitrogen dioxide indoors. Air Qual. Atmos. Health 2022, 15, 479–490. [Google Scholar] [CrossRef]
- Barjoee, S.S.; Malverdi, E.; Kouhkan, M.; Alipourfard, I.; Rouhani, A.; Farokhi, H.; Khaledi, A. Health assessment of industrial ecosystems of Isfahan (Iran) using phytomonitoring: Chemometric, micromorphology, phytoremediation, air pollution tolerance and anticipated performance indices. Urban Clim. 2023, 48, 101394. [Google Scholar] [CrossRef]
- Irga, P.J.; Morgan, A.; Fleck, R.; Torpy, F.R. Phytoremediation of indoor air pollutants from construction and transport by a moveable active green wall system. Atmos. Pollut. Res. 2023, 14, 101896. [Google Scholar] [CrossRef]
- Elmarakby, F.; Eltorky, M.G.; Zaki, A. Efficacy of phytoremediation in improving indoor air quality in homes in Alexandria, Egypt. Biol. Biomed. J. 2024, 2, 92–102. [Google Scholar] [CrossRef]
- Yang, Y.; Su, Y.; Zhao, S. An efficient plant–microbe phytoremediation method to remove formaldehyde from air. Environ. Chem. Lett. 2020, 18, 197–206. [Google Scholar] [CrossRef]
- Kim, T.H.; An, B.R.; Clementi, M. Phytoremediation as adaptive design strategy to improve indoor air quality. Experimental Results Relating to the Application of a Vertical Hydroponic Biofilter. In Sustainability in Energy and Buildings 2020; Springer: Singapore, 2021; pp. 479–489. [Google Scholar]
- Watson, A.S.; Bai, R.S. Phytoremediation for urban landscaping and air pollution control—A case study in Trivandrum city, Kerala, India. Environ. Sci. Pollut. Res. 2021, 28, 9979–9990. [Google Scholar] [CrossRef]
- Bandehali, S.; Miri, T.; Onyeaka, H.; Kumar, P. Current state of indoor air phytoremediation using potted plants and green walls. Atmosphere 2021, 12, 473. [Google Scholar] [CrossRef]
- Cummings, B.E.; Waring, M.S. Potted plants do not improve indoor air quality: A review and analysis of reported VOC removal efficiencies. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 253–261. [Google Scholar] [CrossRef]
- Bandara, W.A.R.T.W.; Dissanayake, C.T.M. Most tolerant roadside tree species for urban settings in humid tropics based on Air Pollution Tolerance Index. Urban Clim. 2021, 37, 100848. [Google Scholar] [CrossRef]
- de Souza, D.M.; da Silva, J.L.; Ludwig, L.D.C.; Petersen, B.C.; Brehm, F.A.; Modolo, R.C.E.; De Marchi, T.C.; Figueiredo, R.; Moraes, C.A.M. Study of the phytoremediation potential of native plant species identified in an area contaminated by volatile organic compounds: A systematic review. Int. J. Phytoremediat. 2023, 25, 1524–1541. [Google Scholar] [CrossRef]
- Sattar, S.; Hussain, R.; Shah, S.M.; Bibi, S.; Ahmad, S.R.; Shahzad, A.; Zamir, A.; Rauf, Z.; Noshad, A.; Ahmad, L. Composition, impacts, and removal of liquid petroleum waste through bioremediation as an alternative clean-up technology: A review. Heliyon 2022, 8, e11101. [Google Scholar] [CrossRef]
- Urionabarrenetxea, E.; Garcia-Velasco, N.; Anza, M.; Artetxe, U.; Lacalle, R.; Garbisu, C.; Becerril, T.; Soto, M. Application of in situ bioremediation strategies in soils amended with sewage sludges. Sci. Total Environ. 2021, 766, 144099. [Google Scholar] [CrossRef]
- Nnaji, N.D.; Onyeaka, H.; Miri, T.; Ugwa, C. Bioaccumulation for heavy metal removal: A review. SN Appl. Sci. 2023, 5, 125. [Google Scholar] [CrossRef]
- Ogundola, A.F.; Adebayo, E.A.; Ajao, S.O. Phytoremediation: The ultimate technique for reinstating soil contaminated with heavy metals and other pollutants. In Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water; Elsevier: Amsterdam, The Netherlands, 2022; pp. 19–49. [Google Scholar]
- Khatoon, H.; Pant, A.; Rai, J.P.N. Plant adaptation to recalcitrant chemicals. In Plant Adaptation Strategies in Changing Environment; Springer: Singapore, 2017; pp. 269–290. [Google Scholar]
- Singh, D. Advances in industrial waste management. In Waste Management and Resource Recycling in the Developing World; Elsevier: Amsterdam, The Netherlands, 2023; pp. 385–416. [Google Scholar]
- Jin, Y.; Yuan, Y.; Liu, Z.; Gai, S.; Cheng, K.; Yang, F. Effect of humic substances on nitrogen cycling in soil-plant ecosystems: Advances, issues, and future perspectives. J. Environ. Manag. 2024, 351, 119738. [Google Scholar] [CrossRef]
- Schwitzguébel, J.P. Phytoremediation of soils contaminated by organic compounds: Hype, hope and facts. J. Soils Sediments 2017, 17, 1492–1502. [Google Scholar] [CrossRef]
- Sengupta, K.; Pal, S. Rhizospheric plant–microbe interactions releasing antioxidants and phytostimulating compounds in polluted agroecosystems. In Antioxidants in Plant-Microbe Interaction; Springer: Singapore, 2021; pp. 157–179. [Google Scholar]
- Odinga, C.A. Assessment of Heavy Metals and Pathogens Removal from Municipal Wastewater Using a Constructed Rhizofiltration System. Ph.D. Thesis, Durban University of Technology, Greyville, Durban, 2018. [Google Scholar]
- Nookongbut, P.; Thiravetyan, P.; Salsabila, S.; Widiana, A.; Krobthong, S.; Yingchutrakul, Y.; Treesubsuntorn, C. Application of Acinetobacter indicus to promote cigarette smoke particulate matter phytoremediation: Removal efficiency and plant–microbe interactions. Environ. Sci. Pollut. Res. 2024, 31, 52352–52370. [Google Scholar] [CrossRef]
- Roy, A.; Mandal, M.; Przybysz, A.; Haynes, A.; Robinson, S.A.; Sarkar, A.; Popek, R. Phytoremediating the air down under: Evaluating airborne particulate matter accumulation by 12 plant species in Australia. Ecol. Res. 2024. [Google Scholar] [CrossRef]
- Yan, Q.; Xu, L.; Duan, Y.; Pan, L.; Wu, Z.; Chen, X. Influence of leaf morphological characteristics on the dynamic changes of particulate matter retention and grain size distributions. Environ. Technol. 2024, 45, 108–119. [Google Scholar] [CrossRef]
- Tripathi, D.P.; Nema, A.K. Air pollution mitigation and suspended particulate matter retention potential of selected plant species across seasonal variation in the urban area. Environ. Sci. Pollut. Res. 2024, 31, 45035–45054. [Google Scholar] [CrossRef]
- Bui, H.T.; Jeong, M.; Park, B.J. Particulate Matter Capture and Air Pollution Tolerance of Six Roadside Plants in Cheongju, South Korea. J. Environ. Sci. Manag. 2024, 27. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Wang, Q.; Zhang, Y.; Wei, C.; Zhang, L.; Li, Z.; Yu, H.; Chang, C. Evaluation of air pollution tolerance index of urban roadside young leaf and the correlation with its capturing capacity for water-insoluble fine particulate matters. Air Qual. Atmos. Health 2024, 1–17. [Google Scholar] [CrossRef]
- Permana, B.H.; Krobthong, S.; Yingchutrakul, Y.; Thiravetyan, P.; Treesubsuntorn, C. Sansevieria trifasciata’s specific metabolite improves tolerance and efficiency for particulate matter and volatile organic compound removal. Environ. Pollut. 2024, 355, 124199. [Google Scholar] [CrossRef]
- Hammad, D.; Thu, K.; Miyazaki, T. Particulate Matter Phytoremediation Effectiveness of Japanese Prunus × Yedoensis Tree Through Spring and Summer Season. In E3S Web of Conferences; EDP Sciences: Tokyo, Japan, 2023; Volume 465, p. 02030. [Google Scholar]
- James, A. Phytoremediation of Urban Air Pollutants: Current Status and Challenges. Urban Ecol. Glob. Clim. Chang. 2022, 8, 140–161. [Google Scholar]
- Nguyen, T.T. Creation of Farm Forestry on Allocated Forestland and Its Contribution to the Livelihoods of Local People in a Mountainous Region of Northeast Vietnam. Ph.D. Thesis, the University of Tsukuba, Tsukuba, Japan, 2018. [Google Scholar]
- Morikawa, H.; Higaki, A.; Nohno, M.; Takahashi, M.; Kamada, M.; Nakata, M.; Toyohara, G.; Okamura, Y.; Matsui, K.; Kitani, S.; et al. More than a 600-fold variation in nitrogen dioxide assimilation among 217 plant taxa. Plant Cell Environ. 1998, 21, 180–190. [Google Scholar] [CrossRef]
- Pettit, T.; Irga, P.J.; Torpy, F.R. The botanical biofiltration of elevated air pollution concentrations associated the Black Summer wildfire natural disaster. J. Hazard. Mater. Lett. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Basharat, Z.; Novo, L.A.; Yasmin, A. Genome editing weds CRISPR: What is in it for phytoremediation? Plants 2018, 7, 51. [Google Scholar] [CrossRef]
- Jusselme, M.D.; Bousserrhine, N.; Abbad-Andaloussi, S.; Brondeau, F.; Balland-Bolou-Bi, C. Phytoremediation: An Ecological Solution for Decontamination of Polluted Urban Soils. In Soil Contamination-Threats and Sustainable Solutions; IntechOpen: London, UK, 2020. [Google Scholar]
- Reinmuth-Selzle, K.; Ackaert, C.; Kampf, C.J.; Samonig, M.; Shiraiwa, M.; Kofler, S.; Yang, H.; Gadermaier, G.; Brandstetter, H.; Huber, C.G.; et al. Nitration of the birch pollen allergen Bet v 1.0101: Efficiency and site-selectivity of liquid and gaseous nitrating agents. J. Proteome Res. 2014, 13, 1570–1577. [Google Scholar] [CrossRef]
- Zhao, F.; Elkelish, A.; Dumer, J.; Lindermayer, C.; Barbro, J.; Rueff, F.; Behrendt, H.; Traidl-Hoffmann, C.; Holzinger, A.; Kofler, W.; et al. Common ragweed (Ambrosia artemisiifolia L.): Allergenicity and molecular characterization of pollen after plant exposure to elevated NO2. Plant Cell Environ. 2016, 39, 147–164. [Google Scholar] [CrossRef]
- Bryce, M.; Drews, O.; Schenk, M.F.; Menzel, A.; Estrella, N.; Weichenmeier, I.; Smulders, M.J.M.; Buters, J.; Ring, J.; Gorg, A.; et al. Impact of urbanization on the proteome of birch pollen and its chemotactic activity on human granulocytes. Int. Arch. Allergy Immunol. 2010, 151, 46–55. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakagawa, M.; Sakamoto, A.; Ohsumi, C.; Matsbara, T.; Morikawa, H. Atmospheric nitrogen dioxide gas is a plant vitalization signal to increase plant size and contents of cell constituents. New Phytol. 2005, 168, 149–154. [Google Scholar] [CrossRef]
- Bidwel, R.G.S.; Bebee, G.P. Carbon monoxide fixation by plant. Can. J. Bot. 1974, 174, 1841–1847. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Almehdi, A.M.; Elsheikh, E.A.; Abouleish, M.Y.; Sheteiwy, M.S.; Galal, T.M. Native desert plants have the potential for phytoremediation of phytotoxic metals in urban cities: Implications for cities sustainability in arid environments. Sci. Rep. 2024, 14, 13761. [Google Scholar] [CrossRef]
- Rabiee, M.; Kaviani, B.; Kulus, D.; Eslami, A. Phytoremediation Potential of Urban Trees in Mitigating Air Pollution in Tehran. Forests 2024, 15, 1436. [Google Scholar] [CrossRef]
- Agarwal, P.; Sarkar, M.; Chakraborty, B.; Banerjee, T. Phytoremediation of air pollutants: Prospects and challenges. Phytomanagement Polluted Sites 2019, 221–241. [Google Scholar] [CrossRef]
- Gong, C.; Xian, C.; Wu, T.; Liu, J.; Ouyang, Z. Role of urban vegetation in air phytoremediation: Differences between scientific research and environmental management perspectives. NPJ Urban Sustain. 2023, 3, 24. [Google Scholar] [CrossRef]
- Hauptvogl, M.; Kotrla, M.; Prčík, M.; Pauková, Ž.; Kováčik, M.; Lošák, T. Phytoremediation potential of fast-growing energy plants: Challenges and perspectives—A review. Pol. J. Environ. Stud. 2019, 29, 505–516. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant growth-promoting microorganisms for environmental sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef]
- Farrow, A.; Miller, K.A.; Myllyvirta, L.; Newport, E.; Son, M. Toxic Air: The Price of Fossil Fuels; Greenpeace Southeast Asia: Bangkok, Thailand, 2020. [Google Scholar]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant–endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2020, 249, 71–131. [Google Scholar]
- Gayathry, G.; Sabarinathan, K.G.; Jayalakshmi, T. Phyllosphere Microbiome in Ecosystem Management and Plant Growth Promotion for Agricultural Sustainability. Int. J. Ecol. Environ. Sci. 2024. [Google Scholar] [CrossRef]
- Thompson, R.; Smith, R.B.; Karim, Y.B.; Shen, C.; Drummond, K.; Teng, C.; Toledano, M.B. Air pollution and human cognition: A systematic review and meta-analysis. Sci. Total Environ. 2023, 859, 160234. [Google Scholar] [CrossRef]
- Pipal, A.S.; Taneja, A. Measurements of Indoor Air Quality: Science and Applications. In Handbook of Metrology and Applications; Springer Nature: Singapore, 2023; pp. 1621–1655. [Google Scholar]
- Sánchez-López, A.S.; González-Chávez, M.D.C.A.; Solís-Domínguez, F.A.; Carrillo-González, R.; Rosas-Saito, G.H. Leaf epiphytic bacteria of plants colonizing mine residues: Possible exploitation for remediation of air pollutants. Front. Microbiol. 2018, 9, 3028. [Google Scholar] [CrossRef]
- Kończak, B.; Wiesner-Sękala, M.; Ziembińska-Buczyńska, A. The European trees phyllosphere characteristics and its potential in air bioremediation. Environ. Pollut. 2024, 349, 123977. [Google Scholar] [CrossRef]
- Mokarram-Kashtiban, S.; Hosseini, S.M.; Tabari Kouchaksaraei, M.; Younesi, H. The impact of nanoparticles zero-valent iron (nZVI) and rhizosphere microorganisms on the phytoremediation ability of white willow and its response. Environ. Sci. Pollut. Res. 2019, 26, 10776–10789. [Google Scholar] [CrossRef]
- Dharmasiri, R.B.N.; Undugoda, L.J.S.; Nilmini, A.H.L.; Nugara, N.N.R.N.; Manage, P.M.; Udayanga, D. Phylloremediation approach to green air: Phenanthrene degrading potential of Bacillus spp. inhabit the phyllosphere of ornamental plants in urban polluted areas. Int. J. Environ. Sci. Technol. 2023, 20, 13359–13372. [Google Scholar] [CrossRef]
- Undugoda, L.; Thambugala, K.; Kannangara, S.; Munasinghe, J.; Premarathna, N.; Dharmasiri, N. Phylloremediation of pyrene and anthracene by endophytic fungi inhabiting tea leaves (Camellia sinensis (L.) Kuntze) in Sri Lanka. N. Z. J. Bot. 2023, 1–14. [Google Scholar] [CrossRef]
- Sharma, A.; Mittal, V.; Grover, R.; Sharma, D.; Gupta, V.; Kumar, K. Applications of Nanotechnology in Phytoremediation. In Phytoremediation: Biological Treatment of Environmental Pollution; Springer: Cham, Switzerland, 2024; pp. 291–313. [Google Scholar]
- Li, H.; Rehman, A.; ur Rahman, S.; Li, K.; Yang, T.; Akuetteh, P.; Khalid, M. Biosynthesized zinc oxide nanoparticles modulate the phytoremediation potential of Pennisetum giganteum and its rhizocompartments associated microbial community structure. J. Clean. Prod. 2024, 434, 140346. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, Z.; Zhu, N.; Yu, Q. Magnetic nanoparticle-assisted colonization of synthetic bacteria on plant roots for improved phytoremediation of heavy metals. Chemosphere 2023, 329, 138631. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation: An eco-friendly sustainable technology for environmental management. In Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Springer: Singapore, 2020; pp. 19–39. [Google Scholar]
- Ugrina, M.; Jurić, A. Current trends and future perspectives in the remediation of polluted water, soil and air—A review. Processes 2023, 11, 3270. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T. Phytostabilization of heavy metals: Understanding of principles and practices. In Plant-Metal Interactions; Springer: Cham, Switzerland, 2019; pp. 263–282. [Google Scholar]
- González-Martín, J.; Kraakman, N.J.R.; Pérez, C.; Lebrero, R.; Muñoz, R. A state–of–the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef]
- Saxena, G.; Kishor, R.; Saratale, G.D.; Bharagava, R.N. Genetically modified organisms (GMOs) and their potential in environmental management: Constraints, prospects, and challenges. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–19. [Google Scholar]
- Jabbar, N.M.; Alardhi, S.M.; Mohammed, A.K.; Salih, I.K.; Albayati, T.M. Challenges in the implementation of bioremediation processes in petroleum-contaminated soils: A review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100694. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Zhang, L.; Xie, D.; Onwosi, C.O.; Muhammad, W.I.; Odoh, C.K.; Sam, K.; Idenyi, J.N. Bioaugmentation as a green technology for hydrocarbon pollution remediation. Problems and prospects. J. Environ. Manag. 2022, 304, 114313. [Google Scholar] [CrossRef]
- Mishra, B.; Varjani, S.; Kumar, G.; Awasthi, M.K.; Awasthi, S.K.; Sindhu, R.; Binod, P.; Rene, E.R.; Zhang, Z. Microbial approaches for remediation of pollutants: Innovations, future outlook, and challenges. Energy Environ. 2021, 32, 1029–1058. [Google Scholar] [CrossRef]
- Mahesh, N.; Balakumar, S.; Danya, U.; Shyamalagowri, S.; Babu, P.S.; Aravind, J.; Kamaraj, M.; Govarthanan, M. A review on mitigation of emerging contaminants in an aqueous environment using microbial bio-machines as sustainable tools: Progress and limitations. J. Water Process Eng. 2022, 47, 102712. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent strategies for bioremediation of emerging pollutants: A review for a green and sustainable environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Misra, M.; Ghosh Sachan, S. Nanobioremediation of heavy metals: Perspectives and challenges. J. Basic Microbiol. 2022, 62, 428–443. [Google Scholar]
- Del Prado-Audelo, M.L.; García Kerdan, I.; Escutia-Guadarrama, L.; Reyna-González, J.M.; Magaña, J.J.; Leyva-Gómez, G. Nanoremediation: Nanomaterials and nanotechnologies for environmental cleanup. Front. Environ. Sci. 2021, 9, 793765. [Google Scholar] [CrossRef]
- Roy, A.; Sharma, A.; Yadav, S.; Jule, L.T.; Krishnaraj, R. Nanomaterials for remediation of environmental pollutants. Bioinorg. Chem. Appl. 2021, 2021, 1764647. [Google Scholar] [CrossRef]
- Odoh, C.K.; Zabbey, N.; Sam, K.; Eze, C.N. Status, progress and challenges of phytoremediation-An African scenario. J. Environ. Manag. 2019, 237, 365–378. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
- Anum, W.; Riaz, U.; Ali, L.; Akhter, L.H.; Ahmed, R.I.; Ali, A. Genetically Modified Plants (GMPs) and Their Potential in Protection, Constraints, Prospects, Challenges, and Opportunities Against Environmental Pollution. In Environmental Pollution Impact on Plants; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 227–243. [Google Scholar]
- Subejano, M.S.E.P.; Daquioag, J.E.L.; Penuliar, G.M. Genetically Modified Organisms in Research and Biotechnology. In Biosafety and Biosecurity; CRC Press: Boca Raton, FL, USA, 2024; pp. 235–258. [Google Scholar]
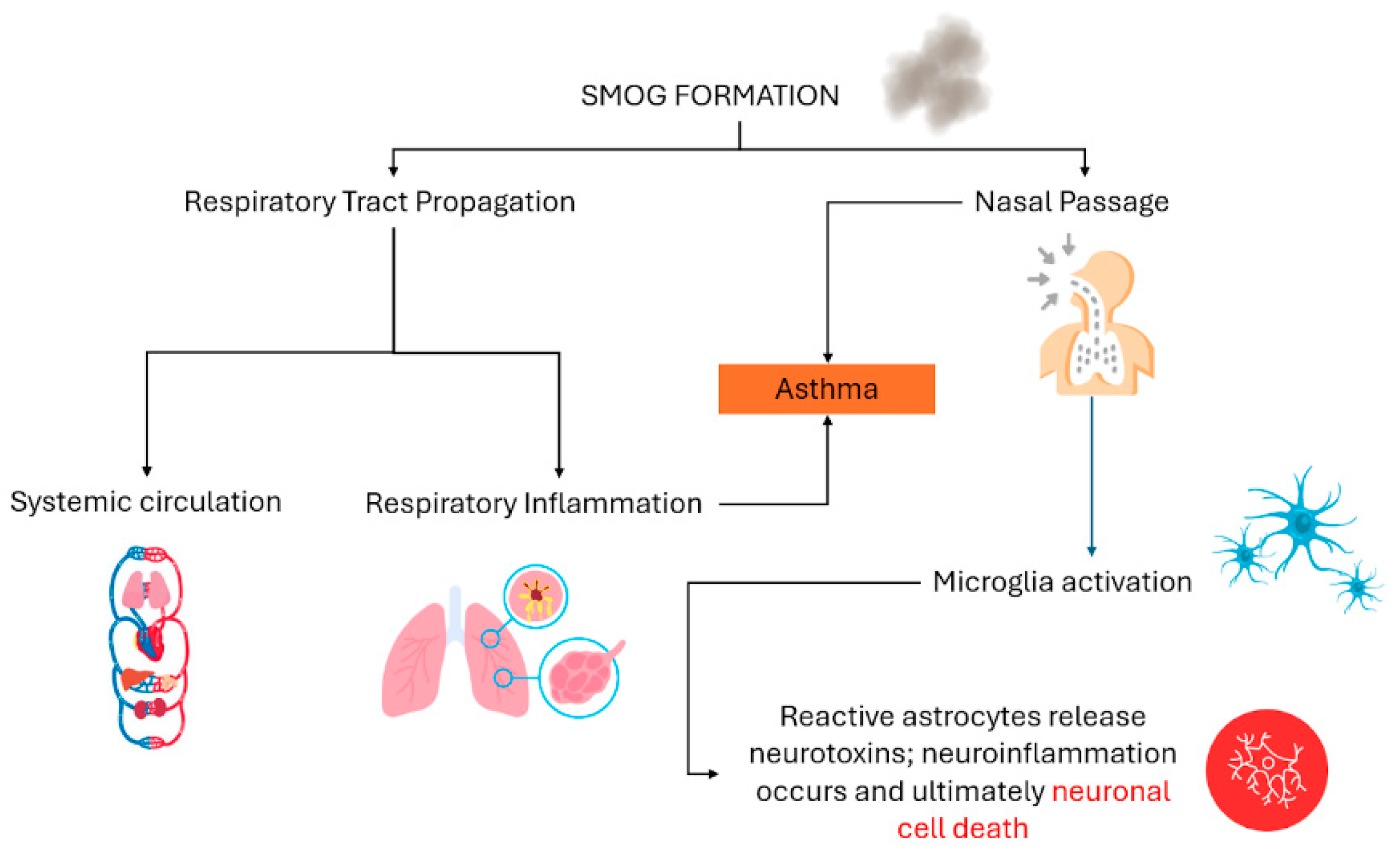

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isha; Ali, S.; Khalid, A.; Naseer, I.A.; Raza, H.; Chang, Y.-C. Bioremediation of Smog: Current Trends and Future Perspectives. Processes 2024, 12, 2266. https://doi.org/10.3390/pr12102266
Isha, Ali S, Khalid A, Naseer IA, Raza H, Chang Y-C. Bioremediation of Smog: Current Trends and Future Perspectives. Processes. 2024; 12(10):2266. https://doi.org/10.3390/pr12102266
Chicago/Turabian StyleIsha, Shakir Ali, Ammara Khalid, Ifrah Amjad Naseer, Hassan Raza, and Young-Cheol Chang. 2024. "Bioremediation of Smog: Current Trends and Future Perspectives" Processes 12, no. 10: 2266. https://doi.org/10.3390/pr12102266
APA StyleIsha, Ali, S., Khalid, A., Naseer, I. A., Raza, H., & Chang, Y.-C. (2024). Bioremediation of Smog: Current Trends and Future Perspectives. Processes, 12(10), 2266. https://doi.org/10.3390/pr12102266









