Behavior of a Mixture of Metals for Competiting Adsorption Sites of Untreated and Alkali-Treated Rice Husk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosorbent
2.2. Reactives
2.3. Biosorbent Treatment
2.4. Rice Husk (Biosorbent) Characterization
2.5. Material Composition
2.5.1. Lignin
2.5.2. Holocellulose
2.5.3. Cellulose
2.5.4. Hemicellulose
2.6. XR Diffraction
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Scanning Electron Microscopy and Elemental Analysis by Energy Dispersive X-ray Spectroscopy
2.9. Adsorption and Desorption Experiments
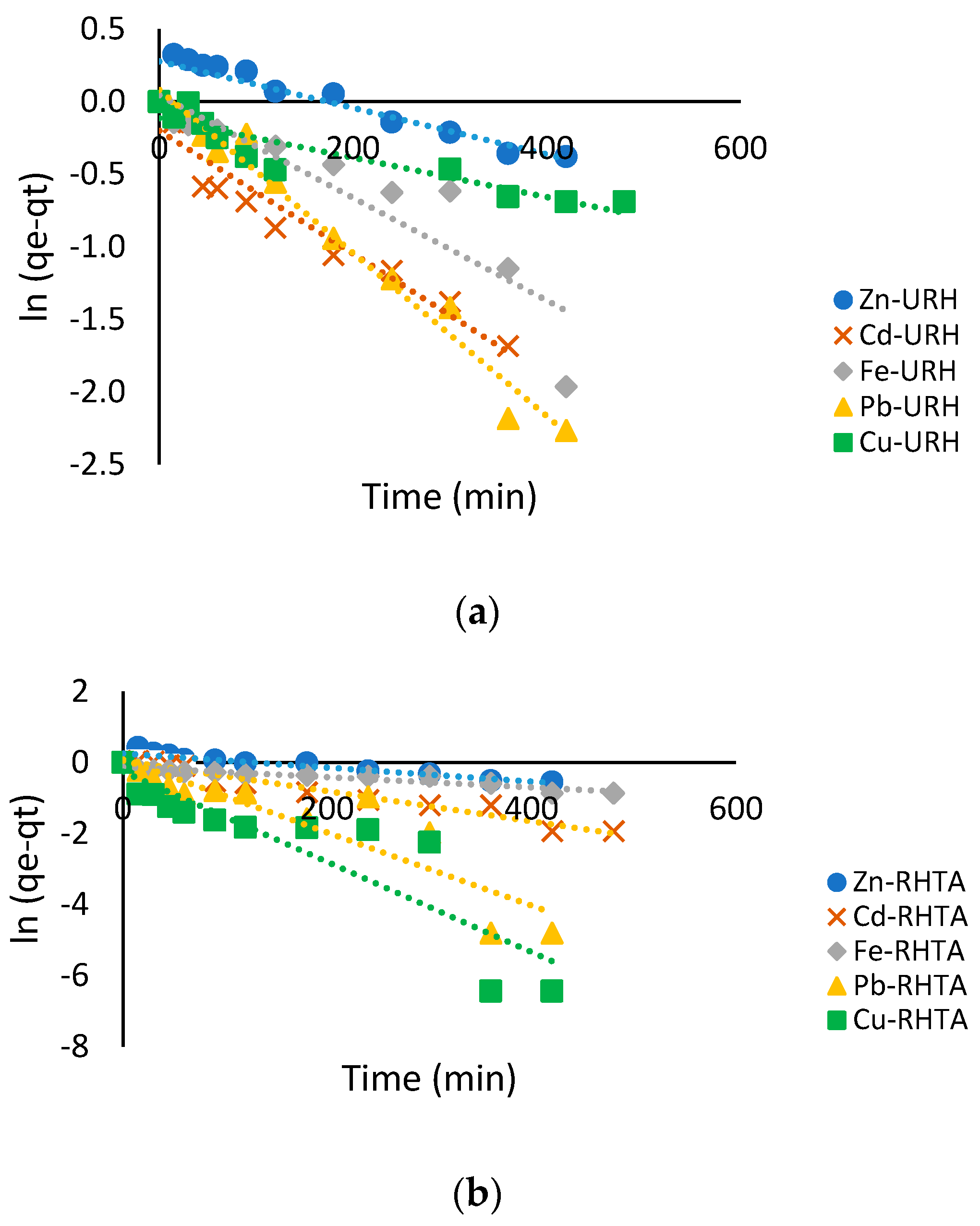
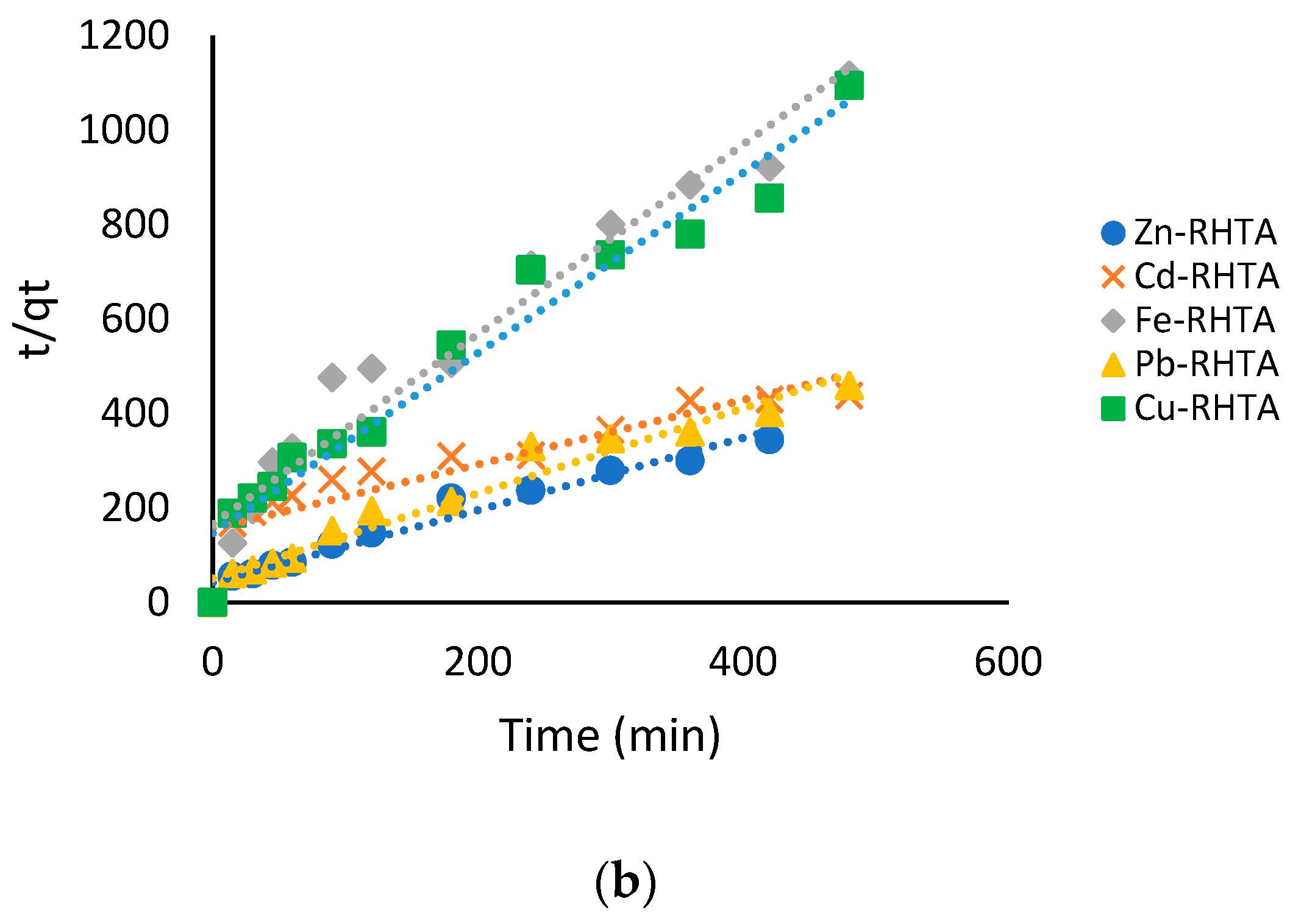
2.10. Adsorption Kinetics
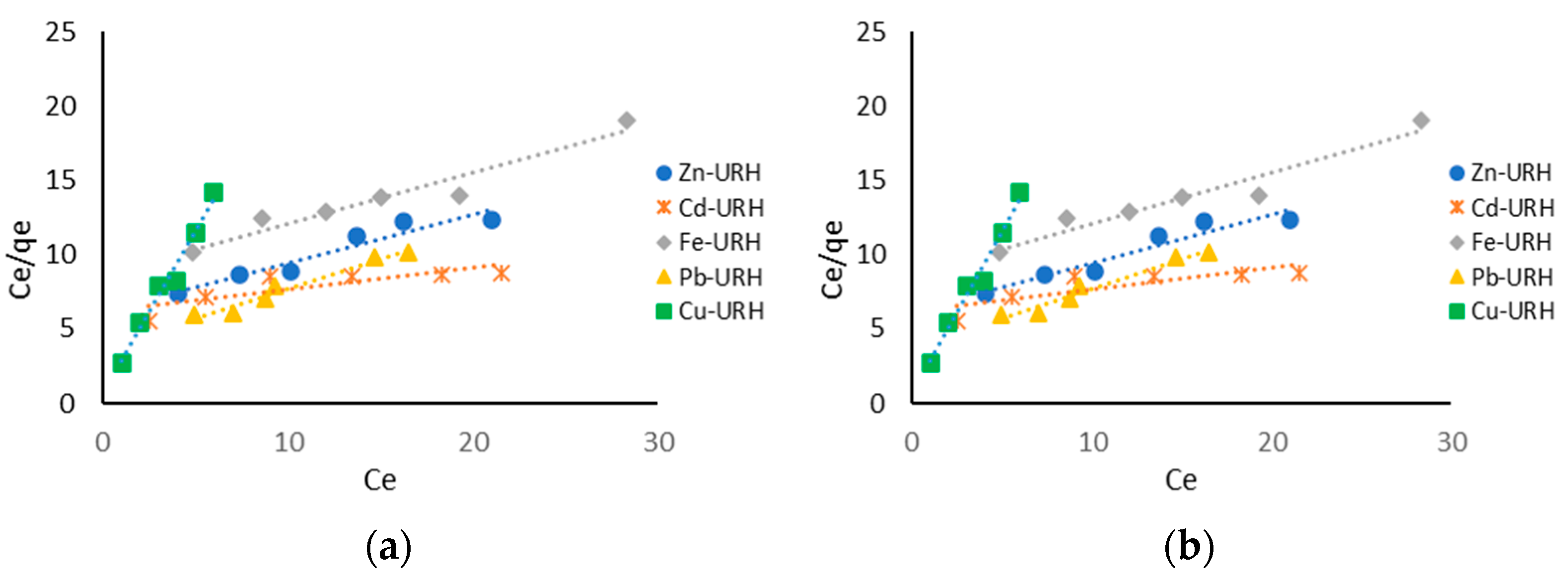
2.11. Adsorption Isotherms
2.12. Metal Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Biosorbent Composition
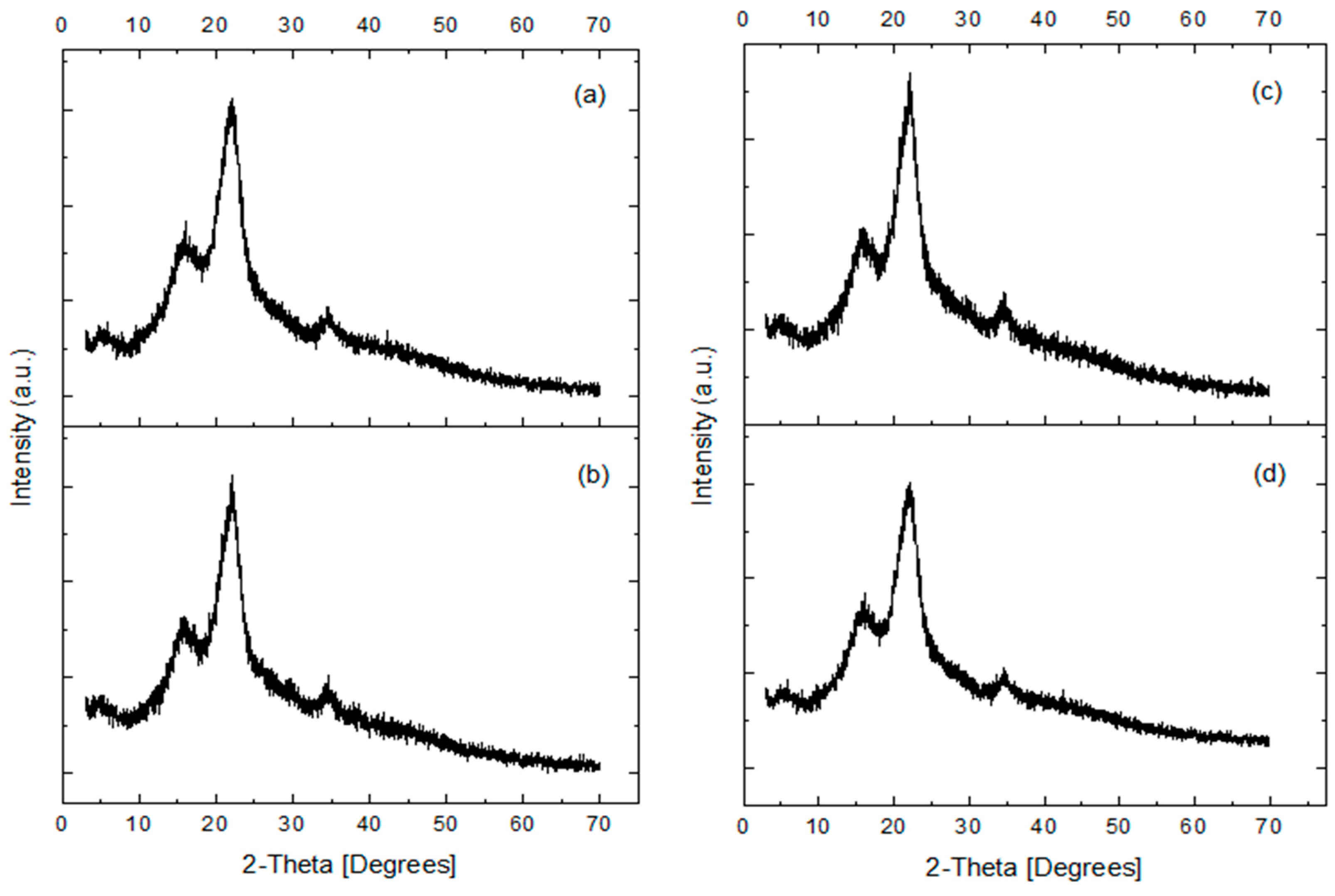
3.2. X-ray Diffraction (XRD)
3.3. Fourier Transform Infrared Spectroscopy (FTIR) (XRD)
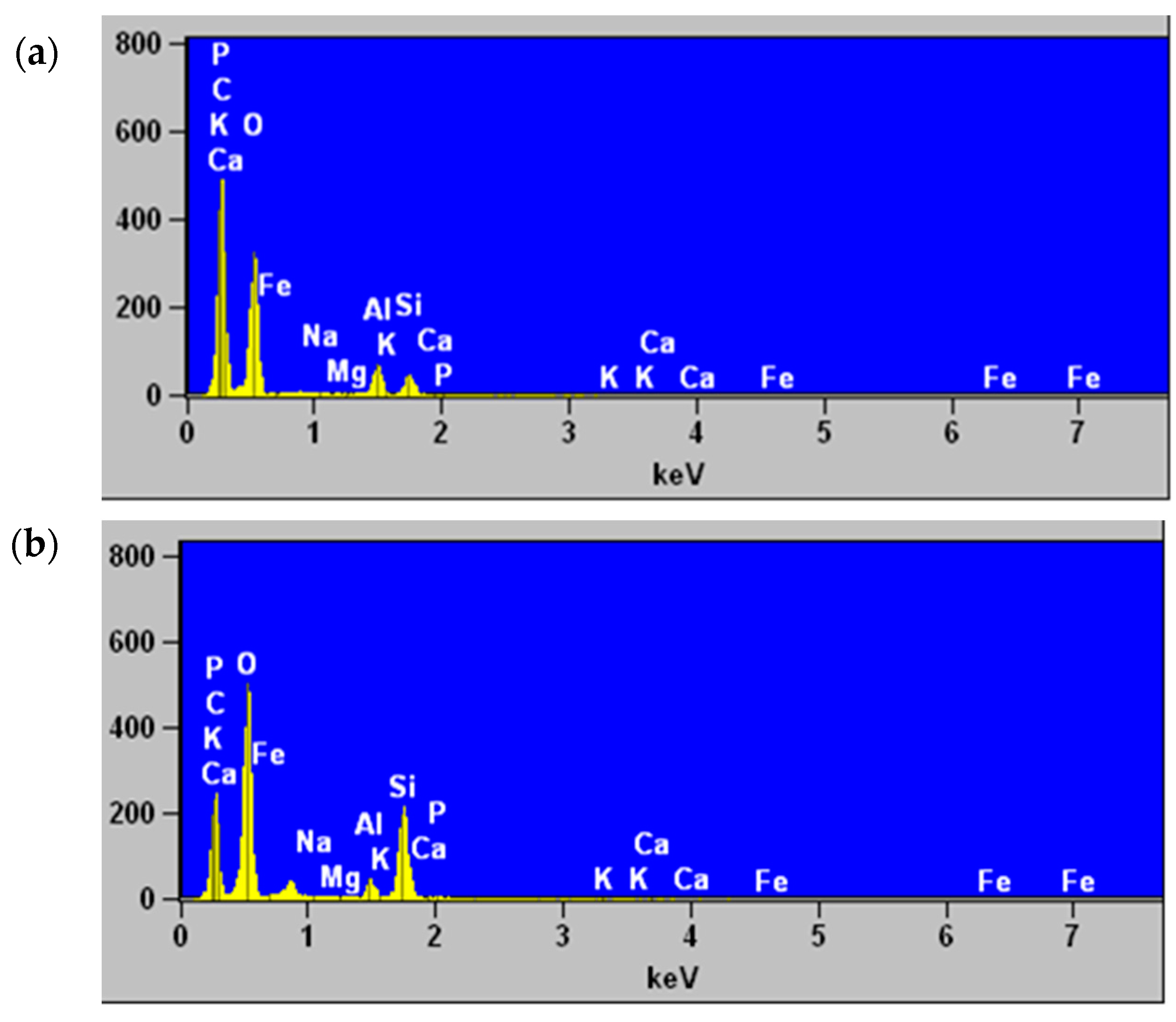
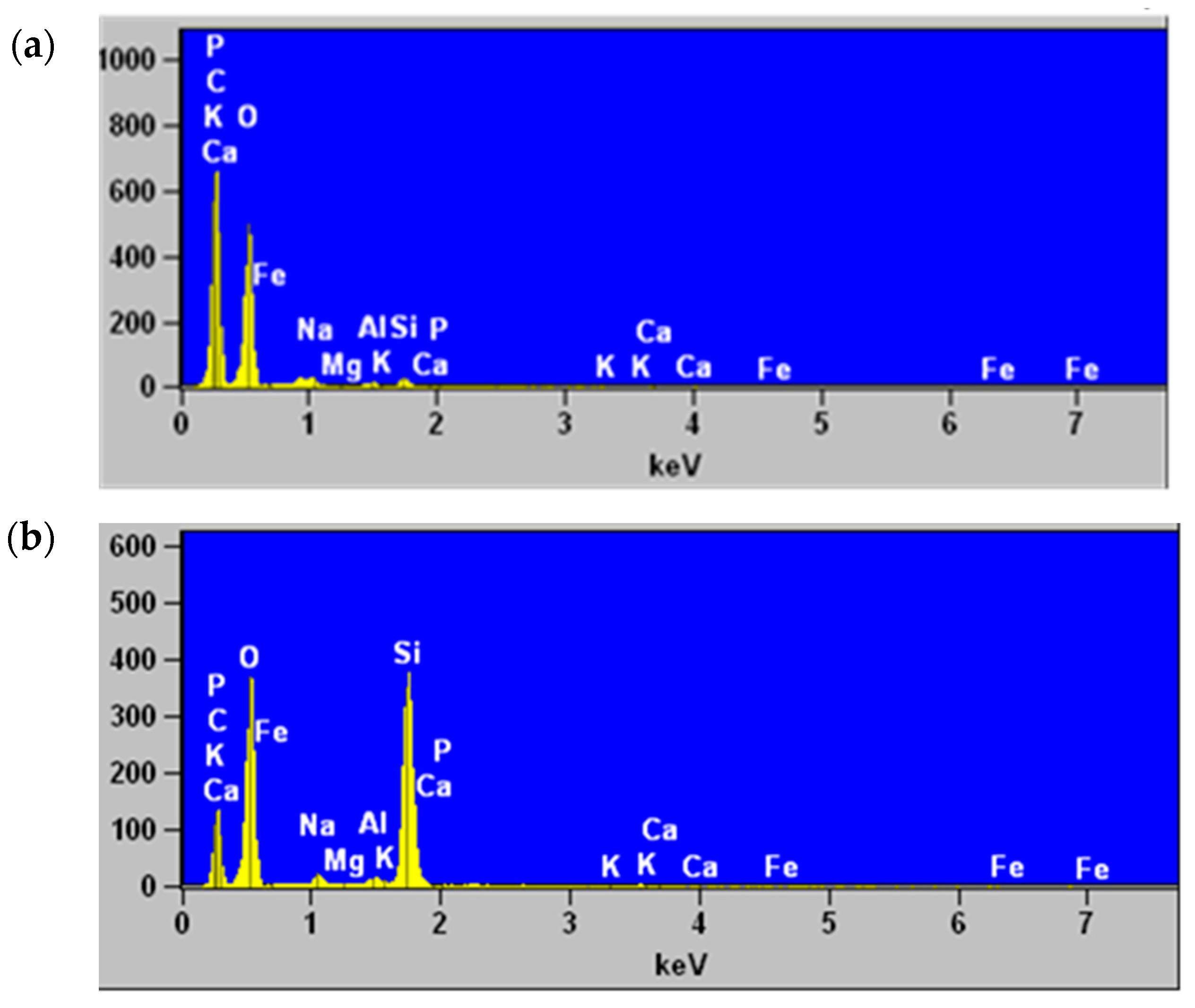
3.4. Scanning Electron Microscopy and Elemental Analysis by Energy Dispersive X-ray Spectroscopy
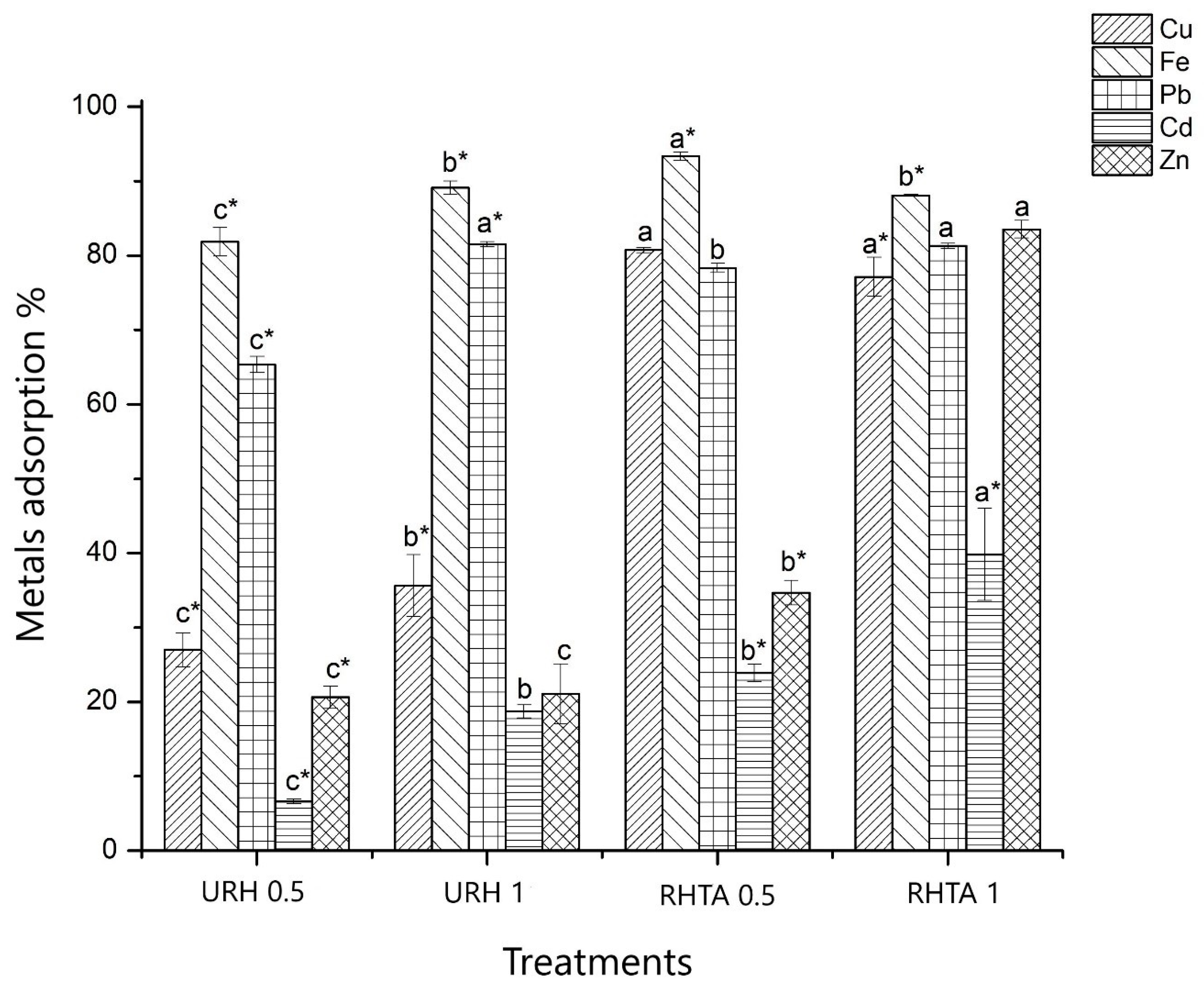
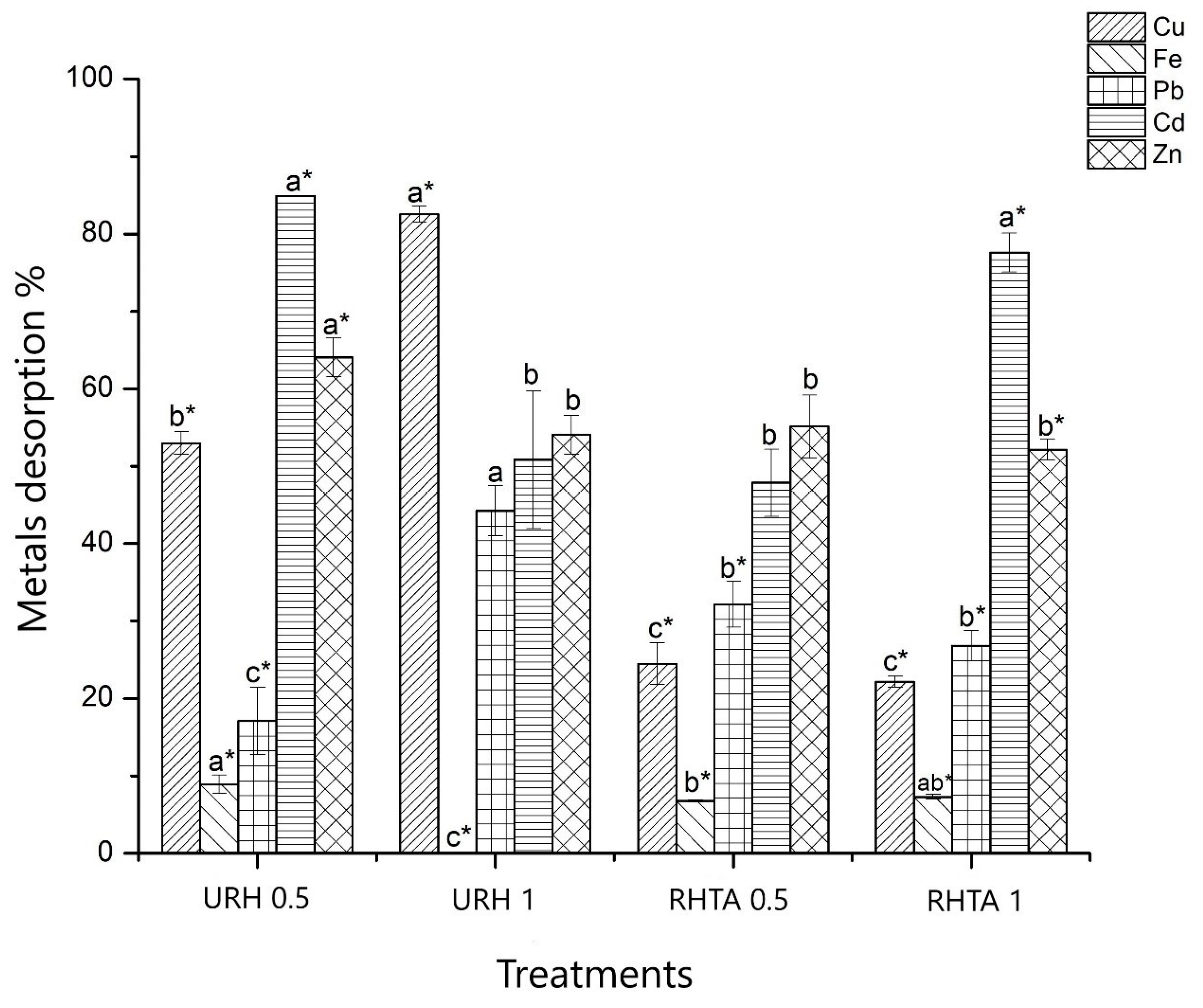
3.5. Adsorption and Desorption of Metals
3.6. Adsorption Isotherms and Kinetics Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Zakir Hossain, S.M.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Shahabudin, M.M.; Musa, S. Occurrence of Surface Water Contaminations: An Overview. IOP Conf. Ser. Earth Environ. Sci. 2018, 140, 012058. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Ashfaq, M.Y.; Khan, M.; Al Disi, Z.; Da’na, D.A.; Shoshaa, R. State-of-the-art adsorption and adsorptive filtration based technologies for the removal of trace elements: A critical review. Sci. Total Env. 2023, 895, 164854. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, N.; Rahimnejad, M.; Pourali, M.; Muallah, S.K. Effective removal of nickel ions from aqueous solution using multi-wall carbon nanotube functionalized by glycerol-based deep eutectic solvent. Colloid. Interface Sci. Commun. 2021, 40, 100347. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Caccamo, F.M.; Carnevale Miino, M.; Durante, A.; Bellazzi, S.; Baldi, M.; Bertanza, G. How to produce an alternative carbon source for denitrification by treating and drastically reducing biological sewage sludge. Membranes 2021, 11, 977. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Sorlini, S.; Milanese, C.; Illankoon, W.A.M.A.N.; Caccamo, F.M.; Calatroni, S. Rice industry by-products as adsorbent materials for removing fluoride and arsenic from drinking water—A review. Appl. Sci. 2022, 12, 3166. [Google Scholar] [CrossRef]
- Quansah, J.O.; Hlaing, T.; Lyonga, F.N.; Kyi, P.P.; Hong, S.H.; Lee, C.G.; Park, S.J. Nascent rice husk as an adsorbent for removing cationic dyes from textile wastewater. Appl. Sci. 2020, 10, 3437. [Google Scholar] [CrossRef]
- Gómez-Aguilar, D.L.; Rodríguez-Miranda, J.P.; Salcedo-Parra, O.J. Fruit peels as a sustainable waste for the biosorption of heavy metals in wastewater: A review. Molecules 2022, 27, 2124. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef] [PubMed]
- Corral-Bobadilla, M.; González-Marcos, A.; Vergara-González, E.P.; Alba-Elías, F. Bioremediation of waste water to remove heavy metals using the spent mushroom substrate of Agaricus bisporus. Water 2019, 11, 454. [Google Scholar] [CrossRef]
- Akhter, F.; Soomro, S.A.; Jamali, A.R.; Chandio, Z.A.; Siddique, M.; Ahmed, M. Rice husk ash as green and sustainable biomass waste for construction and renewable energy applications: A review. Biomass Convers. Biorefinery 2023, 13, 4639–4649. [Google Scholar] [CrossRef]
- Thakur, V.; Sharma, E.; Guleria, A.; Sangar, S.; Singh, K. Modification and management of lignocellulosic waste as an ecofriendly biosorbent for the application of heavy metal ions sorption. Mater. Today Proc. 2020, 32, 608–619. [Google Scholar] [CrossRef]
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost Adsorbents Derived from Agricultural By-products/Wastes for Enhancing Contaminant Uptakes from Wastewater: A Review. Pol. J. Environ. Stud. 2017, 26, 479–510. [Google Scholar] [CrossRef] [PubMed]
- Izwan, S.M.; Sapuan, S.M.; Zuhri, M.Y.M.; Mohamed, A.R. Effects of benzoyl treatment on NaOH treated sugar palm fiber: Tensile, thermal, and morphological properties. J. Mater. Res. Technol. 2020, 9, 5805–5814. [Google Scholar] [CrossRef]
- Zafar, S.; Khan, M.I.; Lashari, M.H.; Khraisheh, M.; Almomani, F.; Mirza, M.L.; Khalid, N. Removal of copper ions from aqueous solution using NaOH-treated rice husk. Emergent Mater. 2020, 3, 857–870. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Khan, M.J. Recent advances in the pretreatment of lignocellulosic biomass for biofuels and value-added products. Curr. Opin. Green. Sustain. Chem. 2019, 20, 18–24. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Cellulose based grafted biosorbents-Journey from lignocellulose biomass to toxic metal ions sorption applications-A review. J. Mol. Liq. 2017, 232, 62–93. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Chavez-Gonzalez, B.; Reynel-Ávila, H.E.; Diaz-Muñoz, L.L.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Lima, E.C.; Tapia-Picazo, J.C.; Lamine, A.B. Application of a heterogeneous physical model for the adsorption of Cd2+, Ni2+, Zn2+ and Cu2+ ions on flamboyant pods functionalized with citric acid. Chem. Eng. J. 2021, 417, 127975. [Google Scholar] [CrossRef]
- Cai, T.; Du, H.; Liu, X.; Tie, B.; Zeng, Z. Insights into the removal of Cd and Pb from aqueous solutions by NaOH–EtOH-modified biochar. Environ. Technol. Innov. 2021, 24, 102031. [Google Scholar] [CrossRef]
- Nizam, N.U.M.; Hanafiah, M.M.; Mahmoudi, E.; Mohammad, A.W.; Oyekanmi, A.A. Effective adsorptive removal of dyes and heavy metal using graphene oxide based Pre-treated with NaOH/H2SO4 rubber seed shells synthetic graphite Precursor: Equilibrium Isotherm, kinetics and thermodynamic studies. Sep. Purif. Technol. 2022, 289, 120730. [Google Scholar] [CrossRef]
- Thi-Quyen, V.; Pham, T.H.; Kim, J.; Thanh, D.M.; Thang, P.Q.; Van Le, Q.; Hoon Jung, S.; Kim, T. Biosorbent derived from coffee husk for efficient removal of toxic heavy metals from wastewater. Chemosphere 2021, 284, 131312. [Google Scholar] [CrossRef]
- Salcedo Sánchez, E.R.; Martínez, J.M.E.; Morales, M.M.; Talavera Mendoza, O.; Alberich, M.V.E. Ecological and health risk assessment of potential toxic elements from a mining area (water and sediments): The San Juan-Taxco River System, Guerrero, Mexico. Water 2022, 14, 518. [Google Scholar] [CrossRef]
- Afzaal, M.; Hameed, S.; Liaqat, I.; Ali Khan, A.A.; Abdul Manan, H.; Shahid, R.; Altaf, M. Heavy metals contamination in water, sediments and fish of freshwater ecosystems in Pakistan. Water Pract. Technol. 2022, 17, 1253–1272. [Google Scholar] [CrossRef]
- Şener, E.; Şener, Ş.; Bulut, C. Assessment of heavy metal pollution and quality in lake water and sediment by various index methods and GIS: A case study in Beyşehir Lake, Turkey. Mar. Pollut. Bull. 2023, 192, 115101. [Google Scholar] [CrossRef] [PubMed]
- El-Degwy, A.A.; Negm, N.A.; El-Tabl, A.S.; Goher, M.E. Assessment of heavy metal pollution in water and its effect on Nile tilapia (Oreochromis niloticus) in Mediterranean Lakes: A case study at Mariout Lake. Appl. Water Sci. 2023, 13, 50. [Google Scholar] [CrossRef]
- AO, A.; PO, O.; PO, A. Evaluation of water pollution monitoring for heavy metal contamination: A case study of Agodi Reservoir, Oyo State, Nigeria. Environ. Monit. Assess. 2022, 194, 675. [Google Scholar] [CrossRef]
- De León-Gómez, H.; Martin del Campo-Delgado, M.A.; Esteller-Alberich, M.V.; Velasco-Tapia, F.; Alva-Niño, E.; Cruz-López, A. Assessment of nitrate and heavy metal contamination of groundwater using the heavy metal pollution index: Case study of Linares, Mexico. Environ. Earth Sci. 2020, 79, 1–19. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Calheiro, D.; Kieling, A.G.; Moraes, C.A.; Rocha, T.L.; Brehm, F.A.; Modolo, R.C. Characterization of rice husk ash produced using different biomass combustion techniques for energy. Fuel 2016, 165, 351–359. [Google Scholar] [CrossRef]
- Soltani, N.; Soltani, S.; Bahrami, A.; Pech-Canul, M.I.; Gonzalez, L.A.; Möller, A.; Tapp, J.; Gurlo, A. Electrical and thermomechanical properties of CVI-Si3N4 porous rice husk ash infiltrated by Al-Mg-Si alloys. J. Alloys Compd. 2017, 696, 856–868. [Google Scholar] [CrossRef]
- Steven, S.; Restiawaty, E.; Pasymi, P.; Bindar, Y. An appropriate acid leaching sequence in rice husk ash extraction to enhance the produced green silica quality for sustainable industrial silica gel purpose. J. Taiwan. Inst. Chem. Eng. 2021, 122, 51–57. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Thapa, M.; Shrestha, R.G.; Maji, S.; Pradhananga, R.R.; Ariga, K. Rice husk-derived high surface area nanoporous carbon materials with excellent iodine and methylene blue adsorption properties. J. Carbon. Res. 2019, 5, 10. [Google Scholar] [CrossRef]
- Chen, S.; Qin, C.; Wang, T.; Chen, F.; Li, X.; Hou, H.; Zhou, M. Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: Adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J. Mol. Liq. 2019, 285, 62–74. [Google Scholar] [CrossRef]
- Bakar, R.A.; Yahya, R.; Gan, S.N. Production of high purity amorphous silica from rice husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef]
- Naseer, A.; Jamshaid, A.; Hamid, A.; Muhammad, N.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Khuram, S.; Shah, N.S. Lignin and lignin based materials for the removal of heavy metals from waste water-an overview. Z. Für Phys. Chem. 2019, 233, 315–345. [Google Scholar] [CrossRef]
- Rafidah, M.S.; Jahimin, A.; Sani, S.M. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C. Biosorption of heavy metals by lignocellulosic biomass and chemical analysis. BioResources 2019, 14, 4952–4995. [Google Scholar] [CrossRef]
- Wijeyawardana, P.; Nanayakkara, N.; Gunasekara, C.; Karunarathna, A.; Law, D.; Pramanik, B.K. Removal of Cu, Pb and Zn from stormwater using an industrially manufactured sawdust and paddy husk derived biochar. Environ. Technol. Innov. 2022, 28, 102640. [Google Scholar] [CrossRef]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+ & Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef] [PubMed]
- Flores-Trujillo, A.K.I.; Mussali-Galante, P.; de Hoces, M.C.; Blázquez-García, G.; Saldarriaga-Noreña, H.A.; Rodríguez-Solís, A.; Tovar-Sánchez, E.; Sánchez-Salinas, E.; Ortiz-Hernández, M.L. Biosorption of heavy metals on Opuntia fuliginosa and Agave angustifolia fibers for their elimination from water. Int. J. Environ. Sci. Technol. 2021, 18, 441–454. [Google Scholar] [CrossRef]
- Bayuo, J.; Pelig-Ba, K.B.; Abukari, M.A. Adsorptive removal of chromium (VI) from aqueous solution unto groundnut shell. Appl. Water Sci. 2019, 9, 107. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Ronda, A.; Pérez, A.; Calero, M. Development and characterization of biosorbents to remove heavy metals from aqueous solutions by chemical treatment of olive stone. Ind. Eng. Chem. Res. 2013, 52, 10809–10819. [Google Scholar] [CrossRef]
- Ponce, J.; Da Silva Andrade, J.G.; Dos Santos, L.N.; Bulla, M.K.; Barros, B.C.B.; Favaro, S.L.; Hioka, N.; Caetano, W.; Batistela, V.R. Alkali pretreated sugarcane bagasse, rice husk and corn husk wastes as lignocellulosic biosorbents for dyes. Carbohydr. Polym. Technol. Appl. 2021, 2, 100061. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Idris, A.; Chandrasekaram, K.; Alias, Y. Efficiency of bronsted acidic ionic liquids in the dissolution and depolymerization of lignin from rice husk into high value-added products. Ind. Crops Prod. 2020, 157, 112885. [Google Scholar] [CrossRef]
- Hafid, H.S.; Omar, F.N.; Zhu, J.; Wakisaka, M. Enhanced crystallinity and thermal properties of cellulose from rice husk using acid hydrolysis treatment. Carbohydr. Polym. 2021, 260, 117789. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Visanko, M. Lignin-rich sulfated wood nanofibers as high-performing adsorbents for the removal of lead and copper from water. J. Hazard. Mater. 2020, 383, 121174. [Google Scholar] [CrossRef]
- Hezam Saeed, A.A.; Harun, N.Y.; Sufian, S.; Afolabi, H.K.; Hussein Al-Qadami, E.H.; Shahirah Roslan, F.A.; Rahim, S.A.; Ghaleb, A.S. Production and characterization of rice husk biochar and kenaf biochar for value-added biochar replacement for potential materials adsorption. Ecol. Eng. Environ. Technol. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885. [Google Scholar] [CrossRef]
- Sultana, S.; Rehan, K.; Rehan, I.; Ali, F.; Waris, S.; Zahoor, M.; Salman, S.M.; Khan, S.; Rehan, M.S. Physicochemical and instrumental characterization of rice husk and its potential use as a low cost adsorbent for mutagenic dye bromophenol blue. Z. Für Phys. Chem. 2021, 235, 1263–1277. [Google Scholar] [CrossRef]
- Niculescu, V.C.; Raboaca, M.S. Efficient rice-husk-derived silica nanocatalysts for organic dye removal from water. Catalysts 2021, 11, 815. [Google Scholar] [CrossRef]
- Tokay, B.; Akpınar, I. A comparative study of heavy metals removal using agricultural waste biosorbents. Bioresour. Technol. Rep. 2021, 15, 100719. [Google Scholar] [CrossRef]
- Figueiredo Severo, F.; Souza da Silva, L.; Costa Moscôso, J.S.; Sarfaraz, Q.; Rodrigues Júnior, L.F.; Ferreria Lopes, A.; Brondani Marzari, L.; Dal Molin, G. Chemical and physical characterization of rice husk biochar and ashes and their iron adsorption capacity. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Amen, R.; Yaseen, M.; Mukhtar, A.; Klemeš, J.J.; Saqib, S.; Ullah, S.; Al-Sehemi, A.G.; Rafiq, S.; Babar, M.; Fatt, C.L.; et al. Lead and cadmium removal from wastewater using eco-friendly biochar adsorbent derived from rice husk, wheat straw, and corncob. Clean. Eng. Technol. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Morcali, M.H.; Zeytuncu, B.; Yucel, O. Platinum uptake from chloride solutions using biosorbents. Mater. Res. 2013, 16, 528–538. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Gahlot, P.; Moustakas, K.; Kazmi, A.A.; Ojha, C.S.P.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [Google Scholar] [CrossRef]
- Ali Redha, A. Removal of heavy metals from aqueous media by biosorption. Arab. J. Basic. Appl. Sci. 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.; Li, H.; Xu, D.; Li, X.; Xiang, L.; Tu, S. Review on rice husk biochar as an adsorbent for soil and water remediation. Plants 2023, 12, 1524. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of heavy metals using rice husk: A review. Int. J. Environ. Res. Dev. 2014, 4, 165–170. [Google Scholar]
- Ebrahimi, M.; Hassanpour, M.; Rowlings, D.W.; Bai, Z.; Dunn, K.; O’Hara, I.M.; Zhang, Z. Effects of lignocellulosic biomass type on nutrient recovery and heavy metal removal from digested sludge by hydrothermal treatment. J. Environ. Manag. 2022, 318, 115524. [Google Scholar] [CrossRef] [PubMed]
- Wonorahardjo, S.; Fajaroh, F.; Joharmawan, R.; Nazriati, N.; Budiasih, E. Cadmium and lead ions adsorption on magnetite, silica, alumina, and cellulosic materials. Sci. Rep. 2023, 13, 4213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption kinetics and isotherm models of heavy metals by various adsorbents: An overview. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1–29. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.J.; Sillanpää, M.; Mtei, K.M. Removal of heavy metals from binary and multicomponent adsorption systems using various adsorbents–a systematic review. RSC Adv. 2023, 13, 13052–13093. [Google Scholar] [CrossRef] [PubMed]
- Neris, J.B.; Luzardo, F.H.M.; Da Silva, E.G.P.; Velasco, F.G. Evaluation of adsorption processes of metal ions in multi-element aqueous systems by lignocellulosic adsorbents applying different isotherms: A critical review. Chem. Eng. J. 2019, 357, 404–420. [Google Scholar] [CrossRef]
- Abdusattar, T.; Hadi, A.; Nuryoto, N.; Kurniawan, T. Kinetic study of ammonium desorption using natural zeolites from Cikalong. World Chem. Eng. J. 2021, 5, 12–17. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Desorption of heavy metals from metal loaded sorbents and e-wastes: A review. Biotechnol. Lett. 2019, 41, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.H.; Wahab, M.M.A. Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef]
- Albis, A.; Cajar, L.V.; Domínguez, M.I. Análisis cinético de la adsorción de Cr (VI) en soluciones acuosas a concentraciones de 10–20 mg/L con el uso de cáscara de yuca amarga (Manihot esculenta). Prospectiva 2015, 13, 64–71. [Google Scholar] [CrossRef]
- Benhouria, A.; Islam, M.A.; Zaghouane-Boudiaf, H.; Boutahala, M.; Hameed, B.H. Calcium alginate–bentonite–activated carbon composite beads as highly effective adsorbent for methylene blue. Chem. Eng. J. 2015, 270, 621–630. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb (II) and Cd (II) by magnetic activated carbon and its mechanism. Sci. Total Env. 2021, 757, 143910. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Aikawa, M.; Tatsumoto, H. Prediction of simultaneous adsorption of Cu(II) and Pb(II) onto activated carbon by conventional Langmuir type equations. J. Hazard. Mater. 2005, 120, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Adsorption of heavy metals from water using banana and orange peels. Water Sci. Technol. 2003, 47, 185–190. [Google Scholar] [CrossRef]
- Low, K.S.; Lee, C.K.; Wong, S.L. Effect of dye modification on the sorption of copper by coconut husk. Environ. Technol. 1995, 16, 877–883. [Google Scholar] [CrossRef]
- Kwon, J.S.; Yun, S.T.; Lee, J.H.; Kim, S.O.; Jo, H.Y. Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic (III) from aqueous solutions using scoria: Kinetics and equilibria of sorption. J. Hazard. Mater. 2010, 174, 307–313. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J. Hazard. Mater. 2006, 134, 257–267. [Google Scholar] [CrossRef]
- Nikolić, M.; Robert, R.J.; Girish, C.R. The adsorption of cadmium, nickel, zinc, copper and lead from wastewater using tea fiber Waste. ARPN J. Eng. Appl. Sci. 2019, 14, 7743–7755. [Google Scholar] [CrossRef]
- Khayyun, T.S.; Mseer, A.H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 170. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, A.K.; Kumar, P.S.; Hoang, T.K.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef] [PubMed]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthés, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]





| Site | Water (mg/L) | Sediment (mg/L) | Reference |
|---|---|---|---|
| San Juan-Taxco River, Mexico | Cu (0.0078), Fe (0.316), Pb (0.0182), Zn (0.2335) | Cu (393.0), Fe (23,223.0), Pb (265.0), Cd (50.4), Zn (4509.0) | [25] |
| River Kabul, Pakistan | Pb (0.810), Cd (0.038) | Pb (29), Cd (4) | [26] |
| Beyşehir Lake, Turkey | Cu (0.0012), Fe (0.0176), Pb (0.0011), Zn (0.0074) | Cu (70.18), Fe (34,100), Pb (16.50), Cd (0.26), Zn (73.92) | [27] |
| Mariout Lake, Egypt | Cu (0.019), Fe (1.145), Pb (0.0596),Cd (0.0099), Zn (0.13) | NA | [28] |
| Agodi Reservoir, Nigeria | Cu (35.93), Fe (254.25), Cd (0.36), Zn (134.52) | NA | [29] |
| Groundwater, Linares, Mexico | Cu (0.0017), Fe (0.01), Pb (0.00013), Cd (0.06), Zn (0.05) | NA | [30] |
| Lignin (%) | Holocellulose (%) | Ash (%) | Humidity (%) | |
|---|---|---|---|---|
| Hemicellulose (%) | Cellulose (%) | |||
| 28.40 | 16.05 | 36.40 | 15.46 | 3.69 |
| Adsorption Kinetics | ||||||
| Pseudo-First-Order | ||||||
| URH | RHTA | |||||
| qe | K1 | R2 | qe | K1 | R2 | |
| Cu | 1.245 | −5.0 × 10−5 | 0.886 | 0.906 | −5.76 × 10−6 | 0.738 |
| Pb | 2.005 | −2.22 × 10−6 | 0.870 | 2.416 | −7.09 × 10−6 | 0.763 |
| Fe | 0.864 | −9.50 × 10−6 | 0.948 | 1.037 | −7.58 × 10−6 | 0.925 |
| Cd | 2.196 | −3.25 × 10−6 | 0.917 | 2.219 | −1.03 × 10−5 | 0.939 |
| Zn | 1.771 | −3.33 × 10−6 | 0.946 | 1.383 | −1.23 × 10−5 | 0.805 |
| Pseudo-Second-Order | ||||||
| URH | RHTA | |||||
| qe | K2 | R2 | qe | K2 | R2 | |
| Cu | 2.323 | 0.022 | 0.994 | 0.700 | 0.026 | 0.919 |
| Pb | 1.479 | 0.020 | 0.978 | 2.799 | 0.003 | 0.886 |
| Fe | 1.461 | 0.018 | 0.974 | 1.488 | 0.006 | 0.951 |
| Cd | 1.740 | 0.004 | 0.891 | 3.308 | 0.001 | 0.913 |
| Zn | 2.487 | 0.007 | 0.930 | 2.569 | 0.011 | 0.983 |
| Adsorption Isotherms Models | ||||||
| Langmuir | ||||||
| URH | RHTA | |||||
| qm, (mg/g) | b, (L/mg) | R2 | qm, (mg/g) | b, (L/mg) | R2 | |
| Cu | 2.684 | 0.118 | 0.910 | 1.627 | 0.152 | 0.914 |
| Pb | 2.470 | 0.117 | 0.949 | 5.769 | 0.049 | 0.863 |
| Fe | 2.921 | 0.035 | 0.863 | 2.715 | 0.036 | 0.884 |
| Cd | 6.850 | 0.024 | 0.720 | 5.032 | 0.039 | 0.537 |
| Zn | 3.040 | 0.059 | 0.957 | 3.136 | 0.058 | 0.784 |
| Freundlich | ||||||
| URH | RHTA | |||||
| Kf, (mg/g) | N | R2 | Kf, (mg/g) | N | R2 | |
| Cu | 0.531 | 2.880 | 0.999 | 0.367 | 2.663 | 0.950 |
| Pb | 0.040 | 0.654 | 0.986 | 0.141 | 1.482 | 0.980 |
| Fe | 0.121 | 1.216 | 0.965 | 0.366 | 1.599 | 0.957 |
| Cd | 0.211 | 1.297 | 0.987 | 0.324 | 1.652 | 0.842 |
| Zn | 0.059 | 0.825 | 0.963 | 0.048 | 0.764 | 0.944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Trujillo, A.K.I.; Morales-Mendoza, A.G.; Rodríguez-Vázquez, R. Behavior of a Mixture of Metals for Competiting Adsorption Sites of Untreated and Alkali-Treated Rice Husk. Processes 2024, 12, 1299. https://doi.org/10.3390/pr12071299
Flores-Trujillo AKI, Morales-Mendoza AG, Rodríguez-Vázquez R. Behavior of a Mixture of Metals for Competiting Adsorption Sites of Untreated and Alkali-Treated Rice Husk. Processes. 2024; 12(7):1299. https://doi.org/10.3390/pr12071299
Chicago/Turabian StyleFlores-Trujillo, Ana Karen Ivanna, Asunción Guadalupe Morales-Mendoza, and Refugio Rodríguez-Vázquez. 2024. "Behavior of a Mixture of Metals for Competiting Adsorption Sites of Untreated and Alkali-Treated Rice Husk" Processes 12, no. 7: 1299. https://doi.org/10.3390/pr12071299
APA StyleFlores-Trujillo, A. K. I., Morales-Mendoza, A. G., & Rodríguez-Vázquez, R. (2024). Behavior of a Mixture of Metals for Competiting Adsorption Sites of Untreated and Alkali-Treated Rice Husk. Processes, 12(7), 1299. https://doi.org/10.3390/pr12071299







