Synthesis of Transparent Bacterial Cellulose Films as a Platform for Targeted Drug Delivery in Wound Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Maintenance Medium
2.2. Production Yield of Bacterial Cellulose and Water Holding Capacity (WHC)
2.3. Drug Loading, Film Sterilization and In Vitro Drug-Release Studies
2.4. Determination of Water Contact Angle, Sorption Index (Q) and Water Vapor Penetration (WVP)
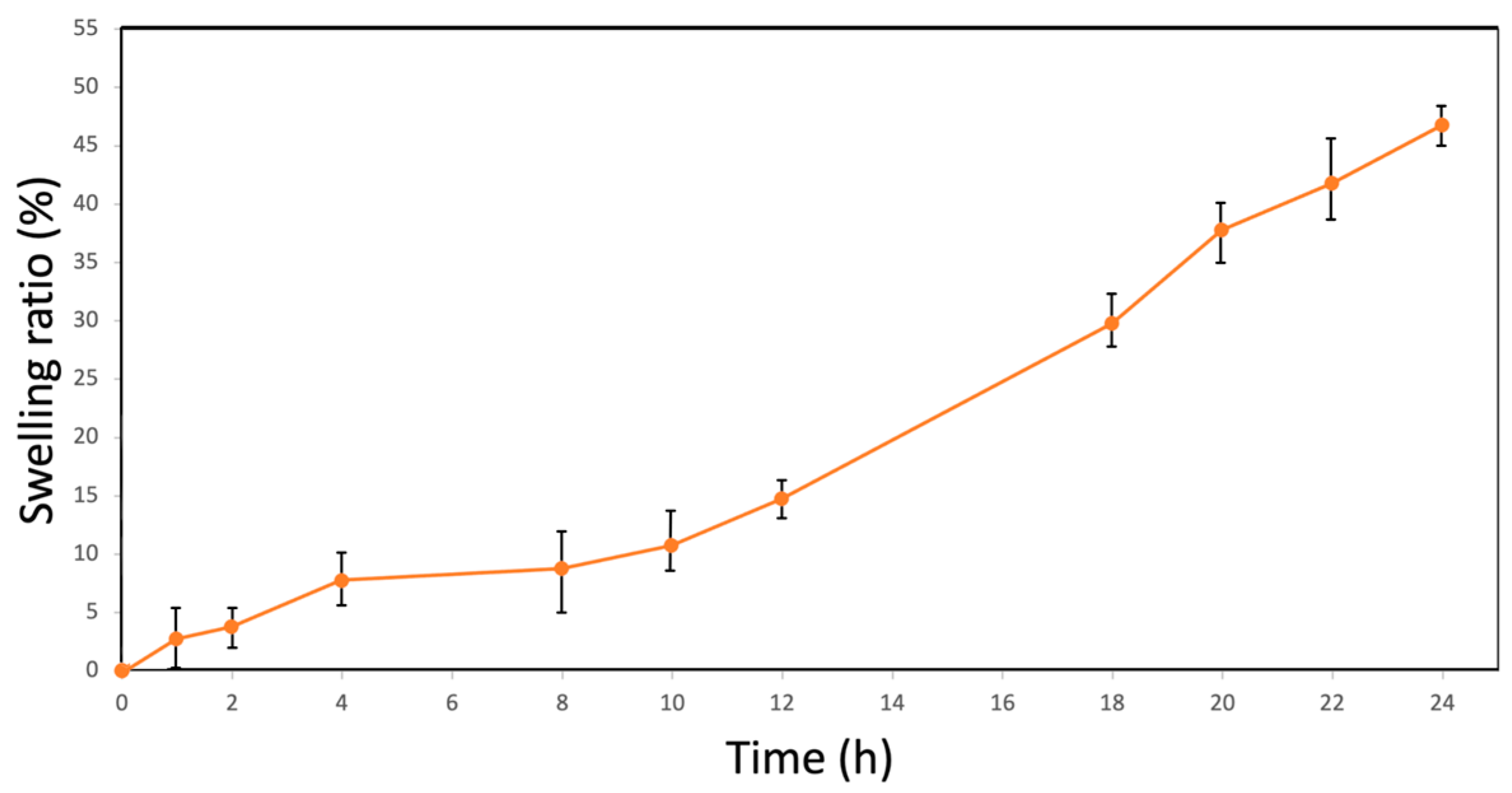
2.5. Swelling Ratio (SR) and Rehydration Ratio (RR)
2.6. Microbial Penetration and Adhesive Strength
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. X-ray Diffractometry (XRD)
2.9. Thermogravimetry Analysis (TGA)
2.10. Scanning Electron Microscopy (SEM)
2.11. Porosity and Measurement of Folding Endurance (FE)
2.12. Statistical Analysis
3. Results and Discussion
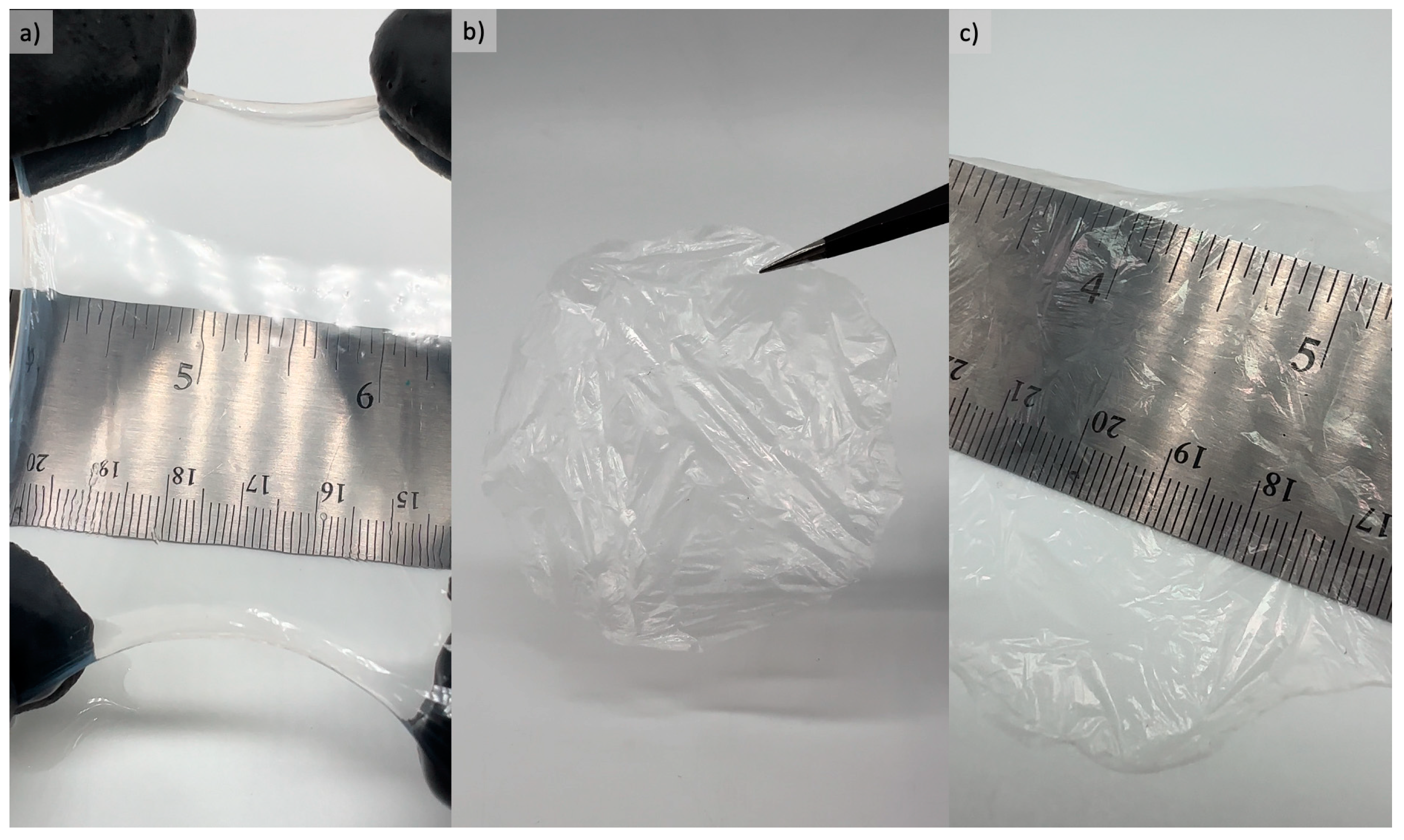
3.1. Hygroscopic Properties of Bacterial Cellulose Films
3.2. Bacterial Cellulose Film Performance Characterization
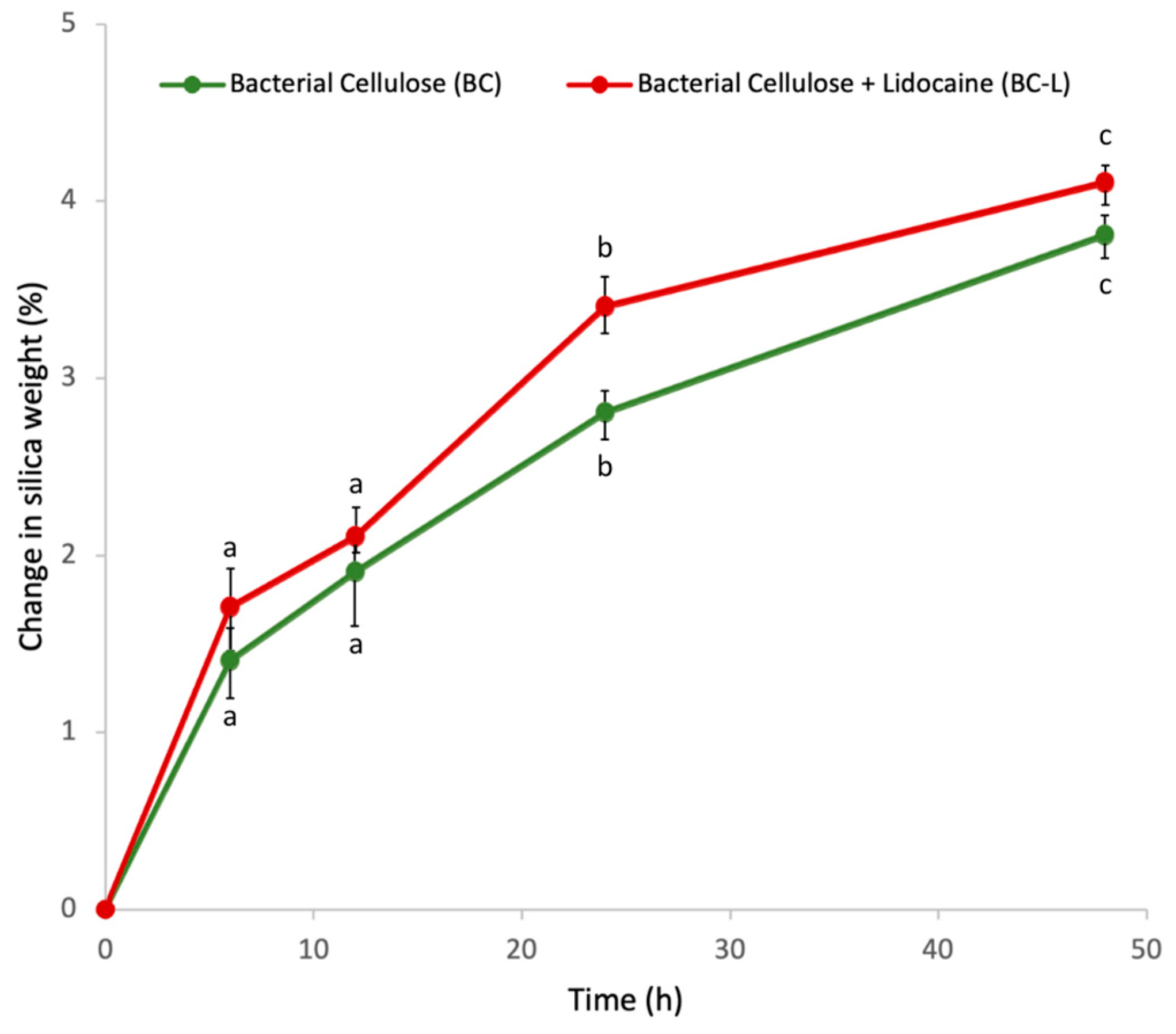
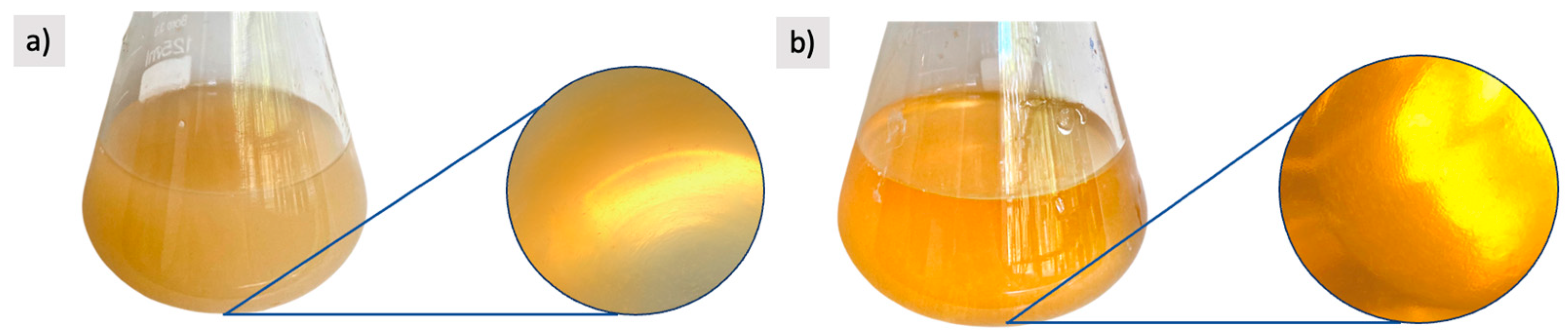
3.3. Assessment of Lidocaine Incorporation and Release
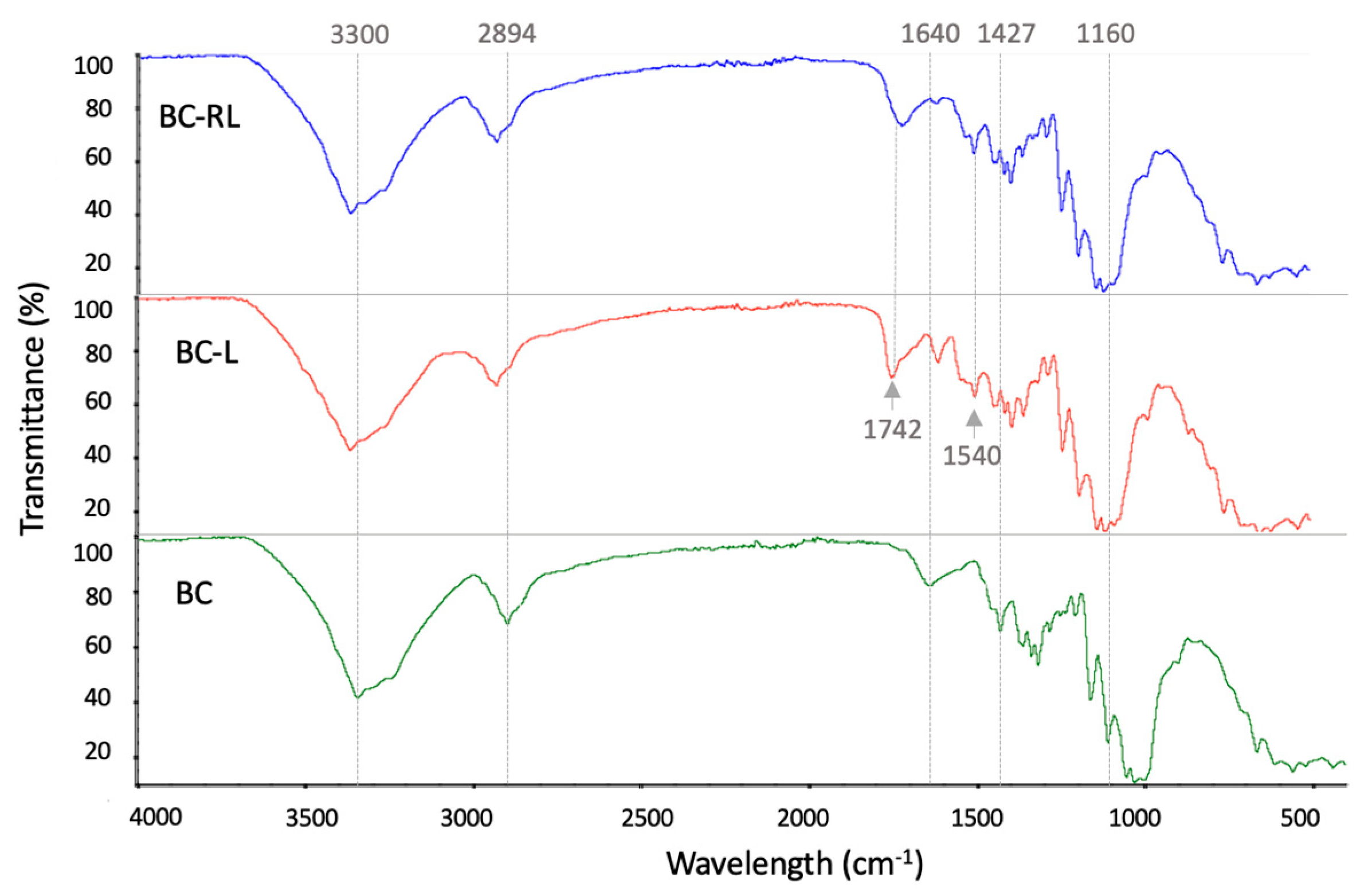
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
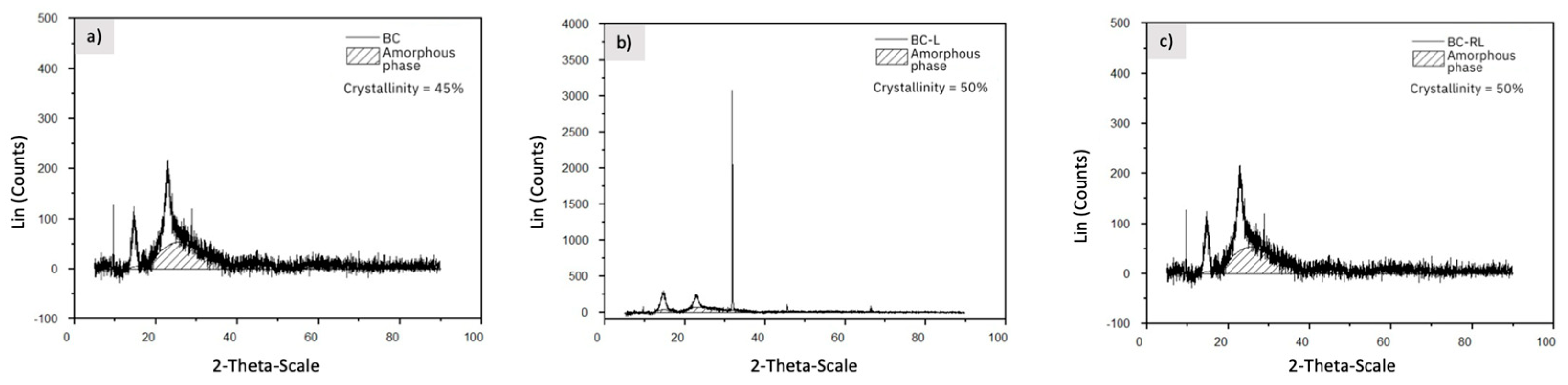
3.5. X-ray Diffraction (XRD) and Crystallinity
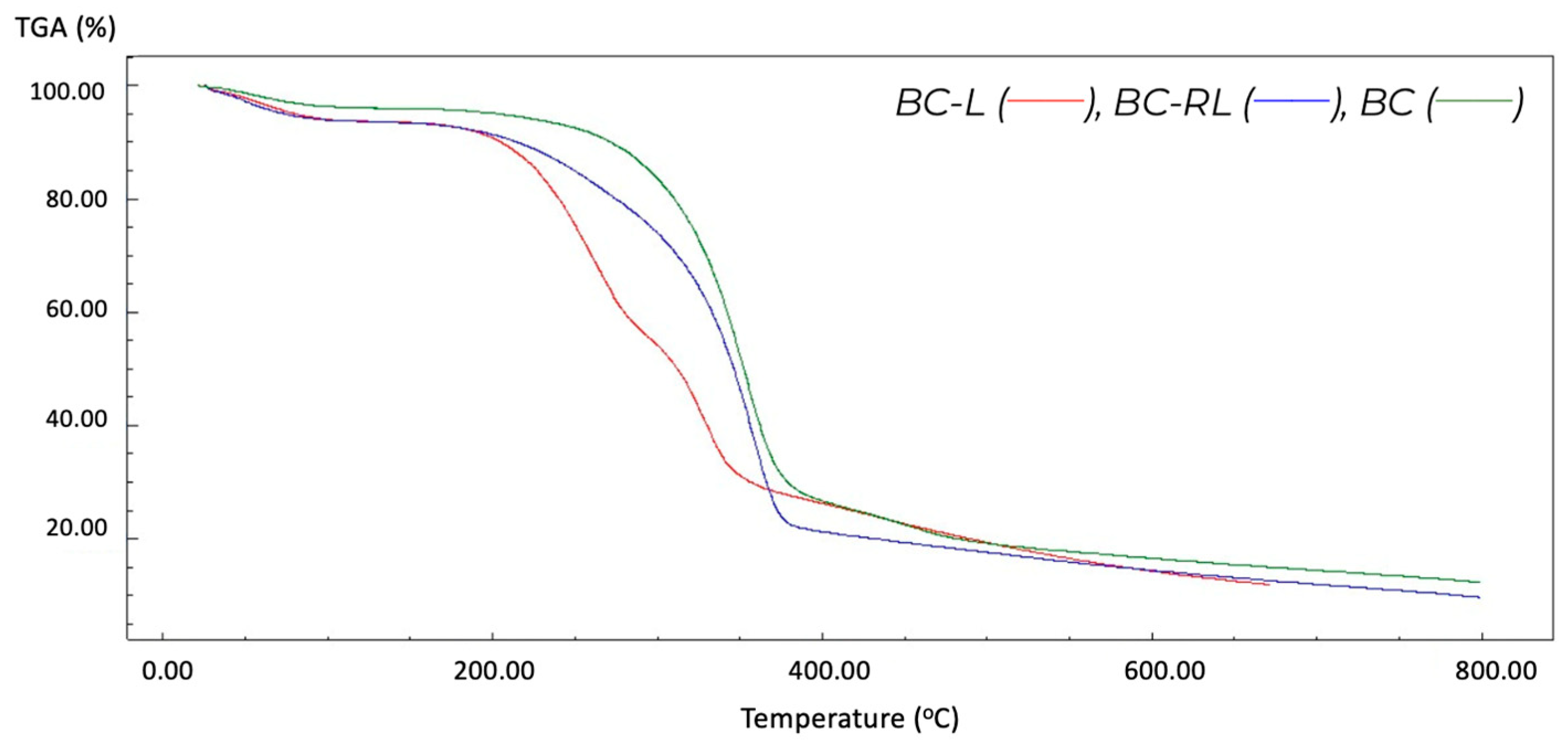
3.6. Thermogravimetric Analysis (TGA)
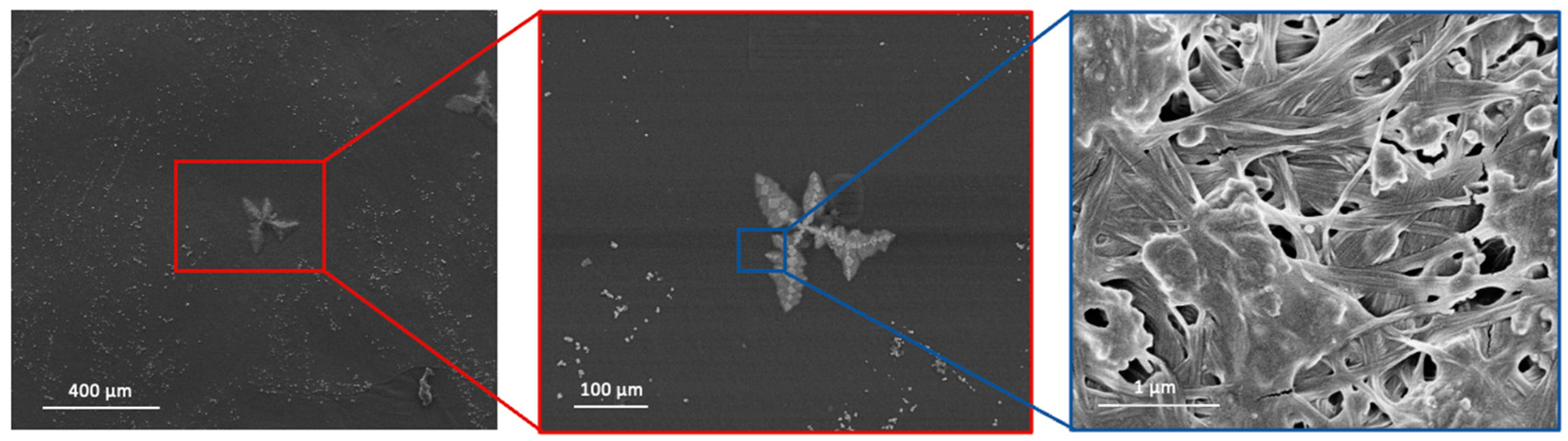
3.7. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef]
- Ahmed, A.; Getti, G.; Boateng, J. Medicated multi-targeted alginate-based dressings for potential treatment of mixed bacterial-fungal infections in diabetic foot ulcers. Int. J. Pharm. 2021, 606, 120903. [Google Scholar] [CrossRef]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional hydrogels for treatment of chronic wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Khodabakhshi, D.; Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Navid, S.; Karbasi, S.; Moshtaghian, J. In vitro and in vivo performance of a propolis-coated polyurethane wound dressing with high porosity and antibacterial efficacy. Colloids Surf. B Biointerfaces 2019, 178, 177–184. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Poblete, J.; Gajardo, F.; Aguayo, C.; Fernández, K. Graphene oxide/polyethylene glycol aerogel reinforced with grape seed extracts as wound dressing. J. Mater. Sci. 2021, 56, 16082–16096. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application: A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Boateng, J.S.; Hafezi, F.; Tabriz, A.G.; Douroumis, D. 3D printed composite dressings loaded with human epidermal growth factor for potential chronic wound healing applications. J. Drug Deliv. Sci. Technol. 2023, 86, 104684. [Google Scholar] [CrossRef]
- Genesi, B.P.; de Melo Barbosa, R.; Severino, P.; Rodas, A.C.; Yoshida, C.M.; Mathor, M.B.; Lopes, P.S.; Viseras, C.; Souto, E.B.; da Silva, C.F. Aloe vera and copaiba oleoresin-loaded chitosan films for wound dressings: Microbial permeation, cytotoxicity, and in vivo proof of concept. Int. J. Pharm. 2023, 634, 122648. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Li, Y.; Zhou, X.; Hui, Z.; Lei, X.; Qiu, L.; Bai, Y.; Wang, C.; Xia, J.; et al. Collagen hydrogel with multiple antimicrobial mechanisms as anti-bacterial wound dressing. Int. J. Biol. Macromol. 2023, 232, 123413. [Google Scholar] [CrossRef]
- Catanzano, O.; Docking, R.; Schofield, P.; Boateng, J. Advanced multi-targeted composite biomaterial dressing for pain and infection control in chronic leg ulcers. Carbohydr. Polym. 2017, 172, 40–48. [Google Scholar] [CrossRef]
- Graça, M.F.; Miguel, S.P.; Cabral, C.S.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Amorim, J.D.; da Silva Junior, C.J.; de Medeiros, A.D.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.; Costa, A.F.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef]
- Hou, S.; Xia, Z.; Pan, J.; Wang, N.; Gao, H.; Ren, J.; Xia, X. Bacterial cellulose applied in wound dressing materials: Production and functional modification—A review. Macromol. Biosci. 2024, 24, 2300333. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Agents that inhibit bacterial biofilm formation. Future Med. Chem. 2015, 7, 647–671. [Google Scholar] [CrossRef]
- Piegerová, A.; Koščová, J.; Schusterová, P.; Nemcová, R.; Kryvtsova, M. In vitro inhibition of biofilm formation by Staphylococcus aureus under the action of selected plant extracts. Folia Vet. 2019, 63, 48–53. [Google Scholar] [CrossRef][Green Version]
- Stanislaw, B.; Alina, K.; Marianna, T.; Halina, K. Bacterial Cellulose. In Biopolymers; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002; Volume 5, p. 37. [Google Scholar] [CrossRef]
- Yun, E.J.; Lee, J.; Kim, D.H.; Kim, J.; Kim, S.; Jin, Y.S.; Kim, K.H. Metabolomic elucidation of the effects of media and carbon sources on fatty acid production by Yarrowia lipolytica. J. Biotechnol. 2018, 272, 7–13. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.H. Effects of minimal media vs. complex media on the metabolite profiles of Escherichia coli and Saccharomyces cerevisiae. Process Biochem. 2017, 57, 64–71. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial cellulose and its applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Ahmed, J.; Gultekinoglu, M.; Edirisinghe, M. Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol. Adv. 2020, 41, 107549. [Google Scholar] [CrossRef]
- Balistreri, G.; Campbell, I.; Li, X.; Amorim, J.; Zhang, S.; Nance, E.; Roumeli, E. Bacterial cellulose nanoparticles as a sustainable drug delivery platform for protein-based therapeutics. RSC Appl. Polym. 2024, 2, 172–183. [Google Scholar] [CrossRef]
- Boyetey, M.J.B.; Torgbo, S.; Sukyai, P. Bio-scaffold for bone tissue engineering with focus on bacterial cellulose, biological materials for hydroxyapatite synthesis and growth factors. Eur. Polym. J. 2023, 194, 112168. [Google Scholar] [CrossRef]
- Chang, W.S.; Chen, H.H. Physical properties of bacterial cellulose composites for wound dressings. Food Hydrocoll. 2016, 53, 75–83. [Google Scholar] [CrossRef]
- Amorim, J.D.; Nascimento, H.A.; Silva Junior, C.J.; Medeiros, A.D.; Silva, I.D.; Costa, A.F.; Vinhas, G.M.; Sarubbo, L.A. Obtainment of bacterial cellulose with added propolis extract for cosmetic applications. Polym. Eng. Sci. 2022, 62, 565–575. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.; Ullah, M.W.; Yang, G. Development and characterization of plant oil-incorporated carboxymethyl cellulose/bacterial cellulose/glycerol-based antimicrobial edible films for food packaging applications. Adv. Compos. Hybrid Mater. 2022, 5, 973–990. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, S.; Adstedt, K.; Kim, M.; Xiong, R.; Yu, J.; Chen, X.; Zhao, X.; Ye, C.; Tsukruk, V.V. Uniformly aligned flexible magnetic films from bacterial nanocelluloses for fast actuating optical materials. Nat. Commun. 2022, 13, 5804. [Google Scholar] [CrossRef]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest advances on bacterial cellulose-based antibacterial materials as wound dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef]
- Di, Z.; Shi, Z.; Ullah, M.W.; Li, S.; Yang, G. A transparent wound dressing based on bacterial cellulose whisker and poly (2-hydroxyethyl methacrylate). Int. J. Biol. Macromol. 2017, 105, 638–644. [Google Scholar] [CrossRef]
- Numata, Y.; Masaki, S.; Tajima, K. Mechanical properties of a bacterial cellulose/polyethylene glycol gel with a peripheral region crosslinked by polyethylene glycol diacrylate. Polym. J. 2016, 48, 317–321. [Google Scholar] [CrossRef]
- Sperotto, G.; Stasiak, L.G.; Godoi, J.P.; Gabiatti, N.C.; De Souza, S.S. A review of culture media for bacterial cellulose production: Complex, chemically defined and minimal media modulations. Cellulose 2021, 28, 2649–2673. [Google Scholar] [CrossRef]
- de Souza, S.S.; Berti, F.V.; de Oliveira, K.P.; Pittella, C.Q.; de Castro, J.V.; Pelissari, C.; Rambo, C.R.; Porto, L.M. Nanocellulose biosynthesis by Komagataeibacter hansenii in a defined minimal culture medium. Cellulose 2019, 26, 1641–1655. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, M.K.; Singh, A. Bacterial cellulose: A smart biomaterial for biomedical applications. J. Mater. Res. 2023, 39, 2–18. [Google Scholar] [CrossRef]
- Trovatti, E.; Freire, C.S.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.; Neto, C.P.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef]
- Park, T.H. Topical Anesthetic versus Lidocaine Mixture for Pain Relief During Keloid Treatment: A Prospective, Split Study. J. Craniofacial Surg. 2023, 34, 10–97. [Google Scholar] [CrossRef]
- Karnina, R.; Arif, S.K.; Hatta, M.; Bukhari, A. Molecular mechanisms of lidocaine. Ann. Med. Surg. 2021, 69, 102733. [Google Scholar] [CrossRef]
- Gupta, A.K.; Sibbald, R.G. Eutectic lidocaine/prilocaine 5% cream and patch may provide satisfactory analgesia for excisional biopsy or curettage with electrosurgery of cutaneous lesions: A randomized, controlled, parallel group study. J. Am. Acad. Dermatol. 1996, 35, 419–423. [Google Scholar] [CrossRef]
- Yang, X.; Wei, X.; Mu, Y.; Li, Q.; Liu, J. A review of the mechanism of the central analgesic effect of lidocaine. Medicine 2020, 99, e19898. [Google Scholar] [CrossRef]
- Kim, H.Y.; Choi, J.B.; Min, S.K.; Chang, M.Y.; Lim, G.M.; Kim, J.E. A randomized clinical trial on the effect of a lidocaine patch on shoulder pain relief in laparoscopic cholecystectomy. Sci. Rep. 2021, 11, 1052. [Google Scholar] [CrossRef]
- Mick, G.; Correa-Illanes, G. Topical pain management with the 5% lidocaine medicated plaster–a review. Curr. Med. Res. Opin. 2012, 28, 937–951. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Adsorptive denitrogenation of model fuel with CuCl-loaded metal–organic frameworks (MOFs). Chem. Eng. J. 2014, 251, 35–42. [Google Scholar] [CrossRef]
- Sarker, M.; Jhung, S.H. Zr-MOF with free carboxylic acid for storage and controlled release of caffeine. J. Mol. Liq. 2019, 296, 112060. [Google Scholar] [CrossRef]
- Sarker, M.; Shin, S.; Jhung, S.H. Synthesis and functionalization of porous Zr-diaminostilbenedicarboxylate metal–organic framework for storage and stable delivery of ibuprofen. ACS Omega 2019, 4, 9860–9867. [Google Scholar] [CrossRef]
- Ferguson, A.; Khan, U.; Walsh, M.; Lee, K.Y.; Bismarck, A.; Shaffer, M.S.; Coleman, J.N.; Bergin, S.D. Understanding the dispersion and assembly of bacterial cellulose in organic solvents. Biomacromolecules 2016, 17, 1845–1853. [Google Scholar] [CrossRef]
- Gudin, J.; Nalamachu, S. Utility of lidocaine as a topical analgesic and improvements in patch delivery systems. Postgrad. Med. 2020, 132, 28–36. [Google Scholar] [CrossRef]
- Tse, C.H.; Comer, J.; Wang, Y.; Chipot, C. Link between membrane composition and permeability to drugs. J. Chem. Theory Comput. 2018, 14, 2895–2909. [Google Scholar] [CrossRef]
- Hamed, D.A.; Maghrawy, H.H.; Abdel Kareem, H. Biosynthesis of bacterial cellulose nanofibrils in black tea media by a symbiotic culture of bacteria and yeast isolated from commercial kombucha beverage. World J. Microbiol. Biotechnol. 2023, 39, 48. [Google Scholar] [CrossRef]
- Tabaii, M.J.; Emtiazi, G. Transparent nontoxic antibacterial wound dressing based on silver nano particle/bacterial cellulose nano composite synthesized in the presence of tripolyphosphate. J. Drug Deliv. Sci. Technol. 2018, 44, 244–253. [Google Scholar] [CrossRef]
- Galdino, C.J.G.; Amorim, J.D.P.; Medeiros, A.D.M.; Cavalcanti, A.K.L.H.; Nascimento, H.A.; Henrique, M.A.; Maranhão, L.J.C.N.; Vinhas, G.M.; Souto Silva, K.K.O.; Costa, A.F.S.; et al. Design of a Naturally Dyed and Waterproof Bio-technological Leather from Reconstituted Cellulose. J. Funct. Biomater. 2022, 13, 49. [Google Scholar] [CrossRef]
- Saska, S.; Teixeira, L.N.; de Oliveira, P.T.; Gaspar, A.M.; Ribeiro, S.J.; Messaddeq, Y.; Marchetto, R. Bacterial cellulose-collagen nano-composite for bone tissue engineering. J. Mater. Chem. 2012, 22, 22102–22112. [Google Scholar] [CrossRef]
- Shezad, O.; Khan, S.; Khan, T.; Park, J.K. Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydr. Polym. 2010, 82, 173–180. [Google Scholar] [CrossRef]
- Padula, C.; Nicoli, S.; Colombo, P.; Santi, P. Single-layer transdermal film containing lidocaine: Modulation of drug release. Eur. J. Pharm. Biopharm. 2007, 66, 422–428. [Google Scholar] [CrossRef]
- Wiberg, K.; Hagman, A.; Burén, P.; Jacobsson, S.P. Determination of the content and identity of lidocaine solutions with UV–visible spectroscopy and multivariate calibration. Analyst 2001, 126, 1142–1148. [Google Scholar] [CrossRef]
- Hou, S.; Hsiu-Ying, Y. Comparison of absorption of aqueous lidocaine and liposome lidocaine following topical application on rabbit vessels. J. Orthop. Res. 1994, 12, 294–297. [Google Scholar] [CrossRef]
- Marin, E.; Rojas, J. Preparation and characterization of crosslinked poly (vinyl) alcohol films with waterproof properties. Int. J. Pharm. Pharm. Sci. 2015, 7, 242–248. [Google Scholar]
- Kamiński, K.; Jarosz, M.; Grudzień, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A.M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef]
- Wittaya-areekul, S.; Prahsarn, C. Development and in vitro evaluation of chitosan–polysaccharides composite wound dressings. Int. J. Pharm. 2006, 313, 123–128. [Google Scholar] [CrossRef]
- Asare, E.O.; Mun, E.A.; Marsili, E.; Paunov, V.N. Nanotechnologies for control of pathogenic microbial biofilms. J. Mater. Chem. B 2022, 10, 5129–5153. [Google Scholar] [CrossRef]
- Jin, S.G.; Kim, K.S.; Kim, D.W.; Kim, D.S.; Seo, Y.G.; Go, T.G.; Youn, Y.S.; Kim, J.O.; Yong, C.S.; Choi, H.G. Development of a novel sodium fusidate-loaded triple polymer hydrogel wound dressing: Mechanical properties and effects on wound repair. Int. J. Pharm. 2016, 497, 114–122. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Zhou, X.; Ruan, C.; Pan, H.; Catchmark, J.M. Bioabsorbable cellulose composites prepared by an improved mineral-binding process for bone defect repair. J. Mater. Chem. B 2016, 4, 1235–1246. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Ikkala, O.; Berglund, L.A. Strong and tough cellulose nanopaper with high specific surface area and porosity. Biomacromolecules 2011, 12, 3638–3644. [Google Scholar] [CrossRef]
- Fei, G.; Wang, Y.; Wang, H.; Ma, Y.; Guo, Q.; Huang, W.; Yang, D.; Shao, Y.; Ni, Y. Fabrication of bacterial cellulose/polyaniline nanocomposite paper with excellent conductivity, strength, and flexibility. ACS Sustain. Chem. Eng. 2019, 7, 8215–8225. [Google Scholar] [CrossRef]
- Medeiros, A.D.; Silva Junior, C.J.; Amorim, J.D.; Durval, I.J.; Damian, R.B.; Cavalcanti, Y.D.; Costa, A.F.; Sarubbo, L.A. Design and Modeling of a Biotechnological Nanofiltration Module Using Bacterial Cellulose Membranes for the Separation of Oily Mixtures. Water 2023, 15, 2. [Google Scholar] [CrossRef]
- van Zyl, E.M.; Kennedy, M.A.; Nason, W.; Fenlon, S.J.; Young, E.M.; Smith, L.J.; Bhatia, S.R.; Coburn, J.M. Structural properties of optically clear bacterial cellulose produced by Komagataeibacter hansenii using arabitol. Biomater. Adv. 2023, 148, 213345. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Juncu, G.; Stoica-Guzun, A.; Stroescu, M.; Isopencu, G.; Jinga, S.I. Drug release kinetics from carboxymethylcellulose-bacterial cellulose composite films. Int. J. Pharm. 2016, 510, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, S.; Sheng, N.; Wang, B.; Wu, Z.; Liang, Q.; Wang, H. Anisotropic bacterial cellulose hydrogels with tunable high mechanical performances, non-swelling and bionic nanofluidic ion transmission behavior. Nanoscale 2021, 13, 8126–8136. [Google Scholar] [CrossRef] [PubMed]
- Clasen, C.; Sultanova, B.; Wilhelms, T.; Heisig, P.; Kulicke, W.M. Effects of different drying processes on the material properties of bacterial cellulose membranes. In Macromolecular Symposia; WILEY-VCH Verlag: Weinheim, Germany, 2006; Volume 244, pp. 48–58. [Google Scholar] [CrossRef]
- Huang, H.C.; Chen, L.C.; Lin, S.B.; Hsu, C.P.; Chen, H.H. In situ modification of bacterial cellulose network structure by adding interfering substances during fermentation. Bioresour. Technol. 2010, 101, 6084–6091. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, self-healing, and tissue-adhesive hydrogel for wound dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velázquez, G.; Vázquez, M. Bacterial cellulose films: Evaluation of the water interaction. Food Packag. Shelf Life 2020, 25, 100526. [Google Scholar] [CrossRef]
- Bainbridge, P.; Browning, P.; Bernatchez, S.F.; Blaser, C.; Hitschmann, G. Comparing test methods for moisture-vapor transmission rate (MVTR) for vascular access transparent semipermeable dressings. J. Vasc. Access 2023, 24, 1000–1007. [Google Scholar] [CrossRef]
- Shi, S.; Wu, H.; Zhi, C.; Yang, J.; Si, Y.; Ming, Y.; Fei, B.; Hu, J. A skin-like nanostructured membrane for advanced wound dressing. Compos. Part B Eng. 2023, 250, 110438. [Google Scholar] [CrossRef]
- Gama, M.; Gatenholm, P.; Klemm, D. (Eds.) Bacterial Nanocellulose: A Sophisticated Multifunctional Material; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Li, Y.; Jiang, H.; Zheng, W.; Gong, N.; Chen, L.; Jiang, X.; Yang, G. Bacterial cellulose–hyaluronan nanocomposite biomaterials as wound dressings for severe skin injury repair. J. Mater. Chem. B 2015, 3, 3498–3507. [Google Scholar] [CrossRef]
- Nascimento, H.A.; Amorim, J.D.; Mfilho, L.E.; Costa, A.F.; Sarubbo, L.A.; Napoleão, D.C.; Maria Vinhas, G. Production of bacterial cellulose with antioxidant additive from grape residue with promising cosmetic applications. Polym. Eng. Sci. 2022, 62, 2826–2839. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Chen, J.; Duan, X.; Guo, B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact. Mater. 2022, 8, 341–354. [Google Scholar] [CrossRef]
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Momoh, F.U.; Boateng, J.S.; Richardson, S.C.; Chowdhry, B.Z.; Mitchell, J.C. Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int. J. Biol. Macromol. 2015, 81, 137–150. [Google Scholar] [CrossRef]
- Singh, B.; Abhishek, D. Design of acacia gum–carbopol–cross-linked-polyvinylimidazole hydrogel wound dressings for antibiotic/anesthetic drug delivery. Ind. Eng. Chem. Res. 2016, 55, 9176–9188. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of mucoadhesive buccal films for local administration of ketoprofen and lidocaine hydrochloride by combining fused deposition modeling and inkjet printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Baroni, D.; Brunetto, G.; Sobral, V.R.V.; da Silva, C.M.G.; Venâncio, P.; Zago, P.W.; Cereda, C.M.S.; Volpato, M.C.; de Araújo, D.R.; et al. Liposomal lidocaine gel for topical use at the oral mucosa: Characterization, in vitro assays and in vivo an-esthetic efficacy in humans. J. Liposome Res. 2015, 25, 11–19. [Google Scholar] [CrossRef]
- Pahlevan, M.; Toivakka, M.; Alam, P. Mechanical properties of TEMPO-oxidised bacterial cellulose-amino acid biomaterials. Eur. Polym. J. 2018, 101, 29–36. [Google Scholar] [CrossRef]
- Ashaduzzaman, M.; Al-Rafin, A.; Mustary, N.; Shamsuddin, S.M. Studies on Interaction of Lidocaine Drug with Natural Cellulosic Fibres. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 4, 36–46. [Google Scholar] [CrossRef]
- Favatela, F.; Horst, M.F.; Bracone, M.; Gonzalez, J.; Alvarez, V.; Lassalle, V. Gelatin/Cellulose nanowhiskers hydrogels intended for the administration of drugs in dental treatments: Study of lidocaine as model case. J. Drug Deliv. Sci. Technol. 2021, 61, 101886. [Google Scholar] [CrossRef]
- Yang, D.; Chen, X.; Li, Z.; Yang, C. Mechanistic Study of Release Characteristics of Two Active Ingredients in Transdermal Patch Containing Lidocaine−Flurbiprofen Ionic Liquid. Pharmaceutics 2022, 14, 2158. [Google Scholar] [CrossRef] [PubMed]
- Babaei, S.; Ghanbarzadeh, Z.M.; Adib, M.; Kouhsoltani, S.; Davaran, H.; Hamishehkar, H. Enhanced skin penetration of lidocaine through encapsulation into nanoethosomes and nanostructured lipid carriers: A comparative study. Die Pharm. Int. J. Pharm. Sci. 2016, 71, 247–251. [Google Scholar] [CrossRef]
- Bakonyi, M.; Berkó, S.; Budai-Szűcs, M.; Kovács, A.; Csányi, E. DSC for evaluating the encapsulation efficiency of lidocaine-loaded liposomes compared to the ultracentrifugation method. J. Therm. Anal. Calorim. 2017, 130, 1619–1625. [Google Scholar] [CrossRef]
- Rodríguez, K.; Sundberg, J.; Gatenholm, P.; Renneckar, S. Electrospun nanofibrous cellulose scaffolds with controlled microar-chitecture. Carbohydr. Polym. 2014, 100, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Silva, N.H.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.; Silvestre, A.J.; Neto, C.P. Biocellulose membranes as supports for dermal release of lidocaine. Biomacromolecules 2011, 12, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Zink, M.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. Bacterial nanocellulose with a shape-memory effect as potential drug delivery system. RSC Adv. 2014, 4, 57173–57184. [Google Scholar] [CrossRef]
- Zhang, Q.; Qi, C.; Wang, X.; Zhu, B.; Li, W.; Xiao, X.; Fu, H.; Hu, S.; Zhu, S.; Xu, W.; et al. Daytime radiative cooling dressings for accelerating wound healing under sunlight. Nat. Chem. Eng. 2024, 1, 301–310. [Google Scholar] [CrossRef]
- Loh, E.Y.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Mohd Amin, M.C. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci. Rep. 2018, 8, 2875. [Google Scholar] [CrossRef]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: Synergistic antimicrobial effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef]
- Onyszko, M.; Markowska-Szczupak, A.; Rakoczy, R.; Paszkiewicz, O.; Janusz, J.; Gorgon-Kuza, A.; Wenelska, K.; Mijowska, E. The cellulose fibers functionalized with star-like zinc oxide nanoparticles with boosted antibacterial performance for hygienic products. Sci. Rep. 2022, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, L.; Wang, S.; Huang, L.; Zhang, Y.; Tian, M.; Li, X.; Zhang, J. Isolation and characterization of bacterial cellulose produced from soybean whey and soybean hydrolyzate. Sci. Rep. 2023, 13, 16024. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Laromaine, A.; Roig, A. Bacterial cellulose films: Influence of bacterial strain and drying route on film properties. Cellulose 2014, 21, 4455–4469. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A.; Rizwan, M.; Azeem, M.K.; Mehmood, A.; Khan, R.U.; Mahmood, H.A. Kinetics and controlled release of lidocaine from novel carrageenan and alginate-based blend hydrogels. Int. J. Biol. Macromol. 2020, 147, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Brassolatti, P.; Kido, H.W.; Bossini, P.S.; Gabbai-Armelin, P.R.; Otterço, A.N.; Almeida-Lopes, L.; Zanardi, L.M.; Napolitano, M.A.; de Avó, L.R.; Forato, L.A.; et al. Bacterial cellulose membrane used as biological dressings on third-degree burns in rats. Bio-Med. Mater. Eng. 2018, 29, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Ghozali, M.; Meliana, Y.; Chalid, M. Synthesis and characterization of bacterial cellulose by Acetobacter xylinum using liquid tapioca waste. Mater. Today Proc. 2021, 44, 2131–2134. [Google Scholar] [CrossRef]
- Ruan, C.; Zhu, Y.; Zhou, X.; Abidi, N.; Hu, Y.; Catchmark, J.M. Effect of cellulose crystallinity on bacterial cellulose assembly. Cellulose 2016, 23, 3417–3427. [Google Scholar] [CrossRef]
- Rodriguez-Chanfrau, J.E.; Santos, M.L.; Riccardi, C.D.; De Olyveira, G.M.; Hernandez-Escalona, M.; Basmaji, P.; Veranes-Pantoja, Y.; Guastaldi, A.C. Chemical modification of bacterial cellulose for use in regenerative medicine. Cellul. Chem. Technol. 2017, 51, 673–680. [Google Scholar]
- Verdugo-Escamilla, C.; Alarcón-Payer, C.; Acebedo-Martínez, F.J.; Fernández-Penas, R.; Domínguez-Martín, A.; Choquesillo-Lazarte, D. Lidocaine Pharmaceutical Multicomponent Forms: A Story about the Role of Chloride Ions on Their Stability. Crystals 2022, 12, 798. [Google Scholar] [CrossRef]
- Masaki, Y.; Tanaka, M.; Nishikawa, T. Physicochemical compatibility of propofol-lidocaine mixture. Anesth. Analg. 2003, 97, 1646–1651. [Google Scholar] [CrossRef]
- Li, G.; Nandgaonkar, A.G.; Habibi, Y.; Krause, W.E.; Wei, Q.; Lucia, L.A. An environmentally benign approach to achieving vectorial alignment and high microporosity in bacterial cellulose/chitosan scaffolds. RSC Adv. 2017, 7, 13678–13688. [Google Scholar] [CrossRef]




| Wet Bacterial Cellulose Yield | Dry Bacterial Cellulose Yield | Moisture Content (MC) |
|---|---|---|
| 77.50 ± 3.71 g/L b | 1.24 ± 0.32 g/L a | 98.4 ± 0.3% c |
| Sample | Temperature Range (°C) | Weight Loss (%) | Residue (%) |
|---|---|---|---|
| BC | 23–180 | 4.25 | 12.38 |
| 180–400 | 68.99 | ||
| 400–800 | 16.37 | ||
| BC-L | 26–160 | 6.15 | 19.42 |
| 160–310 | 46.51 | ||
| 310–380 | 16.37 | ||
| 380–800 | 11.55 | ||
| BC-RL | 27–160 | 6.46 | 9.85 |
| 160–390 | 71.51 | ||
| 390–800 | 12.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, J.D.P.d.; Cavalcanti, Y.d.F.; Medeiros, A.D.M.d.; Silva Junior, C.J.G.d.; Durval, I.J.B.; Costa, A.F.d.S.; Sarubbo, L.A. Synthesis of Transparent Bacterial Cellulose Films as a Platform for Targeted Drug Delivery in Wound Care. Processes 2024, 12, 1282. https://doi.org/10.3390/pr12071282
Amorim JDPd, Cavalcanti YdF, Medeiros ADMd, Silva Junior CJGd, Durval IJB, Costa AFdS, Sarubbo LA. Synthesis of Transparent Bacterial Cellulose Films as a Platform for Targeted Drug Delivery in Wound Care. Processes. 2024; 12(7):1282. https://doi.org/10.3390/pr12071282
Chicago/Turabian StyleAmorim, Julia Didier Pedrosa de, Yasmim de Farias Cavalcanti, Alexandre D’Lamare Maia de Medeiros, Cláudio José Galdino da Silva Junior, Italo José Batista Durval, Andréa Fernanda de Santana Costa, and Leonie Asfora Sarubbo. 2024. "Synthesis of Transparent Bacterial Cellulose Films as a Platform for Targeted Drug Delivery in Wound Care" Processes 12, no. 7: 1282. https://doi.org/10.3390/pr12071282
APA StyleAmorim, J. D. P. d., Cavalcanti, Y. d. F., Medeiros, A. D. M. d., Silva Junior, C. J. G. d., Durval, I. J. B., Costa, A. F. d. S., & Sarubbo, L. A. (2024). Synthesis of Transparent Bacterial Cellulose Films as a Platform for Targeted Drug Delivery in Wound Care. Processes, 12(7), 1282. https://doi.org/10.3390/pr12071282










